Abstract
Diabetes mellitus is the most common endocrine disease worldwide; hyperglycemia is a hallmark of this disease. To alleviate the pain caused by diabetes, developing and utilizing effective diabetic drugs to maintain or recover the function of the residual β-cells is an attractive therapeutic approach. γ-aminobutyric acid (GABA) has been shown to have such effects, but it is easy to have reduced GABA activity under physiological conditions. In the present study, GABA–chitosan nanoparticles (GABA–CS NPs) were prepared, and glucose homeostasis, pancreatic β-cell protection, and anti-inflammatory effects of GABA–CS NPs were investigated in vivo. The results showed that blood glucose levels and IL-1β levels in the GABA–CS NP-administered group were both significantly lower, whereas the PDX1 expression was significantly higher than that of the impaired group (p < 0.01). This indicates that GABA–CS NPs can efficiently maintain glucose homeostasis, protect β-cells, and inhibit inflammation. These nanoparticles have the potential to be applied for future diabetes theranostics.
Introduction
Diabetes mellitus (DM) is the most common endocrine disease worldwide. The International Diabetes Foundation predicted that, by the end of 2015, diabetes will have caused five million deaths and will have cost between USD 673 billion and USD 1197 billion in healthcare spending. If this rise is not halted, by 2040, there will be 642 million people living with the disease.1 China has a high prevalence of people with diabetes.2 Both type 1 DM (T1DM) and type 2 DM (T2DM) are characterized by hyperglycemia; this symptom primarily results from β-cell loss, though the importance of insulin resistance and β-cell dysfunction remains debated in T2DM.3,4 In T1DM, more than 70% of the β-cells are destroyed,5 whereas in T2DM, ∼40–60% of the β-cells are destroyed when T2DM is diagnosed.6 The residual β-cells are overloaded by increased insulin secretion, which accelerates their apoptosis. Therefore, developing and utilizing effective diabetic drugs to maintain or recover the function of the residual β-cells is an attractive approach for diabetes therapeutics.
Recently, γ-aminobutyric acid (GABA) has garnered the interest of researchers as it can affect most neuronal activities and is the predominant neurotransmitter in the central nervous system (CNS).7 It is also present in the peripheral organs; the pancreas has the highest concentration of GABA among the peripheral organs, comparable to that in the CNS.8 Studies have demonstrated that GABA has the effect of regulating glucagon release on α-cells9 and insulin release on β-cells.10 In addition, Soltani et al. reported that GABA exerted protective and regenerative effects on murine pancreatic β-cells.11 Purwana et al. revealed the same effect on human β-cells.10 Most recently, Ben-Othman et al. reported that long-term GABA administration could induce alpha cell-mediated β-like cell neogenesis.12 Further, researchers have also shown that GABA can inhibit inflammation13 and immune responses,14,15 both of which occur in T1DM and T2DM.16
Thus, we tried to control glucose homeostasis using GABA. In consideration of the instability of the drug under physiological conditions, chitosan nanoparticles (CS NPs) were used as a drug carrier to protect and control the release of GABA. CS NPs have the advantage of controlled drug release, which improves drug efficacy, solubility, and stability.17,18 As these particles are scaled by the nanometer, they are able to pass through biological barriers in vivo.19 The final degradation products of the CS NPs in vivo are water and dioxide, which have no adverse effects. In addition, Wu et al. reported that the derivatives of chitosan (CS)-affected murine pancreatic islet β-cell proliferation in vitro.20 In this study, we examined the effect of GABA-loaded CS NPs on glucose homeostasis and protection of pancreatic β-cells by administering GABA–CS NP suspension to mice, followed by multiple low-dose streptozotocin (STZ) injections intraperitoneally (i.p.). The anti-inflammatory effects of GABA–CS NPs in vivo were also investigated.
Results and Discussion
Morphological Characterization of CS NPs
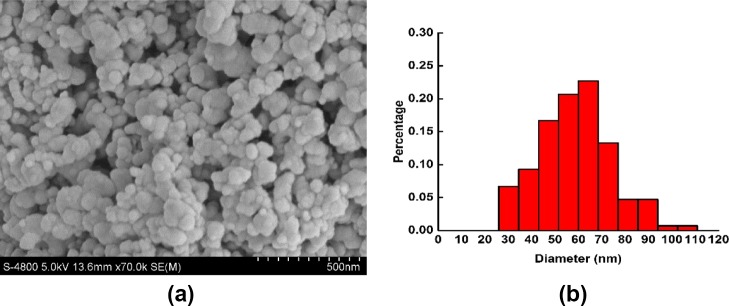
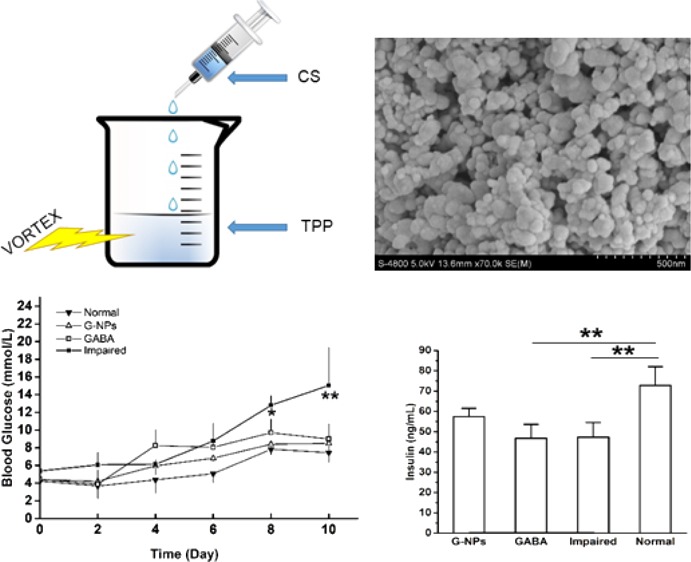
The prepared CS NPs were characterized by scanning electron microscopy (SEM), which can be seen in Figure 1. As shown in Figure 1a, the nanoparticles were spherical with a smooth surface. The average diameter was 50.4 ± 18.7 nm (seen in Figure 1b), which indicates that these nanoparticles can be administered through intravenous injection in future applications.
Figure 1.
SEM (a) and size distribution (b) of CS NPs.
Effect of GABA–CS NPs on Glucose Homeostasis
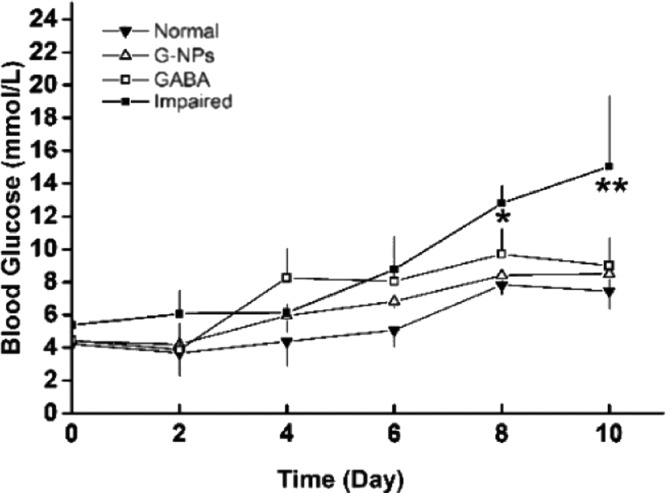
To determine the effect of GABA–CS NPs on blood glucose maintenance, blood glucose levels were measured via a tail vein. Fasting blood glucose concentrations were measured every 2 days in ICR mice. GABA–CS NPs (10 mg per mouse),21 GABA solution (20 μmol per mouse),11 or saline were intragastrically administered to mice on day 3 and day 5. All groups, except for the normal group of mice, were challenged with 40 mg/kg STZ injected intraperitoneally over 4 consecutive days, from day 4. Figure 2 shows that the fasted blood glucose levels of the GABA–CS NP group and the GABA group were both significantly lower than that of the impaired group, after the STZ challenge test. At day 8, the impaired group had reached the diagnostic standard of diabetes, with a blood glucose concentration of 12.80 ± 1.08 mmol/L, whereas the concentrations were 8.40 ± 0.64 and 9.70 ± 1.56 mmol/L for the GABA–CS NP and GABA groups, respectively, both below 10 mmol/L. The levels were significantly lower on day 8 and very significantly lower on day 10, compared with those of the impaired group.
Figure 2.
Effect of GABA–CS NPs on fasting blood glucose in ICR mice (*p < 0.05, **p < 0.01).
Usually, body blood glucose levels should remain in a narrow range. The most important part is hormone regulation, in which insulin secreted by the β-cells and glucagon produced by the α-cells play the role of reducing and elevating the blood glucose level, respectively. Here, the STZ treatment of 40 mg/kg per day for 4 consecutive days was designed to simulate pancreatic β-cell injury due to various factors in daily life. Our results indicate that GABA–CS NP-administered mice could maintain better blood glucose control than the mice administered GABA solution alone.
Effect of GABA–CS NPs on Insulin Secretion
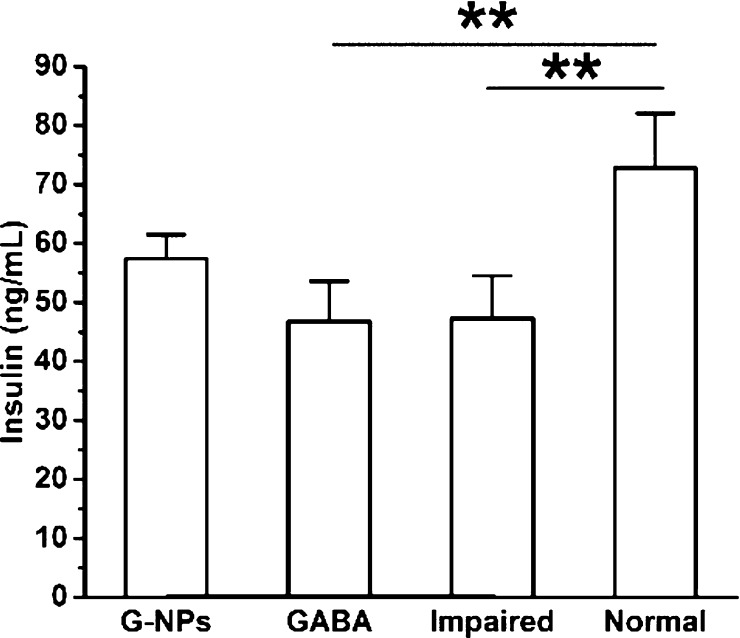
To further examine the effect of GABA–CS NPs on the pancreatic β-cell function, the insulin secretion ability was investigated. As can be seen in Figure 3, on day 14, after multiple low-dose STZ injections intraperitoneally, the serum insulin concentration of the GABA–CS NP group was 57.47 ± 4.08 ng/mL, which was higher than those of the GABA group (46.80 ± 6.83 ng/mL) and the impaired group (47.33 ± 7.21 ng/mL). This indicates that the GABA–CS NP group could maintain serum insulin levels to some extent. While for the GABA and impaired groups, the serum insulin concentrations were similar, indicating that using the drug alone could not stimulate pancreatic β-cells for such a long period because of the instability of GABA under physiological conditions.
Figure 3.
Effect of GABA–CS NPs on serum insulin concentration in ICR mice (*p < 0.05, **p < 0.01).
Effect of GABA–CS NPs on β-Cell Protection
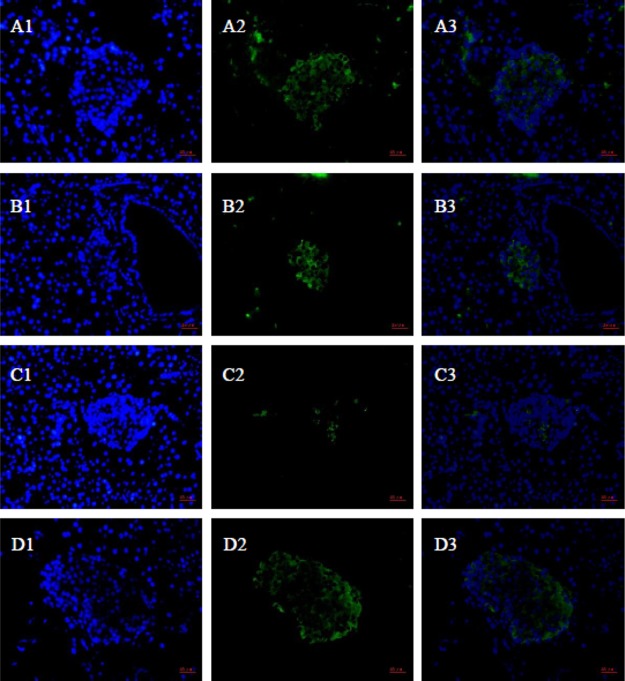
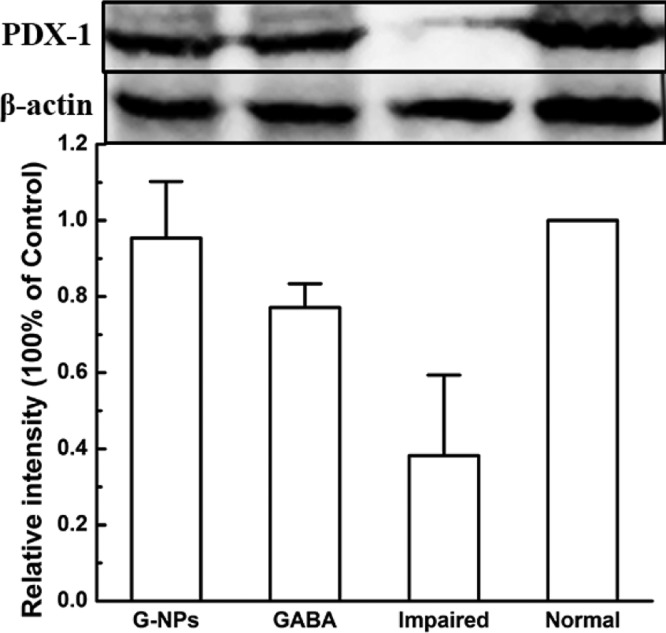
To determine whether GABA–CS NPs can protect β-cells, PDX1 was tested qualitatively and quantitatively by immunofluorescence staining (Figure 4) and immunoblotting (Figure 5), respectively. Though PDX1 is responsible for the pancreatic development, rather than directly for the β-cell development, the PDX1 results can show the difference of the β cell activity by comparing the experimental groups with the normal and impaired groups. As can be seen in Figure 4, there was more PDX1 expression in the pancreas sections of the GABA–CS NP-administered mice than those of the GABA group and impaired group. This indicates that the existence of the nanoparticles exhibited protection efficacy to β-cells, to maintain their activity. Figure 5 further shows that the relative intensity of the GABA–CS NP group was 0.95 ± 0.15, compared with 0.38 ± 0.21 of the impaired group, using western blotting analysis. This indicates that the expression of PDX1 in the GABA–CS NP-administered mice was significantly more than that of the impaired group. This result is in accordance with the result obtained in Figure 4.
Figure 4.
Immunofluorescence of islet β-cells (green) and cell nucleus (blue) ((A) GABA–CS NPs group, (B) GABA group, (C) impaired group, and (D) normal group).
Figure 5.
Effect of GABA–CS NPs on PDX1 expression in ICR mice (western blotting analysis was performed on the pancreas of mice at day 14. *p < 0.05, **p < 0.01).
Combining all of the results above, the preadministration of GABA–CS NPs on day 3 and day 5 significantly diminished the β-cell depletion induced by STZ. As known, both T1DM and T2DM are characterized by β-cell deficiency, whereas the maintenance of β-cell mass is a dynamic process, undergoing both increases and decreases through β-cell renewal and apoptosis.11 Although β-cells were considered as a quiescent cell type which lost most of their proliferate capacity during adulthood,22,23 β-cells are impaired by subtotal pancreatectomy,24 inflammation,25 and some hormones or small molecules10,26,27 that can trigger the proliferation process. On the other hand, the prevention of β-cell apoptosis is also of benefit for β-cell maintenance. For example, the small molecule GABA11 and the carrier material CS,20 both have a proliferative effect on β-cells. In addition, the damage associated with STZ may trigger the proliferation process of β-cells. Although STZ cannot pass through the cell membranes, it can be transported through GLUT2, which is specifically expressed in β-cells. STZ accumulated inside the β-cells fragments the DNA by alkylation and thus triggers the DNA repair mechanism, resulting in cellular energy depletion and ultimately β-cell necrosis.28 GABA protects β-cells from apoptosis by increasing the DNA synthesis,11 which may compensate for the damage from STZ.
Effect of GABA–CS NPs on the Inflammatory Factor
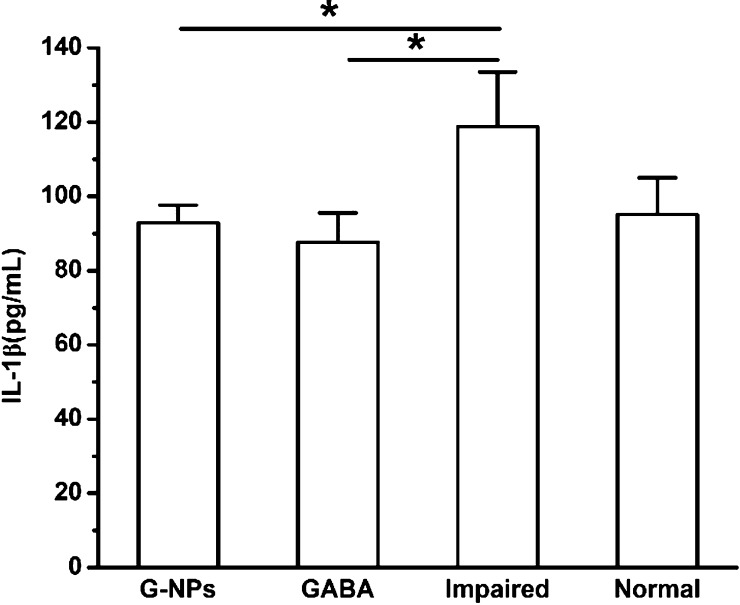
Finally, the serum level of the inflammatory factor IL-1β after 12 days was measured to assess whether the GABA–CS NPs had an effect on inflammation (Figure 6). The IL-1β levels were 92.91 ± 4.70 and 87.62 ± 7.93 pg/mL for the GABA–CS NP group and GABA group, respectively, which was similar to that of the normal group (95.13 ± 9.89 pg/mL) and significantly below that of the impaired group (118.80 ± 14.67 pg/mL). Thus, it can be seen that GABA–CS NPs and GABA inhibit inflammation, which would be beneficial for the recovery of β-cells in diabetes treatment. Soltani et al. also showed the inflammation suppressing effects of GABA on β-cells, where the inflammatory factors, including IL-1β, TNF-α, and INF-γ levels, were decreased.11 As both T1DM and T2DM are associated with inflammation, the anti-inflammatory effect is essential for β-cell protection.
Figure 6.
Effect of GABA–CS NPs on IL-1β levels in ICR mice (*p < 0.05, **p < 0.01).
Conclusion
Maintaining the function of β-cells is an important issue in diabetes therapy. In our opinion, both protection and inhibition of inflammation should be addressed. This study indicates that the administration of GABA–CS NPs can significantly maintain glucose homeostasis by protecting the pancreatic β-cells. At the same time, IL-1β was also reduced by the administration of GABA–CS NPs, which is in accordance with an inhibiting effect on inflammation. Therefore, we anticipate that the GABA–CS NPs could be applied for future diabetes theranostics to improve the activity of pancreatic β-cells and alleviate the inflammation and immune responses caused by diabetes. This carrier system is simple but efficient and is easy to implement in future with intravenous injection.
Materials and Methods
Chemicals
Biochemicals, including GABA, sodium triphosphate (TPP), and STZ were purchased from Sigma-Aldrich Co. LLC, USA. CS was purchased from Aladdin Industrial Co. Ltd., Shanghai. Rabbit antibody PDX1 and β-actin were provided by Cell Signaling Technology Inc., USA, and Bioworld Technology Inc., USA, respectively. Rat anti-insulin antibody and goat antirat IgG conjugated with NL493 were obtained from R&D Systems Inc., USA. 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Solarbio Co., Beijing, China. An insulin ELISA kit was purchased from Millipore KGa, Deutschland. The IL-1β ELISA kit was provided by Excell Biology, Shanghai, China. The poly(vinylidene difluoride) (PVDF) membrane was purchased from Genscript Co., Nanjing, China. Other immunoblotting-related chemicals, including a bicinchoninic acid (BCA) kit and goat antirabbit IgG conjugated with horseradish peroxidase (HRP) were purchased from Beyotime Biotechnology, Shanghai, China. All other reagents were of analytical grade and were purchased locally.
Animals
Experiments were carried out on male ICR mice of 25 ± 2 g body weight, purchased from SLAC Laboratory Co. Ltd., Shanghai. They were housed in separate cages under 12 h light and 12 h dark periods and were maintained on standard food pellets and water ad libitum. All animal care and procedures were performed in accordance with the published guidelines of the China Council on Animal Care (Regulations for the Administration of Affairs Concerning Experimental Animals, approved by the State Council on 31 October, 1988 and promulgated by Decree no. 2 of the State Science and Technology Commission on 14 November, 1988). All efforts were made to minimize the number of animals used and their suffering. ICR mice were divided into four experimental groups: administration of GABA–CS NPs (GABA–CS NPs), administration of GABA (GABA), the impaired group with STZ induction, and the normal group. STZ induction was executed by intraperitoneal injection with 40 mg/kg STZ solution over 4 consecutive days. The normal group was not treated with STZ. Each group consisted of eight animals.
Preparation of GABA–CS NPs
GABA-loaded CS NPs were prepared according to the method described by Shilpa et al. with modifications.21 Briefly, CS was dissolved in 1% acetic acid to obtain a concentration of 1 mg/mL. CS nanoparticles were precipitated from 60 mL CS solution under rapid stirring, with the addition of 30 mL of 1 mg/mL TPP solution. To load GABA to CS, 20 μg of GABA was dissolved in CS solution, and the nanoparticles were precipitated by the addition of TPP solution droplets, making the final concentration as 20 μg/mL CS solution. The precipitated nanoparticles were centrifuged for 10 min at 10 000 rpm. The pellet was washed thoroughly with ultrapure water three times and then resuspended in ultrapure water again, followed by ultrasonic dispersion. CS–GABA powder was obtained by lyophilization 48 h after freezing at −20 °C overnight. The prepared CS NPs were characterized by SEM (S-4800, Hitachi Ltd., Japan) and further analyzed by the software Nanomeasure to determine the size distribution.
Insulin and Inflammatory Factor Detection
Blood samples (∼1 mL) were obtained by the removal of an eyeball in fasted mice on day 14, and the serum was collected and stored at −20 °C. Serum insulin and IL-1β concentrations were measured using ELISA kits.
Immunofluorescence Staining
The isolated pancreas was fixed overnight in 4% formaldehyde solution. Following dehydration in ethanol and clearing with xylene, the pancreas was embedded in paraffin. Pancreatic sections (5 μm) were incubated with rat anti-insulin (1:1000) and DAPI. Insulin was detected with fluorescent NL493-conjugated IgG.
Immunoblotting
The immunoblotting was performed according to the previous method with modifications.29 The isolated pancreas was homogenized in a lysis buffer and incubated on ice for 10 min. The homogenates were centrifuged at 12 000 rpm for 5 min at 4 °C. The supernatants were then collected. The protein concentration was determined by a BCA assay. The proteins were separated using SDS-PAGE and were then transferred to a PVDF membrane. Following blocking in a 5% BSA/TBST solution at room temperature for 1 h, the membranes were incubated with the appropriate primary antibodies at 4 °C overnight (anti-PDX1, 1:1000; anti-insulin, 1:1000; and anti-β-actin, 3000). After washing three times with TBST, the membranes were incubated with an HRP-labeled secondary antibody (1:1000). The blots were washed three times again with TBST, and the immune-reactive bands were detected using an enhanced chemilluminescence method. The results were normalized to the protein expression level of β-actin in each sample.
Statistics
All data were analyzed using SPSS software 22.0 (SPSS Inc., Chicago, USA) and expressed as the mean ± SD. Statistical evaluations were performed with analysis of variance (ANOVA). Student–Newman–Keuls tests were used to compare different groups after ANOVA. A p value less than 0.05 was considered to be statistically significant.
Acknowledgments
The financial support from the National Marine Economic Innovation and Development project (16PYY007SF17), the Science Research Foundation of National Health and Family Planning Commission of PRC and the United Fujian Provincial Health and Education Project for Tacking the Key Research (WKJ2016-2-22), and the Program for New Century Excellent Talents in Fujian Province University (2014FJ-NCET-ZR01) are gratefully acknowledged.
Author Contributions
Y.L. and W.W. contributed equally. Y.L. and W.W. conceived and designed the experiments. W.W. performed the experiments and compiled the draft. Y.L. supervised the project and completed the manuscript. S.W. helped to supervise the project and revise the manuscript. R.L. contributed to the data analysis and technical notes. Hanwen Li, Huihui Li, T.L., and M.W. provided assistance with experiments. All authors have given approval for the final version of the manuscript.
The authors declare no competing financial interest.
References
- Diabetes Atlas. IDF Diabetes Atlas, 7th ed.; Brussels: International Diabetes Federation; 7th Edition Committee, 2015. [Google Scholar]
- Jia W. Diabetes research in China: making progress. Lancet Diabetes Endocrinol. 2017, 5, 9–10. 10.1016/s2213-8587(16)30094-8. [DOI] [PubMed] [Google Scholar]
- Pellegrini S.; Cantarelli E.; Sordi V.; Nano R.; Piemonti L. The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol. 2016, 53, 683–691. 10.1007/s00592-016-0847-z. [DOI] [PubMed] [Google Scholar]
- Kahn S. E.; Cooper M. E.; Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. 10.1016/s0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D.; Chintinne M.; Denys B.; Martens G.; Keymeulen B.; Gorus F. Restoring a functional β-cell mass in diabetes. Diabetes, Obes. Metab. 2008, 10, 54–62. 10.1111/j.1463-1326.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- Butler A. E.; Janson J.; Bonner-Weir S.; Ritzel R.; Rizza R. A.; Butler P. C. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Gladkevich A.; Korf J.; Hakobyan V. P.; Melkonyan K. V. The peripheral GABAergic system as a target in endocrine disorders. Auton. Neurosci. 2006, 124, 1–8. 10.1016/j.autneu.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Gerber J. C.; Hare T. A. Gamma-aminobutyric acid in peripheral tissue, with emphasis on the endocrine pancreas: presence in two species and reduction by streptozotocin. Diabetes 1979, 28, 1073–1076. 10.2337/diab.28.12.1073. [DOI] [PubMed] [Google Scholar]
- Xu E.; Kumar M.; Zhang Y.; Ju W.; Obata T.; Zhang N.; Liu S.; Wendt A.; Deng S.; Ebina Y.; Wheeler M. B.; Braun M.; Wang Q. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006, 3, 47–58. 10.1016/j.cmet.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Purwana I.; Zheng J.; Li X.; Deurloo M.; Son D. O.; Zhang Z.; Liang C.; Shen E.; Tadkase A.; Feng Z.-P.; Li Y.; Hasilo C.; Paraskevas S.; Bortell R.; Greiner D. L.; Atkinson M.; Prud’homme G. J.; Wang Q. GABA promotes human β-cell proliferation and modulates glucose homeostasis. Diabetes 2014, 63, 4197–4205. 10.2337/db14-0153. [DOI] [PubMed] [Google Scholar]
- Soltani N.; Qiu H.; Aleksic M.; Glinka Y.; Zhao F.; Liu R.; Li Y.; Zhang N.; Chakrabarti R.; Ng T.; Jin T.; Zhang H.; Lu W.-Y.; Feng Z.-P.; Prud’homme G. J.; Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 11692–11697. 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Othman N.; Vieira A.; Courtney M.; Record F.; Gjernes E.; Avolio F.; Hadzic B.; Druelle N.; Napolitano T.; Navarro-Sanz S.; Silvano S.; Al-Hasani K.; Pfeifer A.; Lacas-Gervais S.; Leuckx G.; Marroquí L.; Thévenet J.; Madsen O. D.; Eizirik D. L.; Heimberg H.; Kerr-Conte J.; Pattou F.; Mansouri A.; Collombat P. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 2017, 168, 73–85.e11. 10.1016/j.cell.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Tian J.; Lu Y.; Zhang H.; Chau C. H.; Dang H. N.; Kaufman D. L. γ-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J. Immunol. 2004, 173, 5298–5304. 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- Mendu S. K.; Bhandage A.; Jin Z.; Birnir B. Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes. PLoS One 2012, 7, e42959 10.1371/journal.pone.0042959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Chau C.; Hales T. G.; Kaufman D. L. GABA(A) receptors mediate inhibition of T cell responses. J. Neuroimmunol. 1999, 96, 21–28. 10.1016/s0165-5728(98)00264-1. [DOI] [PubMed] [Google Scholar]
- Wan Y.; Wang Q.; Prud’homme G. J. GABAergic system in the endocrine pancreas: a new target for diabetes treatment. Diabetes, Metab. Syndr. Obes.: Targets Ther. 2015, 8, 79–87. 10.2147/DMSO.S50642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. J.; Zeng Z. W.; Xiao R. Z.; Xie T.; Zhou G. L.; Zhan X. R.; Wang S. L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Mukherjee S.; Yi J.; Banerjee P.; Chen Q.; Zhou S. Biocompatible chitosan-carbon dot hybrid nanogels for NIR-imaging-guided synergistic photothermal-chemo therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. 10.1021/acsami.7b06062. [DOI] [PubMed] [Google Scholar]
- Dudhani A. R.; Kosaraju S. L. Bioadhesive chitosan nanoparticles: preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. 10.1016/j.carbpol.2010.02.026. [DOI] [Google Scholar]
- Wu L.; Dong W.; Yang Y.; Han B.; Liu W.; Zhang W. Effects of low molecular weight derivatives of chitosan on the proliferation of pancreatic islet cells. Chin. J. Mar. Drugs 2012, 31, 39–44. 10.13400/j.cnki.cjmd.2012.04.012. [DOI] [Google Scholar]
- Shilpa J.; Roshni B. T.; Chinthu R.; Paulose C. S. Role of GABA and serotonin coupled chitosan nanoparticles in enhanced hepatocyte proliferation. J. Mater. Sci.: Mater. Med. 2012, 23, 2913–2921. 10.1007/s10856-012-4754-8. [DOI] [PubMed] [Google Scholar]
- Vetere A.; Choudhary A.; Burns S. M.; Wagner B. K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discovery 2014, 13, 278–289. 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- Ding L.; Gysemans C.; Mathieu C. β-cell differentiation and regeneration in type 1 diabetes. Diabetes, Obes. Metab. 2013, 15, 98–104. 10.1111/dom.12164. [DOI] [PubMed] [Google Scholar]
- Peshavaria M.; Larmie B. L.; Lausier J.; Satish B.; Habibovic A.; Roskens V.; LaRock K.; Everill B.; Leahy J. L.; Jetton T. L. Regulation of pancreatic β-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 2006, 55, 3289–3298. 10.2337/db06-0017. [DOI] [PubMed] [Google Scholar]
- In’t Veld P.; Lievens D.; De Grijse J.; Ling Z.; Van der Auwera B.; Pipeleers-Marichal M.; Gorus F.; Pipeleers D. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 2007, 56, 2400–2404. 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- Friedrichsen B. N.; Neubauer N.; Lee Y. C.; Gram V. K.; Blume N.; Petersen J. S.; Nielsen J. H.; Møldrup A. Stimulation of pancreatic β-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signaling pathways. J. Endocrinol. 2006, 188, 481–492. 10.1677/joe.1.06160. [DOI] [PubMed] [Google Scholar]
- Kwon D. Y.; Kim Y. S.; Ahn I. S.; Kim D. S.; Kang S.; Hong S. M.; Park S. Exendin-4 potentiates insulinotropic action partly via increasing β-cell proliferation and neogenesis and decreasing apoptosis in association with the attenuation of endoplasmic reticulum stress in islets of diabetic rats. J. Pharmacol. Sci. 2009, 111, 361–371. 10.1254/jphs.09178fp. [DOI] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Liu X.-L.; Luo L.; Mu R.-H.; Liu B.-B.; Geng D.; Liu Q.; Yi L.-T. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Sci. Rep. 2015, 5, 16024. 10.1038/srep16024. [DOI] [PMC free article] [PubMed] [Google Scholar]