Abstract

A concise, metal-free, and gram-scale strategy to convert indoline-2,3-diones to 1,2,4-oxadiazole[4,5-a]indolones through an improved [3 + 2] cycloaddition of α-ketone-lactam with nitrile oxides has been developed. The lactim form of the resonance structure of isatin in protic solvents is the key active dipolarophile that shows chemo- and regioselectivity under experimental and theoretical conditions. This strategy conveniently enabled the assembly of several 1,2,4-oxadiazole[4,5-a]indolines with a broad range of functional groups. Compounds 3a and 4b exhibit cytotoxicity in the NCI/ADR-RES, SKOV3, and OVCAR8 cell lines.
Introduction
Lactams (−NH–CO−) are commonly present in many natural products such as isatins, uracils, and purines. Previous experiments1 and quantum chemistry2 studies have confirmed that lactams have the resonance structure of lactims [−N=C(OH)−] and the tautomeric ratios3 that depend on temperature, solvent, and pH. The C=N double bond of lactim is similar to the C=N moiety of the imine or the imidate ester. In 2012, Jay4 reported the cycloaddition of nitrile imines with an α,β-unsaturated lactam; however, the reactive dipolarophile is an α,β-unsaturated C=C double bond rather than a lactam. Using lactam as a tautomeric dipolarophile (lactim) incorporated with nitrile oxide to prepare a heteropolycyclic system has not been investigated previously (Scheme 1c).
Scheme 1. Synthetic Strategies toward 1,2,4-Oxadiazoles: (a) Common Methods Based on Nitrile. (b) Example of Modern 1,2,4-Oxadiazole Synthesis and (c) Present Work.
1,2,4-Oxadiazoles are important building blocks5 found in natural6 and synthesized products, and they are often used in medicinal chemistry7 and material sciences.8 There are several 1,2,4-oxadiazole pharmaceutical products (Figure 1), such as Translarna,9 used for the treatment of Duchenne muscular dystrophy and cystic fibrosis. Several 1,2,4-oxadiazoles were reported to have antimicrobial, antipsychotic,7a antitumor,7b anticonvulsant,7c antithrombotic, and anti-Alzheimer’s disease7a properties. They are also used in liquid crystals,8b organic light-emitting diodes (OLEDs),8d and fluorogenic chemosensory8a and high-energy materials.8c
Figure 1.
Select examples of biologically active 1,2,4-oxadiazole compounds.
In general, there are two examples of [3 + 2] and modified [4 + 1] strategies to synthesize 1,2,4-oxadiazoles,10 both of which involve a nitrile as a key synthon (Scheme 1a). One pathway exploits the 1,3-dipolar cycloaddition of nitriles with nitrile oxides to directly produce 1,2,4-oxadiazoles [Scheme 1a(1)]. Another major route is based on the prefunctionalization of the nitrile to an amidoxime prepared by the reaction with hydroxylamine and the subsequent reaction with a wide variety of activated substrates, such as carboxylic acids, esters, acid chlorides, or the amidoxime itself, which leads to the formation of 1,2,4-oxadiazoles upon heating [Scheme 1a(2)]. Recent syntheses of 1,2,4-oxadiazoles have focused on alternative methodologies developed to generate only particularly reactive precursors in situ (Scheme 1b).11 Some catalytic pathways have also been exploited to assist the cyclization step or even the initial O-acylation of the amidoxime.12 However, these reactions require organometallics, strongly acidic or basic conditions, and high temperatures that lead to low yields and complicated workups. Reported syntheses of 1,2,4-oxadiazole[4,5-a]indolones are quite rare.13 During the early development of this chemistry, Miller and Scrowston13a reported the syntheses of 9a-ethoxy-9,9a-dihydro-3-(aryl)-1,2,4-oxadiazolo[4,5-a]indol-9-one in 28–62% yield, by reacting nitrile oxides generated in situ in benzene with prefabricated 2-ethoxy-1H-indol-2-one. Other works attempted to increase the reactivity of the C=N double bond of dipolarophiles in imines (−C=N−)13f−13h or imidate esters [−(EtO)C=N−]13j−13l by the prefunctionalization of indolines or imidazoles. The starting materials, such as 2,3,3-trimethyl-3H-indole, are limited because of synthetic difficulties. Little advancement has been made toward the development of a general and reliable method for the synthesis of 1,2,4-oxadiazole[4,5-a]indolones using nitrile oxide dipolar cycloaddition chemistry. Thus, efforts to modernize the synthetic methods are certainly necessary. Isatin represents the first recognized case14 of having tautomeric forms of lactam and lactim. Moreover, most of the substituted isatins are readily accessible.15 In the present work, we not only explored a new, green, efficient, and chemoselective approach to obtain 1,2,4-oxadiazole[4,5-a]indolines but also developed a new type of potentially bioactive heterocyclic system.
Results and Discussion
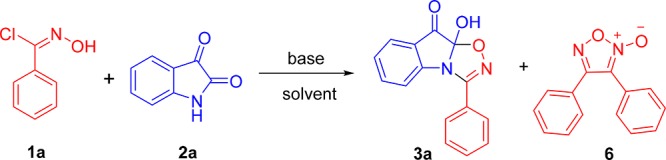
We initially attempted to react indoline-2,3-dione (2a) with in situ-generated nitrile oxide via a fluoride ion16-mediated dehydrochlorination of the N-hydroximoyl chloride (1a). However, both CsF and tetra-n-butylammonium fluoride (TBAF) yielded the dimerized products (6) of phenyl nitrile oxide in aprotic solvents (Table 1, entry 1–2) such as tetrahydrofuran (THF) and dichloromethane (DCM). The less expected compound 3a (entry 3–4, 34%, 31%) was formed in EtOH or MeOH (detected by high-resolution high-performance liquid chromatography–mass spectrometry (HPLC–MS) and proton nuclear magnetic resonance (1H NMR)). When the reaction was mediated by other organic bases such as Et3N and N,N-diisopropylethylamine (DIPEA) instead of the fluoride ion, the yield of 3a was significantly increased in EtOH (entry 7, 12, 73%, 71%) and MeOH (entry 8, 13, 76%, 73%). Meanwhile, dimer 6 was still the main product in THF and DCM (entry 5, 6, 10, 11). These results suggest that the protic solvents can promote the cycloaddition of nitrile oxide with isatin. In addition, an attempt to elevate reaction temperature to increase the rate of [3 + 2] cycloaddition was not successful (entry 9). This may be due to the decomposition of product 3a and dimer 6 under high temperature. Other solvents, such as isopropanol, water, dioxane, and dimethylformamide (DMF) were screened to optimize the [3 + 2] cycloaddition conditions (entry 14–17). To our surprise, the reaction proceeded well to obtain a high yield (70%) of 3a in water (entry 15) and a very high yield (84%) of 3a in isopropanol (entry 14). Further screening using NaHCO3 as a base produced a high yield (72%) of 3a (entry 18). Notably, the cycloaddition reaction can proceed in water, MeOH, EtOH, or isopropanol without any base to obtain a low to moderate yield (40–56%) of 3a (entry 21–24).
Table 1. Optimization Conditions for the Reaction of Indoline-2,3-dione (2a) and N-Hydroximoyl Chloride (1a)a.
| yield
(%)b |
||||||
|---|---|---|---|---|---|---|
| entry | base | solvent | Time (h) | t (°C) | 3a | 6 |
| 1 | CsF | THF | 10 | rt | 3 | 86 |
| 2 | CsF | DCM | 10 | rt | 3 | 90 |
| 3 | CsF | EtOH | 10 | rt | 34 | 65 |
| 4 | TBAF | MeOH | 10 | rt | 31 | 68 |
| 5 | Et3N | THF | 10 | rt | 6 | 78 |
| 6 | EtE3N | DCM | 10 | rt | 5 | 80 |
| 7 | Et3N | EtOH | 10 | rt | 73 | 11 |
| 8 | Et3N | MeOH | 10 | rt | 76 | 15 |
| 9 | Et3N | MeOH | 10 | 80 °C | 30 | 8 |
| 10 | DIPEA | THF | 10 | rt | 4 | 79 |
| 11 | DIPEA | DCM | 10 | rt | 5 | 78 |
| 12 | DIPEA | EtOH | 10 | rt | 71 | 10 |
| 13 | DIPEA | MeOH | 10 | rt | 73 | 13 |
| 14 | Et3N | isopropanol | 5 | rt | 84 | 9 |
| 15 | Et3N | waterc | 5 | rt | 70 | 11 |
| 16 | Et3N | dioxane | 5 | rt | 6 | 86 |
| 17 | Et3N | DMF | 5 | rt | 9 | 89 |
| 18 | NaHCO3 | waterc | 5 | rt | 72 | 12 |
| 19 | NaHCO3 | isopropanol | 5 | rt | 68 | 13 |
| 20 | NaHCO3 | EtOH | 5 | rt | 65 | 11 |
| 21 | d | waterc | 5 | rt | 56 | 12 |
| 22 | d | EtOH | 5 | rt | 45 | 19 |
| 23 | d | MeOH | 5 | rt | 40 | 11 |
| 24 | d | isopropanol | 5 | rt | 41 | 12 |
General conditions: hydroxybenzimidoyl chloride (1a, 0.6 mmol, 1.2 equiv), indoline-2,3-dione (2a, 0.5 mmol), base (1.0 mmol, 2 equiv), and solvent (15 mL).
Isolated yield based on 2a.
Water/isopropanol = 95:5.
No base was used.
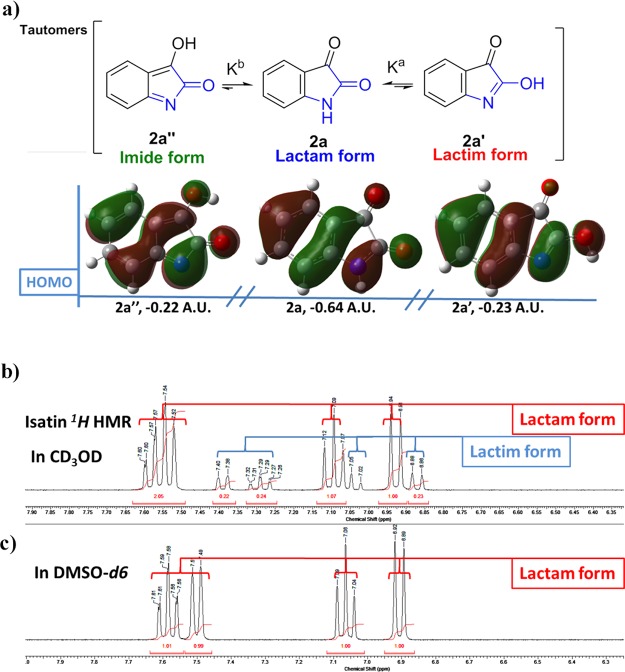
To explore the cause of 1,2,4-oxadiazole[4,5-a]indolone production by isatin and nitrile oxide in different solvents, we first utilized the equilibrium constant17Kn of tautomerization for isatin (Figure 2a, the lactam form, the lactim form, and the imide form) to understand the origins of the reactivity difference in various solvents. The values of Kcacula and Kcacul calculated by the density functional theory analysis are listed in Table S1. The experimental value of Kexp.a was also obtained from the 1H NMR (Figure 2c) of isatin in different solvents. Table S1 shows that the Kcacul values in protic solvents are almost 1000 times greater than those in aprotic solvents; for example, Kcacula in D2O and CD3Cl are 0.13 and 4.89 × 10–3, respectively. These results are consistent with the experimental Kexp. values (Kexp.a in D2O and CDCl3 is 0.18 and 0, respectively). Meanwhile, Kcacul in seven solvents is less than 5.00 × 10–4, indicating that it is difficult for the imine tautomer of isatin to survive in protic and aprotic solvents. These results suggest that the lactim tautomer of isatin is more likely to react with nitrile oxide when the lactam form and lactim form coexist in the solvent.
Figure 2.
(a) Tautomerization of isatin and (b) 1H NMR of isatin in CD3OD and dimethyl sulfoxide (DMSO)-d6 solvent.
On the other hand, there are two regioisomeric pathways (Figure 3, ortho and meta) for nitrile oxide to react with dipolarophile isatin. The cycloaddition of isatin lactim form 2a′ with nitrile oxide in the ortho attack via the transition state TSortho leads to product 3a. Similarly, the cycloaddition of isatin lactim-form 2a′ with nitrile oxide in the meta attack via the transition state TSmeta leads to product 3a′. The corresponding structures and energy profiles are given in Figure 3. As expected, the ortho-cyclization mode is more favorable than the meta mode by about 11.55 kcal·mol–1. Therefore, this cycloaddition shows complete ortho regioselectivity. These results are in agreement with the experimental data in Table 1.
Figure 3.
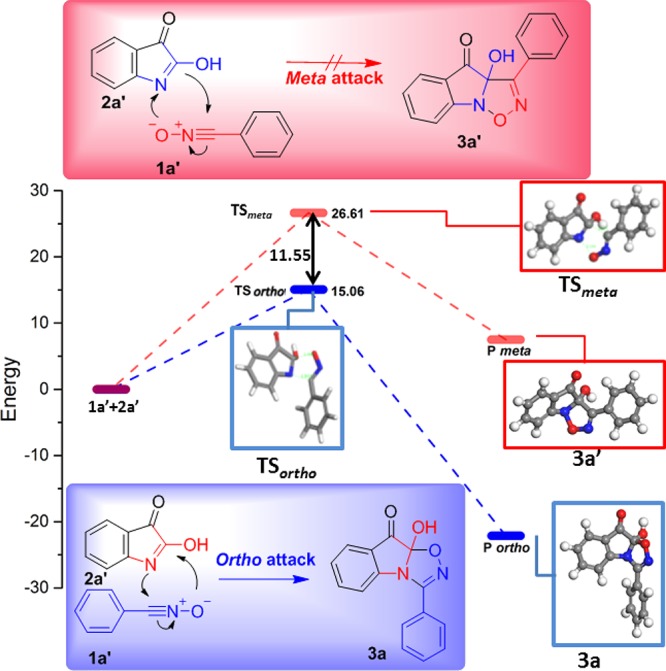
Energy profile in kcal·mol–1 for the 1,3-dipolar cycloaddition reaction between the dipolarophile 1a′ and the dipole 2a′.
Under optimal conditions, hydroxybenzimidoyl chloride (24 mmol, 1.2 equiv)/indoline-2,3-dione (20 mmol)/isopropanol/rt/Et3N (2.0 equiv) over 5 h and various N-hydroximoyl chlorides with electron-deficient or electron-rich groups were selected to investigate the scope of the [3 + 2] cycloaddition reaction. Both electron-rich and electron-deficient nitrile oxide precursors successfully participated in the [3 + 2] cycloaddition to provide 1,2,4-oxadiazolo[4,5-a]indol-9(9aH)-one (Table 2, 3a–4j). Higher yields were observed when R2 = H and N-hydroximoyl chlorides with electron-deficient substrates were used (Table 2, 3b, 3i, 3j, 3k, 3m, with 88, 88, 86, 85, 89% isolated yield), whereas electron-rich methyl or methoxy provided moderate to low yields (Table 2, 3b–3f). In general, the position of the functional group on the aromatic ring did not affect the reaction (Table 2, 3c, 3f–3h). In addition, the aromatic N-hydroximoyl chloride provided 1,2,4-oxadiazolo[4,5-a]indol-9(9aH)-one in good to excellent yields, whereas the heterocyclic or alkyl substrates gave low yields (Table 2, 3d and 3o).
Table 2. Select Examples of [3 + 2] Cycloaddition of in Situ-Generated Nitrile Oxides and Indoline-2,3-dionea.
| entry | 1 (R1) | 2 (R2) | 3 | yieldb (%) |
|---|---|---|---|---|
| 1 | 1a (Ph) | 2a (H) | 3a | 84 |
| 2 | 1b (4-F C6H4) | 2a (H) | 3b | 88 |
| 3 | 1c (4-OCH3 C6H4) | 2a (H) | 3c | 70 |
| 4 | 1d (i-Pr) | 2a (H) | 3d | 46 |
| 5 | 1e (4-CH3 C6H4) | 2a (H) | 3e | 77 |
| 6 | 1f (3-CH3 C6H4) | 2a (H) | 3f | 75 |
| 7 | 1g (2-CH3 C6H4) | 2a (H) | 3g | 79 |
| 8 | 1h (4-Et C6H4) | 2a (H) | 3h | 76 |
| 9 | 1i (3-F C6H4) | 2a (H) | 3i | 88 |
| 10 | 1j (2-F C6H4) | 2a (H) | 3j | 86 |
| 11 | 1k (4-Cl C6H4) | 2a (H) | 3k | 85 |
| 12 | 1l (3-Cl C6H4) | 2a (H) | 3l | 83 |
| 13 | 1m (2-Cl C6H4) | 2a (H) | 3m | 89 |
| 14 | 1n (4-Br C6H4) | 2a (H) | 3n | 82 |
| 15 | 1o (C4H3S)c | 2a (H) | 3o | 64 |
| 16 | 1a (Ph) | 2b (F) | 3p | 72 |
| 17 | 1e (4-CH3 C6H4) | 2b (F) | 3q | 69 |
| 18 | 1c (4-OCH3 C6H4) | 2b (F) | 3r | 70 |
| 19 | 1b (4-F C6H4) | 2b (F) | 3s | 87 |
| 20 | 1a (Ph) | 2c (OCH3) | 3t | 87 |
| 21 | 1e (4-CH3 C6H4) | 2c (OCH3) | 3u | 84 |
| 22 | 1g (2-CH3 C6H4) | 2c (OCH3) | 3v | 78 |
| 23 | 1b (4-F C6H4) | 2c (OCH3) | 3w | 96 |
| 24 | 1j (2-F C6H4) | 2c (OCH3) | 3x | 85 |
| 25 | 1a (Ph) | 2d (CH3) | 3y | 85 |
| 26 | 1e (4-CH3 C6H4) | 2d (CH3) | 3z | 82 |
| 27 | 1g (2-CH3 C6H4) | 2d (CH3) | 4a | 81 |
| 28 | 1b (4-F C6H4) | 2d (CH3) | 4b | 92 |
| 29 | 1i (3-F C6H4) | 2d (CH3) | 4c | 89 |
| 30 | 1j (2-F C6H4) | 2d (CH3) | 4d | 88 |
| 31 | 1k (4-Cl C6H4) | 2d (CH3) | 4e | 80 |
| 32 | 1a (Ph) | 2e (Br) | 4f | 83 |
| 33 | 1e (4-CH3 C6H4) | 2e (Br) | 4g | 81 |
| 34 | 1g (2-CH3 C6H4) | 2e (Br) | 4h | 82 |
| 35 | 1b (4-F C6H4) | 2e (Br) | 4i | 87 |
| 36 | 1k (4-Cl C6H4) | 2e (Br) | 4j | 85 |
General conditions: hydroxybenzimidoyl chloride (1, 24 mmol, 1.2 equiv), indoline-2,3-dione (2, 20 mmol), base (40 mmol, 2 equiv), and isopropanol (50 mL).
Isolated yield based on 2.
C4H3S = thiophene.
Modification of the electronic properties of the indoline-2,3-dione substrate was also explored. Electron-donating groups substituted at C-5 of indoline-2,3-dione were beneficial to the [3 + 2] cycloaddition. When the electron-donating 5-methoxyindoline-2,3-dione reacted with N-hydroxybenzimidoyl chloride, 3t was obtained with a good yield (Table 2, 87%). Furthermore, the combination of the electron-rich indoline-2,3-dione (2c) with the electron-deficient N-hydroxybenzimidoyl chloride (1b) produced 3w with an excellent yield (Table 2, 96%). However, when R2 was an electron-withdrawing group (e.g., R2 = Br), the reaction provided a moderate to low yield (Table 2, 3p–3s, 4f–4j).
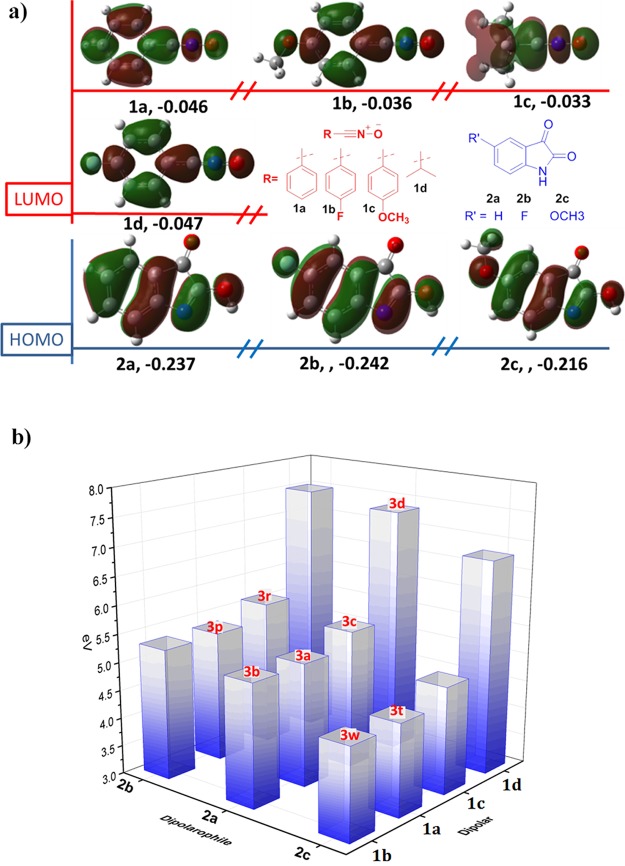
These different reactant activities for this reaction can also be understood by using a frontier molecular orbital (FMO) interaction energy ΔE. The 1,3-dipole cycloaddition is controlled by the highest occupied molecular orbital (HOMO) of dipolarophiles and the lowest unoccupied molecular orbital (LUMO) of dipolars,18a−18c and the chemical reactivity increases with the decrease in the ΔE value (LUMOdipolar – HOMOdipolarophile). Figure 4a shows the computed molecular orbitals of isatin derivatives (lactim form) and nitrile oxides, and Figure 4b shows the ΔE between dipolars (1a, 1b, 1c, 1d) and dipolarophiles (2a, 2b, 2c). The most electron-rich dipole 1b and the most electron-deficient dipolarophile 2c give the lowest ΔE to efficiently obtain the cycloaddition product 3w. On the other hand, the alkyl-substituted nitrile oxide 1d increased the FMO interaction energy, which leads to the reduced cycloaddition activity of 3d.
Figure 4.
(a) Computed LUMOs of dipoles 1a, 1b, 1c, and 1d and the HOMOs of dipolarophiles 2a, 2b, and 2c in eV and calculated using B3LYP/6-31G(d,p)/IEFPCM//M06-2X/6-31G(d,p)/IEFPCM and (b) frontier orbital interaction energy (ΔE = LUMOoxide – HOMOisatin) between dipoles and dipolarophiles in kcal·mol–1.
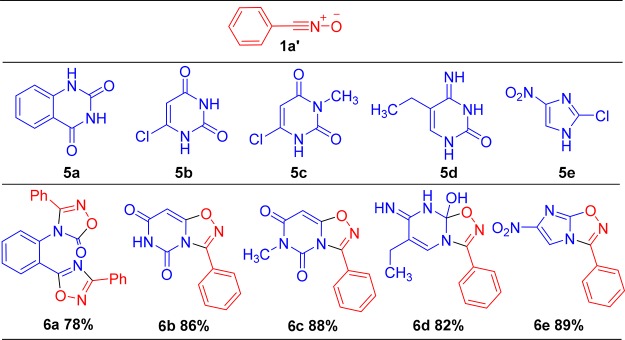
Then, we investigated the cycloaddition of tautomeric-dependent benzouracil and uracil derivatives with nitrile oxide for the synthesis of 1,2,4-oxadiazole. The nitrile oxide reacted with 5a, 5b, 5c, 5d, and 5e, giving the 1,2,4-oxadiazole derivatives 6a, 6b, 6c, 6d, and 6e with 78, 86, 76, 82, and 89% yields, respectively (Scheme 2 and see 1H and 13C NMR in the Supporting Information). This tautomeric-dependent cycloaddition could be employed in either water or alcohol without any base to obtain specific chemo- and regioselectivity.
Scheme 2. Synthesis of 1,2,4-Oxadiazole Derivations.
Reaction conditions: hydroxybenzimidoyl chloride (24 mmol, 1.2 equiv)/dipolarophile (20 mmol)/isopropanol/rt/Et3N (2.0 equiv) over 5 h.
The cytotoxicity of synthetic compounds was determined by the MTT and the colony formation assays against two human ovarian cancer cell lines NCI/ADR-RES (an ovarian cancer cell resistant to doxorubicin) and SKOV3. All compounds were preliminary screened for their cytotoxicity at 10 μM. The results for 3a and 4b are summarized in Table 3. Further structure–activity relationship (SAR) studies are ongoing and have been planned to explore the in vitro biological activities.
Table 3. Cytotoxicity Profile of Compounds (3a and 4b) against Ovarian Cancer Cell Lines, by MTT and Colony Assay.

Inhibition rate (%) at 10 μM.
Image of colonies of cells treated with 3a.
Image of colonies of cells treated with 4b.
Image of colonies of cells treated with (DMSO). Colony formation assay performed to assess the cytotoxic effects on NCI/ADR-RES cancer cell growth at 10 μM.
Conclusions
In summary, we have developed an efficient method for 1,2,4-oxadiazole formation through the [3 + 2] cycloaddition of in situ-generated nitrile oxides and indoline-2,3-dione. The utility of this strategy was demonstrated by the preparation of a broad range of functionalized tricyclic heterocycle containing 1,2,4-oxadiazole in moderate to excellent yields. Potential advantages of our approach versus previously published methods include readily available starting materials, metal-free conditions, and reaction tolerance for broad functional groups. Further applications of this process are currently underway to design novel biologically active compounds for cancer.
Experimental Section
General Experimental Methods
All compounds were fully characterized by infrared (IR), NMR, and high-resolution mass spectrometry (HRMS). NMR spectra were recorded on a Bruker DRX400 (1H: 400 MHz, 13C: 100 MHz), Bruker DRX500 (1H: 500 MHz, 13C: 125 MHz), or Bruker DRX600 (1H: 600 MHz, 13C: 150 MHz) instruments using deuterated CDCl3 and DMSO-d6 as solvents. Chemical shifts (δ) are expressed in parts per million, and J values are given in hertz. IR spectra were recorded on a Fourier transform infrared (FT-IR) Thermo Nicolet Avatar 360 instrument using a KBr pellet. Reactions were monitored by thin-layer chromatography (TLC) using silica gel GF254. The melting points were determined using an XT-4A melting point apparatus and were uncorrected. HRMS was performed on an Agilent liquid chromatography/mass selective detector time-of-flight instrument. All chemicals and solvents were used as received without further purification, unless otherwise stated. Column chromatography was performed on silica gel (200–300 mesh). Benzaldehyde, hydroxylamine hydrochloride, N-chiorosuccinimide, and indolone (2a–e) were purchased from Adamas-Beta Corporation Limited.
General Procedure for the Synthesis of Intermediate Benzaldehyde Oxime
Substituted benzaldehyde (50 mmol), hydroxylamine hydrochloride (50 mmol), and K2CO3 (50 mmol) were dissolved in 50 mL methanol into a 125 mL round-bottom flask. The mixture was stirred at room temperature for 3 h and monitored by TLC. After the reaction was completed, the solvent was removed with a rotary evaporator, and then, water was added to the residue, extracted with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate, concentrated by a rotary evaporator to yield intermediate benzaldoxime (90–96% yield).
General Procedure for the Synthesis of Hydroxybenzimidoyl Chloride 1a–o
Benzaldoxime (50 mmol) and N-chiorosuccinimide (50 mmol) were dissolved in 40 mL DMF and placed into a 125 mL round-bottom flask, and the mixture was stirred at room temperature for 2–4 h. The completion of the reaction was monitored by TLC. Water was added, and the mixture was extracted with ethyl acetate, dried over Na2SO4, and concentrated and purified by flash column chromatography to yield the intermediate 1a–o (92–95% yield).
General Procedure for the Synthesis of 3a–4j
Compound 1a–o (24 mmol), substituted isatin 2a–e (20 mmol), and Et3N (40 mmol) were dissolved in 50 mL isopropanol and placed into a 125 mL round-bottom flask and stirred at room temperature for 5 h. Progress of the reaction was monitored by TLC. The mixture was evaporated by a rotary evaporator, extracted with ethyl acetate, dried over Na2SO4, and concentrated and purified by flash column chromatography (petroleum ether/ethyl acetate (PE/EA) = 5:1) to yield the compound 3a–4j (46–96% yield). The products were further characterized by FT-IR, NMR, and HRMS and were in good agreement with the target structures.
9a-Hydroxy-3-phenyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3a)
Yellow solid; 84% yield; mp = 180.5–182.3 °C; IR (KBr) νmax: 3367, 3212, 3060, 1749, 1627, 1576, 1475, 1448, 1353, 1333, 1286, 1256, 1217, 1160, 1118, 1093, 1070, 1029, 1013, 971, 938, 857, 812, 754, 685, 660, 645, 618, 492 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.95 (1H, s), 7.81 (2H, d, J = 7.2 Hz), 7.66–7.46 (5H, m), 7.14–7.10 (1H, m), 6.98 (1H, d, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.35, 159.31, 143.75, 134.10, 132.96, 129.77, 127.19, 126.58, 123.74, 121.82, 121.24, 111.91, 106.44; HRMS (ESI+) m/z: calcd for C15H10N2O3Na [M + Na]+ 289.0584; found 289.0673.
3-(4-Fluorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3b)
Yellow solid; 88% yield; mp = 193.5–195.8 °C; IR (KBr) νmax: 3414, 3216, 1752, 1630, 1604, 1512, 1476, 1413, 1354, 1283, 1258, 1240, 1217, 1157, 1118, 1097, 1069, 1018, 966, 941, 864, 840, 812, 754, 719, 689, 660, 625, 506, 493 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.95 (1H, s), 7.89–7.85 (2H, m), 7.61 (1H, d, J = 7.2 Hz), 7.50–7.39 (3H, m), 7.12 (1H, t, J = 7.6 Hz), 6.98 (3H, d, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.28, 166.03, 163.54, 158.64, 143.76, 134.12, 129.99, 129.90, 126.61, 123.73, 121.13, 118.41, 117.18, 116.96, 111.91, 106.56; HRMS (ESI+) m/z: calcd for C15H9FN2O3Na [M + Na]+ 307.0489; found 307.0501.
9a-Hydroxy-3-(4-methoxyphenyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3c)
Yellow solid; 70% yield; mp = 196.2–198.3 °C; IR (KBr) νmax: 3437, 3161, 3104, 2838, 1738, 1626, 1607, 1513, 1474, 1423, 1346, 1309, 1283, 1256, 1221, 1177, 1118, 1091, 1069, 1019, 1004, 971, 926, 835, 815, 759, 722, 688, 666, 643, 625, 611, 563, 518, 502, 486, 471, 456, 422 cm–1; 1H NMR (600 MHz, DMSO-d6): δ 10.92 (1H, s), 7.75–7.74 (2H, m), 7.58 (1H, d, J = 7.4 Hz), 7.48 (1H, t, J = 7.8 Hz), 7.13–7.10 (3H, m), 6.98 (1H, d, J = 7.8 Hz), 3.84 (3H, s); 13C NMR (150 MHz, DMSO-d6): δ 170.50, 162.81, 159.17, 143.72, 133.99, 129.11, 126.48, 123.71, 123.21, 121.43, 115.22, 113.87, 111.87, 106.11, 56.00; HRMS (ESI+) m/z: calcd for C16H12N2O4Na [M + Na]+ 319.0689; found 319.0692.
9a-Hydroxy-3-isopropyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3d)
Yellow solid; 46% yield; mp = 111.2–112.5 °C; IR (KBr) νmax: 3550, 3475, 3412, 3303, 2978, 2938, 2879, 1751, 1717, 1622, 1471, 1384, 1331, 1300, 1244, 1198, 1158, 1121, 1074, 1037, 971, 943, 879, 829, 811, 767, 690, 615, 489 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.83 (1H, s), 7.49–7.43 (2H, m), 7.10 (1H, t, J = 7.6 Hz), 6.94 (1H, d, J = 8.0 Hz), 2.89–2.82 (1H, m), 1.22 (6H, d, J = 7.2 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.53, 165.18, 143.44, 133.71, 126.06, 123.60, 121.92, 111.75, 105.49, 24.68, 19.07, 19.03; HRMS (ESI+) m/z: calcd for C12H12N2O3Na [M + Na]+ 255.0740; found 255.0742.
9a-Hydroxy-3-(4-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3e)
Yellow solid; 77% yield; mp = 160.7–162.9 °C; IR (KBr) νmax: 3438, 3192, 3160, 3036, 1759, 1627, 1606, 1515, 1473, 1350, 1329, 1303, 1281, 1257, 1214, 1119, 1097, 1071, 1023, 1008, 969, 931, 884, 859, 827, 811, 752, 716, 689, 663, 627, 503, 484 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.93 (1H, s), 7.68 (2H, d, J = 8.0 Hz), 7.58 (1H, d, J = 7.2 Hz), 7.47 (1H, t, J = 7.6 Hz), 7.36 (2H, d, J = 8.0 Hz), 7.13–7.09 (1H, m), 6.97 (1H, d, J = 8.0 Hz), 2.38 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.41, 159.34, 143.72, 143.17, 134.03, 130.28, 127.15, 126.51, 123.72, 121.33, 118.98, 111.88, 106.26, 21.62; HRMS (ESI+) m/z: calcd for C16H12N2O3Na [M + Na]+ 303.0740; found 303.0836.
9a-Hydroxy-3-(3-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3f)
Yellow solid; 75% yield; mp = 197.8–198.8 °C; IR (KBr) νmax: 3446, 3220, 3058, 1750, 1627, 1607, 1588, 1476, 1358, 1336, 1282, 1256, 1215, 1158, 1118, 1071, 1030, 967, 941, 884, 813, 787, 748, 688, 665, 618, 488 cm–1; 1H NMR (600 MHz, DMSO-d6): δ 10.94 (1H, s), 7.63–7.59 (3H, m), 7.50–7.45 (3H, m), 7.13 (1H, t, J = 5.0 Hz), 6.97 (1H, d, J = 7.8 Hz), 2.37 (3H, s); 13C NMR (150 MHz, DMSO-d6): δ 170.41, 159.41, 143.77, 139.36, 134.10, 133.60, 129.68, 127.57, 126.56, 124.40, 123.76, 121.78, 121.34, 111.94, 106.39, 21.22; HRMS (ESI+) m/z: calcd for C16H12N2O3Na [M + Na]+ 303.0740; found 303.0742.
9a-Hydroxy-3-(2-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3g)
Yellow solid; 79% yield; mp = 170.5–173.1 °C; IR (KBr) νmax: 3318, 2980, 2922, 2276, 1752, 1734, 1625, 1494, 1471, 1395, 1325, 1274, 1248, 1210, 1121, 1078, 1064, 1019, 968, 932, 882, 855, 843, 818, 759, 749, 717, 690, 661, 620, 486, 442 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.93 (1H, s), 7.71 (1H, d, J = 8.0 Hz), 7.61 (1H, d, J = 7.6 Hz), 7.53–7.42 (3H, m), 7.38–7.35 (1H, m), 7.12 (1H, t, J = 7.6 Hz), 6.78 (1H, d, J = 8.0 Hz), 2.54 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.49, 159.46, 143.75, 138.61, 133.99, 132.35, 132.00, 129.22, 126.84, 126.48, 123.73, 121.41, 120.99, 111.90, 105.47, 22.07; HRMS (ESI+) m/z: calcd for C17H13NO3Na [M + Na]+ 303.0740; found 303.0860.
3-(4-Ethylphenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3h)
White solid; 76% yield; mp = 172.0–173.1 °C; IR (KBr) νmax: 3443, 3178, 2966, 2934, 2874, 1736, 1623, 1510, 1471, 1417, 1346, 1313, 1278, 1249, 1209, 1122, 1089, 1074, 1008, 971, 926, 843, 814, 757, 727, 686, 662, 647, 627, 534, 486; 1H NMR (400 MHz, DMSO-d6): δ 10.95 (1H, s), 7.72 (2H, d, J = 8.4 Hz), 7.58 (1H, d, J = 7.6 Hz), 7.49 (1H, t, J = 7.6 Hz), 7.42–7.40 (2H, m), 7.12 (1H, t, J = 7.6 Hz), 6.99 (1H, d, J = 8.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.41, 159.33, 149.27, 143.72, 134.03, 129.13, 127.27, 126.49, 123.72, 121.36, 119.23, 111.89, 106.27, 28.64, 15.64; HRMS (ESI+) m/z: calcd for C17H14N2O3Na [M + Na]+ 317.0897; found 317.0896.
3-(3-Fluorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3i)
Yellow solid; 88% yield; mp = 201.0–202.5 °C; IR (KBr) νmax: 3459, 3229, 2273, 1745, 1622, 1585, 1474, 1454, 1408, 1339, 1307, 1280, 1251, 1214, 1158, 1117, 1093, 1071, 1023, 969, 950, 873, 839, 811, 788, 755, 687, 653, 621, 523, 487, 455, 443; 1H NMR (400 MHz, DMSO-d6): δ 10.97 (1H, s), 7.67–7.58 (4H, m), 7.53–7.46 (2H, m), 7.12 (1H, t, J = 7.6 Hz), 6.98 (1H, d, J = 8.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.16, 163.72, 161.28, 158.56, 158.53, 143.79, 138.80, 134.19, 132.22, 132.14, 126.67, 125.12, 123.87, 123.78, 123.75, 123.48, 123.46, 123.20, 121.00, 120.09, 119.88, 114.08, 113.83, 112.65, 111.94, 106.79; HRMS (ESI+) m/z: calcd for C15H9FN2O3Na [M + Na]+ 307.0489; found 307.0490.
3-(2-Fluorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3j)
Yellow solid; 86% yield; mp = 180.5–182.3 °C; IR (KBr) νmax: 3416, 3105, 1735, 1623, 1497, 1471, 1411, 1339, 1314, 1284, 1254, 1226, 1164, 1117, 1075, 1018, 974, 929, 849, 822, 767, 753, 728, 688, 661, 622, 551, 485, 418; 1H NMR (400 MHz, DMSO-d6): δ 10.96 (1H, s), 7.83–7.79 (1H, m), 7.73–7.69 (1H, m), 7.63 (1H, d, J = 7.2 Hz), 7.51–7.47 (2H, m), 7.40 (1H, t, J = 7.6 Hz), 7.15–7.11 (1H, m), 6.99 (1H, d, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.21, 161.28, 158.72, 156.13, 156.07, 143.79, 135.16, 135.08, 134.17, 129.96, 126.65, 125.78, 125.74, 123.78, 121.05, 117.54, 117.34, 111.95, 110.14, 110.02, 105.80; HRMS (ESI+) m/z: calcd for C15H9FN2O3Na [M + Na]+ 307.0489; found 307.0488.
3-(4-Chlorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3k)
Yellow solid; 85% yield; mp = 234.2–236.3 °C; IR (KBr) νmax: 3451, 3217, 1745, 1653, 1626, 1597, 1493, 1474, 1405, 1347, 1282, 1253, 1210, 1157, 1120, 1095, 1068, 1023, 1009, 970, 838, 813, 759, 743, 714, 688, 660, 644, 620, 486 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.96 (1H, s), 7.81 (2H, d, J = 8.0 Hz), 7.62 (3H, t, J = 8.4 Hz), 7.48 (1H, t, J = 7.6 Hz), 7.12 (1H, t, J = 7.2 Hz), 6.98 (1H, d, J = 8.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.21, 158.69, 143.77, 137.67, 134.16, 129.96, 128.97, 126.64, 123.75, 121.04, 120.67, 111.93, 106.69; HRMS (ESI+) m/z: calcd for C15H9ClN2O3Na [M + Na]+ 323.0194; found 323.0324.
3-(3-Chlorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3l)
White solid; 83% yield; mp = 262.5–263.5 °C; IR (KBr) νmax: 3453, 3190, 3120, 1746, 1623, 1569, 1474, 1433, 1408, 1333, 1281, 1252, 1214, 1159, 1120, 1076, 1020, 969, 934, 888, 861, 813, 782, 768, 749, 682, 621, 493, 428; 1H NMR (400 MHz, DMSO-d6): δ 10.98 (1H, s), 7.80–7.78 (2H, m), 7.74–7.72 (1H, m), 7.64–7.59 (2H, m), 7.52–7.48 (1H, m), 6.99 (1H, t, J = 7.6 Hz), 6.99 (1H, d, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.12, 158.38, 143.79, 134.44, 134.21, 132.83, 126.70, 126.64, 125.87, 123.75, 120.97, 111.94, 106.81; HRMS (ESI+) m/z: calcd for C15H9ClN2O3Na [M + Na]+ 323.0194; found 323.0194.
3-(2-Chlorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3m)
Yellow solid; 89% yield; mp = 173.1–174.1 °C; IR (KBr) νmax: 3417, 3223, 3117, 1744, 1621, 1483, 1469, 1433, 1406, 1332, 1271, 1247, 1203, 1166, 1155, 1120, 1079, 1053, 1013, 971, 933, 869, 842, 812, 771, 757, 731, 689, 663, 650, 615, 519, 487; 1H NMR (400 MHz, DMSO-d6): δ 10.98 (1H, s), 7.86 (1H, d, J = 1.2 Hz), 7.84–7.64 (3H, m), 7.57–7.48 (2H, m), 7.15 (1H, t, J = 7.6 Hz), 7.00 (1H, d, J = 8.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.12, 157.68, 143.72, 134.13, 134.07, 132.70, 131.65, 131.46, 128.31, 126.55, 123.78, 121.18, 120.86, 111.95, 106.14; HRMS (ESI+) m/z: calcd for C15H9ClN2O3Na [M + Na]+ 323.0194; found 323.0193.
3-(4-Bromophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3n)
Yellow solid; 82% yield; mp = 262.5–263.5 °C; IR (KBr) νmax: 3443, 3215, 1737, 1627, 1592, 1488, 1473, 1400, 1344, 1309, 1284, 1254, 1213, 1180, 1117, 1090, 1067, 1018, 1005, 969, 855, 828, 811, 751, 721, 708, 686, 641, 617, 485, 472; 1H NMR (400 MHz, DMSO-d6): δ 10.97 (1H, s), 7.80–7.73 (4H, m), 7.62 (1H, d, J = 7.2 Hz), 7.51–7.47 (1H, m), 7.13 (1H, t, J = 7.6 Hz), 6.99 (1H, d, J = 7.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.20, 158.81, 143.76, 134.18, 132.90, 129.08, 126.66, 126.58, 123.76, 121.02, 111.94, 106.70; HRMS (ESI+) m/z: calcd for C15H9BrN2O3Na [M + Na]+ 366.9689; found 366.9688.
9a-Hydroxy-3-(thiophen-3-yl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3o)
Yellow solid; 64% yield; mp = 166.8–168.7 °C; IR (KBr) νmax: 3445, 3219, 3116, 1737, 1625, 1518, 1471, 1435, 1331, 1298, 1280, 1252, 1209, 1156, 1116, 1087, 1071, 1026, 973, 936, 842, 813, 792, 757, 675, 617, 484, 419 cm–1; 1H NMR (600 MHz, DMSO-d6): δ 8.24 (1H, s), 7.81–7.76 (1H, m), 7.72–7.58 (1H, m), 7.49–7.47 (2H, m), 7.13–7.11 (1H, m), 6.97 (1H, d, J = 7.8 Hz); 13C NMR (150 MHz, DMSO-d6): δ 170.37, 156.36, 143.76, 134.08, 130.47, 129.65, 126.55, 125.74, 123.74, 122.32, 121.22, 111.91, 106.08; HRMS (ESI+) m/z: calcd for C13H8N2O3SNa [M + Na]+ 295.0148; found 295.0151.
7-Fluoro-9a-hydroxy-3-phenyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3p)
Yellow solid; 72% yield; mp = 234.3–236.1 °C; IR (KBr) νmax: 3478, 3203, 3070, 1750, 1628, 1577, 1496, 1481, 1448, 1352, 1282, 1256, 1217, 1185, 1120, 1106, 1093, 1060, 1028, 1013, 944, 899, 859, 818, 798, 770, 748, 704, 685, 655, 633, 592, 499, 468 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.00 (1H, s), 7.83–7.80 (2H, m), 7.68–7.64 (2H, m), 7.60–7.56 (2H, m), 7.38–7.33 (1H, m), 7.02–6.98 (1H, m); 13C NMR (100 MHz, DMSO-d6): δ 170.46, 160.15, 159.24, 157.76, 139.94, 139.92, 132.98, 129.74, 127.25, 122.72, 122.64, 121.74, 120.75, 120.52, 114.56, 114.31, 113.20, 113.12, 106.22; HRMS (ESI+) m/z: calcd for C15H9FN2O3Na [M + Na]+ 307.0489; found 307.0491.
7-Fluoro-9a-hydroxy-3-(4-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3q)
Yellow solid; 69% yield; mp = 233.0–234.5 °C; IR (KBr) νmax: 3447, 3198, 3072, 1753, 1630, 1612, 1496, 1483, 1409, 1352, 1309, 1281, 1257, 1217, 1184, 1119, 1100, 1059, 1024, 1011, 945, 901, 860, 817, 799, 749, 702, 655, 635, 614, 592, 487 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.97 (1H, s), 7.69 (2H, d, J = 8.4 Hz), 7.64–7.62 (1H, m), 7.39–7.32 (3H, m), 7.00–6.97 (1H, dd, J = 4, 8.8 Hz), 2.39 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.51, 160.14, 159.28, 157.75, 143.20, 139.92, 139.90, 130.25, 127.21, 122.79, 122.71, 120.69, 120.46, 118.89, 114.48, 114.23, 113.17, 113.09, 106.05, 21.62; HRMS (ESI+) m/z: calcd for C16H11FN2O3Na [M + Na]+ 321.0646; found 321.0649.
7-Fluoro-9a-hydroxy-3-(4-methoxyphenyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3r)
Yellow solid; 70% yield; mp = 253.5–254.7 °C; IR (KBr) νmax: 3551, 3477, 3234, 1741, 1635, 1616, 1515, 1481, 1424, 1352, 1311, 1260, 1207, 1174, 1119, 1093, 1059, 1022, 1004, 930, 903, 827, 799, 704, 618, 592, 478 cm–1; 1H NMR (500 MHz, DMSO-d6): δ 10.95 (1H, s), 7.74 (2H, d, J = 9.0 Hz), 7.63–7.61 (1H, dd, J = 2.5, 7.5 Hz), 7.36–7.32 (1H, m), 7.11 (2H, d, J = 9.0 Hz), 7.00–6.97 (1H, dd, J = 4, 8.5 Hz), 3.85 (3H, s); 13C NMR (125 MHz, DMSO-d6): δ 170.60, 162.84, 159.91, 159.11, 158.00, 139.91, 129.17, 122.87, 122.81, 120.63, 120.44, 115.20, 114.41, 114.21, 113.77, 113.15, 113.09, 105.90, 56.00; HRMS (ESI+) m/z: calcd for C16H11FN2O4Na [M + Na]+ 337.0595; found 337.0597.
7-Fluoro-3-(4-fluorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3s)
Yellow solid; 87% yield; mp = 193.4–195.2 °C; IR (KBr) νmax: 3449, 3207, 3071, 1747, 1633, 1603, 1511, 1492, 1413, 1349, 1279, 1211, 1184, 1157, 1115, 1088, 1061, 1020, 1009, 947, 930, 910, 877, 847, 816, 798, 747, 729, 703, 653, 614, 591, 512, 489, 420 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.99 (1H, s), 7.90–7.86 (2H, m), 7.68–7.65 (1H, m), 7.43 (2H, t, J = 8.8 Hz), 7.37–7.32 (1H, m), 7.01–6.98 (1H, dd, J = 4.0, 8.8 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.38, 166.04, 163.55, 160.15, 158.56, 157.76, 139.94, 130.06, 129.97, 122.61, 122.53, 120.79, 120.55, 118.34, 118.31, 117.17, 116.95, 114.60, 114.35, 113.20, 113.12, 106.33; HRMS (ESI+) m/z: calcd for C15H8F2N2O3Na [M + Na]+ 325.0395; found 325.0393.
9a-Hydroxy-7-methoxy-3-phenyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3t)
Red solid; 87% yield; mp = 159.1–161.9 °C; IR (KBr) νmax: 3250, 3056, 2957, 2938, 2835, 1746, 1633, 1613, 1578, 1493, 1450, 1440, 1354, 1301, 1276, 1251, 1213, 1178, 1130, 1094, 1079, 1030, 1013, 993, 917, 901, 877, 860, 805, 767, 745, 727, 703, 688, 655, 606, 523, 496, 457 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.75 (1H, s), 7.81 (2H, d, J = 7.2 Hz), 7.66–7.63 (1H, m), 7.57 (2H, t, J = 7.6 Hz), 7.27 (1H, d, J = 3.6 Hz), 7.06–7.03 (1H, m), 6.90 (1H, d, J = 7.6 Hz), 3.73 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.49, 159.27, 156.31, 136.76, 132.89, 129.72, 127.21, 122.13, 121.91, 119.75, 112.71, 112.24, 106.77, 56.25; HRMS (ESI+) m/z: calcd for C16H12N2O4Na [M + Na]+ 319.0689; found 319.0715.
9a-Hydroxy-7-methoxy-3-(4-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3u)
Red solid; 84% yield; mp = 195.5–198.4 °C; IR (KBr) νmax: 3296, 1753, 1630, 1613, 1560, 1495, 1443, 1410, 1345, 1303, 1279, 1205, 1177, 1142, 1089, 1073, 1026, 1011, 989, 932, 883, 856, 823, 774, 743, 726, 703, 652, 637, 612, 491 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.73 (1H, s), 7.69 (2H, d, J = 8.4 Hz), 7.37 (2H, d, J = 8.0 Hz), 7.25 (1H, d, J = 2.4 Hz), 7.05–7.03 (1H, dd, J = 8.8, 2.8 Hz), 6.90 (1H, d, J = 8.8 Hz), 3.73 (3H, s), 2.38 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.54, 159.31, 156.29, 143.10, 136.74, 130.24, 127.17, 122.21, 119.66, 119.07, 112.68, 112.17, 106.59, 56.23, 21.61; HRMS (ESI+) m/z: calcd for C17H14N2O4Na [M + Na]+ 333.0846; found 333.0902.
9a-Hydroxy-7-methoxy-3-(2-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3v)
Red solid; 78% yield; mp = 161.2–163.4 °C; IR (KBr) νmax: 3428, 3194, 3105, 2972, 2935, 1735, 1617, 1495, 1470, 1437, 1381, 1328, 1304, 1283, 1215, 1173, 1139, 1127, 1074, 1030, 1016, 933, 901, 881, 849, 821, 786, 763, 730, 712, 657, 585, 523, 502, 441 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.73 (1H, s), 7.72 (1H, d, J = 7.6 Hz), 7.53–7.50 (1H, m), 7.43 (1H, d, J = 7.6 Hz), 7.37 (1H, t, J = 7.6 Hz), 7.28 (1H, d, J = 2.8 Hz), 7.06–7.03 (1H, m), 6.90 (1H, d, J = 8.4 Hz), 3.74 (3H, s), 2.54 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.62, 159.42, 156.31, 138.62, 136.77, 132.31, 131.97, 129.26, 126.80, 122.30, 121.06, 119.58, 112.69, 112.22, 105.77, 56.25, 22.11; HRMS (ESI+) m/z: calcd for C17H14N2O4Na [M + Na]+ 333.0846; found 333.0984.
3-(4-Fluorophenyl)-9a-hydroxy-7-methoxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3w)
Red solid; 96% yield; mp = 196.5–198.4 °C; IR (KBr) νmax: 3289, 2928, 2844, 1752, 1633, 1606, 1561, 1511, 1495, 1442, 1413, 1348, 1302, 1279, 1208, 1178, 1158, 1091, 1071, 1025, 883, 841, 812, 775, 744, 727, 703, 650, 635, 614, 513, 486 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.75 (1H, s), 7.89–7.85 (2H, m), 7.43–7.39 (2H, m), 7.27 (1H, d, J = 2.4 Hz), 7.06–7.03 (1H, m), 6.90 (1H, d, J = 8.4 Hz), 3.74 (3H, d, J = 5.6); 13C NMR (100 MHz, DMSO-d6): δ 170.41, 166.00, 163.50, 158.60, 156.30, 136.77, 130.00, 129.91, 122.01, 119.76, 118.51, 117.14, 116.91, 112.70, 112.28, 106.88, 56.23; HRMS (ESI+) m/z: calcd for C16H11FN2O4Na [M + Na]+ 337.0595; found 337.0642.
3-(2-Fluorophenyl)-9a-hydroxy-7-methoxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3x)
Red solid; 85% yield; mp = 160.5–161.0 °C; IR (KBr) νmax: 3418, 1735, 1619, 1496, 1439, 1340, 1304, 1228, 1180, 1125, 1074, 1028, 821, 764, 659, 586; 1H NMR (400 MHz, DMSO-d6): δ 10.76 (1H, s), 7.83–7.79 (1H, m), 7.74–7.69 (1H, m), 7.51–7.46 (1H, m), 7.43–7.39 (1H, m), 7.31 (1H, d, J = 2.8 Hz), 7.07–7.04 (1H, m), 6.91 (1H, d, J = 8.8 Hz), 3.74 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.35, 161.28, 158.72, 156.32, 156.08, 156.01, 136.79, 135.07, 134.99, 129.98, 125.72, 125.69, 121.96, 119.80, 117.50, 117.29, 112.74, 112.32, 110.26, 110.14, 106.10, 56.26; HRMS (ESI+) m/z: calcd for C16H11FN2O4Na [M + Na]+ 337.0595; found 337.0597.
9a-Hydroxy-7-methyl-3-phenyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3y)
Yellow solid; 85% yield; mp = 153.8–155.4 °C; IR (KBr) νmax: 3195, 3106, 1734, 1628, 1575, 1493, 1451, 1409, 1351, 1294, 1251, 1212, 1178, 1130, 1089, 1064, 1031, 1015, 943, 927, 891, 847, 819, 771, 703, 686, 654, 590, 578, 490, 460, 434 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.85 (1H, s), 7.80 (2H, d, J = 7.6 Hz), 7.64 (1H, t, J = 7.6 Hz), 7.56 (2H, t, J = 15.2 Hz), 7.42 (1H, s), 7.27 (1H, d, J = 8.0 Hz), 6.87 (1H, d, J = 8.0 Hz); 13C NMR (100 MHz, DMSO-d6): δ 170.37, 159.24, 141.18, 134.24, 133.08, 132.92, 129.75, 127.16, 126.89, 121.85, 121.34, 111.65, 106.64, 20.74; HRMS (ESI+) m/z: calcd for C16H12N2O3Na [M + Na]+ 303.0740; found 303.0730.
9a-Hydroxy-7-methyl-3-(4-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (3z)
Yellow solid; 82% yield; mp = 188.7–190.8 °C; IR (KBr) νmax: 3201, 3036, 2921, 1748, 1628, 1500, 1410, 1355, 1295, 1259, 1219, 1184, 1132, 1101, 1065, 1010, 944, 908, 861, 820, 752, 709, 655, 636, 589, 489, 457 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.84 (1H, s), 7.68 (2H, d, J = 8.0 Hz), 7.39–7.34 (3H, m), 7.26 (1H, d, J = 8.0 Hz), 6.86 (1H, d, J = 8.0 Hz), 2.37 (3H, s), 2.25 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.45, 159.29, 143.10, 141.17, 134.16, 133.02, 130.24, 127.11, 126.79, 121.44, 119.03, 111.62, 106.47, 21.59, 20.73; HRMS (ESI+) m/z: calcd for C17H14N2O3Na [M + Na]+ 317.0897; found 317.0962.
9a-Hydroxy-7-methyl-3-(2-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4a)
Yellow solid; 81% yield; mp = 138.1–141.7 °C; IR (KBr) νmax: 3197, 3115, 2971, 2923, 1751, 1632, 1617, 1494, 1454, 1410, 1333, 1295, 1281, 1251, 1215, 1187, 1137, 1077, 1016, 951, 933, 880, 853, 822, 805, 759, 718, 703, 656, 592, 502, 461, 445 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.82 (1H, s), 7.70 (1H, d, J = 7.6 Hz), 7.51 (1H, d, J = 7.2 Hz), 7.43 (2H, d, J = 7.2 Hz), 7.36 (1H, t, J = 7.6 Hz), 7.27 (1H, d, J = 8.0 Hz), 6.87 (1H, d, J = 8.0 Hz), 2.54 (3H, s), 2.26 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.52, 159.39, 141.20, 138.59, 134.14, 133.05, 132.32, 131.99, 129.19, 126.82, 121.48, 121.01, 111.63, 105.64, 22.11, 20.76; HRMS (ESI+) m/z: calcd for C17H14N2O3Na [M + Na]+ 317.0897; found 317.0955.
3-(4-Fluorophenyl)-9a-hydroxy-7-methyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4b)
Red solid; 92% yield; mp = 204.7–206.9 °C; IR (KBr) νmax: 3231, 2923, 1752, 1685, 1631, 1606, 1512, 1499, 1414, 1357, 1293, 1255, 1238, 1219, 1183, 1158, 1130, 1112, 1098, 1069, 1016, 941, 905.45, 864, 816, 750, 732, 719, 703, 652, 637, 615, 590, 508, 495 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.85 (1H, s), 7.88–7.85 (2H, m), 7.43–7.38 (3H, m), 7.27 (1H, d, J = 7.6 Hz), 6.86 (1H, d, J = 8.0 Hz), 2.25 (3H, m); 13C NMR (100 MHz, DMSO-d6): δ 170.29, 166.01, 163.52, 158.56, 141.19, 134.27, 133.07, 129.96, 129.87, 126.92, 121.21, 118.43, 117.17, 116.95, 111.65, 106.75, 20.73; HRMS (ESI+) m/z: calcd for C16H11FN2O3Na [M + Na]+ 321.0646; found 321.0769.
3-(3-Fluorophenyl)-9a-hydroxy-7-methyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4c)
Red solid; 89% yield; mp = 166.5–168.7 °C; IR (KBr) νmax: 3418, 1747, 1627, 1587, 1496, 1452, 1405, 1338, 1291, 1253, 1220, 1182, 1136, 1094, 1068, 1022, 943, 875, 840, 815, 781, 760, 703, 682, 593, 461; 1H NMR (400 MHz, DMSO-d6): δ 10.86 (1H, s), 7.67–7.61 (3H, m), 7.59–7.53 (1H, m), 7.45 (1H, s), 7.30–7.28 (1H, dd, J = 0.8, 8.0 Hz), 6.87 (1H, d, J = 8.0 Hz), 2.27 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.15, 163.72, 161.28, 158.49, 158.45, 141.20, 134.37, 133.12, 132.26, 132.17, 127.01, 123.88, 123.80, 123.49, 123.46, 121.07, 120.10, 119.89, 114.07, 113.82, 111.69, 106.97, 20.75; HRMS (ESI+) m/z: calcd for C16H11FN2O3Na [M + Na]+ 321.0646; found 321.0647.
3-(2-Fluorophenyl)-9a-hydroxy-7-methyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4d)
Yellow solid; 88% yield; mp = 192.3–193.5 °C; IR (KBr) νmax: 3419, 1738, 1627, 1493, 1463, 1409, 1354, 1341, 1294, 1252, 1215, 1185, 1164, 1119, 1075, 1035, 1016, 929, 895, 824, 768, 702, 653, 591, 550, 455; 1H NMR (400 MHz, DMSO-d6): δ 10.86 (1H, s), 7.82–7.78 (1H, m), 7.72–7.70 (1H, m), 7.50–7.45 (2H, m), 7.42–7.38 (1H, m), 7.28 (1H, d, J = 8.0 Hz), 6.87 (1H, d, J = 8.0 Hz), 2.27 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.24, 161.27, 158.71, 156.06, 156.00, 141.22, 135.11, 135.02, 134.31, 133.11, 129.92, 126.95, 125.75, 125.71, 121.13, 117.52, 117.32, 111.68, 110.17, 110.05, 105.99, 20.75; HRMS (ESI+) m/z: calcd for C16H11FN2O3Na [M + Na]+ 321.0646; found 321.0648.
3-(4-Chlorophenyl)-9a-hydroxy-7-methyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4e)
Yellow solid; 80% yield; mp = 226.3–227.4 °C; IR (KBr) νmax: 3199, 2922, 1754, 1629, 1598, 1495, 1405, 1349, 1292, 1255, 1216, 1181, 1130, 1100, 1067, 1008, 940, 904, 834, 813, 787, 754, 731, 714, 702, 652, 636, 590, 526, 491 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 10.85 (1H, s), 7.81 (2H, d, J = 8.4 Hz), 7.63 (2H, d, J = 8.4 Hz), 7.43 (1H, s), 7.27 (1H, d, J = 8.0 Hz), 6.86 (1H, d, J = 8.0 Hz), 2.25 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.22, 158.62, 141.20, 137.64, 134.32, 133.09, 129.95, 128.95, 126.95, 121.12, 120.70, 111.67, 106.89, 20.74; HRMS (ESI+) m/z: calcd for C16H11ClN2O3Na [M + Na]+ 337.0350; found 337.0470.
7-Bromo-9a-hydroxy-3-phenyl-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4f)
Yellow solid; 83% yield; mp = 194.1–196.2 °C; IR (KBr) νmax: 3187, 3145, 3114, 3060, 2857, 1745, 1618, 1575, 1496, 1475, 1447, 1350, 1312, 1210, 1134, 1094, 1072, 1050, 1029, 1014, 932, 878, 858, 814, 766, 730, 692, 649, 570, 533, 485, 456 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.10 (1H, s), 7.91 (1H, d, J = 2 Hz), 7.80 (2H, d, J = 7.2 Hz), 7.67–7.63 (2H, m), 7.56 (2H, t, J = 7.6 Hz), 6.94 (1H, d, J = 8.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 169.99, 159.24, 143.00, 136.69, 132.95, 129.70, 129.51, 127.27, 123.53, 121.75, 115.21, 113.97, 105.94; HRMS (ESI+) m/z: calcd for C15H9BrN2O3Na [M + Na]+ 366.9689; found 366.9814.
7-Bromo-9a-hydroxy-3-(4-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4g)
Yellow solid; 81% yield; mp = 210.7–213.1 °C; IR (KBr) νmax: 3221, 3190, 3060, 2921, 1755, 1627, 1513, 1473, 1446, 1409, 1351, 1308, 1266, 1208, 1130, 1101, 1079, 1051, 1025, 1011, 940, 909, 888, 862, 819, 748, 731, 702, 689, 653, 630, 535, 492 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.08 (1H, s), 7.87 (1H, s), 7.69–7.64 (3H, m), 7.37 (2H, d, J = 8.0 Hz), 6.94 (1H, d, J = 8.4 Hz), 2.38 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.04, 159.28, 143.18, 142.99, 136.64, 130.23, 129.42, 127.23, 123.60, 118.90, 115.18, 113.95, 105.76, 21.63; HRMS (ESI+) m/z: calcd for C16H11BrN2O3Na [M + Na]+ 380.9845; found 380.9776.
7-Bromo-9a-hydroxy-3-(2-tolyl)-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4h)
Red solid; 82% yield; mp = 138.4–140.4 °C; IR (KBr) νmax: 3197, 3113, 2975, 2922, 2847, 1763, 1619, 1498, 1476, 1440, 1398, 1330, 1265, 1247, 1211, 1134, 1087, 1069, 1052, 1019, 954, 933, 884, 852, 822, 807, 762, 719, 704, 689, 665, 653, 572, 535, 506, 457, 446 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.08 (1H, s), 7.92 (1H, s), 7.92–7.65 (2H, m), 7.52 (1H, t, J = 7.6 Hz), 7.43 (1H, d, J = 7.6 Hz), 7.36 (1H, t, J = 7.6 Hz), 6.94 (1H, d, J = 8.4 Hz), 2.53 (3H, s); 13C NMR (100 MHz, DMSO-d6): δ 170.15, 159.33, 143.01, 138.69, 136.60, 132.36, 131.94, 129.42, 129.29, 126.77, 123.70, 120.90, 115.20, 113.96, 104.95; HRMS (ESI+) m/z: calcd for C16H11BrN2O3Na [M + Na]+ 380.9845; found 380.9933.
7-Bromo-3-(4-fluorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4i)
Yellow solid; 87% yield; mp = 232.5–233.3 °C; IR (KBr) νmax: 3220, 3191, 3060, 1758, 1629, 1605, 1512, 1472, 1447, 1414, 1353, 1295, 1266, 1210, 1158, 1131, 1111, 1099, 1081, 1052, 1020, 939, 910, 888, 862, 840, 818, 748, 719, 690 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.10 (1H, s), 7.91–7.85 (3H, m), 7.67–7.64 (1H, m), 7.43–7.39 (2H, m), 6.94 (1H, d, J = 8.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 169.92, 166.02, 163.53, 158.56, 143.00, 136.70, 130.07, 129.98, 129.55, 123.43, 118.36, 117.12, 116.90, 115.21, 113.96, 106.05; HRMS (ESI+) m/z: calcd for C15H8BrFN2O3Na [M + Na]+ 384.9595; found 384.9671.
7-Bromo-3-(4-chlorophenyl)-9a-hydroxy-[1,2,4]oxadiazolo[4,5-a]indol-9(9aH)-one (4j)
Yellow solid; 85% yield; mp = 229.6–231.2 °C; IR (KBr) νmax: 3452, 1755, 1625, 1598, 1493, 1477, 1442, 1404, 1344, 1263, 1207, 1134, 1096, 1051, 1010, 883, 816, 757, 700, 647, 537 cm–1; 1H NMR (400 MHz, DMSO-d6): δ 11.11 (1H, s), 7.91 (1H, s), 7.80 (2H, d, J = 8.4 Hz), 7.66–7.60 (3H, m), 6.93 (1H, d, J = 8.4 Hz); 13C NMR (100 MHz, DMSO-d6): δ 169.84, 158.62, 143.00, 137.66, 136.72, 129.87, 129.56, 129.03, 123.36, 120.62, 115.22, 113.96, 106.17; HRMS (ESI+) m/z: calcd for C15H8BrClN2O3Na [M + Na]+ 400.9299; found 400.9412.
3-Phenyl-4-(2-(3-phenyl-1,2,4-oxadiazol-5-yl)phenyl)-1,2,4-oxadiazol-5(4H)-one (6a)
White solid; 78% yield; mp = 165.1–167.4 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.32 (1H, d, J = 6.8 Hz), 8.05–8.04 (3H, m), 7.98–7.94 (1H, m), 7.87–7.84 (1H, m), 7.65–7.63 (3H, m), 7.48 (1H, t, J = 7.6 Hz), 7.35 (2H, d, J = 8.0 Hz), 7.28 (2H, d, J = 7.2 Hz); 13C NMR (100 MHz, DMSO-d6): δ 172.5, 168.6, 158.5, 158.1, 135.3, 132.5, 132.4, 132.1, 131.1, 130.6, 129.9, 129.7, 129.5, 129.0, 128.3, 127.4, 126.0, 123.1, 121.8; HRMS (ESI+) m/z: calcd for C22H14N4O3Na [M + Na]+ 405.0964; found 405.0953.
3-Phenyl-5H-[1,2,4]oxadiazolo[4,5-c]pyrimidine-5,7(6H)-dione (6b)
White solid; 86% yield; mp = 132.5–134.1 °C; 1H NMR (400 MHz, DMSO-d6): δ 11.55 (1H, s), 7.77–7.75 (2H, m), 7.67–7.63 (1H, m), 7.57–7.53 (2H, m), 5.59 (1H, s); 13C NMR (100 MHz, DMSO-d6): δ 164.07, 163.94, 154.56, 143.69, 132.39, 130.78, 128.47, 121.59, 73.74; HRMS (ESI+) m/z: calcd for C11H7N3O3Na [M + Na]+ 252.0385; found 252.0373.
6-Methyl-3-phenyl-5H-[1,2,4]oxadiazolo[4,5-c]pyrimidine-5,7(6H)-dione (6c)
White solid; 88% yield; mp = 153.2–155.8 °C; IR (KBr) νmax: 3416, 3109, 1728, 1703, 1674, 1659, 1563, 1491, 1439, 1360, 1342, 1263, 1233, 1185, 1136, 1040, 1001, 978, 893, 851, 833, 785, 765, 746, 709, 691, 677, 659, 621, 544, 475, 445, 408 cm–1; 1H NMR (600 MHz, DMSO-d6): δ 7.77 (2H, d, J = 7.2 Hz), 7.68–7.66 (1H, m), 7.57 (2H, t, J = 7.8 Hz), 5.80 (1H, s), 3.15 (3H, s); 13C NMR (150 MHz, DMSO-d6): δ 162.57, 162.39, 154.62, 144.41, 132.42, 130.81, 128.49, 121.60, 73.33, 27.99; HRMS (ESI+) m/z: calcd for C12H10N3O3 [M + H]+ 244.0717; found 262.0723.
6-Ethyl-7-imino-3-phenyl-7,8-dihydro-8aH-[1,2,4]oxadiazolo[4,5-a]pyrimidin-8a-ol (6d)
White solid; 82% yield; mp = 131.1–132.9 °C; 1H NMR (400 MHz, CDCl3): δ 7.97–7.87 (2H, m), 7.64–7.53 (1H, m), 7.47 (3H, t, J = 7.3 Hz), 7.27 (2H, d, J = 13.2 Hz), 2.06 (3H, q, J = 7.4 Hz), 0.98 (4H, t, J = 7.4 Hz); 13C NMR (100 MHz, CDCl3): δ 154.26, 144.44, 130.63, 129.10, 127.56, 127.19, 126.33, 122.69, 117.31, 21.22, 14.67; HRMS (ESI+) m/z: calcd for C13H14N4O2Na [M + Na]+ 281.1014; found 281.1003.
6-Nitro-3-phenylimidazo[1,2-d][1,2,4]oxadiazole (6e)
White solid; 89% yield; mp = 187.2–189.5 °C; IR (KBr) νmax: 3416, 3148, 1999, 1636, 1604, 1584, 1533, 1484, 1452, 1354, 1306, 1291, 1278, 1154, 1132, 1073, 1028, 970, 880, 852, 790, 771, 753, 722, 686, 670, 644, 480, 456; 1H NMR (400 MHz, DMSO-d6): δ 9.28 (1H, s), 8.15–8.13 (2H, m), 7.77–7.75 (1H, m), 7.71–7.67 (2H, m); 13C NMR (100 MHz, DMSO-d6): δ 158.80, 152.59, 151.33, 133.86, 130.13, 127.96, 121.14, 110.43; HRMS (ESI+) m/z: calcd for C10H6N4O3Na [M + Na]+ 253.0332; found 253.0337.
General Computation Methods
All computations were carried out with Gaussian 09. Reactants, transition stats, and products were optimized with the density-functional M06-2X1 using the 6-31G(d) basis set with an ultrafine grid, consisting of 590 radial shell and 99 grid points per shell.2 M06-2X has been found to give reliable energetics for cycloadditions involving main group elements.3 Normal vibrational mode analysis confirmed all stationary points to be minima (no imaginary frequencies) or transition states (one imaginary frequency). Zero-point energy and thermal corrections were computed from unscaled frequencies for the standard state of 1 M and 298.15 K. Truhlar’s quasiharmonic correction was applied for entropy calculations by setting all frequencies less than 100 cm–1.4 Input structures for these computations were generated using GaussView.
General Procedure for Biological Activity
Cytotoxicity of 3a and 3b was determined by the MTT assay against three human ovarian cancer cell lines, NCI/ADR-RES, SKOV3, and OVCAR8. All compounds were preliminary screened for their cytotoxic activity at 10 μM. We also investigated the level of colony formation to assess the effects of cytotoxicity on the NCI/ADR-RES cancer cell growth at 10 μM.
Cell Lines
NCI/ADR-RES is an ovarian cancer cell resistant to doxorubicin.
SKOV3 and OVCAR8 are ovarian cancer cells.
MTT Assay
The evaluation of cytotoxicity was based on the reduction of MTT dye by viable cells to give purple formazan products, which can be measured spectrophotometrically at 540 nm. One hundred eighty microliters of cancer cells were seeded into 96-well plates at 2000 cell/well and incubated at 37 °C overnight before the indicated treatments. After 72 h, 20 μL of the MTT solution (3 mg/mL) was added and incubated again for 3 h. After removal of the media and the solubilization of the formazan crystals in 150 μL of DMSO, absorbance was measured at 570 nm. Percentage of cell growth inhibition is expressed as 1 – [(A – B)/(C – B)] × 100% (A, B, and C were the absorbance values from experimental, blank, and control cells, respectively).
Colony Assay
Colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. This technique was also performed to confirm the activity. Briefly, cells were plated in 96-well plates at a density of 200 cells/well and allowed to attach overnight. The next day, the corresponding compounds were added and allowed to incubate for 24 h. After exposure, cells were changed with new media and cultured until colonies were formed (7–10 days). Cells were subsequently washed and stained with a solution of crystal violet for 30 min. After staining, the cells were thoroughly washed with water. Colonies were imaged on the inverted fluorescence microscope.
Acknowledgments
The authors gratefully acknowledge the financial support of the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13095) and the NSFC (nos. 21262043, 21662044, 20902079, U1202221, and 21262042) and thank the High Performance Computing Center at Yunnan University for use of the high performance computing platform.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00490.
1H NMR of isatin in different solvents, equilibrium constants (Kn) of tautomerization, Cartesian coordinates and energy, and 1H NMR and 13C NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Ji Y.; Yang X.; Qian Y. RSC Adv. 2014, 4, 49535–49540. 10.1039/c4ra09081k. [DOI] [Google Scholar]; b Taylor P. J.The “Basicity Method” for Estimating Tautomer Ratio: A Radical Re-Appraisal. In Tautomerism: Methods and Theories; Wiley-VCH Verlag GmbH & Co. KGaA, 2013; Vol. 12, pp 305–335. [Google Scholar]; c Oszczapowicz J.Basicity, H-Bonding, Tautomerism and Complex Formation of Imidic Acid Derivatives. In Amidines and Imidates; John Wiley & Sons, Ltd., 1991; Vol. 2, pp 623–688. [Google Scholar]; d Buśko-Oszczapowicz I.; Oszczapowicz J.. Detection and Determination of Imidic Acid Derivatives. In Amidines and Imidates; John Wiley & Sons, Ltd., 1991; Vol. 2, pp 231–299. [Google Scholar]
- Kemnitz C. R.; Loewen M. J. J. Am. Chem. Soc. 2007, 129, 2521–2528. 10.1021/ja0663024. [DOI] [PubMed] [Google Scholar]
- a Wong M. W.; Leung-Toung R.; Wentrup C. J. Am. Chem. Soc. 1993, 115, 2465–2472. 10.1021/ja00059a048. [DOI] [Google Scholar]; b Balabin R. M. J. Chem. Phys. 2009, 131, 154307. 10.1063/1.3249968. [DOI] [PubMed] [Google Scholar]
- Chandanshive J. Z.; González P. B.; Tiznado W.; Bonini B. F.; et al. Tetrahedron 2012, 68, 3319–3328. 10.1016/j.tet.2012.02.068. [DOI] [Google Scholar]
- a Ritter T.; Carreira E. M. Angew. Chem., Int. Ed. 2005, 44, 936–938. 10.1002/anie.200461934. [DOI] [PubMed] [Google Scholar]; b Tang Y.; Gao H.; Mitchell L. A.; Parrish D. A.; et al. Angew. Chem., Int. Ed. 2016, 55, 1147–1150. 10.1002/anie.201509985. [DOI] [PubMed] [Google Scholar]
- Carbone M.; Li Y.; Irace C.; Mollo E.; et al. Org. Lett. 2011, 13, 2516–2519. 10.1021/ol200234r. [DOI] [PubMed] [Google Scholar]
- a Takahashi H.; Riether D.; Bartolozzi A.; Bosanac T.; et al. J. Med. Chem. 2015, 58, 1669–1690. 10.1021/jm501185j. [DOI] [PubMed] [Google Scholar]; b Budriesi R.; Cosimelli B.; Ioan P.; Ugenti M. P.; et al. J. Med. Chem. 2009, 52, 2352–2362. 10.1021/jm801351u. [DOI] [PubMed] [Google Scholar]; c Touaibia M.; Djimdé A.; Cao F.; Boilard E.; et al. J. Med. Chem. 2007, 50, 1618–1626. 10.1021/jm060082n. [DOI] [PubMed] [Google Scholar]
- a Dickmeis M.; Cinar H.; Ritter H. Angew. Chem., Int. Ed. 2012, 51, 3957–3959. 10.1002/anie.201107608. [DOI] [PubMed] [Google Scholar]; b Koyama Y.; Miura K.; Cheawchan S.; Seo A.; et al. Chem. Commun. 2012, 48, 10304–10306. 10.1039/c2cc35158g. [DOI] [PubMed] [Google Scholar]; c Li Q.; Cui L.-S.; Zhong C.; Jiang Z.-Q.; et al. Org. Lett. 2014, 16, 1622–1625. 10.1021/ol5002494. [DOI] [PubMed] [Google Scholar]; d Tang Y.; Gao H.; Mitchell L. A.; Parrish D. A.; et al. Angew. Chem., Int. Ed. 2016, 55, 3200–3203. 10.1002/anie.201600068. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wei H.; He C.; Zhang J.; Shreeve J. M. Angew. Chem., Int. Ed. 2015, 54, 9367–9371. 10.1002/anie.201503532. [DOI] [PubMed] [Google Scholar]
- Lentini L.; Melfi R.; Di Leonardo A.; Spinello A.; et al. Mol. Pharmaceutics 2014, 11, 653–664. 10.1021/mp400230s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kesornpun C.; Aree T.; Mahidol C.; Ruchirawat S.; et al. Angew. Chem., Int. Ed. 2016, 55, 3997–4001. 10.1002/anie.201511730. [DOI] [PubMed] [Google Scholar]; b Pace A.; Buscemi S.; Piccionello A. P.; Pibiri I. Adv. Heterocycl. Chem. 2015, 116, 85–136. 10.1016/bs.aihch.2015.05.001. [DOI] [Google Scholar]; c Pace A.; Pierro P. Org. Biomol. Chem. 2009, 7, 4337–4348. 10.1039/b908937c. [DOI] [PubMed] [Google Scholar]
- a Bhat S. V.; Robinson D.; Moses J. E.; Sharma P. Org. Lett. 2016, 18, 1100–1103. 10.1021/acs.orglett.6b00203. [DOI] [PubMed] [Google Scholar]; b Kotipalli T.; Kavala V.; Konala A.; Janreddy D.; et al. Adv. Synth. Catal. 2016, 358, 2652–2660. 10.1002/adsc.201600274. [DOI] [Google Scholar]; c Mercalli V.; Massarotti A.; Varese M.; Giustiniano M.; et al. J. Org. Chem. 2015, 80, 9652–9661. 10.1021/acs.joc.5b01676. [DOI] [PubMed] [Google Scholar]; d Ovdiichuk O. V.; Hordiyenko O. V.; Arrault A. Tetrahedron 2016, 72, 3427–3435. 10.1016/j.tet.2016.04.069. [DOI] [Google Scholar]
- a Bolotin D. S.; Kulish K. I.; Bokach N. A.; Starova G. L.; et al. Inorg. Chem. 2014, 53, 10312–10324. 10.1021/ic501333s. [DOI] [PubMed] [Google Scholar]; b Zora M.; Kivrak A.; Kelgokmen Y. J. Organomet. Chem. 2014, 759, 67–73. 10.1016/j.jorganchem.2014.02.018. [DOI] [Google Scholar]; c Andersen T. L.; Caneschi W.; Ayoub A.; Lindhardt A. T.; et al. Adv. Synth. Catal. 2014, 356, 3074–3082. 10.1002/adsc.201400487. [DOI] [Google Scholar]; d Melekhova A. A.; Smirnov A. S.; Novikov A. S.; Panikorovskii T. L.; Bokach N. A.; Kukushkin V. Y. ACS Omega 2017, 2, 1380–1391. 10.1021/acsomega.7b00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Miller D. J.; Scrowston R. M.; Kennewell P. D.; Westwood R. Tetrahedron 1994, 50, 5159–5168. 10.1016/s0040-4020(01)90426-0. [DOI] [Google Scholar]; b Huang X.; Pissarnitski D.; Li H.; Asberom T.; et al. Tetrahedron Lett. 2012, 53, 6451–6455. 10.1016/j.tetlet.2012.09.070. [DOI] [Google Scholar]; c Li X.; Zheng A.; Liu B.; Li G.; et al. J. Heterocycl. Chem. 2011, 48, 776–779. 10.1002/jhet.578. [DOI] [Google Scholar]; d Altuğ C.; Dürüst Y.; Elliott M. C.; Kariuki B. M.; et al. Org. Biomol. Chem. 2010, 8, 4978–4986. 10.1039/c0ob00286k. [DOI] [PubMed] [Google Scholar]; e Corsaro A.; Pistara V.; Rescifina A.; Chiacchio M. A.; et al. Heterocycles 2005, 65, 1079–1097. 10.3987/com-05-10342. [DOI] [Google Scholar]; f Dawood K. M. Heteroat. Chem. 2004, 15, 432–436. 10.1002/hc.20037. [DOI] [Google Scholar]; g Corsaro A.; Perrini G.; Pistarà V.; Quadrelli P.; et al. Tetrahedron 1996, 52, 6421–6436. 10.1016/0040-4020(96)00276-1. [DOI] [Google Scholar]; h Coutouli-Argyropoulou E.; Malamidou-Xenikaki E.; Mentzafos D.; Terzis A. J. Heterocycl. Chem. 1990, 27, 1185–1189. 10.1002/jhet.5570270505. [DOI] [Google Scholar]; i Malamidou-Xenikaki E.; Coutouli-Argyropoulou E. Tetrahedron 1990, 46, 7865–7872. 10.1016/s0040-4020(01)90084-5. [DOI] [Google Scholar]; j Luheshi A.-B. N.; Smalley R. K.; Kennewell P. D.; Westwood R. Tetrahedron Lett. 1990, 31, 123–126. 10.1016/s0040-4039(00)94351-x. [DOI] [Google Scholar]; k Hemming K.; Luheshi A.-B. N.; Redhouse A. D.; Smalley R. K.; et al. Tetrahedron 1993, 49, 4383–4408. 10.1016/s0040-4020(01)85755-0. [DOI] [Google Scholar]; l Hemming K.; Khan M. N.; O’Gorman P. A.; Pitard A. Tetrahedron 2013, 69, 1279–1284. 10.1016/j.tet.2012.12.007. [DOI] [Google Scholar]
- a Sumpter W. C. Chem. Rev. 1944, 34, 393–434. 10.1021/cr60109a003. [DOI] [Google Scholar]; b da Silva J. F. M.; Garden S. J.; Pinto A. C. J. Braz. Chem. Soc. 2001, 12, 273–324. 10.1590/s0103-50532001000300002. [DOI] [Google Scholar]
- a Zi Y.; Cai Z.-J.; Wang S.-Y.; Ji S.-J. Org. Lett. 2014, 16, 3094–3097. 10.1021/ol501203q. [DOI] [PubMed] [Google Scholar]; b Huang P.-C.; Gandeepan P.; Cheng C.-H. Chem. Commun. 2013, 49, 8540–8542. 10.1039/c3cc44435j. [DOI] [PubMed] [Google Scholar]; c Chen S.; Liu Z.; Shi E.; Chen L.; et al. Org. Lett. 2011, 13, 2274–2277. 10.1021/ol200716d. [DOI] [PubMed] [Google Scholar]; d Chouhan M.; Senwar K. R.; Sharma R.; Grover V.; et al. Green Chem. 2011, 13, 2553–2560. 10.1039/c1gc15416h. [DOI] [Google Scholar]
- a Boruah M.; Konwar D. Synth. Commun. 2012, 42, 3261–3268. 10.1080/00397911.2011.558665. [DOI] [Google Scholar]; b Dubrovskiy A. V.; Larock R. C. Org. Lett. 2010, 12, 1180–1183. 10.1021/ol902921s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassaee M. Z.; Arshadi S.; Haerizade B. N.; Vessally E. J. Mol. Struct.: THEOCHEM 2005, 731, 29–37. 10.1016/j.theochem.2005.02.087. [DOI] [Google Scholar]
- a Xie S.; Lopez S. A.; Ramström O.; Yan M.; et al. J. Am. Chem. Soc. 2015, 137, 2958–2966. 10.1021/ja511457g. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Aronoff M. R.; Gold B.; Raines R. T. Org. Lett. 2016, 18, 1538–1541. 10.1021/acs.orglett.6b00278. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sayin K.; Kurtoglu N.; Kose M.; Karakas D.; et al. J. Mol. Struct. 2016, 1119, 413–422. 10.1016/j.molstruc.2016.04.097. [DOI] [Google Scholar]; d Khojastehnezhad A.; Eshghi H.; Moeinpour F.; Bakavoli M.; et al. Struct. Chem. 2016, 27, 1041–1047. 10.1007/s11224-015-0703-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.