Abstract
Brassica oleracea crops are exposed to seasonal changes in temperature because of their biennial life cycle. Extreme temperatures (cold and heat) affect the photosynthetic activity and the yield of cabbage (B. oleracea capitata group) and kale (B. oleracea acephala group). We studied the relationship among antioxidant defenses, photosynthesis, and yield under extreme temperatures in both crops. Under these conditions, the plants increase the antioxidant defenses, responding to an increment in reactive oxygen species (ROS). The accumulation of ROS in chloroplasts decreases the chlorophyll content and provokes photoinhibition that leads to a low fixation of CO2 and loss of dry weight. Low temperatures especially increase the antioxidant defenses and decrease the chlorophyll content compared to the heat conditions. However, dry weight losses are higher when plants are grown under heat than under cold conditions, probably because of the inactivation of Rubisco and/or the associated enzymes. Both crops were more resilient to cold than to heat temperatures, the capitata group being more resistant.
1. Introduction
Environmental changes cause metabolic responses in plants focused on maintaining homeostasis inside cells. When the equilibrium between energy generation and consumption to maintain plant defenses is broken, the growth and development of plants can be compromised leading even to death after a long-term exposure.1 Among the environmental stresses, high and low temperatures can have devastating effects on plants, leading to important economic losses in the field of agriculture.
Brassica oleracea crops such as broccoli, kale, cauliflower, cabbage, and so forth are cultivated worldwide, making the species the most outstanding from an economical point of view.2 During their vegetative cycle, biennial species, such as B. oleracea, are exposed to summer temperatures that can reach more than 40 °C and also to winter temperatures that can drop below 0 °C. The physiological and morphological responses of the two cultivars of B. oleracea, cabbage (capitata group) and kale (acephala group), subjected to extreme temperatures, were studied by Rodríguez et al.2 Extreme temperatures affected the photosynthetic activity and yield of both crops. One of the possible causes of these adverse effects is the oxidative stress.
When the environmental temperature overpasses the physiological threshold of a plant, the concentration of reactive oxygen species (ROS) can increase inside the cells.3 ROS are currently produced in plants, and they are needed for cellular signaling; however, under stress conditions and when the antioxidant defenses are overcome, their concentration can increase to harmful levels producing oxidative stress. They can seriously disrupt the normal metabolism of plants through the oxidation of membrane lipids, proteins, and nucleic acids,4 thus fatally affecting the plant metabolism and limiting the growth and yield.5 The coordinated action of the antioxidant defenses is necessary to protect the plants against the high concentration of ROS.1
Different enzymes and metabolites participate in the antioxidant defense system. Ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase, and peroxiredoxins are the antioxidant enzymes which participate in protecting the cells against an excess of ROS.4 Glutathione is a metabolite which protects the photosynthetic apparatus.6 The principal function of glutathione is to maintain the intracellular redox balance, detoxifying ROS, xenobiotics, and heavy metals.5,7 Phenolic compounds are able to scavenge ROS capacity,8 neutralizing singlet and triplet oxygen, or decomposing peroxides.9
Understanding the bases of tolerance to temperature stress would help in designing the strategies for sustainable crop yield under these environmental conditions. In this paper, we study the relationship of the antioxidant defenses of kale and cabbage subjected to temperature stress to several physiological parameters and leaf yield.
2. Results and Discussion
2.1. Thermal Stress Increases Antioxidant Defenses
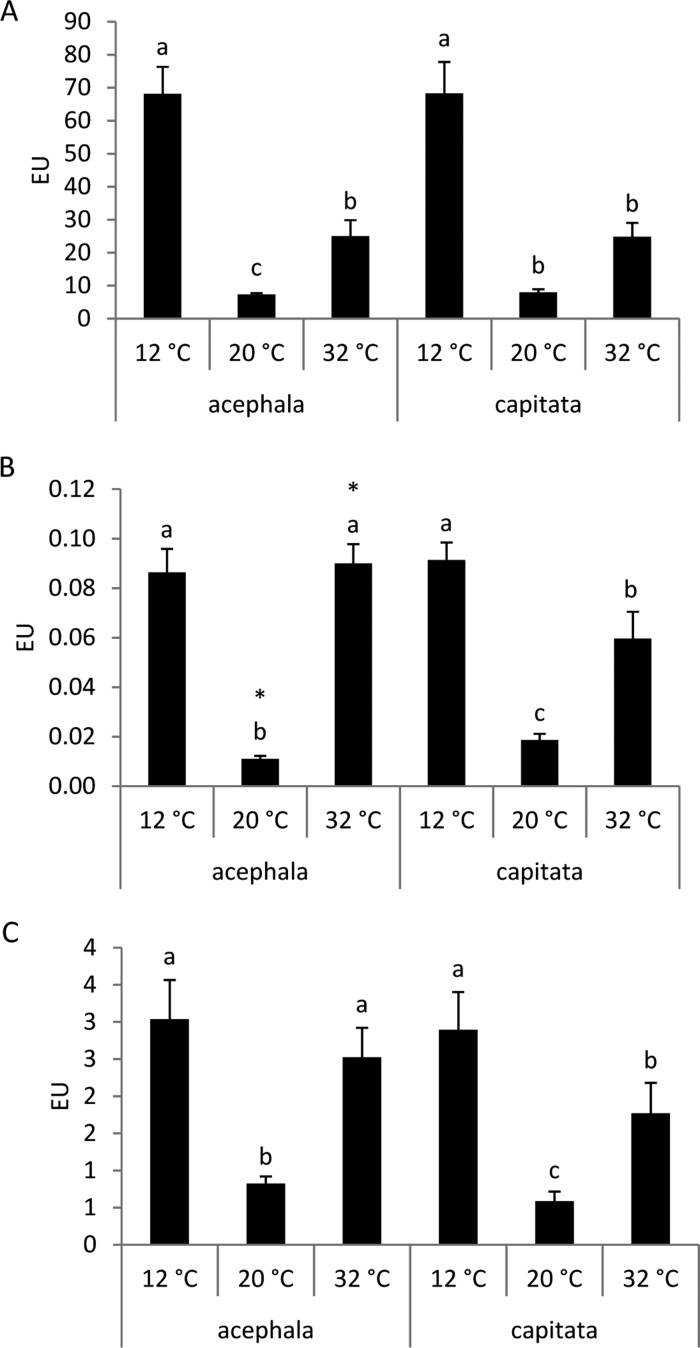
Significant changes occur in antioxidant defenses in plants subjected to environmental stresses, including enzymatic and nonenzymatic antioxidants. The association of the antioxidant enzymes (SOD, CAT, and GR) and several others enables to keep the oxidative damages on the plant intracellular components to a very low level.7 For this reason, we measured the activity of the three antioxidant enzymes (SOD, CAT, and GR) under two conditions of thermal stress (12 and 32 °C) and under the optimal temperature (20 °C). Cold and heat produced a significant increase of enzymatic activities compared to the values observed under the control temperature, confirming that the plants under stressful conditions undergo oxidative stress (Figure 1). The most active enzymes in response to environmental stressful conditions are CAT and SOD.10 SOD is usually considered as the first line of defense against oxidative stress.11 This enzyme catalyzes the partitioning of O2– into either an ordinary molecular O2 or into H2O2, which is also damaging, but less so, and is degraded by other enzymes such as APX or CAT. Although both enzymes degrade H2O2, the role of CAT is mainly focused in neutralizing the excess of ROS during stressful conditions, whereas APX is more involved in the fine modulation of ROS for signaling.4 CAT neutralizes H2O2 by transforming it into H2O and O2. SOD and CAT are important antioxidant defense systems in nearly all living cells exposed to oxygen. Another important antioxidant enzyme is GR. GR catalyzes the reduction of glutathione disulfide (GSSG) to reduced glutathione (GSH), which plays a significant role in maintaining the GSH level and redox state and protecting the cells against ROS.12
Figure 1.
Activities of (A) CAT, (B) SOD, and (C) GR enzymes in the seedlings of B. oleracea var. acephala and B. oleracea var. capitata grown under three temperatures: 12, 20, and 32 °C. The significant differences among temperatures (p ≤ 0.05) are indicated by different letters. The significant differences between the crops at a given temperature are indicated by an asterisk (p ≤ 0.05).
In general terms, the activity of these enzymes was significantly higher under cold than under control conditions (Figure 1). An increment in the CAT and SOD activities compared to the control temperatures was also observed in different species of the Brassica genus subjected to cold conditions. The activity of these enzymes has been associated to the resilience to low temperatures in canola (Brassica napus L.) cultivars,10,13 especially in the cold-adapted ones.14 However, although we recently reported that cabbage showed a higher stability under cold conditions than kale based on the physiological parameters,2 we did not observe any differences between the two crops under cold conditions for any of the three enzymatic activities measured.
The enzymatic activity of CAT, SOD, and GR was significantly higher under heat than under control conditions (Figure 1) for both crops. The activity of these enzymes has also been associated to the resilience to heat stress in Brassica species. The CAT activity was correlated to the tolerance against heat stress in three genotypes of Indian mustard which belong to Brassica juncea.5 Lin et al.4 found that the heat stress enhanced the enzymatic activity of CAT in broccoli and Chinese cabbage; however, the SOD activity was not affected. The CAT and GR activities under heat conditions were not significantly different between acephala and capitata. However, SOD was higher in the acephala variety compared to the capitata cultivar.
Cold and heat temperatures increased the antioxidant activity of CAT, SOD, and GR, compared to control. On the contrary, Zhang et al.11 studied the responses of canola under contrasting temperature regimes, 35/30 and 15/10 °C in comparison with the optimal temperature (25/20 °C) at an early seedling stage. The antioxidant enzymes responded to high and low temperatures differently. Under high temperatures, the SOD activity was reduced, whereas the CAT activity increased. Under low temperatures, however, the SOD activities increased, whereas those of CAT remained unchanged.11
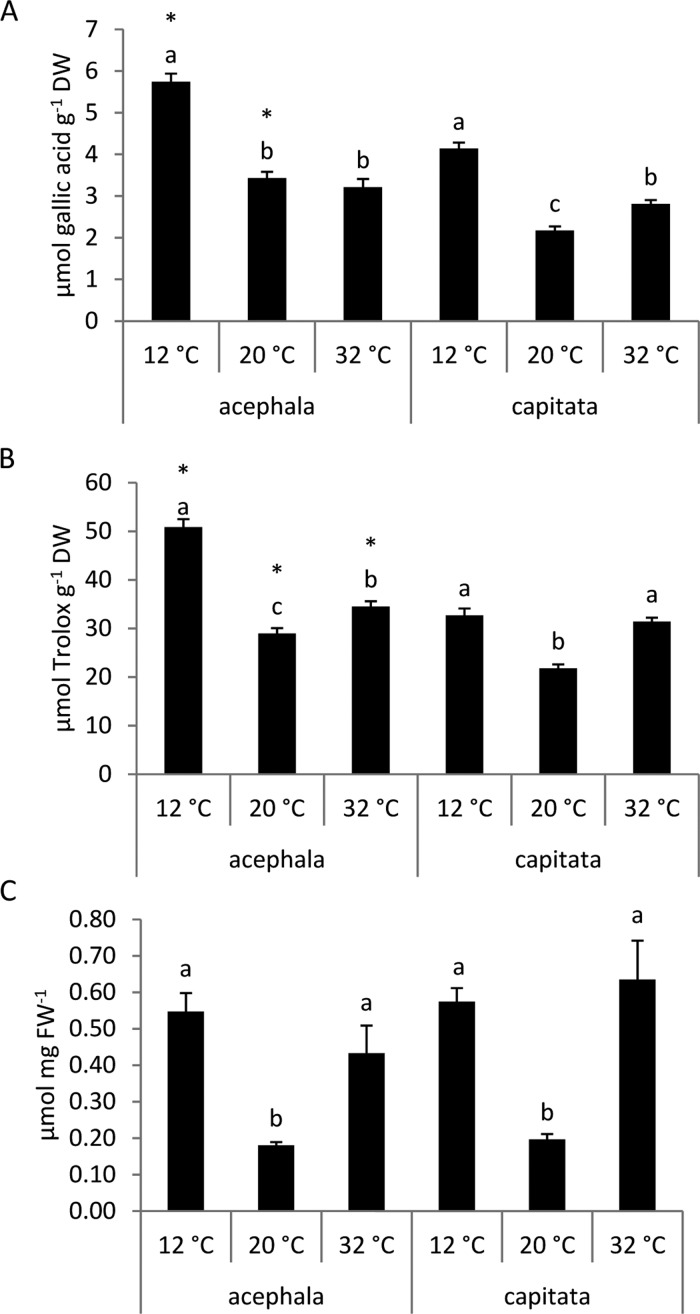
The antioxidant defenses neutralize ROS, keeping their concentration to nonharmful levels under environmental stress.1 The antioxidant defense system includes both enzymatic and nonenzymatic components. Phenolic compounds and glutathione are recognized as the important antioxidant metabolites.1 The phenolic compounds are considered to be mainly responsible for antioxidant capacity in the Brassica crops. They have higher antioxidant activity in vitro than the vitamins or carotenoids8 and can neutralize ROS because of their structure, by donating electrons.15
The plants showed the highest phenolic content at 12 °C (Figure 2A). At 32 °C, the capitata variety showed a higher phenolic content compared to the control conditions, whereas the acephala variety did not show significant differences. The content of phenolic compounds was lower in the capitata variety compared to that in acephala at 12 and 20 °C (Figure 2A). The content of soluble phenolic acids and anthocyanins increased in winter oilseed rape leaves with cold treatment.16 Plants can accumulate phenolic compounds as a response to temperature stress by regulating the anabolism and catabolism of these metabolites.1 The antioxidant potential, measured with the ABTS assay, was significantly higher at 12 °C and at 32 °C compared to control for both crops. The acephala variety had more antioxidant activity than the capitata one under the three temperatures (Figure 2B).
Figure 2.
(A) Total phenolic content, (B) antioxidant potential (ABTS), and (C) glutathione content in the seedlings of B. oleracea var. acephala and B. oleracea var. capitata grown under three temperatures: 12, 20, and 32 °C. The significant differences among temperatures (p ≤ 0.05) are indicated by different letters. The significant differences between the crops at a given temperature are indicated by an asterisk (p ≤ 0.05).
Glutathione is an antioxidant metabolite which is involved in the protection of the photosynthetic apparatus.6 A high glutathione content would protect the photosynthetic apparatus against ROS generated by temperature stress. The glutathione content was not significantly different between 12 and 32 °C, but both temperatures significantly differed from control (Figure 2C). Phenolic compounds and antioxidant activity, which is associated to this kind of compounds in Brassica crops,17 and glutathione increased under temperature stress, indicating an increase in ROS. A significant increase in the level of glutathione was observed in the seedlings of broccoli subjected to cold conditions.10 The content of glutathione increased significantly after heat stress in the seedlings of B. juncea.5
2.2. Antioxidant Defenses Are Related to Chlorophyll Content and Leaf Weight
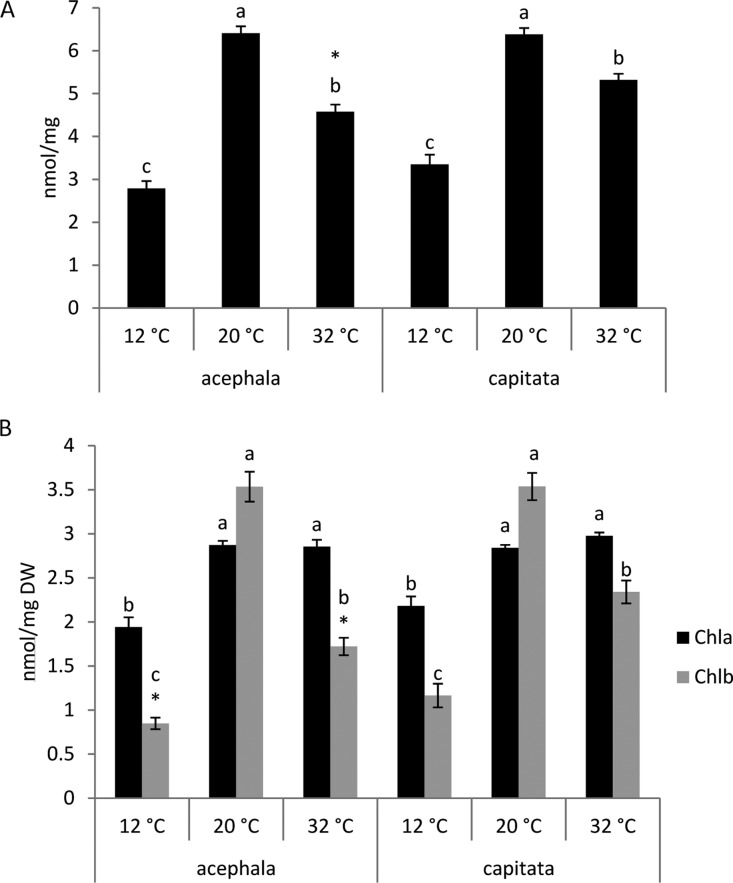
The total amount of chlorophyll (Chl) was higher under control conditions, followed by heat and then cold for both varieties (Figure 3A). The concentration of Chl a and Chl b suffers a reduction at 12 °C compared to control in both crops (Figure 3B). Under heat conditions, the concentration of Chl b decreased with respect to control, but the concentration of Chl a was similar to that of the control (Figure 3B).
Figure 3.
(A) Total Chl concentration and (B) Chl a and Chl b concentrations in the seedlings of B. oleracea var. acephala and B. oleracea var. capitata grown under three temperatures: 12, 20, and 32 °C. The significant differences among temperatures (p ≤ 0.05) are indicated by different letters. The significant differences between the crops at a given temperature are indicated by an asterisk (p ≤ 0.05).
The concentration of Chl b was higher than Chl a under 20 °C, but this relationship was inverted when the stress temperatures were considered (Figure 3B). Similarly, the Chl a/b ratio increased with higher temperature in canola.18 Chl a is the pigment that acts in the light requiring reactions of photosynthesis. Chl b is an accessory pigment and acts indirectly in photosynthesis by transferring the light it absorbs to Chl a. An increase in the Chl a/b ratio under stress conditions may result from the faster degradation of Chl b.19 The concentration of Chl b was higher in capitata than in acephala at 12 and 32 °C.
To study the relationship between the antioxidant parameters and the content of Chl, the correlation analysis was performed. Generally speaking, the concentration of the total Chl and Chl b were negatively and significantly correlated with the level of antioxidant activities and the amount of antioxidant compounds (Table 1), whereas the Chl a content was significantly and negatively correlated only with the CAT activity and the content of phenolic compounds. As mentioned before, Chl b has a faster degradation than Chl a under stress conditions. The degradation may be due to the accumulation of ROS in the chloroplast. Photosynthesis is a well-established source of ROS in plants.6 The reaction centers of PSI and PSII in the chloroplast thylakoids are the major sites of ROS generation.20 One of the key factors that influences the balance between the damage of the photosynthetic machinery and the restoration of its activity is the relationship between the strength of the oxidative stress and the activity of the antioxidant system.21
Table 1. Pearson’s Correlation Coefficients between Antioxidant-Related Traits and Chlorophyll Contents and between These Traits and Leaf Weight, Taken from Ref (2)a.
| traits | CAT | SOD | GR | PHENOLICS | ABTS | glutathione | Chl | Chl a | Chl b | FWEIGHT | DWEIGHT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAT | 1.000 | 0.799 | 0.893 | 0.831 | 0.744 | 0.720 | –0.967 | –0.924 | –0.922 | –0.882 | 0.089 |
| p-value | 0.057 | 0.016 | 0.041 | 0.090 | 0.106 | 0.002 | 0.009 | 0.009 | 0.020 | 0.867 | |
| SOD | 1.000 | 0.969 | 0.565 | 0.661 | 0.822 | –0.897 | –0.577 | –0.957 | –0.895 | –0.486 | |
| p-value | 0.002 | 0.243 | 0.153 | 0.045 | 0.016 | 0.231 | 0.003 | 0.016 | 0.328 | ||
| GR | 1.000 | 0.747 | 0.789 | 0.798 | –0.966 | –0.726 | –0.994 | –0.878 | –0.272 | ||
| p-value | 0.088 | 0.062 | 0.057 | 0.002 | 0.102 | <0.0001 | 0.022 | 0.603 | |||
| PHENOLICS | 1.000 | 0.926 | 0.442 | –0.839 | –0.896 | –0.766 | –0.905 | –0.436 | |||
| p-value | 0.008 | 0.380 | 0.037 | 0.016 | 0.076 | 0.013 | 0.388 | ||||
| ABTS | 1.000 | 0.565 | –0.829 | –0.747 | –0.808 | –0.561 | 0.166 | ||||
| p-value | 0.242 | 0.041 | 0.088 | 0.052 | 0.247 | 0.754 | |||||
| glutathione | 1.000 | –0.744 | –0.429 | –0.812 | –0.528 | 0.404 | |||||
| p-value | 0.090 | 0.396 | 0.050 | 0.282 | 0.427 | ||||||
| Chl | 1.000 | 0.873 | 0.984 | 0.882 | 0.052 | ||||||
| p-value | 0.023 | 0.000 | 0.020 | 0.922 | |||||||
| Chl a | 1.000 | 0.770 | 0.664 | –0.416 | |||||||
| p-value | 0.073 | 0.150 | 0.412 | ||||||||
| Chl b | 1.000 | 0.905 | 0.221 | ||||||||
| p-value | 0.013 | 0.674 | |||||||||
| FWEIGHT | 1.000 | 0.323 | |||||||||
| p-value | 0.533 | ||||||||||
| DWEIGHT | 1.000 |
CAT: catalase, SOD: superoxide dismutase, GR: glutathione reductase, Chl: chlorophyll content, Chl a: chlorophyll a content, Chl b: chlorophyll b content, FWEIGHT: fresh weight, DWEIGHT: dry weight.
In this case, there is an increase in antioxidant defenses when plants are grown under stressful conditions, coinciding with the decrement in Chl b content, probably because of the accumulation of ROS in the chloroplasts. The accumulation of ROS may also decrease PSII activity and stimulate gene expression, particularly with regard to the acclimation and defense genes.2,18 Rodriguez et al.2 studied the physiological and morphological changes of plants subjected to temperature stress in the same experiment. The maximum quantum yield of primary photochemistry (φP0), equivalent to the Fv/Fm parameter,2 was used to monitor the performance of PSII activity. This parameter was significantly reduced under heat and cold compared to control conditions2 in both crops, suggesting that the stress temperatures caused photoinhibition.
When photoinhibition takes place, the accumulation of the reduced electron acceptors may increase the generation of ROS, which can induce oxidative injuries.19 These oxidative injuries could enhance Chl b degradation or the inhibition of its biosynthesis, damage PSII components, inactivate chloroplast enzymes, especially those participating in CO2 assimilation,19 and could further explain the reductions in Fv/Fm and Chl content in the temperature-stressed plants of this work.
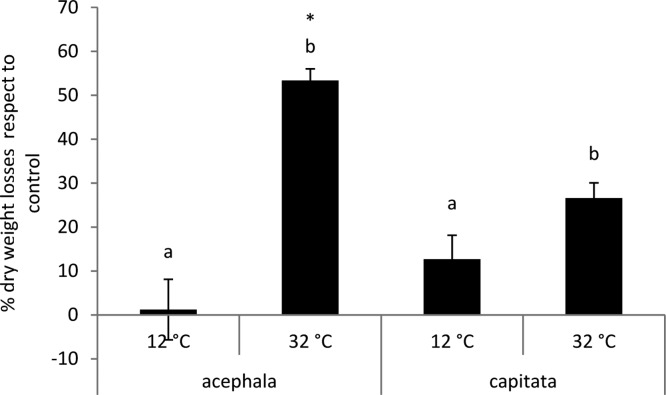
The correlations computed with the data obtained from the present work (antioxidant defenses and content of Chl) and the leaf yield (fresh and dry weight) obtained by Rodríguez et al.2 were computed. The fresh weight was positively correlated to the Chl b content and negatively to the different antioxidant systems (Table 1). However, the dry weight was not correlated to the antioxidant defenses or the Chl content. Enhanced photosynthesis does not always lead to increased yield in crops because of several possible reasons, including the inhibition of the fixation of CO2.22,23 We have found that at 12 and 32 °C there was a loss in the dry weight of both varieties compared to control (Figure 4). The losses are significantly higher at 32 °C compared to those at 12 °C. However, the contents of Chl and Chl b were higher at 32 °C compared to those at 12 °C; therefore, we would expect a higher photosynthetic rate and also a higher fixation of CO2 at 32 °C, translated into dry weight. The inhibition of photosynthesis caused by high temperatures is often attributed to the inactivation of membrane-associated proteins, particularly the complex of photosystem II.24 However, the fixation of CO2 can be inhibited at other levels. Photosynthesis can be limited by Rubisco capacity for carboxylation when the plants are exposed to heat stress. The evidence that the inactivation of Rubisco is an early event in the inhibition of photosynthesis at elevated temperatures was presented by several authors (reviewed by ref (25)). Of the various reactions associated with CO2 fixation, the one that appears to be most sensitive to inhibition by heat is the activation of Rubisco by Rubisco activase.24
Figure 4.
Loss of dry weight expressed as percentage with respect to control in the seedlings of B. oleracea var. acephala and B. oleracea var. capitata grown under 12 and 32 °C. The significant differences among temperatures (p ≤ 0.05) are indicated by different letters. The significant differences between the crops at a given temperature are indicated by an asterisk (p ≤ 0.05).
Acephala loses more dry weight at 12 and 32 °C than capitata; however, only the differences under heat conditions are significant (Figure 4). Under cold conditions, the antioxidant activity of the acephala and capitata varieties is similar. However, under heat conditions, the acephala variety shows a significantly higher SOD activity than capitata (Figure 1B). This, together with a higher GR activity (Figure 1C) and a lower glutathione content (although these two parameters showed nonsignificant differences), may indicate that more O2– is being produced in the acephala variety compared to the capitata one under heat conditions, as SOD, GR, and glutathione are involved in the detoxification of this ROS through the Mahler cycle. This may be translated into a lower content of total Chl and Chl b. These results are in agreement with those of Rodríguez et al.,2 who found, based on the morphological and physiological parameters, that the capitata variety was less sensitive to the changes in air temperature than the acephala one.
3. Conclusions
The relationship among antioxidant defenses, Chl content, and leaf yield was studied in two B. oleracea crops. Under stress temperatures, the plants increase the antioxidant defenses, confirming that they suffer oxidative stress. The accumulation of ROS in chloroplasts would lead to a decrement in Chl and Chl b and to a reduction in the Fv/Fm ratio which suggests the presence of photoinhibition that produced a loss of dry weight compared to control conditions. The losses are higher when plants are grown under heat than under cold conditions, probably because of the inactivation of Rubisco and/or the associated enzymes with heat. Therefore, the B. oleracea crops seem to be more resilient to cold than to heat stress, which is probably related to the origin of domestication of these crops in the Atlantic coast of Europe, where normally temperatures in summer are mild. Coinciding with previous studies, the acephala variety is less resilient to temperature stress compared to capitata.
4. Experimental Section
4.1. Plant Material and Growth Conditions
We measured the antioxidant defenses and Chl content with the same assay as that used in ref (2). Two local populations of B. oleracea were obtained from the Brassica seed bank of the Misión Biológica de Galicia (CSIC-Spain), one cabbage (MBG-BRS0072, B. oleracea capitata group) and one kale (MBG-BRS00464, B. oleracea acephala group).
The plants were grown in sterilized peat (Gramoflor GmbH & Co. KG, Vechta, Germany) using multipot trays. The fluorescent light was provided (228 μmol m–2 s–1) in cycles of 14 h light/10 h dark. The plants were watered as needed. The thresholds of high and low temperatures were established by Rodríguez et al;2 then, the heat conditions were set at 32 ± 1 °C and cold conditions at 12 ± 1 °C; above or below these temperatures, the growth and survival of plants were seriously compromised. The control condition was set up at 20 ± 1 °C, and the plants were grown at constant day/night temperatures under the three conditions.
4.2. Enzyme Extraction and Assays
Using a precooled mortar and pestle, the third leaf of 10 individual plants per population was homogenized in 10 mL of chilled 100 mM phosphate buffer, pH 7.0, containing 20 μL of 0.2 M phenylmethylsulfonyl fluoride at the four-leaf stage. The extracts were centrifuged at 4000 rpm for 3 min, and then the supernatants were used to study the enzyme activity. All procedures were performed at 4 °C.
The CAT (EC: 1.11.1.6) activity was measured according to the method used in ref (26). The samples were diluted 1:10, and then 200 μL of the dilutions were added to 100 μL of 100 mM phosphate buffer, pH 7.0, 30 mM H2O2. The absorbance of the reaction was monitored during 1 min at 240 nm. An extinction coefficient of 39.4 M–1 cm–1 was employed to calculate the enzymatic activity. The data are expressed as enzymatic units (EU), defined as the decrease of micromoles of H2O2 per milligram of protein per minute.
The GR (EC: 1.6.4.2) activity was measured following ref (27). The reaction mixture contained 100 mM phosphate buffer, pH 7.0, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.75 μM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), 1 μM nicotinamide adenine dinucleotide phosphate (NADPH), 10 μM GSSG, and the enzyme extract in a final volume of 300 μL. The absorbance of the reaction was monitored for 2 min at 412 nm. An extinction coefficient of 6.2 mM–1 cm–1 was employed to calculate the enzymatic activity. EU is defined as micromoles of TNB per milligram of protein per minute.
The SOD (EC: 1.15.1.1) activity was measured following ref (28). The reaction mixture contained 100 mM phosphate buffer, pH 7.0, 65 mM nitrotetrazolium blue chloride (NBT), 2 mM riboflavin, 20 μL enzyme extract, and 1 μL tetramethylethylenediamine in a final volume of 300 μL. Different concentrations of the enzyme extracts were assayed. The reaction mixture was exposed to light of 350 μmol m–2 s–1 for 15 min. The SOD activity was assayed by measuring its ability to inhibit the photochemical reduction of NBT. EU is defined as the amount of enzyme extract required to cause 50% inhibition of the rate of NBT reduction at 560 nm. The protein concentration was measured using the Bradford reagent and bovine serum albumin as standards.29
4.3. Antioxidant Potential Assays
The general approach to measure the antioxidant potential was done following ref (15). Leaves of 10 individual plants per population were harvested at the four-leaf stage in liquid N2 and then kept at −80 °C. The samples were freeze-dried for 72 h (BETA 2-8 LD plus, Christ) and then powdered with an IKA-A10 mill (IKA-Werke GmbH & Co. KG). The leaf extracts were obtained by adding 1 mL of 80% aqueous methanol to 10 mg of the leaf sample and leaving it in dark for 24 h. The extracts were centrifuged afterward (3700 rpm, 5 min). The antioxidant activity and the content of phenolic compounds were measured in the methanolic extracts using ABTS+ assay and Folin reagent, respectively.
ABTS+ assay was performed following ref (30). ABTS+ was generated by the oxidation of 7 mM ABTS with 2.45 mM K2S2O8 at room temperature for 16 h. The stock solution of ABTS+ was diluted in water to reach an absorbance between 0.8 and 1.0 at 734 nm. Then, 20 μL of each methanolic extract was mixed to 250 mL of the ABTS+ solution, and the absorbance was recorded after 30 min of incubation under dark conditions. The results were standardized to Trolox equivalents per gram of dry weight. The total phenolic content was determined following ref (31). A 50 μL of each extract was mixed with 50 μL of 0.5 M Folin reagent. The reaction was neutralized after 5 min with 200 μL of 20% Na2CO3. After 2 h of incubation in dark conditions, the absorbance of the reaction was measured at 760 nm. The results are given as micromoles of gallic acid equivalent per gram of dry weight.
4.4. Glutathione Determination
Using a precooled mortar and pestle, the third leaf of 10 individual plants per population at the four-leaf stage was homogenized in a mixture of 1 mL of chilled 100 mM phosphate buffer, 2 mM of EDTA, pH 7.4, and 1 mL of 2 M HClO4. The samples were centrifuged at 4000 rpm for 3 min. The pH values of the resultant supernatants were neutralized with 2 M KOH and 0.3 M 3-(N-morpholino)propanesulfonic acid to reach the pH value of 6–7. The samples were conserved at −80 °C for at least 1 h. In a 96-well microplate, 200 μL of 100 mM phosphate buffer, 2 mM of EDTA, pH 7.4, 10 μL of NADPH (4 mg/mL in 0.5% NaHCO3), 15 μL of DTNB (1 mg/mL in 0.5% NaHCO3), and 50 μL of the extract solution were added. The reaction started after the addition of 25 μL of GR (2 EU/mL in 100 mM phosphate buffer). The total glutathione (GSH + GSSG) concentration was measured using 100 μM of GSSG standard.
4.5. Chl Quantification
One hundred milligrams of the frozen leaf tissue was extracted in 1 mL of acetone overnight at 4 °C and centrifuged at 3500 rpm for 10 min. The Chl was quantified from the supernatant spectrophotometrically. The quantification of Chl a and b was performed as described in ref (32).
The enzymatic activity, phenolic, glutathione, and Chl content, as well as the antioxidant potential assay, were monitored spectrophotometrically (Spectra MR; Dynex Technologies, Chantilly, VA). Three replicates were made for each measurement. The reagents were obtained from Sigma-Aldrich Chemie GmbH (Taufkirchen, Germany).
4.6. Dry Weight Loss
The percentage of dry weight loss at 12 and 32 °C was computed with respect to the control temperature taken into account from the data from ref (2).
4.7. Statistical Analysis
Analyses of variance were performed using the GLM procedure of SAS.33 The temperatures (12, 20, 32 °C) and populations (cabbage and kale) were considered as fixed effects, and individual plants were considered random factors. Fisher’s protected least significant difference test was employed to compare among different temperatures, whereas Student’s t test was used to perform mean comparisons between crops. The correlation analysis between the biochemical traits obtained in this work and the physiological and morphological traits obtained by Rodríguez et al.2 were performed with the CORR procedure of SAS.33
Acknowledgments
We thank Rosaura Abilleira and Juan Carlos Fernández for their support in the laboratory work. This work was financially supported by two grants of the Spanish National Plan for Research and Development (AGL2012-35539 and AGL2015-66256-C2-1-R).
Glossary
Abbreviations
- PMSF
phenylmethylsulfonyl fluoride
- EDTA
ethylenediaminetetraacetic acid
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- NADPH
nicotinamide adenine dinucleotide phosphate
- GSSG
glutathione disulfide
- NBT
nitrotetrazolium blue chloride
- TEMED
tetramethylethylenediamine
- ABTS
(2,29-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid))
- MOPS
3-(N-morpholino)propanesulfonic acid
- GSH
reduced glutathione
- Trolox
(6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid)
The authors declare no competing financial interest.
References
- Awasthi R.; Bhandari K.; Nayyar H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. 10.3389/fenvs.2015.00011. [DOI] [Google Scholar]
- Rodríguez V. M.; Soengas P.; Alonso-Villaverde V.; Sotelo T.; Cartea M. E.; Velasco P. Effect of temperature stress on the early vegetative development of Brassica oleracea L. BMC Plant Biol. 2015, 15, 145. 10.1186/s12870-015-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Lin K.-H.; Huang H.-C.; Lin C.-Y. Cloning, expression and physiological analysis of broccoli catalase gene and Chinese cabbage ascorbate peroxidase gene under heat stress. Plant Cell Rep. 2010, 29, 575–593. 10.1007/s00299-010-0846-4. [DOI] [PubMed] [Google Scholar]
- Wilson R. A.; Sangha M. K.; Banga S. S.; Atwal A. K.; Gupta S. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J. Environ. Biol. 2014, 35, 383–387. [PubMed] [Google Scholar]
- Foyer C. H.; Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. 10.1104/pp.110.166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raseetha S.; Leong S. Y.; Burritt D. J.; Oey I. Understanding the degradation of ascorbic acid and glutathione in relation to the levels of oxidative stress biomarkers in broccoli (Brassica oleracea L. italica cv. Bellstar) during storage and mechanical processing. Food Chem. 2013, 138, 1360–1369. 10.1016/j.foodchem.2012.09.126. [DOI] [PubMed] [Google Scholar]
- Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT--Food Sci. Technol. 2007, 40, 1–11. 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Cartea M. E.; Francisco M.; Soengas P.; Velasco P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowska R.; Hanus-Fajerska E. J.; Kołton A.; Kamińska I.; Grabowska A.; Kunicki E. The effect of seedling chilling on glutathione content, catalase and peroxidase activity in Brassica oleracea L. var. italica. Acta Soc. Bot. Pol. 2013, 82, 243–248. 10.5586/asbp.2013.020. [DOI] [Google Scholar]
- Zhang J.; Jiang F.; Yang P.; Li J.; Yan G.; Hu L. Responses of canola (Brassica napus L.) cultivars under contrasting temperature regimes during early seedling growth stage as revealed by multiple physiological criteria. Acta Physiol. Plant. 2015, 37, 7. 10.1007/s11738-014-1748-9. [DOI] [Google Scholar]
- Ding S. H.; Chen S.; Lu C. M. Research progress on functions of glutathione reductase in chloroplasts of plants. Zhiwu Shengli Xuebao 2016, 52, 1703–1709. [Google Scholar]
- Moieni-Korbekandi Z.; Karimzadeh G.; Sharifi M. Evaluation of total soluble protein and antioxidant activities in two spring cultivars of canola (Brassica napus L.) in response to low temperature. Int. J. Agric. Crop Sci. 2013, 5, 401–409. [Google Scholar]
- Fahimirad S.; Karimzadeh G.; Ghanati F. Cold-induced changes of antioxidant enzymes activity and lipid peroxidation in two canola (Brassica napus L.) cultivars. J. Plant Physiol. Breed. 2013, 3, 1–11. [Google Scholar]
- Sotelo T.; Cartea M. E.; Velasco P.; Soengas P. Identification of antioxidant capacity -related QTLs in Brassica oleracea. PLoS One 2014, 9, e107290 10.1371/journal.pone.0107290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecka D.; Boudet A.-M.; Kacperska A. Phenylpropanoid and anthocyanin changes in low-temperature treated winter oilseed rape leaves. Plant Physiol. Biochem. 1999, 37, 491–496. 10.1016/s0981-9428(99)80054-0. [DOI] [Google Scholar]
- Soengas P.; Cartea M. E.; Francisco M.; Sotelo T.; Velasco P. New insights into antioxidant activity of Brassica crops. Food Chem. 2012, 134, 725–733. 10.1016/j.foodchem.2012.02.169. [DOI] [PubMed] [Google Scholar]
- Slauenwhite K. L. I.; Qaderi M. M. Single and interactive effects of temperature and light quality on four canola cultivars. J. Agron. Crop Sci. 2013, 199, 286–298. 10.1111/jac.12014. [DOI] [Google Scholar]
- Cui L.; Li J.; Fan Y.; Xu S.; Zhang Z. High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Bot. Stud. 2006, 47, 61–69. [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavski V. D.; Lyubimov V. Y.; Shabnova N. I.; Balakhnina T. I.; Kosobryukhov A. A. Heat-induced impairments and recovery of photosynthetic machinery in wheat seedlings. Role of light and prooxidant-antioxidant balance. Physiol. Mol. Biol. Plants 2009, 15, 115–122. 10.1007/s12298-009-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. 10.1104/pp.110.165076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. P.; Zhu X. G.; Naidu S. L.; Ort D. R. Can improvement in photosynthesis increase crop yields?. Plant, Cell Environ. 2006, 29, 315–330. 10.1111/j.1365-3040.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- Salvucci M. E.; Osteryoung K. W.; Crafts-Brandner S. J.; Vierling E. Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol. 2001, 127, 1053–1064. 10.1104/pp.010357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirevska-Kepova K.; Feller U. Heat sensitivity of Rubisco, Rubisco activase and Rubisco binding protein in higher plants. Acta Physiol. Plant. 2004, 26, 103–114. 10.1007/s11738-004-0050-7. [DOI] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Smith I. K.; Vierheller T. L.; Thorne C. A. Assay of glutathione-reductase in crude tissue-homogenates using 5,5’-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Tewari R. K.; Kumar P.; Neetu; Sharma P. N. Signs of oxidative stress in the chlorotic leaves of iron starved plants. Plant Sci. 2005, 169, 1037–1045. 10.1016/j.plantsci.2005.06.006. [DOI] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Samarth R. M.; Panwar M.; Kumar M.; Soni A.; Kumar A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008, 106, 868–873. 10.1016/j.foodchem.2007.05.005. [DOI] [Google Scholar]
- Dewanto V.; Wu X.; Adom K. K.; Liu R. H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Sims D. A.; Gamon J. A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. 10.1016/s0034-4257(02)00010-x. [DOI] [Google Scholar]
- SAS . SAS OnlineDoc, version 9.2; SAS Institute: Cary, NC, 2008.