Abstract

It is shown here that controlled mixing of a gelator, drug, solvent, and antisolvent in a microfluidic channel leads to faster setting gels and more robust materials with longer release profiles than the physical gels of the same composition obtained using random mixing in solution. The system is similar to a related gelator system we had studied previously, but we were unable to apply the same gelling procedure because of the instability of the colloid caused by the small structural modification (length of the alkyl chain in the bis-imidazolium head group). This situation holds true for the gels formed with varying compositions and under different conditions (gelator/drug ratio, solvent proportion, and flow rates), with the most significant differences being the improved gel rheology and slower drug release rates. Very importantly, the gels (based on a previously unexplored system) have a higher water content ratio (water/EtOH 4:1) than others in the family, making their medicinal application more attractive. The gels were characterized by a variety of microscopy techniques, X-ray diffraction and infrared spectroscopy, and rheology. Salts of the antiinflammatory drugs ibuprofen and indomethacin were successfully incorporated into the gels. The diffraction experiments indicate that these composite gels with relatively short alkyl chains in the gelator component contrast to previous systems, in that they exhibit structural order and the presence of crystalline areas of the drug molecule implying partial phase separation (even though these drug crystallites are not discernible by microscopy). Furthermore, the release study with the gel incorporating ibuprofenate showed promising results that indicate a possible drug delivery vehicle application for this and related systems.
Introduction
The control of nanostructure in soft matter systems is important in a number of areas,1−4 among them in the preparation of materials for controlled drug delivery.5−9 Gels can be chemical (comprising a covalent network) or physical (or supramolecular, comprising noncovalent bonds).10−13 Polymeric chemical gels comprise covalently bonded networks of fibers that cannot be redissolved, and the structures are thermally irreversible, which make them limited where relatively rapid degradation is preferred. Hence, physical (macro)molecular gels, where noncovalent interactions lead to gelation and are generally characterized by their structural reversibility under thermal and mechanical stress, could be a better choice for drug delivery applications.14,15 Supramolecular gels can be formed at low temperature, with the majority of the solvent component trapped between the entangled and intertwined nanofibrous networks that are often held together by London dispersion forces between the alkyl chains of the gelator.
Gemini-imidazolium amphiphiles are one such kind of supramolecular material16 existing as colloids17−19 and that can form gels20 useful for drug delivery, where the cationic nature of the head group provides a location for interaction with drug molecules.21,22 Previously, we have explored the gelation ability in bulk of gemini-imidazolium amphiphiles related to compounds 1–3 (Figure 1),21,22 comprising two imidazolium rings linked through a bis-methylenebenzene spacer (either 1,3- or 1,4-substituted) and bearing n-octadecyl chains. These gels were stable and capable of incorporating anionic drugs as drug delivery systems mainly targeting topical applications. However, in the course of our studies on related compounds bearing shorter alkyl chains, we found that the bulk gel formation (usually carried out in sample pots in our experiments) sometimes led to a qualitatively inhomogeneous material and the gel formation—achieved by mixing aqueous and ethanolic solutions—could be poorly reproducible. For this reason, we sought a controlled mixing protocol that could provide a reproducibly more homogeneous material. In doing so, some intriguing effects of mixing on supramolecular composite gel formation have been discovered.
Figure 1.
Structures of the imidazolium-based gelators 1·2Br–3·2Br and the sodium salts of ibuprofen and indomethacin.
The mixing technique we used involved combining distinct components under laminar flow in a microfluidic chip, in which small volumes of fluids are combined in a channel with micrometric dimensions—generally from tens to hundreds of micrometers.23,24 Microfluidics has a growing range of applications in various fields, from combinatorial materials synthesis25,26 to biomedicine (drug delivery, separation, and diagnostic devices).27−29 Among the many advantages of the continuous preparation of materials using microfluidics30 are the parallel and consecutive reactions in a single system, low reagent consumption, and the highly controlled and steady mixing, which can be useful for the preparation of nanomaterials.31 The mixing in the chips is characterized by a low Reynolds number, and therefore, the flow is laminar; mixing of fluids occurs only at the interface through diffusion with the viscous force dominant and no lateral convection. In narrow channels, the surface area to volume ratio is high and the heat and mass transfer rate increases compared with other conditions because of the steeper temperature and concentration gradients.32,33 It has been demonstrated that the self-assembly of amphiphiles can be controlled using a microfluidic system to determine the flow condition,31 but insomuch as we are aware, excepting the fabrication of polymer microgels by gelation in droplet microfluidics,34 there is no such experiment on bulk gelling systems.
The initial hypothesis for our study was that inside the microchannel, the dominant interfacial forces, enhanced heat—and mass diffusion—transfer, can be exploited to promote the self-assembly process of the supramolecular gelators with the drug molecule with different flow parameters to obtain stable gels efficiently incorporating the drug. The basis for this hypothesis is the observed influence of flow on the formation of nanoscale aggregates in solution.35 Our purpose is to show that microfluidic systems can be used as a platform for supramolecular gel formation for materials for drug delivery applications. Here, we demonstrate this proof of principle for the gelator molecules 1–3 when combined with the sodium salts of nonsteroidal antiinflammatory drugs ibuprofen and indomethacin (Figure 1).
Results and Discussion
The gelator molecules (prepared using methods described elsewhere21,36,37) that contain between 14 and 16 carbon atoms in their aliphatic chains were first explored as gelators on their own for mixtures of ethanol in water. The putative gel mixtures were prepared by adding water (that acts as an antisolvent here) to the gelator dissolved in ethanol, swirling the mixture and leaving it undisturbed at room temperature for some time, because gels do not form immediately. Under these conditions, chaotic mixing takes place, and it is not possible to control reproducibly the combination of the two liquids. To obtain the optimum solvent mixture for gel formation, gelation experiments with different concentrations of the gelator and different proportions of ethanol and water were attempted. For the gels with drug, different aqueous concentrations of the sodium salt of either ibuprofen or indomethacin were mixed with the ethanolic solution of the gelator. Some of the different conditions tested for the gel formation incorporating these drugs are shown in Table 1 (see Table S1 in the Supporting Information for more details). Compound 1·2Br formed the qualitatively less homogeneous (large fibers were observed in the only gel that formed) and less stable gel and was not taken forward in the subsequent studies, whereas both 2·2Br and 3·2Br formed more homogeneous and stable gels. The time for the gels to set properly was rather long (several hours to days, see Table 1), an aspect that is improved dramatically by the subsequent microfluidic preparation routes (see below).
Table 1. Selected Experimental Conditions and Results of Bulk Mixing Gelation Experiments Using Gelators 1·2Br–3·2Br at a Concentration of 10 mg/mLa.
| gelator | ethanol/water ratio (v/v) | drug | gelator/drug ratio (mol/mol) | gel formation | gelation time (h) |
|---|---|---|---|---|---|
| 1·2Br | 1:1 | none | no | ||
| 3:7 | none | no | |||
| 1:4 | none | yes | 24 | ||
| 2·2Br | 1:1 | none | no | ||
| 3:7 | none | partial | 24 | ||
| 1:4 | none | yes | 5 | ||
| 1:4 | Ibu | 1:2 | no | ||
| 1:4 | Ibu | 1:1 | yes | 24 | |
| 1:4 | Ibu | 2:1 | yes | 24 | |
| 1:4 | Ind | 1:1 | no | ||
| 1:4 | Ind | 2:1 | yes | 120 | |
| 3·2Br | 1:1 | none | no | ||
| 3:7 | none | partial | 120 | ||
| 1:4 | none | yes | 24 | ||
| 1:4 | Ibu | 1:2 | no | ||
| 1:4 | Ibu | 1:1 | no | ||
| 1:4 | Ibu | 2:1 | yes | 24 |
Ibu—ibuprofen (sodium salt); Ind—indomethacin (sodium salt).
The ethanol/water ratio is the dominant factor determining gel formation, with the length of the alkyl chains playing a lesser, though significant, role in gelation ability across the series studied here. None of the gelators are able to immobilize a 1:1 volume mixture of the solvents, whereas all of them gel in a 1:4 mixture of ethanol and water at a concentration of 10 mg/mL. The analogous gelator with alkyl chains bearing 18 carbon atoms21 does not form gels at this high water content at this concentration (the maximum aqueous content is 2:3 ethanol/water), although it does gel this mixture at a concentration of only 5 mg/mL. In the present study, the higher ratio of water possible for the gel formation probably arises from the fact that the amphiphiles with shorter chains are less hydrophobic and thus more soluble in the medium. On the other hand, the shorter chains tend to lead to thicker fibers, as seen visually because of the much greater visible light scattering (see photographs of a selection of the gels, Supporting Information, Figure S1).
Another striking difference between the gels reported here and the analogue with longer hydrocarbon chains is their ability to have higher drug loadings while maintaining an apparently homogeneous phase. Previously, only gels with a maximum molar ratio of 4:1 (gelator/ibuprofen sodium salt) could be obtained.21 Instead, for gelator 2·2Br, equimolar amounts of gelator and ibuprofen (sodium salt) result in the formation of a gel in a 1:4 ethanol/water mixture. For the sodium salt of indomethacin in combination with 2·2Br, the equimolar mixture does not form a gel, whereas the 2:1 mixture does. The more favorable gel formation in the presence of the drug molecules as a result of shortening of the alkyl chain in the gelator (whereas the polar head group is identical) is an interesting and potentially useful effect. The gels prepared from compounds 2·2Br and 3·2Br take longer to form than their octadecyl homologue.21 Importantly, though, the higher content of the drug in the gel achieved using these gelators compared with previous ones represents a clear advantage regarding drug loading.
During the preparation of these gels, occasionally, the reproducibility of gel formation was not perfect, and it was clear that occasionally inhomogeneous gels formed, sometimes with a nongelated solvent present. To address this lack of reproducibility, we turned to microfluidic mixing.
The microfluidic chips we employed comprised four symmetric inlet channels that converge and continue as a single channel, the microreactor, as seen in Figure 2. They were obtained by the replica molding technique (soft lithography) of a microfabricated mold using poly(dimethylsiloxane) (PDMS).26,27 An outlet solvent proportion 20:80 ethanol/water and a total gelator concentration 10 mg/mL were used in all the microfluidic experiments. Different flow conditions were tried to obtain a stable gel loaded with drug. The flow rates for every experiment are specified for Inlet 1, Inlet 2, Inlet 3, and Inlet 4 in μL/min in Table 2.
Figure 2.

Photograph showing the inlets and outlet of the microfluidic setup and the experimental configurations for the gelation experiments.
Table 2. Microfluidic Mixing Gelation Experimentsa.
| flow rates (μL/min) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| gelator | gelator/drug ratio (mol/mol) | drug | configuration | Inlet 1 water | Inlet 2 ethanol | Inlet 3 ethanol | Inlet 4 water | gel formation | gelation time (h) |
| 2·2Br | none | 160 | 40 | 40 | 160 | yes | 1 | ||
| 1:1 | Ibu | 1 | 320 | 80 | 80 | 320 | no | ||
| 1:1 | Ibu | 1 | 160 | 40 | 40 | 160 | no | ||
| 1:1 | Ibu | 1 | 80 | 20 | 20 | 80 | yes | 5–6 | |
| 1:1 | Ibu | 1 | 40 | 10 | 10 | 40 | partial | 24 | |
| 2:1 | Ibu | 2 | 160 | 40 | 40 | 160 | yes | 5 | |
| 1:1 | Ibu | 2 | 160 | 40 | 40 | 160 | no | ||
| 1:1 | Ibu | 2 | 80 | 20 | 20 | 80 | no | ||
| 2:1 | Ind | 2 | 160 | 40 | 40 | 160 | yes | 2–3 | |
| 3·2Br | none | 160 | 40 | 40 | 160 | yes | 18 | ||
| 1:2 | Ibu | 1 | 160 | 40 | 40 | 160 | no | ||
| 1:1 | Ibu | 1 | 160 | 40 | 40 | 160 | no | ||
| 2:1 | Ibu | 2 | 160 | 40 | 40 | 160 | yes | <24 | |
Configuration 1: Inlets 1 and 4 = water + drug, Inlets 2 and 3 = ethanol + gelator; configuration 2: Inlets 1 and 4 = water, Inlets 2 and 3 = ethanol + gelator + drug; Ibu—ibuprofen (sodium salt); Ind—indomethacin (sodium salt). Experiments gave a final water/ethanol ratio of 80:20, so the final concentration of the gelator is 10 mg/mL.
Because ibuprofen sodium salt is soluble in both ethanol and water, two experimental configurations were used. In configuration 1, the gelator was dissolved in ethanol and the drug in the water streams (these conditions resemble the ones used in the bulk mixing gelation experiments), whereas in configuration 2, both gelator and drug were dissolved in ethanol. The inlet channels in the center (Inlets 2 and 3) were connected to the ethanol solution containing gelator or containing gelator and drug, and the outer inlet channels (Inlets 1 and 4) were connected to water with drug or water, respectively. A selection of experimental conditions is shown in Table 2 (see the Supporting Information, Table S2, for more information on gelation outcome and gelation time).
Visual comparison of the gels prepared in bulk solution with those obtained using microfluidic mixing indicated that the latter showed better quality; no excess solvent was ever visible, the gels were more homogeneous (to the naked eye), had better stability, and most importantly showed better drug-loading capacity (up to a gelator/drug ratio of 1:1 for 2.2Br). Additionally, the gelation process after microfluidic mixing is faster when compared to the bulk gelation in all cases (on the order of hours compared with days). This order of magnitude increase in the speed of gelation is, for example, for the 2:1 2.2Br/indomethacin sample going from 120 h without microfluidic mixing to only 2–3 h with microfluidic mixing. This effect is presumably a result of the faster self-assembly arising from a more homogeneously distributed material and nucleation sites in the mixture emerging from the microfluidic channel. The nuclei grow homogeneously in three dimensions to form an intertwined fibrous network with apparently uniform distribution that cannot be achieved by simple mixing in bulk. Without any drug, the gels of 2·2Br and 3·2Br were formed at flow rates of 160 μL/min in the outer inlets (water) and 40 μL/min in the middle inlets (gelator in ethanol) to maintain the preferred 4:1 solvent proportion. When preparing gels incorporating drug with configuration 2 with a 2:1 gelator/drug molar ratio, and at flow rates 160/40/40/160, 3·2Br formed a gel with ibuprofen (sodium salt) only, whereas 2·2Br formed a gel with both ibuprofen (sodium salt) and indomethacin (sodium salt). Increasing the molar ratio of drug to 1:1 did not result in the formation of a gel with ibuprofen (sodium salt). Therefore, different flow rates were tried, and it was found that 2·2Br did form a gel with ibuprofen (sodium salt) with a 1:1 molar ratio using configuration 1 at a flow rate (80/20/20/80). Photographs of the different outcomes obtained by changing the flow rates with a 1:1 molar ratio of 2·2Br and ibuprofen (sodium salt) using either configuration 1 or 2 are shown in Figure 3.
Figure 3.

Photographs of the outcomes from the attempted gel formation with a 1:1 mixture of 2·2Br and ibuprofen (sodium salt) configuration 1 at flow rates (A) 320:80:80:320, (B) 160:40:40:160, (C) 80:20:20:80, and (D) 40:10:10:40 and configuration 2 (E) 160:40:40:160 and (F) 80:20:20:80.
Forming the gel with 2·2Br and ibuprofen (sodium salt) at 1:1 molar ratio, using configuration 1 and the initially tested flow rates (160/40/40/160) and (320/80/80/320), resulted in a turbid white suspension (Figure 3A,B). Lower flow rates were then attempted: gel formation occurred at flow rates (80/20/20/80) (Figure 3C), and further reducing the flow rates to half (40/10/10/40) resulted in the partial formation of a gel that had poor mechanical stability (Figure 3D). This evidence suggests that the residence time inside the microchannel plays a very important role in the evolution of the self-assembly of the gelator in the presence of a drug molecule to form a stable gel structure. The lack of stable gels at slower flow rates is in no doubt partly caused by the formation of agglomerates inside the channels. Although this solid gets dispersed in the solvent before collecting from the outlet, a nonhomogeneous partial gel with a high amount of solvent not trapped inside is formed because of the changing concentration of the gelator in the effluent from the chip. Using mixing configuration 2, no gel formation was observed. Instead, turbid solutions with a small amount of deposits at the bottom were obtained (Figure 3E,F). This observation suggests that a homogeneous mixture of ibuprofen (sodium salt) and gelator in the ethanolic solution does not favor the formation of long fibers that lead to gelation (vide infra).
The samples from the experiments where no gel was formed under different flow rates were analyzed by dynamic light scattering (DLS) (to determine the size distribution of the particles present in the suspension) and scanning electron microscopy (SEM) (to analyze the deposits obtained in the outlet). DLS indicated that in all cases the particles had diameters of around 1 μm (see Figure S3 in the Supporting Information). The SEM images (Figure 4) show that the output from the higher flow rates (in configuration 1) contains phase-separated deposits that have a very heterogeneous fibrous structure with some particles that could come from the liquid phase while drying (Figure 4A,B). The SEM images of the partial gel that formed under lower flow rates (half of the flow rates which formed a gel in configuration 1) show that it comprises fibers with smaller width (when compared to the case that formed a gel) and drug precipitate was also seen (Figure 4C). Whereas in configuration 2 with the same flow rates of configuration 1 which formed a gel, also contains the nonhomogeneous morphology with fibers, particles that could come from the liquid phase and crystals of drug due to phase separation (Figure 4D).
Figure 4.

SEM images of output mixtures obtained with the microfluidic system of 2·2Br and ibuprofen (sodium salt) (1:1) from experiments with configuration 1 (A) 320:80:80:320, (B) 160:40:40:160, and (C) 80:20:20:80 and configuration 2 (D) 80:20:20:80. Images were taken with 4000× magnification; the scale bar represents 20 μm in all images.
Atomic force microscopy (AFM) and SEM were used to determine the structural differences between the gels formed in bulk or microfluidic mixing conditions (Figures 5 and 6). AFM in the intermittent (“tapping”) mode of the xerogels of both 2·2Br and 3·2Br shows that the fibers of both colloids are not single fibers but co-parallel bundles of narrower fibers that are joined laterally. The gels formed by 2·2Br contain fibers that are much wider than those formed by 3·2Br, under both bulk and microfluidic mixing conditions. For gelator 2·2Br, the average width of the fibers is similar in both cases, approximately 450–650 nm, although the bulk gelation experiment tends to produce fiber aggregates of lower order, whereas those from microfluidic mixing can be several microns wide and composed of several individual fibers, as seen clearly in the SEM images (Figure 5). For the gels formed by 3·2Br, a more pronounced effect is seen: in the bulk sample, the polydisperse fibers have widths of around 50–250 nm, whereas from microfluidic mixing, more uniform 200–300 nm wide fibers are generated. Once again, a co-alignment of individual fibers to give tapes comprising multiple joined fibers is observed under microfluidic mixing conditions.
Figure 5.
Representative AFM (left and colored) and SEM (right and grayscale) images of gels of 2·2Br and 3·2Br from gelation in solution and microfluidic mixed systems. The scale bar is 1 μm in all images.
Figure 6.
SEM images of the gels obtained with both bulk (A–D) and microfluidic mixing (E–H) obtained with 2·2Br (A,E), 2·2Br with ibuprofen (sodium salt) 1:1 molar ratio (B,F) and 2:1 molar ratio (C,G) and with indomethacin (sodium salt) 2:1 molar ratio (D,H). Images were taken with 4000× magnification; scale bars represent 20 μm.
Comparison of the morphology of the gels formed with drugs presents a trend (Figure 6) similar to that of the pure gelators. The microfluidic mixing leads to fibrous networks observed in the xerogels by SEM that have much greater lateral interfiber connections, a feature especially prominent for the samples incorporating drugs. All the gels are composed of fibers irrespective of the presence and type of drug, but the morphology and dimensions of the fibers differ in each case: the width of the fibers of the gel without any drug is smaller when compared to that of the gels with drugs both in bulk and microfluidics gelation. This confirms the influence of drug in the assembly and consequently in the morphology of the fibers. It should also be noted that the gels of 2·2Br with drugs (both ibuprofen sodium salt and indomethacin sodium salt) obtained with the microfluidic system show much wider fiber bundles (from a mean value of approximately 150 nm to 1 to 2 μm, depending on the case, the visual appearance of the gels also gives support to this observation). The fibers of gels from 3·2Br are smaller, fused with each other and with poorly defined edges (Supporting Information, Figure S4) when compared to gels from 2·2Br where the fibers are relatively wider and more distinct.
The difference in morphology of the gels is echoed in the mechanical stability and viscoelastic properties of the materials. Rheological studies of the gels of pure 2·2Br and 2·2Br containing ibuprofen sodium salt prepared under both bulk and microfluidic mixing (configuration 1, flow in inlets 80:20:20:80) conditions show important differences in the materials. The use of microfluidic mixing increases both the resistance to deformation, as seen in the G′ and G″ values (storage and loss modulus) (Figure 7), and the resistance to rupture, as seen in the critical stress values (Table 3). In the case of the gel formed solely with 2·2Br (without drug), critical stress is increased up to 24% when using a microfluidic mixing platform as compared to the bulk process.
Figure 7.

Stress sweep tests of pure 2·2Br gels (A,B) and the same gelator with ibuprofen sodium salt (C,D) prepared by bulk mixing (A,C) or microfluidics (B,D).
Table 3. Rheological Properties of Gels from 2·2Br and Incorporating Ibuprofen Sodium Salt (Ibu).
| drug | mixing method | critical stress (Pa) | crossover (Pa) |
|---|---|---|---|
| none | bulk | 10 | 45.8 |
| none | microfluidic | 12.4 | 36.8 |
| Ibu | bulk | 1.76 | 8.2 |
| Ibu | microfluidic | 4.18 | 19.2 |
The mechanical properties of the gel are also affected by the incorporation of a drug. The gel incorporating ibuprofen sodium salt prepared by the bulk process shows 82% less resistance to rupture when compared to the gel without drug. However, using microfluidic mixing, the gels with sodium ibuprofenate are more than twice resistant to rupture, and four times more flexible, as seen in G′ values, when compared to the bulk process gel with ibuprofen sodium salt (Figure 7). All these data show that the supramolecular differences found in gels as a result of using the microfluidic mixing significantly enhance their mechanical and viscoelastic properties.
The surrounding of the drug molecule in the gel formed with an equimolar amount of 2·2Br and ibuprofen sodium salt using the microfluidic system (at 80:20:20:80 flow ratios) was analyzed by infrared (IR) spectroscopy and powder X-ray diffraction (PXRD) experiments. The IR spectra show, besides the peaks that correspond to the gelator, the presence of a peak at 1714 cm–1 that corresponds to the carboxylate group of ibuprofen sodium salt (Supporting Information, Figure S5), but no shifts in the signals from either compound corresponding to the possible host–guest inclusion were witnessed, implying that any contact between the gelator and the drug must be at the interfaces between the materials. The PXRD (Figure 8) study confirmed this hypothesis. The diffractograms of the gel formed by 2·2Br with ibuprofen sodium salt (1:1 molar ratio), from both bulk and microfluidic mixing experiments, show a peak at about 3.5 in 2θ that corresponds to the crystalline ibuprofen sodium salt,21 suggesting that the drug crystallized in the gel without incorporating into the bulk structure of the gel fibers. However, as presented above, drug crystals are not visible in the images obtained by SEM (or by optical microscopy), and thus, a layered structure must be present in the fibers with areas of gelator and drug, presumably held together by electrostatic interactions at the interfaces between the materials. Interestingly, the peak that corresponds to ibuprofen sodium salt is more intense in the gel from microfluidics (Figure 8B), which suggests that the fiber growth occurs along this crystallographic direction of the drug. The gelator has similar relative intensities in diffraction peaks, which suggests that the fiber growth is favored under both bulk and microfluidic mixing conditions.
Figure 8.
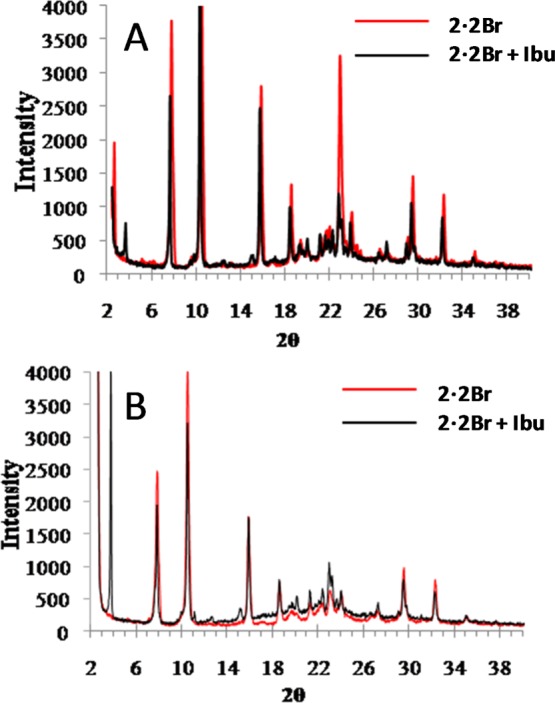
Powder X-ray diffractograms of xerogels formed with 2·2Br, without drug and with ibuprofen sodium (1:1 molar ratio) prepared (A) in bulk solution and (B) using microfluidic at a flow rate ratio of 80:20:20:80 in configuration 1.
These results concerning long-range order in the gels contrast with the ones seen with gels prepared using the analogous gelator containing 18 carbon atoms in the alkyl chain, where no drug crystallinity could be observed by PXRD (albeit it at a lower drug loading).21 Although the higher drug loading used in these gels may contribute to this factor, the morphology of the drug is clearly modified and the crystalline areas are indistinguishable from those of the gelator.
The ability of the drug-loaded gels to release the incorporated compound was studied in materials with the highest ibuprofen sodium salt content. Both, the gel prepared with mixing in the microfluidic platform (flow rate ratio of 80:20:20:80 in configuration 1) and the gel obtained with bulk preparation, were studied for comparison purposes. The release was performed in vitro using a Franz-cell system, with a receptor phase with pH 7.4 all maintained at 37 °C to simulate human physiological conditions. The amount of the released drug was calculated as a percentage of the total amount of the drug present in the gel. Three different kinetic models that can describe the drug release from hydrogels (first-order release, Peppas–Korsmeyer and Higuchi) were used to fit the experimental data obtained. The Akaike information criterion (AIC) was calculated to determine which model presents the best adjustment (see the Supporting Information, Table S3). The release profiles for the two gels are shown in Figure 9.
Figure 9.
Drug release profiles, at pH 7.4 and 37 °C, of ibuprofen sodium salts from the gels of 2·2Br obtained by microfluidics and bulk gelation processes.
The most important difference found between the gels is the release kinetics. The gel prepared in bulk follows first-order release kinetics, whereas that obtained with the microfluidic system follows a Higuchi model, which resembles a first-order model during the first hours, but does not reach a plateau, suggesting that the drug could keep releasing with time. Regarding the speed of the drug release, the bulk prepared gel shows a faster release, with a constant KD of 0.017 h–1. The maximum amount of ibuprofen salt that can be released is around 55%, and after 40 h, half of this value is already reached. The gel prepared using microfluidics, on the other hand, shows a slower release profile. The KD is 3.08 h–1, and after 48 h only 20% of the total drug is released. The release of 50% of the total amount of drug would take around 260 h. Even though the initial release seems faster, afterward, it slows down. The release is constant, but the amount being released is very small and the time interval is very wide.
All these differences found in the drug release profiles between microfluidics and bulk prepared gels could be explained by the morphological and rheological differences between them. With regard to the previous studies with gelators with a similar structure, but with longer alkyl chains, it was found that more than 75% of ibuprofen salt was released under similar conditions, although loading of the drug was much lower (gelator/drug ratio 4:1).21 In the present case, the bulk gel can release up to 50%. It is sure that these gels are able to load a much higher amount of the drug (gelator/drug ratio 1:1) when compared to the previously described ones, which means that the amount of the drug released per weight of the composite is approximately five times higher. Furthermore, the effect of microfluidic mixing produces a different release profile to bulk mixing and therefore is a useful tool to employ in the preparation of gel-derived release systems.
Conclusions
The gels described here show the dramatic effect that the chain length of the gelator can have on the incorporation of guest drug compounds and their morphology and consequently on their release characteristics. These effects are true for samples prepared in bulk solution mixing conditions as well as using a microfluidic system to mix the two solution components of the far-from-equilibrium system. In microfluidic mixing conditions, the flow rates and residence time inside the microchannel greatly influence the gel formation. We show an order of magnitude increase in the speed of gelation in certain cases, for example, the 2:1 gelator/indomethacin sample gels in 120 h without microfluidic mixing and only 2–3 h with microfluidic mixing. The effect of diffusive mixing taking place at the early stages of assembly—as is the case for liposome systems38—is propagated into the formation of the gel, even in the presence of drugs that are incorporated. These effects may be of interest for the preparation of other composite materials, such as gel–nanoparticle materials,39 and for the use of this kind of mixing in additive manufacturing applications of soft materials.40 The gels formed under microfluidic mixing here were found to be more stable, consistent, and with better drug incorporation than the gels from bulk gelation, and the combination of gels for vehicles and microfluidics for mixing are promising variables in the search for materials for drug delivery.
Experimental Section
General Methods
The SEM images of the xerogels were acquired by the electron microscopy service in the ICMAB-CSIC on a Quanta FEI 200 FEG-ESEM system. The dry gels on the carbon tape were coated with gold before imaging to avoid the charging of the samples. All the images were taken at 15 kV, with a spot size of 3–4 and a working distance of ≈ 10 mm under high vacuum conditions. AFM images were recorded by the scanning probe microscopy service at ICMAB-CSIC on a PicoSPM system (molecular imaging). The intermittent contact mode was used close to resonance frequencies of the silicon cantilevers (nanosensors, FM type force constant 1.2–3.5 N/m and tip diameter 5 nm) of around 60–70 kHz. All the images were recorded under atmospheric conditions.
The IR spectra of dry gels were obtained with spectrometer PerkinElmer Spectrum One FT-IR, energy range: 450–4000 cm–1 using the universal attenuated total reflectance accessory (U-ATR).
XRD measurements were performed with a Siemens D-5000 X-ray diffractometer. The source was a DRX ceramic tube (λ Cu Kα = 1.540560 Å and λ Cu Kα 2 = 1.544390 Å) with a voltage and current of 45 kV and 35 mA, respectively. The gel was mounted on a glass slide and dried and was scanned from 2θ = 2.5 to 2θ = 75°.
DLS was performed using Zetasizer Nano ZS, Malvern Instruments for the samples from microfluidics which formed turbid solution at room temperature. Only the solution was taken in the cuvette.
The rheological characterization was performed at 32 °C using a HAAKE RheoStress 1 rheometer (Thermo Fisher Scientific, Karlsruhe, Germany) connected to a Thermo Haake Phoenix II + Haake C25P temperature controller. The rheological studies were carried out on freshly prepared samples in the native state. The oscillatory test was conducted with a plate–plate setup (Haake PP60 Ti, 60 mm diameter, 1 mm gap between plates), by performing oscillatory stress sweeps between 0.1 and 100 Pa at 1 Hz, to determine the resistance to deformation, related to storage modulus (G′) and loss modulus (G″), and the phase shift (δ). Both the viscoelastic moduli are defined as follows: G′ = τ0/γ0 cos δ and G″ = τ0/γ0 sin δ (where τ0 and γ0 are the amplitudes of stress and strain).
Microfabrication of Microfluidic Chips
The microchannels were formed by transferring the complementary structure of a silicon master (fabricated by the standard photolithography of SU-8 epoxy resist on the silicon substrate) to PDMS and bonding the latter to glass. The length, width, and height of the microreactor in the chip are 10 mm, 250 μm, and 50 μm, respectively. Fabrication of the PDMS chips was carried out in the Nanoquim platform class 10000 (ISO7) clean room facility (ICMAB-CSIC scientific and technical services) under controlled room conditions (T = 21 °C, ΔP = 20 mbar and RH ≤ 45%). PDMS (20 g) and curing agent (1.8 g) were taken and mixed well. The mixture was degassed with vacuum for 30 min. Then the mixture was poured without any bubbles onto a mold which was placed on the silicon master containing the structure (complementary structure of microchannels). It was then cured in an oven at 150 °C for 10 min. PDMS got solidified, and holes were made at the ends of the inlet and outlet channels for the external connection. The surface of PDMS which contains the structure (channels) and a side of a glass slide were exposed to nitrogen plasma for 1 min and 30 s. PDMS was placed on the glass slide (with the sides exposed to plasma), pressed well, and kept on a hot plate at 75 °C overnight for the bonding to occur.
Bulk Gelation
For all bulk gelation experiments without any drug, the gelator was dissolved in ethanol, and water was added (v/v ratio depends on the experiment, see Table 1) and mixed well. For the gel with ibuprofen (sodium salt), the drug was dissolved in water and added to the ethanolic solution of the gelator and mixed well. For the gels with indomethacin (sodium salt), both gelator and drug were dissolved in ethanol and water was added to the solution and mixed well by stirring by hand. All the experiments were done at room temperature and were kept undisturbed after mixing until the formation of gels.
Mixing Using a Microfluidic System for Gelation
Experiments using the microfluidic chips described above were carried out by taking the solutions in the syringe (volume and concentration depend on the need of the experiment) that were connected to the holes of the inlet channels of the PDMS chip through a tube of 1 mm diameter. The flow rates (see Table 2) were controlled by neMESYS low-pressure syringe pumps, Cetoni Automation and Microsystems GmbH, Germany. The output of the experiments was collected with a small outlet tube (1 mm in diameter) connected to the hole in the outlet channel. The neMESYS user interface software was used to control the syringe pumps.
Drug Release Experiments
For the release study, two gels were chosen: the gel obtained using the microfluidic system (configuration 1 flow rate ratios 80:20:20:80), and the corresponding gel obtained in bulk conditions with an ethanol/water proportion of 20:80 and a gelator/drug molar ratio of 1:1. The release of the drugs from the gels was performed in a Microette transdermal diffusion system (Microette plus-Hanson Research) with vertically assembled Franz-type diffusion cells (crown glass). Dialysis membranes (Cellu Sep T3 dialysis membrane, MWCO 12 000–14 000 Da, MFPI, USA), previously hydrated in water/methanol 1:1, were placed in the Franz-type diffusion cells. Known weights of gel were placed into the donor compartment onto the dialysis membranes. The dialysis membrane and the donor container were put onto the glass receptor chamber, and the assembly was fixed with a joint. The receptor chamber contained phosphate-buffered saline 100 mM pH 7.4, which complies with the SINK conditions. The Franz-type cells were connected to a controlled temperature system, with a heating bath set to 37 °C. Samples were taken at given time intervals, and the sample taken was replaced by an equal volume of the receptor solution.
Drug determination in samples was done by high-performance liquid chromatography in a Waters LC module I, in a Waters Spherisorb 5 μm ODS-2 (4.6 mm × 150 mm) column. The mobile phase consisted of acetonitrile/water (acidified to pH 3 with phosphoric acid) 65:35, with a flow rate of 1.5 mL min–1, and the detection wavelength was set to 220 nm. The data were collected using Millennium32 version 4.0.0 software from Waters Corp. All data were calculated as the average ± standard deviation of three replicates. A nonlinear least-squares regression was performed using the WinNonLin software (WinNonlin Professional edition version 3.3, Pharsight Corp., USA), and the model parameters were calculated. Modelistic parameters were statistically compared by using Statgraphics software version 5.1.
Acknowledgments
Support by MINECO (project TEC2014-51940-C2-2-R) is acknowledged. The authors thank Judith Oró and Maite Simón (Scientific Experimental Platforms, ICMAB-CSIC) for their help with SEM and AFM, respectively, Lyda Halbaut Bellowa and Ana Calpena (Universitat de Barcelona) for the rheology studies, and Cristina Oliveras-Gonzalez for her guidance in synthesis. D.L. thanks the CONACYT for a predoctoral grant. D.B.A. thanks GSK, EPSRC (EP/NO24818/1), and the University of Nottingham for funding. G.S. thanks SASTRA University, India for the financial support and the opportunity to be a part of the Semester Abroad Programme.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01800.
Additional photographs of the gels; graphs showing size distribution of fibers; additional SEM images and IR spectra; tables’ conditions for all the experiments performed to obtain gels using 1·2Br, 2·2Br, and 3·2Br in bulk solution conditions; and conditions for all the experiments performed to obtain gels using 2·2Br and 3·2Br using microfluidic mixing and kinetic models used to fit the data for the release of ibuprofen (sodium salt) from the gels and the respective AIC parameter (PDF)
Author Present Address
¶ Division of Pharmaceutical Chemistry and Technology, Faculty of Pharmacy, FI-00014 University of Helsinki, Helsinki, Finland (G.S.).
Author Present Address
∇ Vall d’Hebron Institute of Oncology (VHIO), Psg. Vall d’Hebron 119-129, 08035 Barcelona (M.R.).
Author Present Address
○ Department d’Enginyeries: Electrònica, Universitat de Barcelona, C/Martí i Franquès 1, 08028 Barcelona, Spain (R.R.-T.).
Author Present Address
◊ School of Pharmacy, The University of Nottingham, University Park, Nottingham, NG7 2RD, England, UK (L.P.-G.).
The authors declare no competing financial interest.
Supplementary Material
References
- Du X.; Zhou J.; Shi J.; Xu B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. 10.1021/acs.chemrev.5b00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.; Tan X.; Wang Z.; Zhang X. Supramolecular Polymers: Historical Development, Preparation, Characterization, and Functions. Chem. Rev. 2015, 115, 7196–7239. 10.1021/cr500633b. [DOI] [PubMed] [Google Scholar]
- Cornwell D. J.; Smith D. K. Expanding the scope of gels—combining polymers with low-molecular-weight gelators to yield modified self-assembling smart materials with high-tech applications. Mater. Horiz. 2015, 2, 279–293. 10.1039/c4mh00245h. [DOI] [Google Scholar]
- Voorhaar L.; Hoogenboom R. Supramolecular polymer networks: hydrogels and bulk materials. Chem. Soc. Rev. 2016, 45, 4013–4031. 10.1039/c6cs00130k. [DOI] [PubMed] [Google Scholar]
- Hoare T. R.; Kohane D. S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. 10.1016/j.polymer.2008.01.027. [DOI] [Google Scholar]
- Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- Karavasili C.; Fatouros D. G. Smart materials: in situ gel-forming systems for nasal delivery. Drug Discovery Today 2016, 21, 157–166. 10.1016/j.drudis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Fakhari A.; Subramony J. A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Controlled Release 2015, 220, 465–475. 10.1016/j.jconrel.2015.11.014. [DOI] [PubMed] [Google Scholar]
- McKenzie M.; Betts D.; Suh A.; Bui K.; Kim L.; Cho H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. 10.3390/molecules201119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah D. J.; Weiss R. G. Organogels and low molecular mass organic gelators. Adv. Mater. 2000, 12, 1237–1247. . [DOI] [Google Scholar]
- Sangeetha N. M.; Maitra U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]
- Functional Molecular Gels; Escuder B., Miravet F., Eds.; RSC, 2014. [Google Scholar]
- Terech P.; Weiss R. G. Low molecular mass gelators of organic liquids and the properties of their gels. Chem. Rev. 1997, 97, 3133–3159. 10.1021/cr9700282. [DOI] [PubMed] [Google Scholar]
- Weiss R. G. The Past, Present, and Future of Molecular Gels. What Is the Status of the Field, and Where Is It Going?. J. Am. Chem. Soc. 2014, 136, 7519–7530. 10.1021/ja503363v. [DOI] [PubMed] [Google Scholar]
- Skilling K. J.; Citossi F.; Bradshaw T. D.; Ashford M.; Kellam B.; Marlow M. Insights into low molecular mass organic gelators: a focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. 10.1039/c3sm52244j. [DOI] [PubMed] [Google Scholar]
- Amabilino D. B.; Smith D. K.; Steed J. W. Supramolecular Materials. Chem. Soc. Rev. 2017, 46, 2404–2420. 10.1039/c7cs00163k. [DOI] [PubMed] [Google Scholar]
- Casal-Dujat L.; Rodrigues M.; Yagüe A.; Calpena A. C.; Amabilino D. B.; González-Linares J.; Borràs M.; Pérez-García L. Gemini imidazolium amphiphiles for the synthesis, stabilization, and drug delivery from gold nanoparticles. Langmuir 2012, 28, 2368–2381. 10.1021/la203601n. [DOI] [PubMed] [Google Scholar]
- Casal-Dujat L.; Griffiths P. C.; Rodríguez-Abreu C.; Solans C.; Rogers S.; Pérez-García L. Nanocarriers from dicationic bis-imidazolium amphiphiles and their interaction with anionic drugs. J. Mater. Chem. B 2013, 1, 4963–4971. 10.1039/c3tb20289e. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.; Calpena A. C.; Amabilino D. B.; Ramos-López D.; de Lapuente J.; Pérez-García L. Water-soluble gold nanoparticles based on imidazolium gemini amphiphiles incorporating piroxicam. RSC Adv. 2014, 4, 9279–9287. 10.1039/c3ra44578j. [DOI] [Google Scholar]
- D’Anna F.; Vitale P.; Marullo S.; Noto R. Geminal Imidazolium Salts: A New Class of Gelators. Langmuir 2012, 28, 10849–10859. 10.1021/la301319u. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.; Calpena A. C.; Amabilino D. B.; Garduño-Ramírez M. L.; Pérez-García L. Supramolecular gels based on a gemini imidazolium amphiphile as molecular material for drug delivery. J. Mater. Chem. B 2014, 2, 5419–5429. 10.1039/c4tb00450g. [DOI] [PubMed] [Google Scholar]
- Limón D.; Amirthalingam E.; Rodrigues M.; Halbaut L.; Andrade B.; Garduño-Ramírez M. L.; Amabilino D. B.; Pérez-García L.; Calpena A. C. Novel nanostructured supramolecular hydrogels for the topical delivery of anionic drugs. Eur. J. Pharm. Biopharm. 2015, 96, 421–436. 10.1016/j.ejpb.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Park J. I.; Saffari A.; Kumar S.; Günther A.; Kumacheva E. Microfluidic Synthesis of Polymer and Inorganic Particulate Materials. Annu. Rev. Mater. Res. 2010, 40, 415–443. 10.1146/annurev-matsci-070909-104514. [DOI] [Google Scholar]
- Mark D.; Haeberle S.; Roth G.; von Stetten F.; Zengerle R. Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. 10.1039/b820557b. [DOI] [PubMed] [Google Scholar]
- Watts P.; Haswell S. J. Microfluidic combinatorial chemistry. Curr. Opin. Chem. Biol. 2003, 7, 380–387. 10.1016/s1367-5931(03)00050-4. [DOI] [PubMed] [Google Scholar]
- deMello A. J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. 10.1038/nature05062. [DOI] [PubMed] [Google Scholar]
- Fujii T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61–62, 907–914. 10.1016/s0167-9317(02)00494-x. [DOI] [Google Scholar]
- Nisar A.; Afzulpurkar N.; Mahaisavariya B.; Tuantranont A. MEMS-based micropumps in drug delivery and biomedical applications. Sens. Actuators, B 2008, 130, 917–942. 10.1016/j.snb.2007.10.064. [DOI] [Google Scholar]
- Oh J. K.; Drumright R.; Siegwart D. J.; Matyjaszewski K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. 10.1016/j.progpolymsci.2008.01.002. [DOI] [Google Scholar]
- Jahn A.; Reiner J. E.; Vreeland W. N.; DeVoe D. L.; Locascio L. E.; Gaitan M. Preparation of nanoparticles by continuous-flow microfluidics. J. Nanopart. Res. 2008, 10, 925–934. 10.1007/s11051-007-9340-5. [DOI] [Google Scholar]
- Jahn A.; Vreeland W. N.; Gaitan M.; Locascio L. E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. 10.1021/ja0318030. [DOI] [PubMed] [Google Scholar]
- McDonald J. C.; Duffy D. C.; Anderson J. R.; Chiu D. T.; Wu H.; Schueller O. J. A.; Whitesides G. M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. . [DOI] [PubMed] [Google Scholar]
- McDonald J. C.; Whitesides G. M. Poly(dimethylsiloxane) as a Material for Fabricating Microfluidic Devices. Acc. Chem. Res. 2002, 35, 491–499. 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- Rossow T.; Heyman J. A.; Ehrlicher A. J.; Langhoff A.; Weitz D. A.; Haag R.; Seiffert S. Controlled Synthesis of Cell-Laden Microgels by Radical-Free Gelation in Droplet Microfluidics. J. Am. Chem. Soc. 2012, 134, 4983–4989. 10.1021/ja300460p. [DOI] [PubMed] [Google Scholar]
- Sorrenti A.; Rodriguez-Trujillo R.; Amabilino D. B.; Puigmartí-Luis J. Milliseconds Make the Difference in the Far-from-Equilibrium Self-Assembly of Supramolecular Chiral Nanostructures. J. Am. Chem. Soc. 2016, 138, 6920–6923. 10.1021/jacs.6b02538. [DOI] [PubMed] [Google Scholar]
- Casal-Dujat L.; Penon O.; Rodríguez-Abreu C.; Solans C.; Pérez-García L. Macrocyclic ionic liquid crystals. New J. Chem. 2012, 36, 558–561. 10.1039/c2nj20934a. [DOI] [Google Scholar]
- Casal Dujat des Allumes L.Ph.D. Thesis, Universitat de Barcelona, 2010. [Google Scholar]
- Jahn A.; Vreeland W. N.; DeVoe D. L.; Locascio L. E.; Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir 2007, 23, 6289–6293. 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.; Genç A.; Arbiol J.; Amabilino D. B.; Pérez-García L. In situ template synthesis of gold nanoparticles using a bis-imidazolium amphiphile-based hydrogel. J. Colloid Interface Sci. 2015, 446, 53–58. 10.1016/j.jcis.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Sommer M. R.; Alison L.; Minas C.; Tervoort E.; Rühs P. A.; Studart A. R. 3D printing of concentrated emulsions into multiphase biocompatible soft materials. Soft Matter 2017, 13, 1794–1803. 10.1039/c6sm02682f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






