Abstract
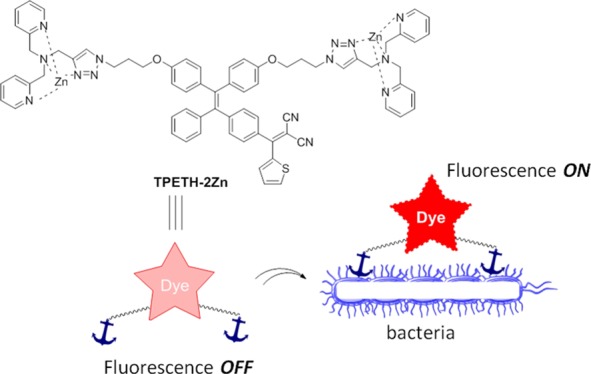
The emergence of drug-resistant bacterial pathogens highlights an urgent need for new therapeutic options. Photodynamic therapy (PDT) has emerged as a potential alternative to antibiotics to kill bacteria, which has been used in clinical settings. PDT employs photosensitizers (PSs), light, and oxygen to kill bacteria by generating highly reactive oxygen species (ROS). PDT can target both external and internal structures of bacteria, which does not really require the PSs to enter bacteria. Therefore, bacteria can hardly develop resistance to PDT. However, most of the PSs reported so far are hydrophobic and tend to form aggregates when they interact with bacteria. The aggregation could cause fluorescence quenching and reduce ROS generation, which generally compromises the effects of both imaging and therapy. In this contribution, we report on a Zn(II)-tetradentate-coordinated red-emissive probe with aggregation-induced emission characterization. The probe could selectively image bacteria over mammalian cells. Moreover, the probe shows potent phototoxicity to both Gram-negative bacteria (Escherichia coli) and Gram-positive bacteria (Bacillus subtilis).
Introduction
The emergence of drug-resistant bacterial pathogens highlights an urgent need for new therapeutic options.1 The dominant resistance mechanisms include alteration of antibiotic targets, active efflux of antibiotics from the cell, and antibiotic modification catalyzed by enzymes.2 Many pathogens simultaneously harbor several mechanisms to achieve antibiotic resistance. One comprehensive approach to overcome resistance is to develop antibiotic adjuvants that contain both antibiotics and other bioactive molecules.3 These adjuvants change the physiology of antibiotic-insensitive cells, incur a synergetic effect, and finally improve the treatment efficiency and inhibit resistance. The alternative choice is through photodynamic therapy (PDT). PDT has emerged as a good and potential alternative to antibiotics to kill bacteria that has been used in clinical settings.4 PDT employs photosensitizers (PSs), light, and oxygen to kill bacteria by generating highly reactive oxygen species (ROS).5 PDT can target both external and internal structures of bacteria, which does not really require the PSs to enter bacteria.6 Therefore, bacteria can hardly develop resistance to PDT.5a,7 In addition, many PSs could aid in image-guided antibacterial studies, as they also have intrinsic fluorescence. However, most of the PSs reported so far are hydrophobic and tend to form aggregates when they interact with bacteria. The aggregation could cause fluorescence quenching and reduce ROS generation,8 which generally compromises the effects of both imaging and therapy.
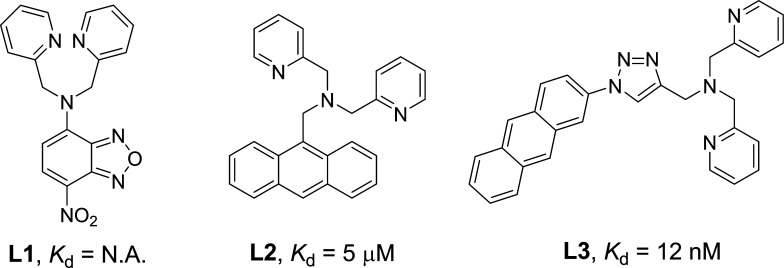
Fluorogens with aggregation-induced emission (AIE) characteristics have recently attracted great attention in biological sensing, imaging, and therapeutic applications.9 AIE fluorogens (AIEgens) generally show very weak fluorescence as free molecules but can become highly emissive in the aggregated state.10 Moreover, strong ROS generation has been observed for some AIEgens, which are effective for cancer-cell ablation under light irradiation.11 So far, several positively charged AIEgens have been reported to image and kill bacteria on the basis of electrostatic interactions.12 To endow the probes with a positive charge, ammonium salts13a and the zinc(II)-dipicolylamine (ZnDPA) coordination complex13b,13c are often employed, among which ZnDPA is the most popular as it has a stronger binding affinity with bacteria due to its higher positive charge.13b,13c In our previous work, we developed a zinc(II)-coordinated PS (AIE-ZnDPA) with AIE and excited-state intramolecular proton transfer characteristics to selectively image and kill bacteria over mammalian cells.14 Although successful, the probe has several limitations. First, the used AIEgen absorbs mainly in the UV region, which makes it difficult for the probe to produce ROS when white light is used as the light source. Moreover, fluorogens with short-wavelength absorption and emission possess severe interference from sample autofluorescence.15 Second, AIE-ZnDPA used DPA to coordinate zinc(II), which served as an anchor to the negatively charged bacterial membrane. However, the binding between DPA and zinc(II) is weak, with a Kd of around 5 μM (Figure 1).16 As Kd is the ratio between ([free ligand] × [ion]) and [the ligand–ion binding complex], a higher Kd indicates that more free ligands exist in solution (eq 1 in Supporting Information). As the ligand–ion complex is the recognition element in the probe, a larger amount of probes are needed to bind with bacteria. This can also lead to a higher background signal, as more unbound free probes exist in the detection system. To design a better zinc(II)-binding ligand, we reviewed the literature and found that (1) electron-withdrawing groups can decrease the electron density on the ligand, which significantly reduces its binding affinity (L1 vs L2; Figure 1)17 and that (2) additional binding positions could significantly increase the binding affinity (L2 vs L3; Figure 1).18 We are specifically interested in L3 because it could be readily synthesized by Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) and shows a very low Kd value, in the nanomolar range.18a Taking all of the above considerations into account, in this work, we employ CuAAC to decorate a red-emissive AIE PS (TPETH) to develop a new probe for bacterial detection and killing. The probe is almost nonemissive in aqueous solution, but it becomes highly emissive after binding to the membrane of bacteria. In addition, the probe can efficiently generate ROS to kill bacteria.
Figure 1.
Selected zinc(II)-binding ligands and their apparent dissociation constants.
Results and Discussion
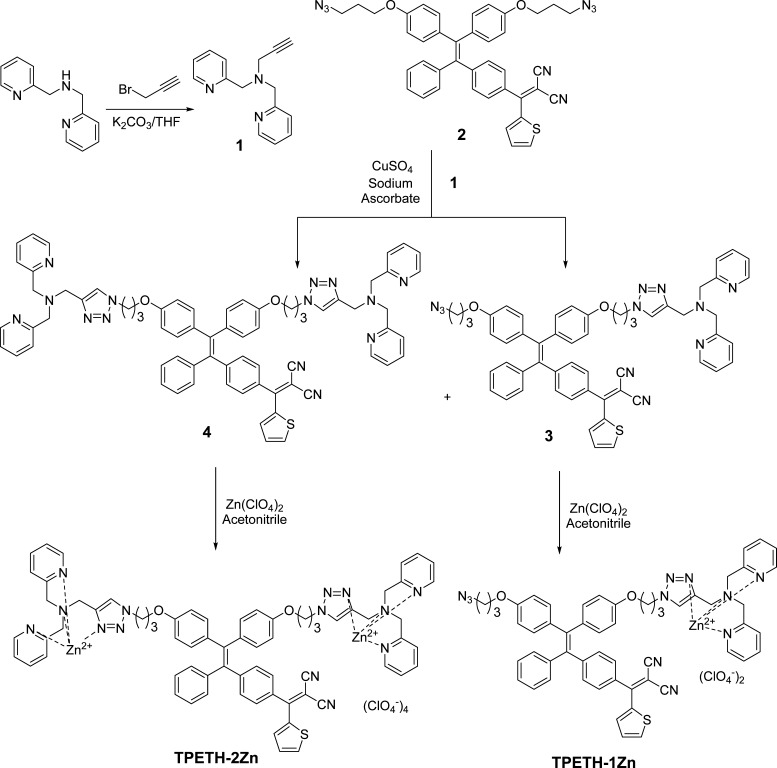
The synthetic route to the probes is shown in Scheme 1. Briefly, alkylation of bis-2-picolylamine with propargyl bromide generated 1. A CuAAC-mediated click reaction between 1 and 2 yielded intermediates 3, with one binding motif, and 4, with two binding motifs. Intermediates 3 and 4 were treated with zinc perchlorate to yield the probes TPETH-1Zn and TPETH-2Zn. All compounds were characterized by NMR and high-resolution mass spectrometry (HRMS) to confirm their high purities and right structures (Figures S1–S15).
Scheme 1. Synthetic Route to the Probes.
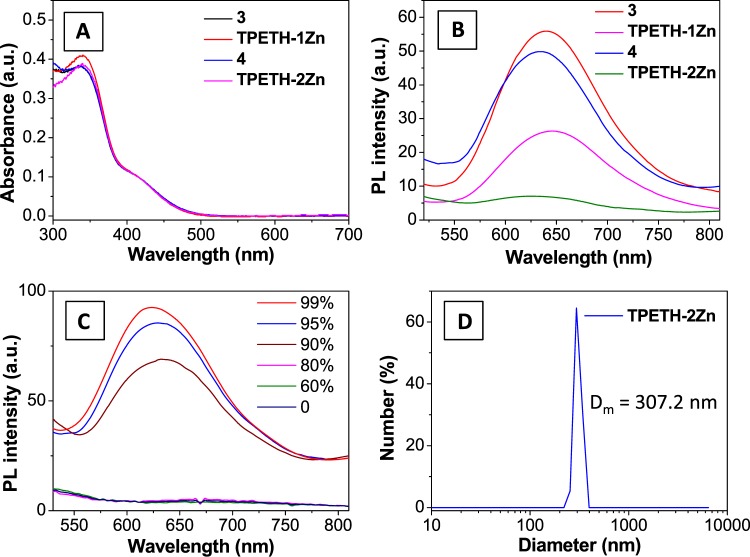
With the probes and their intermediates at hand, we first measured their photophysical properties. Compounds 3, 4, TPETH-1Zn, and TPETH-2Zn have similar absorption spectra in dimethyl sulfoxide (DMSO) (Figure 2A). However, their fluorescent properties are very different. As shown in Figure 2B, 3 and 4 are highly emissive in the mixture of water and DMSO (v/v = 100/1), whereas TPETH-1Zn has moderate emission and TPETH-2Zn is only weakly emissive under the same conditions. This indicates that the binding between 3 or 4 and zinc(II) increases the probe solubility in aqueous media, leading to decreased background signals. The increased aqueous solubility after zinc(II) binding was further confirmed by dynamic light scattering experiments. Both 3 and 4 have a mean hydrodynamic diameter of around 300 nm, whereas TPETH-1Zn exhibits a mean size of around 30 nm after zinc(II) binding, but no hydrodynamic size can be detected for TPETH-2Zn (Figure S16), clearly indicating that zinc(II) binding could largely improve the aqueous solubility of the probe. Following this fluorescence change after binding with zinc(II), we measured the dissociation constant (Kd) of TPETH-1Zn by fitting the fluorescence titration data with a 1:1 association equation (Figure S17A). The Kd is calculated to be 16.7 nM for TPETH-1Zn (Figure S17B), which indicates that the ligands in TPETH-2Zn can bind to Zn(II) tightly.
Figure 2.
(A) Ultraviolet–visible (UV–vis) absorption spectra of 3, TPETH-1Zn, 4, and TPETH-2Zn in DMSO. (B) Photoluminescence (PL) spectra of 3, TPETH-1Zn, 4, and TPETH-2Zn in DMSO/water (v/v = 1/100). (C) PL spectra of TPETH-2Zn in a mixture of acetonitrile and ethyl ether, with various percentages of ethyl ether. (D) Hydrodynamic diameter measured with laser light scattering for TPETH-2Zn (10 μM) in a mixture of acetonitrile/ether (v/v = 1/100).
Although TPETH-2Zn is almost nonemissive in aqueous media, its fluorescence is intensified in the mixture of acetonitrile and ethyl ether when the ratio of ether increases (Figure 2C). When the ether-to-acetonitrile ratio is above 90%, TPETH-2Zn becomes insoluble and emits bright red fluorescence and forms aggregates with an average diameter of 307 nm (Figure 2D). These results indicate that TPETH-2Zn is an AIEgen, which could turn on its fluorescence upon aggregate formation, such as upon binding to a bacterial surface.
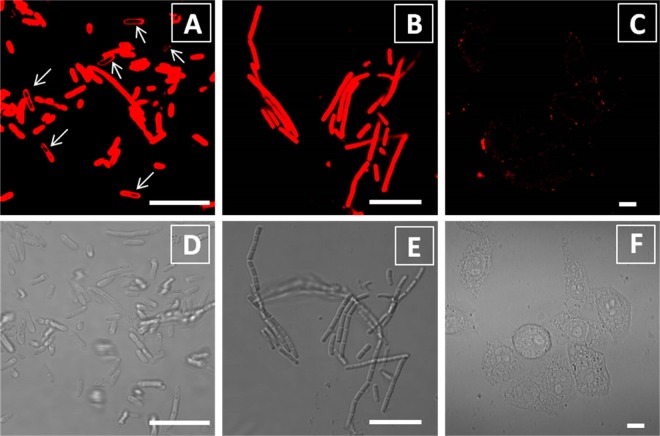
After confirming that TPETH-2Zn was not emissive in aqueous medium but was able to fluoresce upon aggregate formation, we evaluated its performance in real-time imaging of bacteria. Without any washing steps, the probe emits strong red emission upon interaction with bacteria (Escherichia coli and Bacillus subtilis), which is significantly brighter than that in PBS only (Figure S18). The fluorescence increase is dependent on the probe concentration. The confocal image (Figure 3) indicates that both E. coli and B. subtilis are stained by TPETH-2Zn after 30 min of incubation. It is noted that no washing is required for image acquisition. For B. subtilis, the probe lights up the whole bacterial cell, including the membrane and inner parts. For E. coli, although the probe stains the whole bacterial cell in the majority of E. coli, it stains only the membrane in some E. coli (arrow in Figure 3A). This small difference indicates that it is easier for the probe to penetrate into B. subtilis than into E. coli, as the latter contains an extra outer membrane.19 On the other hand, TPETH-2Zn only slightly stains HeLa cells under the same conditions (Figures 3C and S19), indicating that the probe prefers bacteria to mammalian cells. Together, these data demonstrate that E. coli and B. subtilis, with negatively charged membranes, could anchor, uptake, and aggregate the positively charged probe, which restricts the intramolecular motion, leading to fluorescence.
Figure 3.
Confocal (A–C) and bright-field (D–F) images of E. coli (A, D) B. subtilis (B, E), and HeLa cells (C, F) after incubation with the TPEPH-2Zn probe (20 μM) for 30 min without any washing steps. The red signal is collected above 590 nm upon excitation at 458 nm. All images share the same scale bar of 10 μm.
As reported, TPETH also has good efficiency to generate singlet oxygen species (1O2) under light irradiation.20 Next, we tested whether TPETH-2Zn has a similar ability to generate singlet oxygen. 9,10-Anthracenediylbis(methylene)dimalonic acid (ABDA), which quickly decomposes upon reaction with 1O2, was used as an indicator of 1O2 generation. White light irradiation of the mixture of TPETH-2Zn and ABDA in water greatly diminishes the absorbance of ABDA. After light irradiation for 2 min, around 90% of ABDA was consumed (Figure S20). The singlet oxygen quantum yield for TPETH-2Zn is 77.6%, measured using Rose Bengal as the reference.21 It should be noted that TPETH-2Zn exhibited a similar singlet oxygen generation ability even after binding to bacteria (Figure S21), indicating that the design of AIE PS could offer an effective singlet oxygen generation ability in the aggregated state.
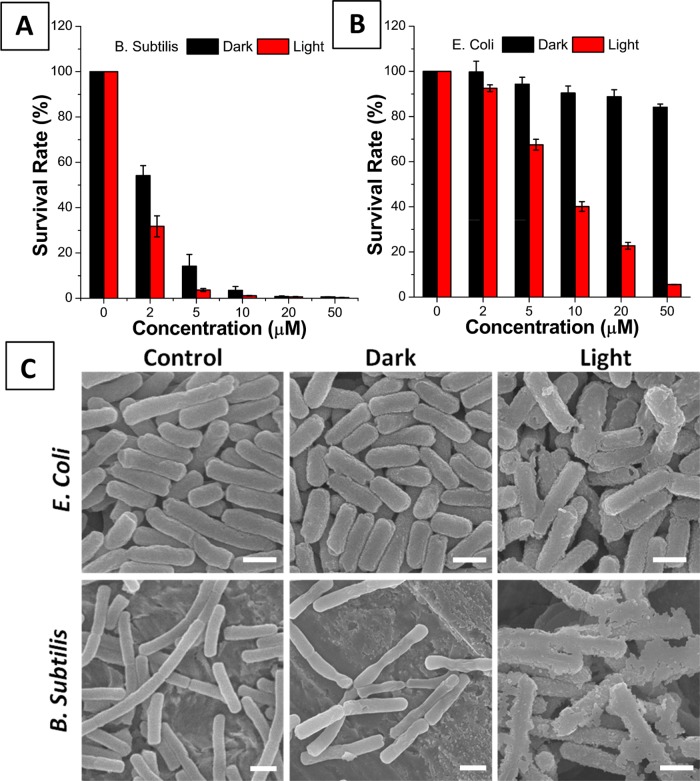
Finally, we applied TPETH-2Zn to photoablate bacteria. In the dark, the probe shows little toxicity toward E. coli but high toxicity toward B. subtilis, as 20 μM TPETH-2Zn could kill nearly all of the B. subtilis but only less than 10% of the E. coli (Figure 4A,B). The same trend was also observed in our previous report, mainly because E. coli contains an extra outer protective membrane as an effective barrier to PSs.14,19 Under irradiation with white light (100 mW cm–2), 10 μM TPETH-2Zn could photoinactivate all of the B. subtilis and 60% of the E. coli (Figures 4A,B, S22, and S23). Scanning electron microscopy (SEM) results indicate that the membranes of bacteria are damaged after probe treatment and light irradiation (Figure 4C). It should be noted that under the same experimental conditions the previous probe (AIE-ZnDPA, 10 μM) could only photoinactivate about 80% of the B. subtilis and 10% of the E. coli, which is around fivefold less toxicity than that of TPETH-2Zn. This toxicity difference could partially be ascribed to the lower Kd of TPETH-2Zn than that of AIE-ZnDPA, which favors bacterial binding in this case. It should be noted that TPETH-2Zn could not cause any dark or light toxicity toward HeLa cells (Figure S24), indicating that the AIE probe could selectively kill bacteria over mammalian cells due to the different binding affinities.
Figure 4.
Survival rates of (A) B. subtilis and (B) E. coli treated with TPEPH-2Zn at different concentrations, with or without light irradiation. (C) SEM images of E. coli and B. subtilis treated with TPEPH-2Zn, with or without light irradiation. All images share the same scale bar of 1 μm.
Conclusions
In conclusion, we used click chemistry (CuAAC) to readily synthesize a light-up AIE probe (TPETH-2Zn) that tightly binds zinc(II), selectively images bacteria over mammalian cells, and potently exerts phototoxicity to bacteria. The AIE probe, TPETH-2Zn, is not emissive in aqueous solution but is able to turn on its emission upon aggregate formation. As most bacteria contain significant amounts of anionic phospholipids, which make their membrane negatively charged,22TPETH-2Zn could tightly bind to the bacterial surface, but not mammalian cell membranes, and fluoresce. Benefiting from its low background signal, TPETH-2Zn does not require additional washing steps, which largely simplifies the imaging procedure and detection sensitivity. Possessing excellent singlet oxygen generation ability even after binding to bacteria, TPETH-2Zn potentially serves as an effective theranostic probe for killing bacteria, which shall open new opportunities for the treatment of bacterial infections.
Methods
Materials and Instruments
All the chemicals were purchased from a commercial vendor and used directly without further purification. Dry dichloromethane was distilled over CaH2. All 1H NMR and 13C NMR spectra were recorded on a Bruker ACF-400 MHz NMR spectrometer with CDCl3 or (CD3)2SO (DMSO-d6) as the solvent. Chemical shifts are described in parts per million, which are referenced according to residual solvent for 1H NMR (7.26 ppm for CDCl3 and 2.50 ppm for DMSO-d6) and 13C NMR (77.0 ppm for CDCl3 and 40.0 ppm for DMSO-d6). HRMS (ESI) spectra were recorded by the AmaZon X LC-MS. UV–vis absorption spectra were recorded on a Shimadzu Model UV-1700 spectrometer. PL spectra were recorded on a Perkin-Elmer LS 55 spectrofluorometer.
Synthesis of 3 and 4
To the solution of 1 (60 mg, 0.087 mmol) and 2 (50 mg, 0.21 mmol) in DMSO (2.0 mL) were added copper sulfite (1.6 mg, 0.01 mmol) and sodium ascorbate (4.0 mg, 0.02 mmol) in water (0.1 mL). The mixture was stirred at room temperature for 24 h. Then, water (20 mL) was added to quench the reaction. The precipitated solid was purified by chromatography to give product 4 as a red oil (5.0 mg). 1H NMR (400 MHz, CDCl3) δ 8.59 (m, 4H), 7.82 (dd, J1 = 1.2 Hz, J2 = 4.8 Hz, 1H), 7.71–7.74 (m, 4H), 7.64–7.65 (m, 3H), 7.11–7.24 (m, 11 H), 7.03–7.06 (m, 2H), 6.87–6.94 (m, 4H), 6.60–6.64 (m, 4H), 4.56 (t, J = 6.8 Hz, 4H), 4.11–4.14 (m, 12H), 3.92 (m, 4H), 2.34–2.37 (m, 4H); HRMS (ESI) calcd for [M + Na]+: 1185.4793, found: 1185.4795. Product 3 is a red oil (20 mg). 1H NMR (400 MHz, CDCl3) δ 8.53 (m, 2H), 7.79–7.81 (m, 1H), 7.72 (m, 1H), 7.62–7.66 (m, 3H), 7.55–7.57 (m, 2H), 7.11–7.19 (m, 9H), 7.04–7.04 (m, 2H), 6.88–6.95 (m, 4H), 6.61–6.65 (m, 4H), 4.53 (t, J = 6.8 Hz, 2H), 3.97 (t, J = 5.6 Hz, 2H), 3.87–3.93 (m, 8H), 3.48 (t, J = 6.8 Hz, 2H), 2.33–2.36 (m, 2H), 1.99–2.02 (m, 2H); HRMS (ESI) calcd for [M + H]+: 926.3708, found: 926.3704.
Synthesis of TPETH-1Zn
To the solution of 3 (1.9 mg) in acetonitrile (2.0 mL) was added zinc perchlorate hexahydrate (0.82 mg). The mixture was stirred at room temperature for 30 min. Then, all of the solvent was removed under reduced pressure to give the product without further purification. 1H NMR (400 MHz, CD3CN) δ 8.72 (m, 2H), 8.10–8.15 (tt, J1 = 1.2 Hz, J2 = 7.6 Hz, 2H), 7.97–8.00 (m, 1H), 7.94 (m, 1H), 7.65–7.68 (m, 3H), 7.58–7.60 (m, 2H), 7.21–7.29 (m, 3H), 7.13–7.1 (m, 5H), 7.05–7.08 (m, 2H), 6.93–6.95 (m, 1H), 6.87–6.90 (m, 2H), 6.80–6.82 (m, 1H), 6.69–6.71 (m, 2H), 6.53–6.55 (m, 2H), 4.58–4.62 (m, 2H), 4.23 (s, 4H), 3.95–3.99 (dt, J1 = 1.2 Hz, J2 = 6.0 Hz, 2H), 3.95 (t, J = 6.0 Hz, 2H), 3.41–3.48 (m, 2H), 2.30 (m, 2H); HRMS (ESI) calcd for [M – ClO4]+: 1088.2406, found: 1088.2401.
Synthesis of TPETH-2Zn
To the solution of 4 (5.0 mg) in acetonitrile (2.0 mL) was added zinc perchlorate hexahydrate (2.3 mg). The mixture was stirred at room temperature for 30 min. Then, all of the solvent was removed under reduced pressure to give the product without further purification. 1H NMR (400 MHz, CD3CN) δ 9.02 (brs, 4H), 8.06 (m, 4H), 7.98 (dd, J1 = 1.2 Hz, J2 = 5.2 Hz, 2H), 7.92 (m, 2H), 7.62–7.66 (m, 5H), 7.53 (d, J = 7.6 Hz, 3H), 7.22–7.26 (m, 3H), 7.10–7.18 (m, 6H), 7.03–7.06 (m, 2H), 6.76–6.85 (m, 4H), 6.54–6.56 (m, 4H), 4.62 (m, 4H), 4.16 (brs, 8H), 4.02 (brs, 4H), 3.95 (m, 4H); HRMS (ESI) calcd for [M – 2ClO4]2+: 744.1222, found: 744.1221.
Bacterial Labeling
The bacterial suspensions (E. coli or B. subtilis) at an OD600 of 0.2 in 1× phosphate buffered saline (PBS) buffer were incubated with TPETH-2Zn (20 μM) for 30 min at room temperature. After incubation, the bacteria were harvested by centrifugation at 5000 rpm for 5 min, the unbound TPETH-2Zn in the supernatant was discarded, and the bacteria were re-suspended in PBS buffer for absorbance measurements. To study the fluorescence of TPETH-2Zn upon binding to bacteria, these bacteria were transferred to a 96-well plate in a volume of 100 μL/well, with an OD600 of 0.1. Designated concentrations of TPETH-2Zn were then added into each well. After 30 min of incubation, the fluorescence intensity at 650 nm upon excitation at 458 nm was recorded. PBS was used as a negative control.
Confocal Imaging Experiments
The prepared bacterial (B. subtilis and E. coli) solutions were incubated with 20 μM TPETH-2Zn in the dark for 30 min at 37 °C. Individual aliquots of 10 μL of the prepared suspension were spotted on polylysine-pretreated glass slides and immobilized with coverslips. The specimens were imaged immediately under a confocal laser scanning microscope (CLSM; Zeiss). Excitation wavelength: 458 nm (1% laser power); emission filter: LP590 nm. For confocal images of HeLa cells, the HeLa cells were seeded in an eight-well chamber at 4 × 104 cells/mL. After overnight culturing, the HeLa cells were incubated with TPETH-2Zn at a concentration of 20 μM for 30 min. The cells were immediately imaged under a CLSM without washing, using the same setup for bacterial imaging.
Antimicrobial Assay
The antibacterial activity of TPETH-2Zn was studied by plating the diluted bacterial suspensions on a solid Luria–Bertani (LB) agar plate. The bacteria were harvested and re-suspended in 1× PBS with an OD600 of 0.5. Then, 90 μL of the bacterial suspension was added to a 96-well plate and TPETH-2Zn was added to each well to achieve final concentrations of 0, 2, 5, 10, 20, and 50 μM. The total volume was kept at 100 μL, and the plate was incubated in the dark at 37 °C for 30 min. For the photodynamic antibacterial assay, the samples in the 96-plate were illuminated with white light for 5 min at 100 mW cm–2. After irradiation, the bacterial suspensions were serially diluted 0.5–1 × 105-fold with 1× PBS. A portion (100 μL) of the diluted bacterial cells was spread on the solid LB agar plate and incubated at 37 °C for 16 h. The colonies formed were counted, and the survival rates of the bacteria were determined from colony-forming unit counting on the solid LB agar plate with the control in the dark without TPETH-2Zn treatment. For the control in the dark, the irradiation step was replaced by incubation in the dark for 6 min.
SEM Measurements
On the basis of the antimicrobial experiments, the concentration of TPETH-2Zn was determined to be 20 μM for SEM measurements. The bacteria were centrifuged at 5000 rpm for 5 min to remove the 1× PBS, followed by irradiation. They were then suspended in and fixed with 2.5% glutaraldehyde for 2–3 h at room temperature. The glutaraldehyde was removed by centrifugation, and the bacterial pellets were re-suspended in sterile water; then, 10 μL of the bacterial suspension was spotted onto the SEM conducting paste. After natural drying in air, the bacteria were dehydrated with a series of graded ethanol solutions (30, 50, 70, 80, 90, and 100% for 6 min). After drying overnight, the specimens were coated with platinum before SEM measurements.
Acknowledgments
The authors thank SMART (R279-000-378-592), the Ministry of Education (R279-000-391-112), Singapore NRF Investigatorship (R279-000-444-281), and the Institute of Materials Research and Engineering of Singapore (IMRE/14-8P1110) for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00564.
Synthesis of compounds 1 and 2; preparation of the bacterial solution and cell culture; binding constant determination; 1H NMR, 13C NMR, and HRMS spectra; fluorometric titration of 3 with ZnSO4; hydrodynamic diameters of 3, 4, and TPETH-1Zn in aqueous media; time-dependent bleaching of ADPA caused by TPETH-2Zn with white light irradiation, with or without the presence of bacteria; plate photographs of E. coli and B. subtilis on LB agar plates supplemented with TPETH-2Zn, with or without white light irradiation; cell viability of TPETH-2Zn-treated HeLa cells, with or without white light irradiation (PDF)
Author Contributions
§ G.F. and C.-J.Z. contributed equally to this work.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Arias C. A.; Murray B. E. Antibiotic-Resistant Bugs in the 21st Century — A Clinical Super-Challenge. N. Engl. J. Med. 2009, 439–443. 10.1056/nejmp0804651. [DOI] [PubMed] [Google Scholar]; b Livermore D. M. Has the era of untreatable infections arrived?. J. Antimicrob. Chemother. 2009, 64, i29–i36. 10.1093/jac/dkp255. [DOI] [PubMed] [Google Scholar]
- Blair J. M. A.; Webber M. A.; Baylay A. J.; Ogbolu D. O.; Piddock L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- a Kalan L.; Wright G. D. Antibiotic adjuvants: multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13, e5 10.1017/S1462399410001766. [DOI] [PubMed] [Google Scholar]; b Gonzales P. R.; Pesesky M. W.; Bouley R.; Ballard A.; Biddy B. A.; Suckow M. A.; Wolter W. R.; Schroeder V. A.; Burnham C. D.; Mobashery S.; Chang M.; Dantas G. Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat. Chem. Biol. 2015, 11, 855. 10.1038/nchembio.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W.; Stapletion P. D.; Luzio J. P. New ways to treat bacterial infections. Drug Discovery Today 2002, 7, 1086–1091. 10.1016/S1359-6446(02)02498-4. [DOI] [PubMed] [Google Scholar]
- a Tavares A.; Carvalho C. M. B.; Faustino M. A.; Neves M. G. P. M. S.; Tomé J. P. C.; Tomé A. C.; Cavaleiro J. A. S.; Cunha Â.; Gomes N. C. M.; Alves E.; Almeida A. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Shao Q.; Xing B. Enzyme responsive luminescent ruthenium(II) cephalosporin probe for intracellular imaging and photoinactivation of antibiotics resistant bacteria. Chem. Commun. 2012, 48, 1739–1741. 10.1039/C1CC16165B. [DOI] [PubMed] [Google Scholar]
- Almeida A.; Faustino M. A. F.; Tomé J. P. C. Photodynamic inactivation of bacteria: finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. 10.4155/fmc.15.59. [DOI] [PubMed] [Google Scholar]
- a Maisch T. Revitalized Strategies Against Multi-Resistant Bacteria: Antimicrobial Photodynamic Therapy and Bacteriophage Therapy. Anti-Infect. Agents Med. Chem. 2007, 6, 145–150. 10.2174/187152107780361634. [DOI] [Google Scholar]; b Maisch T. A New Strategy to Destroy Antibiotic Resistant Microorganisms: Antimicrobial Photodynamic Treatment. Mini-Rev. Med. Chem. 2009, 9, 974–983. 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- a Bennett L. E.; Ghiggino K. P.; Henderson R. W. Singlet oxygen formation in monomeric and aggregated porphyrin c. J. Photochem. Photobiol., B 1989, 3, 81–89. 10.1016/1011-1344(89)80022-3. [DOI] [PubMed] [Google Scholar]; b Park S. Y.; Baik H. J.; Oh Y. T.; Oh K. T.; Youn Y. S.; Lee E. S. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew. Chem., Int. Ed. 2011, 50, 1644–1647. 10.1002/anie.201006038. [DOI] [PubMed] [Google Scholar]
- a Shi H.; Kwok R. T. K.; Liu J.; Xing B.; Tang B. Z.; Liu B. Real-Time Monitoring of Cell Apoptosis and Drug Screening Using Fluorescent Light-Up Probe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. 10.1021/ja3064588. [DOI] [PubMed] [Google Scholar]; b Chen S.; Hong Y.; Liu Y.; Liu J.; Leung C. W. T.; Li M.; Kwok R. T. K.; Zhao E.; Lam J. W. Y.; Yu Y.; Tang B. Z. Full-Range Intracellular pH Sensing by an Aggregation-Induced Emission-Active Two-Channel Ratiometric Fluorogen. J. Am. Chem. Soc. 2013, 135, 4926–4929. 10.1021/ja400337p. [DOI] [PubMed] [Google Scholar]; c Li Y.; Yu H.; Qian Y.; Hu J.; Liu S. Amphiphilic Star Copolymer-Based Bimodal Fluorogenic/Magnetic Resonance Probes for Concomitant Bacteria Detection and Inhibition. Adv. Mater. 2014, 26, 6734–6741. 10.1002/adma.201402797. [DOI] [PubMed] [Google Scholar]; d Hu F.; Huang Y.; Zhang G.; Zhao R.; Yang H.; Zhang D. Targeted Bioimaging and Photodynamic Therapy of Cancer Cells with an Activatable Red Fluorescent Bioprobe. Anal. Chem. 2014, 86, 7987–7995. 10.1021/ac502103t. [DOI] [PubMed] [Google Scholar]
- a Ding D.; Li K.; Liu B.; Tang B. Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441. 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]; b Liang J.; Tang B. Z.; Liu B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. 10.1039/C4CS00444B. [DOI] [PubMed] [Google Scholar]
- a Zhang C.-J.; Hu Q.; Feng G.; Zhang R.; Yuan Y.; Lu X.; Liu B. Image-guided combination chemotherapy and photodynamic therapy using a mitochondria-targeted molecular probe with aggregation-induced emission characteristics. Chem. Sci. 2015, 6, 4580–4586. 10.1039/C5SC00826C. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yuan Y.; Zhang C.-J.; Gao M.; Zhang R.; Tang B. Z.; Liu B. Specific Light-Up Bioprobe with Aggregation-Induced Emission and Activatable Photoactivity for the Targeted and Image-Guided Photodynamic Ablation of Cancer Cells. Angew. Chem., Int. Ed. 2015, 54, 1780–1786. 10.1002/anie.201408476. [DOI] [PubMed] [Google Scholar]
- a Zhao E.; Chen Y.; Wang H.; Chen S.; Lam J. W. Y.; Leung C. W. T.; Hong Y.; Tang B. Z. Light-Enhanced Bacterial Killing and Wash-Free Imaging Based on AIE Fluorogen. ACS Appl. Mater. Interfaces 2015, 7, 7180–7188. 10.1021/am509142k. [DOI] [PubMed] [Google Scholar]; b Feng G.; Yuan Y.; Hu F.; Zhang R.; Xing B.; Zhang G.; Zhang D.; Liu B. A light-up probe with aggregation-induced emission characteristics (AIE) for selective imaging, naked-eye detection and photodynamic killing of Gram-positive bacteria. Chem. Commun. 2015, 51, 12490–12493. 10.1039/C5CC03807C. [DOI] [PubMed] [Google Scholar]
- a Maisch T.; Bosl C.; Szeimies R.-M.; Lehn N.; Abels C. Photodynamic Effects of Novel XF Porphyrin Derivatives on Prokaryotic and Eukaryotic Cells. Antimicrob. Agents Chemother. 2005, 49, 1542–1552. 10.1128/AAC.49.4.1542-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Leevy W. M.; Johnson J. R.; Lakshmi C.; Morris J.; Marquez M.; Smith B. D. Selective recognition of bacterial membranes by zinc(II)-coordination complexes. Chem. Commun. 2006, 42, 1595–1597. 10.1039/b517519d. [DOI] [PubMed] [Google Scholar]; c Leevy W. M.; Gammon S. T.; Jiang H.; Johnson J. R.; Maxwell D. J.; Jackson E. N.; Marquez M.; Piwnica-Worms D.; Smith B. D. Optical Imaging of Bacterial Infection in Living Mice Using a Fluorescent Near-Infrared Molecular Probe. J. Am. Chem. Soc. 2006, 128, 16476–16477. 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Hu Q.; Feng G.; Tomaczak N.; Liu R.; Xing B.; Tang B. Z.; Liu B. A Multifunctional Probe with Aggregation-Induced Emission Characteristics for Selective Fluorescence Imaging and Photodynamic Killing of Bacteria Over Mammalian Cells. Adv. Healthcare Mater. 2015, 4, 659–663. 10.1002/adhm.201400654. [DOI] [PubMed] [Google Scholar]
- a Guo Z.; Zhu W.; Tian H. Dicyanomethylene-4H-pyran chromophores for OLED emitters, logic gates and optical chemosensors. Chem. Commun. 2012, 48, 6073–6084. 10.1039/c2cc31581e. [DOI] [PubMed] [Google Scholar]; b Wang X.; Morales A. R.; Urakami T.; Zhang L.; Bondar M. V.; Komatsu M.; Belfield K. D. Folate Receptor-Targeted Aggregation-Enhanced Near-IR Emitting Silica Nanoprobe for One-Photon in Vivo and Two-Photon ex Vivo Fluorescence Bioimaging. Bioconjugate Chem. 2011, 22, 1438–1450. 10.1021/bc2002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva S. A.; Zavaleta A.; Baron D. E.; Allam O.; Isidor E. V.; Kashimura N.; Percarpio J. M. A fluorescent photoinduced electron transfer sensor for cations with an off-on-off proton switch. Tetrahedron Lett. 1997, 38, 2237–2240. 10.1016/S0040-4039(97)00332-8. [DOI] [Google Scholar]
- Atilgan S.; Ozdemir T.; Akkaya E. U. A Sensitive and Selective Ratiometric Near IR Fluorescent Probe for Zinc Ions Based on the Distyryl–Bodipy Fluorophore. Org. Lett. 2008, 10, 4065–4067. 10.1021/ol801554t. [DOI] [PubMed] [Google Scholar]
- a Huang S.; Clark R. J.; Zhu L. Highly Sensitive Fluorescent Probes for Zinc Ion Based on Triazolyl-Containing Tetradentate Coordination Motifs. Org. Lett. 2007, 9, 4999–5002. 10.1021/ol702208y. [DOI] [PubMed] [Google Scholar]; b Walkup G. K.; Burdette S. C.; Lippard S. J.; Tsien R. Y. A New Cell-Permeable Fluorescent Probe for Zn2+. J. Am. Chem. Soc. 2000, 122, 5644–5645. 10.1021/ja000868p. [DOI] [Google Scholar]
- a Wu T.; McCandlish A. C.; Gronenberg L. S.; Chng S. S.; Silhavy T. J.; Kahne D. Identification of a protein complex that assembles lipopolysaccharide in the outer membrane of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 11754–11759. 10.1073/pnas.0604744103. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Pereira M. A.; Faustino M. A. F.; Tomé J. P. C.; Neves M. G. P. M. S.; Tomé A. C.; Cavaleiro J. A. S.; Cunha Â.; Almeida A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. 10.1039/c3pp50408e. [DOI] [PubMed] [Google Scholar]
- Yuan Y.; Zhang C.-J.; Kwok R. T. K.; Xu S.; Zhang R.; Wu J.; Tang B. Z.; Liu B. Light-Up Probe for Targeted and Activatable Photodynamic Therapy with Real-Time In Situ Reporting of Sensitizer Activation and Therapeutic Responses. Adv. Funct. Mater. 2015, 25, 6586–6595. 10.1002/adfm.201502728. [DOI] [Google Scholar]
- Xu S.; Yuan Y.; Cai X.; Zhang C.-J.; Hu F.; Liang J.; Zhang G.; Zhang D.; Liu B. Tuning the singlet-triplet energy gap: a unique approach to efficient photosensitizers with aggregation-induced emission (AIE) characteristics. Chem. Sci. 2015, 6, 5824–5830. 10.1039/C5SC01733E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C.; Wilkinson S. G.. Microbial Lipids; Academic Press: New York, 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.