Abstract
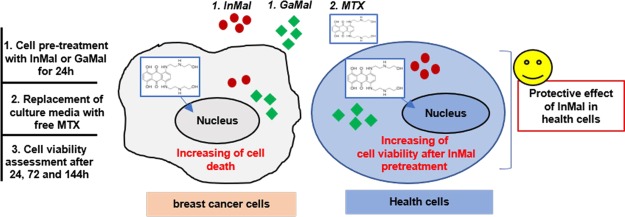
In this study, we aimed to investigate in vitro whether the synthetized indium maltolate (InMal) and gallium maltolate (GaMal) could exert either a toxic effect toward breast cancer cell line MDA-MB-231 or an agonistic activity with mitoxantrone (MTX) in comparison to fibroblast cell line NIH-3T3. Both GaMal and InMal reduced viability of MDA-MB-231, and at a lesser extent of NIH3-T3, in a dose- and time-dependent mode, the outcome was more effective in comparison to MTX sole exposure. Both GaMal and InMal toxicity was reverted by iron citrate addition on NIH3-T3, not on MDA-MB-231, showing indirectly that gallium and indium’s mechanisms of action may include iron targeting. The agonistic activity against MDA-MB-231 survival was shown pretreating with 100 μM InMal for 24 h followed by medium exchange with MTX at 10 ng mL–1 or vice-versa but not with co-incubation of both compounds. In particular, InMal pretreating resulted more protective to MTX subsequent exposure.
1. Introduction
Considering the metallic elements, gallium (group IIIa of the periodic table) has shown efficacy in the treatment of several apparently different disorders.1 In recent years, gallium maltolate (GaMal) has gained the same popularity as antimicrobial agents2−4 and antineoplastic drugs for the treatment of scarcely responding tumors (e.g. hepatocellular carcinoma and lymphomas)5,6 together with other gallium compounds that can play a significant role as antineoplastic both in vitro and in vivo.7−12 Gallium is particularly effective against some lymphatic and urothelial cancers, because of its ability to reach high concentrations in these sites.1 Gallium may inhibit DNA synthesis through substitution of Ga3+ for Fe3+ in the M2 subunit of ribonucleotide reductase, thus blocking its action; furthermore, gallium seems to follow biochemical pathways similar to those for iron absorption and metabolism in proliferating cells.1 Its action is partially attributed to this ability to produce species that are deprived of the biological action of the corresponding iron complexes.2−7 One of the reasons which has given GaMal so much popularity is the absence of the typical side effects of antineoplastic agents;13 therefore, a therapy in which the effect of gallium complexes is potentiated by the presence of classical antineoplastic could in theory guarantee a dose reduction of the classic cytotoxic drug with a significant decrement of side effects.
Anthracyclines are among the most active and widely used antineoplastics,14 but their clinical use is limited by adverse events, particularly by cardiotoxicity and by the development of tumor cell resistance.15−17 In particular, mitoxantrone (MTX), an aminoanthraquinone derived from classical anthracyclines, is widely used for its action against several cancers, despite its side effects such as cardiotoxicity, severe myelosuppression, stomatitis, high grade mucositis, and alopecia.18 These side effects put a limit to the dose that can be administered to patients,19 typically around 10 mg m–2 every day for up to five consecutive days.20
Bernstein et al.,21 demonstrated that at the administered doses investigated, GaMal was very well-tolerated by all the human subjects, with no reports of serious treatment-related adverse events; again, Bernstein et al.22 showed that a patient, with an advanced hepatocellular carcinoma, when treated with GaMal, has greatly increased his quality of life, mainly because of a large reduction in pain. Furthermore, in recent years GaMal has been the subject of studies in combination with known chemotherapeutics, with the purpose to obtain the same anticancer action and less side effects.23,24
Searching for a metal with chemical properties comparable to gallium, we considered indium, another metallic element of group 13 (IIIa), widely studied in the field of cell labeling, both in detection and diagnosis of infections and inflammatory lesions,25−31 but so far unexplored for antitumor activity.32 The isotopically labeled indium maltolate (InMal) is one of the compounds recently studied,33 along with its biodistribution, both in vitro and in vivo.34 The toxicity of indium compounds is poorly established, and although existing data indicate that indium is more toxic than gallium, toxicity in human (in particular teratogenicity) develops only at high levels of exposure.35
Starting from these considerations and from the chemical properties of group IIIa metallic elements, indium(III) maltolate (InMal) and GaMal were synthesized and tested at increasing doses and incubation times for their in vitro ability of killing cancerous cells such as MDA-MB-231 in comparison to a non-neoplastic cell line, NIH-3T3. MDA-MB-231, a triple negative breast cancer cell line and a perfect model for chemotherapy,36 was selected as one of the classic target of MTX.37 IC50 values, apoptosis observations, quantitative determination of gallium and indium cell uptake, and toxicity reversion with the addition of iron citrate, on the basis of the proposed in vivo action mechanism of orally administered Ga, which bounds to serum transferrin,1 were also determined. Finally, the synergic effect of both Ga or InMal and MTX was investigated to evaluate the lower dose to be used for MTX therapeutic treatments in combination with metallic complexes.
2. Results and Discussion
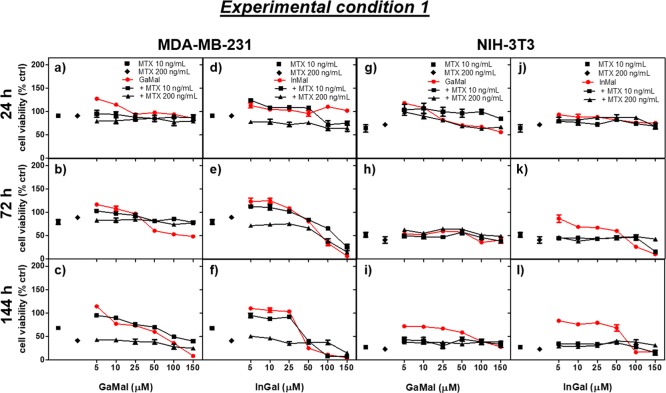
At first, the synthetized GaMal and InMal complexes were physicochemical characterized (Figures S1–S3) and tested for stability assessments (section 2.1) before in vitro biological assays (sections 2.2 and 2.3). MDA-MB-231 and NIH-3T3 cell lines were employed for the study to evaluate antitumor activity of both metallic complexes. Dose- and time-dependence cytotoxicity of GaMal or InMal (Figure 1 and Table S1) was evaluated in apoptosis observations (Figures 2 and 3), IC50 values (Table 1), and cell uptake. Both cell types were treated with increasing concentrations of GaMal or InMal and co-incubated with iron citrate to indirectly evaluate similarity in the indium sand gallium’s mechanisms of action (Figure S4).
Figure 1.
Dose- and time-dependence cell viability due to the addition of GaMal and InMal alone or each one co-incubated with MTX (10 or 200 ng mL–1) (Experiment 1, see Experimental Section) on both MDA-MB-231 (a–f) and NIH-3T3 (g–l), respectively. Results of MTT test are expressed as percentage related to untreated cells (no metal complexes or MTX addition) set as 100%. Bars represent the mean values ± SEM (standard error of the means) of results from three experiments (n = 3, p < 0.05).
Figure 2.
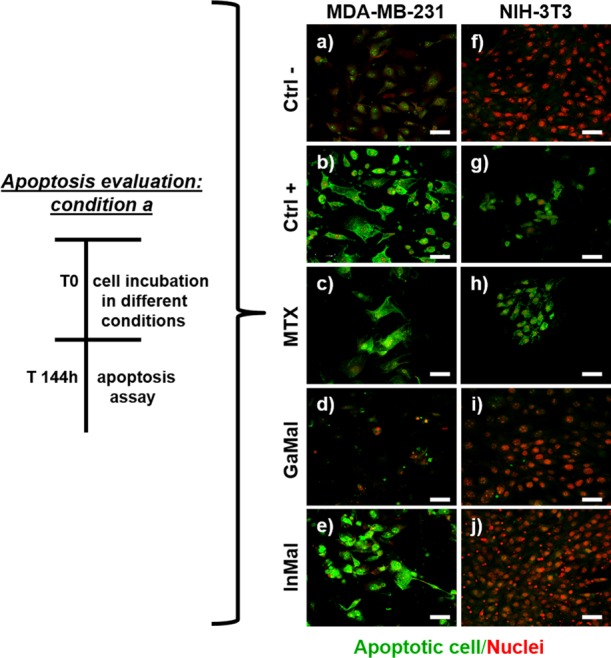
CLSM images of the apoptosis assay. MDA-MB-231 and NIH-3T3 cells were cultured for 144 h under the following conditions: negative control without substances (a,f); positive control with H2O2 (b,g); with 10 ng mL–1 MTX (c,h); with 50 μM GaMal (d,i); with 50 μM InMal (e,j). PSVue480 reagent was used to evaluate apoptotic cells (in green) in MDA-MB-231 and NIH-3T3 cells, respectively. Nuclei were stained with PI (red). CLSM images were magnified at 40×, scale bar: 50 μm.
Figure 3.
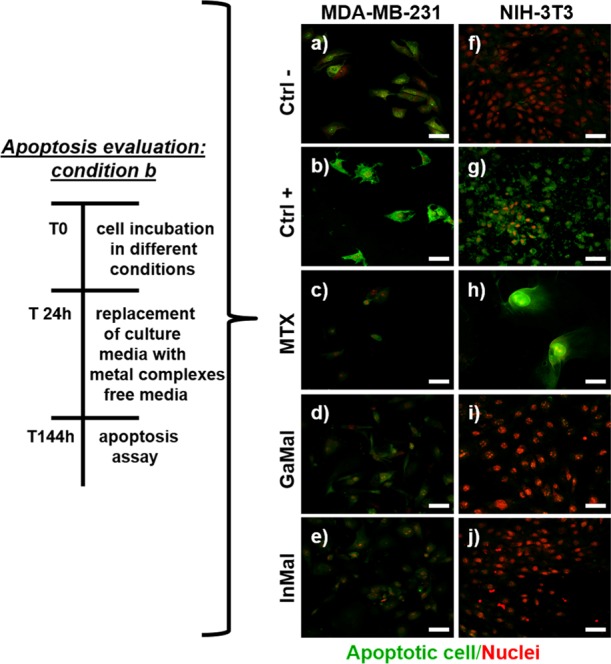
CLSM images of the apoptosis assay. MDA-MB-231 and NIH-3T3 cells were cultured for 24 h under the following conditions: negative control without substances (a,f); positive control with H2O2 (b,g); with 10 ng mL–1 MTX (c,h); with 50 μM GaMal (d,i); with 50 μM InMal (e,j). After 24 h, the cell culture media were replaced with metal complexes free media and incubated up to 144 h. PSVue480 reagent was used to evaluate apoptotic cells (in green) in both MDA-MB-231 and NIH-3T3 cells, respectively. Nuclei were stained with PI (red). CLSM images were magnified at 40×, scale bar: 50 μm.
Table 1. IC50 Values Were Determined for Both Cell Types Following GaMal or InMal Treatment.
| IC50 [mM] |
||
|---|---|---|
| metal complexes | MDA-MB-231 | NIH-3T3 |
| GaMala | 90 | 87 |
| InMala | 32 | 74 |
| GaMalb | >150 | >150 |
| InMal | 120 | >150 |
Cell viability assessed immediately after contact with metal complexes.
Cell viability assessed at 144 h but following medium replacement at 24 h.
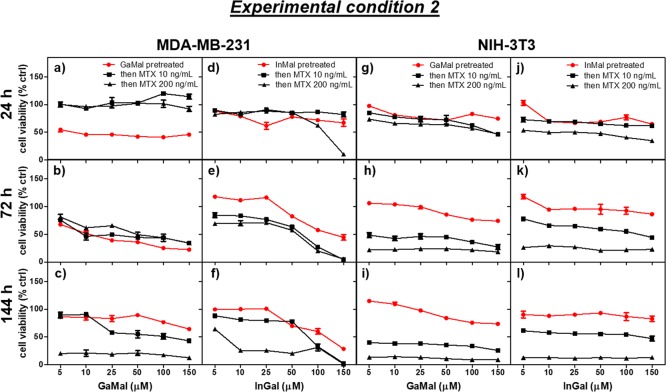
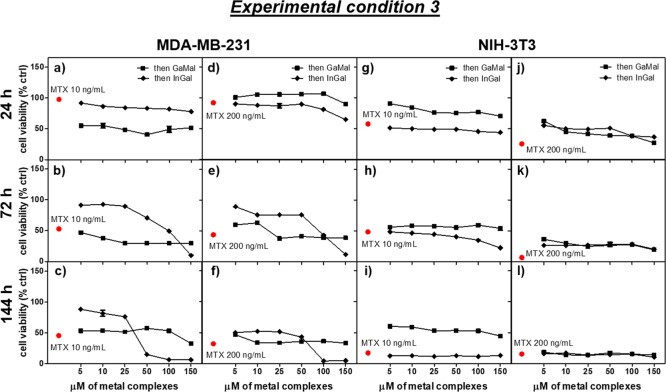
Three types of experiments were performed to determine the synergic effects of the metal complexes with MTX on both MDA-MB-231 and NIH-3T3 cell lines: (1) dose- and time-dependence cytotoxicity of GaMal or InMal co-incubated with MTX (experimental condition 1, Figure 1 and Table S2); (2) pretreatment of both cell types with GaMal or InMal for 24 h subsequently replaced with MTX without metal complexes (experimental condition 2, Figure 4 and Table S3); (3) pretreatment of both cell types with MTX for 24 h subsequently replaced with GaMal or InMal without MTX (experimental condition 3, Figure 5 and Table S4).
Figure 4.
Dose- and time-dependence cell viability exerted by the addition of MTX (10 or 200 ng mL–1) on either MDA-MB-231 (a–f) and NIH-3T3 cells (g–l) pretreated for 24 h with GaMal (a–c and g–i) or InMal (d–f and j–l), respectively (Experiment 2, see Experimental Section). The control is represented by GaMal pretreated cells for 24 h and then incubated for the indicated time with culture media without MTX. Results of MTT test are expressed as a percentage related to untreated cells (no metal complexes or MTX addition) set as 100%. Bars represent the mean values ± SEM of results from the three experiments (n = 3, p < 0.5).
Figure 5.
Dose- and time-dependence cell viability exerted by the addition of GaMal or InMal on MDA-MB-231MDA-MB-231(a–f) and NIH-3T3 (g–l), both pretreated for 24 h with MTX (10 or 200 ng mL–1), respectively (experiment 3, see Experimental Section). The control is represented by both MTX-pretreated cell types for 24 h and then incubated for the time indicated with culture media without metal complexes. Results of MTT treatments were expressed as a percentage related to untreated cells (no metal complexes or MTX) set as 100%. Bars represent the mean values ± SEM of results from three experiments (n = 3, p < 0.05).
2.1. GaMal and InMal Stability Assessments
GaMal and InMal complexes stability was evaluated by high performance liquid chromatography (HPLC) at time 0 and after 14 days. Complexes were dissolved in NIH-3T3 and MDA-MB-231 culture media and in physiological solution as a control. Results indicated that both maltolate metal complexes were still stable 14 days after their dissolution in culture media (degradation < 10%), thus assuring the stability of the compounds for the entire period of the performed experiments (data not shown).
2.2. Dose- and Time-Dependence Viability of GaMal or InMal Treated Cells
At first, maltol, the starting material for the synthesis of the two complexes, showed no cytotoxicity in the whole concentration range explored (data not shown), as previously reported by Sakagami et al.38 Furthermore, the concentrations of the metal complexes employed in this study were chosen close to or less than those produced in plasma by standard GaMal dose.21,22
GaMal or InMal dose- (5, 10, 25, 50, 100, and 150 μM) and time- (24, 72, and 144 h) dependence viability was investigated for MDA-MB-231 (Figure 1a–c,d–f; Table S1) and NIH-3T3 cells (Figure 1g–i,j–l; Table S1), respectively. The data are presented as viability percent of untreated cells set as 100% (no addition of metal complexes or drug). In particular, the viability percent for both cell lines treated with the highest and the lowest concentrations (5 or 150 μM) of GaMal or InMal, respectively, was also reported in Table S1.
In brief, at either GaMal or InMal lowest concentrations (<25 μM) a slight reduction in MDA-MB-231 viability was observed at any time (Figure 1a–f), whereas at the highest concentration (150 μM) for each one of the metal complexes cell viability showed the lowest values (<10%) at 144 h (Figure 1c,f). Although a rather similar dose- and time-dependence viability was observed on NIH-3T3 (Figure 1g–i,j–l), it is remarkable to highlight that cells treated with 150 μM GaMal (Figure 1i) at 144 h showed a 3-fold higher viability in comparison to MDA-MB-231 (Figure 1c). In summary, viability of both MDA-MB-231 and NIH-3T3 cells was consistently reduced at longer incubation times with high doses of InMal in comparison to GaMal treatment.
To determine whether GaMal or InMal treatment induced apoptosis on the cell lines, PSVue480 reagents and propidium iodide (PI) staining were performed in two different experimental conditions (condition a and b, Figures 2 and 3, respectively). In the first condition, the GaMal or InMal (50 μM) added to the culture medium of both cell types was kept throughout the incubation time (144 h) (Figure 2). In the second condition, MAD-MB-231 or NIH-3T3 cells were treated with GaMal or InMal (50 μM) for 24 h followed by removal and replacement with culture medium without metallic complexes up to 144 h (Figure 3). A comparison with MTX treated cells was also performed (Figure 2c,h; Figure 3c,h). In the same day as the dye exclusion test, confocal laser scanning microscopy (CLSM) analyses were performed on untreated cells (negative control (Figure 2a,f; Figure 3a,f), H2O2 (positive control, Figure 2b,g; Figure 3b,g), GaMal (Figure 2d,i; and Figure 3d,i), InMal (Figure 2e,j; and Figure 3e,j), or MTX treated cells (Figure 2c,h; Figure 3c,h). As expected, MAD-MB-231 and NIH-3T3 cells exhibited markedly green fluorescence after H2O2 treatment in both experimental conditions, showing a high level of apoptosis. A quite similar trend for MTX treated cells (at concentration 10 ng mL–1) was observed. As expected, no apoptosis was observed in MDA-MB-231 and NIH3T3 untreated cells (Figure 2a,f; Figure 3a,f).
CLSM analysis of both cell lines treated with GaMal or InMal without (experimental condition a) or with (experimental condition b) medium exchange (Figures 2 and 3) showed interesting differences. MDA-MB-231 and NIH-3T3 cells treated for 144 h with GaMal (experimental condition a) showed a moderate apoptosis for the breast cancer cells with nuclei and cytoplasm partially compromised (Figure 2d), whereas no membrane damage was observed for the fibroblast cell line (Figure 2i). On the contrary, both cell types treated with InMal showed some manifest differences: MDA-MB-231 cells were strongly positive after staining with PSVue480 reagents, whereas NIH-3T3 cells were negative (Figure 2j), indicating that InMal treatment did induce a strong apoptosis, especially in the breast cancer cell line (Figure 2e).
The results obtained by treating both cell lines with GaMal followed by culture media replacement (experimental condition b) showed only degradation of cytoplasm for MDA-MB-231, whereas no apoptosis was observed for NIH-3T3 cells except for the presence of some vacuoles in the cytoplasm. On the contrary, InMal treatment followed by culture media replacement showed no apoptosis or only slight cytoplasm degradation on MDA-MB-231, whereas no membrane damage was observed on NIH-3T3 (Figure 3).
In conclusion, the IC50 values were calculated for both metal complexes to perform a quantitative comparison of experimental conditions a and b as reported in Table 1. At short incubation time (experimental condition a), the IC50 of InMal was consistently lower in comparison to GaMal on MDA-MB-231 cells (32 μM vs 90 μM); at longer incubation time, InMal treatment maintained a strong cytostatic action with IC50 of 120 μM, whereas for GaMal IC50 > 150 μM was calculated (experimental condition b). A similar IC50 for both the experimental conditions was calculated for NIH-3T3 with both metal complexes: interestingly, the IC50 was higher in experimental condition b compared to a (Table 1). Furthermore, the therapeutic index of the two metal complexes, calculated as the ratio between the IC50 value of NIH-3T3 and MDA-MB-231 cells, was approximately 1 for GaMal, and 2.3 for InMal, making the latter more advantageous. Notably, the IC50 values for MTX were >200 ng mL–1 (i.e. >0.45 μM) for MDA-MB-231 and 15 ng mL–1 (i.e. 0.034 μM) for NIH-3T3.37
Considering these results, GaMal and InMal were more effective than the standard drug MTX in reducing the viability of the neoplastic cell lines and at the same time in preserving the viability of the NIH-3T3 cell line (red lines in Figure 1).
Inductively coupled plasma (ICP) analysis was performed as indicated in Experimental Section to quantitatively determine Ga and In cell uptakes. For MDA-MB-231 cells, the absolute amounts of gallium and indium were 25.5 and 45.2 ng, respectively, whereas for NIH-3T3 52 and 190 ng (in 5 × 106 cells for both lines). Because 2.44 × 108 cells occupy a volume of 1 cm339 and the density of cells is near 1 g cm–3, an internal concentration of Ga 18 μM and In 20 μM for the MDA-MB-231 cells was calculated, showing a ratio between cell internal content and culture medium of 40% for Ga and 36% for In, respectively. However, the internal concentration of Ga 37.5 μM and In 83.5 μM was determined for the NIH-3T3 cells. A ratio between cell internal content and culture medium of 167% for Ga and 75% for In was determined, respectively. These data suggest that both metal complexes can penetrate both cell lines but, unexpectedly, the greatest concentration was found in the non-neoplastic cells, but with a reduced toxicity. We may argue that the toxicity of the metal complexes is quite selective for the neoplastic cells, reasonably because of their enhanced metabolism and turnover.40 Moreover, non-neoplastic cells have a reparatory mechanism that can prevent a cascade-reaction toward cell death when the homeostasis is perturbed with the metal complex, while cancerous cells present metabolic dysregulations that ultimately could lead to cell death when perturbation are introduced40 (see also section 2.3).
2.3. Cell Recovery by Iron Citrate in the Copresence of GaMal or InMal
Gallium compounds were reported to inhibit tumor cell growth by targeting iron homeostasis.1,12 Therefore, we tested whether the reduced viability of InMal treated cells could be reversed by the contemporary addition of iron(III) citrate (50 and 500 μM) and we compared these results with those obtained in the same conditions with GaMal. At longer incubation times (144 h), no reversibility in cell viability of MDA-MB-231 treated with either InMal or GaMal (50 μM) in the co-presence of the lowest iron(III) citrate concentration was observed; nevertheless, a supplementary reduction in cell viability was detected (>15%) at higher doses of iron citrate (500 μM). As previously reported,12 in the in vivo situation gallium has a unique mechanism of action because it can interpose itself between proteins and processes in lieu of iron and disrupt critical iron-dependent steps in cell function. Because of similar results obtained with InMal treatments, we indirectly hypothesized that indium and gallium’s mechanisms of action include iron targeting. Differently from NIH-3T3, cell death was only sensibly reduced following InMal or GaMal (50 μM) treatment in the presence of 250 μM iron(III) citrate (>20%) (Figure S4).
These in vitro test results are important because they would suggest that if GaMal or InMal are co-incubated with high iron(III) citrate doses, a selective rescue effect on non-neoplastic cells could be predicted, whereas the toxicity toward neoplastic cells seems to be conserved. The role of iron in cell viability and proliferation is well-known, as certain malignant cells need a greater requirement for iron than normal cells.41 Furthermore, proteins involved in iron import, export, and storage may be altered in cancer cells as previously reported.12
2.4. Evaluation of the Synergic Effects of the Metallic Complexes with MTX
2.4.1. Dose- and Time-Dependence Viability of Cells Co-Incubated with Either GaMal or InMal and MTX
Viability of both cell lines was monitored in culture media with increasing concentrations as previously reported for each metal complex in the copresence of MTX at 10 and 200 ng mL–1, respectively (Figure 1 and Table S2). For MTX exposure, the chosen concentrations were the highest and lowest doses of a chemotherapeutic treatment respectively. MTX cell treatment at both doses and incubation times showed a higher reduction in cell viability on NIH3T3 (<10%) than on MDA-MB-231 (around 35–40%) (as reported in Table S1); cell viability was dose- and time-dependent.
The co-incubation of increasing concentrations of both metallic complexes with 10 ng mL–1 MTX determined a high reduction in cell viability on both MDA-MB-231 and NIH-3T3: also, the data appeared lower in comparison to MTX treatment alone but greater with respect to sole metallic complexes exposure with higher doses and longer incubation times for both GaMal and InMal (Figure 1). The trend was quite similar using 200 ng mL–1 MTX. The viability percent for both cell lines treated with the highest and lowest concentrations (5 or 150 μM) of GaMal or InMal, respectively, in the presence of MTX was also reported in Table S2.
Summing up the results of Experiment 1 (Figure 1 and Table S2), the combined addition of increasing concentrations of GaMal and MTX at 10 or 200 ng mL–1 did not show synergic effect on both cell lines, with viability comparable to the one obtained by MTX treatment alone. At the most elevated concentrations and incubation times, InMal was more toxic than MTX alone but with cell viability comparable to the sole InMal exposure on MDA-MB-231, whereas no great differences were observed on NIH-3T3.
As previously reported, when more than one chemotherapeutic is administered, a direct combination of them could not always lead to a synergic effect, but antagonism or null addition of the effects may be obtained.42 Even if the mechanism of action of the administered chemotherapeutics is different, no certainty of the synergic effect may be expected. In our experimental setup, the co-incubation of metallic complexes with MTX was not beneficial to reduce cell viability. To better evaluate the synergic/antagonistic effect of MTX and the metallic complexes, we investigated whether cells pretreatment with maltolate complexes or MTX followed by medium replacement and supplementation with each one of the mentioned compounds and drug could improve the reduction in viability of neoplastic cells without showing an important toxic effect on NIH-3T3.
2.4.2. Dose- and Time-Dependence Viability of Pretreated GaMal or InMal Cells with MTX
As indicated in Experimental Section, both cell types were incubated with GaMal or InMal with increasing concentrations for 24 h; then, the culture medium was removed and replaced with no metal complexes (as a control) or supplemented with MTX either at 10 or 200 ng mL–1 and incubated up to 144 h (Figure 4 and Table S3).
MDA-MB-231 and NIH-3T3 cell lines pretreated with increasing concentrations of GaMal or InMal followed by replacement with fresh medium without metallic complexes (controls) showed some differences (Figure 4). GaMal pretreatment determined an increment in cells viability at longer incubation times (144 h) on MDA-MB231 (Figure 4c), whereas on NIH-3T3 (Figure 4i) the cell survival was marginally reduced; InMal showed a dose- and time-dependent cytotoxicity on MDA-MB-231 (Figure 4d–f), whereas on NIH-3T3 cells (Figure 4j–l) no manifest reduction in cell viability was observed.
The pretreatment of both cell lines with GaMal or InMal for 24 h, followed by medium exchange and supplementation with MTX at both 10 and 200 ng mL–1 was different with respect to their controls (Figure 4). On both MDA-MB-231 and NIH-3T3 cell lines, the sequential treatment reduced cell viability because of the addition of MTX but with some clear differences. MTX at 10 ng mL–1 was less cytotoxic on GaMal pretreated MDA-MB-231 (Figure 4a–c) in comparison with NIH3T3 cells either at shorter or longer incubation times (Figure 4g–i); InMal pretreated MDA-MB-231 cells (Figure 4d–f) did not reduce viability at lower concentration of InMal, but cells were more sensibly affected at higher concentrations, whereas for NIH-3T3 the cell survival was quite higher (around 50–70%) at all doses and incubation times (Figure 4j–l). At the higher MTX concentration (200 ng mL–1), the dose- and time-dependent viability was greatly reduced on GaMal pretreated NIH-3T3 than on MDA-MB-231 cells (Figure 4); survivability of MDA-MB-231 was significantly reduced at higher concentrations of InMal pretreatment for longer incubation time if compared to NIH-3T3 cell, whose values were more constant at all doses and incubation times even if low (Figure 4). The viability percent for both cell lines treated with the highest and lowest concentrations (5 or 150 μM) of GaMal or InMal, respectively, followed by MTX incubation was also reported in Table S3.
As a conclusion of experimental condition 2 (Figure 4 and Table S3), the addition of 10 ng mL–1 MTX exerted its synergic effect more clearly on MDA-MB-231 pretreated with higher doses (>50 μM) and longer incubation times (144 h) of InMal (viability < 2–5%), whereas a lower reduction on NIH-3T3 cell viability at all doses and times (viability > 55%) was detected. NIH-3T3 InMal pretreatment showed to be more protective to the subsequent addition of MTX at lower dose. On the contrary, the GaMal pretreatment reduced twice NIH-3T3 cell viability.
2.4.3. Dose- and Time-Dependence Viability of MTX Pretreated Cells with GaMal or InMal
The previously reported experiment in section 2.4.2 was repeated changing the sequence of the drugs addition (experimental condition 3). Both MDA-MB-231 and NIH-3T3 cells were pretreated for 24 h with 10 or 200 ng mL–1 MTX; then, the culture medium was removed and replaced with the new culture medium alone (as a control) or supplemented with increasing concentrations of GaMal or InMal, respectively (Figure 5 and Table S4).
MTX cell pretreatment followed by new culture media replacement (control) presented at both doses and incubation time a higher reduction in cell viability on NIH-3T3 (<3%) than on MDA-MB-231 (around 35–40%) (Figure 5).
On MDA-MB-231, the MTX pretreatment at both concentrations showed some differences: at 10 ng mL–1 MTX, the cell viability with GaMal was reduced at 40–50% without great differences in doses and times (Figure 5a–c), whereas with InMal the viability was quite high at low doses at any time (100%) and drastically reduced with high concentrations of the metallic complex at 144 h (Figure 5f). At higher MTX pretreatment, the cell survival with GaMal was even greater at short incubation time and similar to the sole MTX treatment at any time and doses (Figure 5d–f); with InMal addition, the viability was dose- and time-dependent, reaching a very low survivability with high concentration of InMal at 144 h (Figure 5f).
On NIH-3T3, pretreatment with MTX 10 ng mL–1 followed by GaMal addition determined a higher cell survival at any time and doses in comparison to MTX, whereas with InMal the values of cell viability were similar to sole MTX treatment (Figure 5g–i). At MTX 200 ng mL–1, the cell viability with GaMal or InMal was dose- and time-dependent for both metallic complexes even if the values were slightly higher in comparison to sole MTX action (Figure 5j–l).
The viability percent for both cell lines treated with MTX and followed by the highest and lowest concentrations (5 or 150 μM) of GaMal or InMal, respectively, was also reported in Table S4.
In conclusion in our experimental conditions, the synergic action against MDA-MB-231 survival was observed with MTX pretreatment at 10 ng mL–1 followed by exposure to InMal at high doses and incubation times (>50 μM and 144 h).
3. Conclusions
Both metallic complexes showed a higher dose- and time-dependence toxicity on MDA-MB-231 than on NIH-3T3 cells, independently of their internal uptake: 2-fold Ga and 4-fold In were greater in NIH-3T3 in comparison with MDA-MB-231 cells. In general, InMal was more toxic than GaMal. In fact, the InMal calculated IC50 value was 3-fold lower than GaMal on MDA-MB-231 with respect to NIH-3T3 at shorter incubation times; the IC50 value was a 1-fold lower than GaMal on the neoplastic cells in comparison with the fibroblast cell line at 144 h, making InMal an efficient anticancer compound. The GaMal IC50 value calculated for the first time on MDA-MB-231 cell line was 4-fold higher in comparison to the IC50 value previously detected on four hepatocellular carcinoma cell lines.37 The apoptosis tests (PSVue480) showed that both these metal complexes produced cytostatic rather than cytotoxic effects, clearly manifested on NIH-3T3 cells. Interestingly, the co-incubation of the complexes with iron citrate did not revert the toxicity toward MDA-MB-231 but exert a significant protective effect toward NIH-3T3 cells, indicating that InMal may act on iron metabolism. Further investigations need to be performed at a deeper level.
No synergic effect of either GaMal or InMal co-incubated with the well-known cytotoxic MTX was detected on both cell types: cell viability was quite similar to the sole metallic complexes treatment, even though it was reduced in comparison to MTX exposure alone. On the contrary, a synergic effect was observed when pretreating both cell types with InMal followed by culture medium replacement and supplementation with MTX at 10 ng mL–1 or vice versa. The main conclusion drawn by these data showed the necessity to administer the metallic complexes and MTX not in contemporary but separately.
Gallium and indium malonate represent an important advance in the development of therapeutic metallic-based drugs. Further investigation into GalMal and InMal antineoplastic activity and toxicity in tumor-bearing animal models with/without MTX treatment needs to be pursued to determine whether these agents should be advanced to clinical trials and used with other chemotherapeutics. Moreover, the unique mechanism of action that excludes the induction of classical apoptotic path may reasonably prove that drugs can be effective against multidrug resistant cancers, that is, when classical antineoplastic agents failed. The side effect profile of the two complexes is also very interesting; as from the small amount of literature and data available, it appears such that GaMal is neither myelotoxic nor prone to cause classical side effects correlated with chemotherapy, and paradoxically, GaMal showed some antibiotic and mucoprotective effects that can counteract the side effects of chemotherapy.1−9
4. Experimental Section
4.1. Material Preparation and Physicochemical Characterization
4.1.1. Synthesis of GaMal
GaMal was made by a modification of a known procedure:21,43 gallium nitrate nonahydrate (Ga(NO3)3·9H2O corresponding to 7 g gallium, 0.10 mol) was dissolved in 200 mL water; the solution was brought at 70 °C and 45 g maltol (0.36 mol) was carefully added in 5 g portions; each addition is done when the precedent was completely dissolved. A clear solution was obtained. The pH of the resulting solution was raised to approximately 8 by the gradual addition of 2 M NaOH. The solution was heated to approximately 70 °C for 10 min and then cooled to 5 °C. The resulting white to light beige crystalline precipitate was filtered, dispersed in 100 mL boiling water, then cooled and filtered again. The solid was treated with 100 mL boiling ethanol, cooled to 5 °C, filtered, additionally washed with 100 mL ethanol, and finally dried at 50 °C. Yield: 84% (from gallium). Gallium content (by inductively coupled plasma-optical emission spectrometry (ICP-OES)): 15.2%; theoretical: 15.7%. 1H NMR, 13C NMR (Figure S1a,b) and IR data (Figure S3a) obtained are in line with the literature:81H NMR (DMSO-d6): 2.35 (s, 3H, CH3), 6.78 (d, J3 = 5, 1 Hz, 1H, −CH–C=O), 8.33 (d, J3 = 5, 1 Hz, 1H, −C–O–C–CH) ppm; 13C NMR (DMSO-d6): 14.74 (CH3), 108.63 (−CH=CH–C=O), 149.08 (−C–O–C–(CH3)), 151.12 (−C–O–Ga), 155.68 (O–CH=C (CH3)), 175.08 (−C=O) ppm. In particular, in the 1H NMR performed in the nonproton exchanging solvent DMSO-d6, the OH proton at 8.7 ppm44 of maltol is accordingly absent from the spectrum of the complexes. The other change on complex formation is the deshielding of protons 5 and 6 and to a lesser extent of the methyl protons. This is a natural consequence of electron donation from the chelating oxygen atoms. IR spectra of the complexes shows the disappearance of the broad band at 3250 cm–1 attributable to the maltol free −OH, involved in the complexation of Ga or In.
4.1.2. Synthesis of InMal
InMal was prepared similarly to GaMal following a known procedure:44,45 to 45 mmol indium nitrate dissolved in 100 mL water and kept to 90 °C, 20.2 g of maltol (0.16 mol) was added in 5 g portions; the solution was made alkaline with 2 M sodium hydroxide (pH ≈ 9) and warmed for 5 min, and then, the precipitate was collected at room temperature and dried naturally overnight. The solid obtained was recrystallized from methanol/water. Yield: 90% (from indium). Indium content (by ICP-OES): 22.9%; theoretical: 23.4%. 1H NMR, 13C NMR (Figure S2a,b) and IR data obtained for InMal (Figure S3b), are in line with the literature:44,451H NMR (DMSO-d6): 2.39 (s, 3H, CH3), 6.80 (d, J3 = 5, 1 Hz, 1H, −CH–C=O), 8.33 (d, J3 = 5, 1 Hz, 1H, −C–O–C–CH) ppm; 13C NMR (DMSO-d6): 14.57 (CH3), 109.68 (−CH=CH–C=O), 149.06 (−C–O–C–(CH3)), 152.91(−C–O–Ga), 154.59 (O–CH=C (CH3)), 175.32 (−C=O) ppm. The same considerations reported for GaMal regarding the NMR spectra (see above) also apply for InMal. IR spectra of the complexes shows the disappearance of the broad band at 3250 cm–1 attributable to the maltol free −OH, involved in the complexation of Ga or In.
4.1.3. Physicochemical Characterization of GaMal and InMal
PerkinElmer Optima 3300 Dual Vision ICP-OES was used, following standard procedures suggested by the manufacturer.
1H and 13C NMR spectra were registered on a 300 MHz Bruker AVANCE instrument measurements were performed in DMSO-d6 solutions at 20 °C. Chemical shift values are given in δ units with reference to internal reference TMS (see Figures S1 and S2).
The Fourier transform infrared (FTIR) spectra were recorded on powder samples using a Nicolet FT-IR iS10 spectrophotometer (Nicolet, Madison, WI) equipped with an attenuated total reflectance sample cell (Smart iTR with ZnSe dish). The spectra (4000–600 cm–1) were collected adding 56 scans, at a resolution of 2 cm–1. The background consists of 56 scans, collected under the same conditions (see Figure S3).
4.1.4. Stability of GaMal and InMal
Stability tests were performed by HPLC studies of 50 μM GaMal or InMal solutions in MDA-MB-231 or NIH-3T3 culture media and in physiological solution (H2O with 0.9% NaCl) at time 0 and after 14 days, following the diminishing of the peak of the complex. HPLC was performed on 20 cm C-18 columns using a 60:40 acetonitrile/water eluent, flux 1 mL min–1, detection at 260 nm.
4.2. Biological Characterizations
4.2.1. Cell Types and Culture Conditions
The human breast cancer cell line, MDA-MB-231 (HTB26),46 as well as the murine fibroblast cell line, NIH-3T3 (CRL1658), were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). MDA-MB-231 cells were cultured in Leibovitz’s medium (Invitrogen) supplemented with 10% p/v Fetal Bovine Serum (Biowest), 0.5% antibiotics (penicillin and streptomycin, Lonza), and 0.2% p/v Fungizone (EuroClone). NIH-3T3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) modified medium with 4.5 g L–1 glucose (Invitrogen), supplemented with 10% Bovine Calf Serum (Sigma-Aldrich) and 1% p/v l-glutamine (Lonza). Both cell lines were incubated at 37 °C with 5% CO2, routinely trypsinized after confluence, then counted, and seeded into wells.
4.2.2. Cell Viability Assay
MDA-MB-231 and NIH-3T3 cells were seeded at 1 × 104 viable cells/well on 96-well plates and incubated for 4 h (to allow cells to attach to the well). At first, maltol, the starting material for the synthesis of the two complexes, was tested for cytotoxicity in the whole concentration range used for the experiment. The nontoxic effect of maltol was the starting point to determine dose- and time-dependence cytotoxicity of GaMal or InMal. Both cell types were cultured with increasing concentrations (from 5 to 150 μM) of each metal complex and incubated for three time points, respectively (24, 72, and 144 h).
MTX, GaMal, and InMal cytotoxicity were evaluated by MTT assay.47 In brief, the culture medium was replaced by 100 μL Leibovitz’s (for MDA-MB-231 cells) or DMEM high glucose (for NIH-3T3 cells) supplemented with 10 μL of a 5 mg mL–1 solution of MTT in 1× phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4), and the cell cultures were incubated for 3 h. Viable cells are able to reduce MTT into formazan crystals. After the addition of 100 μL of solution C (2-propanol and HCl 0.04%), the well plate containing the cultured cells was incubated at 37 °C for 30 min. Aliquots of 100 μL were sampled, and the absorbance was measured at 595 nm with a microplate reader (Bio-Rad Laboratories, Hercules, CA). A standard curve of cell viability for both either MDA-MB-231 cells and NIH-3T3 cells was used to convert the results into control percent (no treatment with metal complexes or drug).
4.2.3. Assessment of Apoptosis
To measure apoptosis, MDA-MB-231 and NIH-3T3 cells were labeled using the PSVue480 cell stain according to the manufacturer’s instructions (Molecular Targeting Technologies, Inc.). Briefly, the cells were seeded on glass slides (Thermo Scientific) at a density of 1 × 104 cells per well and incubated with H2O2 (positive control; 100 mM), without maltolate complexes (negative control), with MTX 200 ng mL–1, and with GaMal or InMal for 144 h (experimental condition a) or for 24 h followed by medium replacement without compounds and further incubation up to 144 h (experimental condition b). All samples were prepared, following the seeding procedure reported previously. At the end of incubation time, the cells were stained with PSVue480 solution prepared as follows: a 2 mM solution of preweighed apo-PSS480 was prepared in DMSO until the solid apo-PSS480 was fully dissolved; an equal volume of 4.2 mM zinc nitrate solution was then added, and the solution was placed in a water bath at 37 °C and shaken for 30 min to ensure complete complex formation, obtaining a clear orange solution of 1 mM stock PSVue480 in 1:1 DMSO. The samples were stained with 10 mM PSVue480 by gently shaking for 2 h at room temperature and finally washed with TES buffer, consisting of 5 mM TES (n-[tris(hydroxymethyl)]-2-aminoethanesulfonic acid, Sigma-Aldrich) and 145 mM NaCl in distilled water. Samples were then counterstained with a PI solution (2 mg mL–1) to target the cellular nuclei and observed with a CLSM system (Leica TCS SPII Microsystems, Bensheim, Germany) at 40× magnification.48
4.2.4. Ga and In Uptake Studies by ICP Analysis
To this end, MDA-MB-231 and NIH-3T3 cells were seeded at 5 × 104 viable cells in a 250 mL flask, incubated for 72 h with the appropriate culture medium (15 mL) containing 50 μM of one of the chosen metal complex. The medium was removed, and the cells carefully washed three times with physiological solution and trypsinized as described above. The cells were centrifuged at about 1200 rpm for 3 min, suspended in NaCl 0.9% (5 mL) and centrifuged again; the procedure was repeated twice. The cells, suspended in 5 mL NaCl 0.9%, were counted in a Burke chamber (10 μL cell suspension +10 μL Trypan Blue, 0.4%), and a volume corresponding to 5 × 106 cells was centrifuged (3 min, 12 000 rpm). The precipitate was digested with 250 μL ultrapure nitric acid (65% p/p) and after dilution to 3 mL, gallium or indium content was determined by ICP-mass spectrometry (MS) following standard procedures suggested by the manufacturer (PerkinElmer ICP-MS-DRCe: inductively coupled plasma equipped with a mass spectrometric detector and a direct reaction cell).
4.2.5. Reversion of Toxicity of GaMal and InMal by iron(III) Citrate
It was reported that in vivo Ga(III) follows the route of cellular uptake of Fe(III) and antagonize in part its effect.1,12 To this end, both MDA-MB-231 and NIH-3T3 cells were incubated with a solution of ferric citrate (FeCit 250 μM, Sigma-Aldrich) and 50 μM GaMal or InMal at different times (24, 72, and 144 h) to ascertain any competition or antagonism between these substances (Figure S4).
4.2.6. Evaluation of the Synergic Activity of Metallic Complexes with MTX
To determine the synergic activity of the metal complexes with MTX treatment, three types of experiments were performed:
Experiment 1: both cell types were cultured with increasing concentration of GalMal or InMal in the presence of MTX at two different concentrations (10 or 200 ng mL–1) for three different time points, respectively (24, 72, and 144 h).
Experiment 2: both cell types were cultured with increasing concentrations (from 5 to 150 μM) of GaMal or InMal for 24 h. Then, the culture media was removed, changed with new culture media containing MTX (10 or 200 ng mL–1), and incubated for three different time points, respectively (24, 72, and 144 h). As a control, GaMal- or InMal-treated cells of both cell lines were incubated with new culture media, free of any compounds.
Experiment 3: both cell types were cultured with MTX (10 or 200 ng mL–1) for 24 h. Then, the drug was removed and changed with increasing concentrations (from 5 to 150 μM) of GaMal or InMal added in the culture media and incubated for three different time points, respectively (24, 72, and 144 h). As a control, MTX-treated cells of both cell lines were incubated with new culture media, free of any compounds.
Cell viability was determined as previously indicated in section 4.2.2.
4.3. Statistical Analysis
Each experiment consisted of three replicates for each condition and was repeated three times. Results were expressed as the mean ± standard deviation. To compare the results between the control and each treated sample, the one-way ANOVA test was applied (p < 0.05).
Acknowledgments
This study was supported by “Project SAL-45” financed by the Regione Lombardia (2010) and a project financed by the Fondazione Alma Mater Ticinensis (2010). The authors would like to acknowledge financial support from the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (grant: PRIN 2011, NanoMed prot. 2010FPTBSH_009). This research was also performed with the support of Pavia University’s Crowdfunding on breast tumor studies (2015, https://universitiamo.eu/en/campaigns/tumore-seno-diagnosi- cura-nanoparticelle-doro). N.B. thanks the support by Fondazione Umberto Veronesi Fellowship—grant 2017. We are grateful to Dr. P. Vaghi (Centro Grandi Strumenti, University of Pavia, Italy) for the technical assistance in the CLSM studies and to Marta Bordoni (University of Pavia) for precious help in correcting the manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b02026.
Representative viability percent of both MDA-MB-231 and NIH-3T3 cell types: treated with GaMal or InMal or MTX for 24 and 144 h, respectively, treated with two concentrations of GaMal or InMal in the co-presence of MTX for 24 and 144 h, respectively, pretreated with two concentrations of GaMal or InMal for 24 h, followed by medium removal and supplementation with MTX for 24 and 144 h, respectively, and pretreated with MTX for 24 h, followed by culture medium removal and replacement with two concentrations of GaMal or InMal for 24 and 144 h, respectively; 13C NMR and 1H NMR spectra of GaMal in DMSO-d6 and InMal in DMSO-d6; IR spectra of GaMal, InMal, and maltol; and representative viability percent of both MDA-MB-231 and NIH-3T3 cell types pretreated with MTX for 24 h, followed by culture medium removal and replacement with two concentrations of GaMal or InMal for 24 and 144 h, respectively (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bernstein L. R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–682. [PubMed] [Google Scholar]
- Fecteau M.-E.; Aceto H. W.; Bernstein L. R.; Sweeney R. W. Comparison of the antimicrobial activities of gallium nitrate and gallium maltolate against Mycobacterium avium subsp. paratuberculosis in vitro. Vet. J. 2014, 202, 195–197. 10.1016/j.tvjl.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Sampieri F.; Allen A. L.; Alcorn J.; Clark C. R.; Vannucci F. A.; Pusterla N.; Mapes S. M.; Ball K. R.; Dowling P. M.; Thompson J.; et al. Efficacy of gallium maltolate against Lawsonia intracellularis infection in a rabbit model. J. Vet. Pharmacol. Ther. 2014, 37, 571–578. 10.1111/jvp.12132. [DOI] [PubMed] [Google Scholar]
- Arnold C. E.; Bordin A.; Lawhon S. D.; Libal M. C.; Bernstein L. R.; Cohen N. D. Antimicrobial activity of gallium maltolate against Staphylococcus aureus and methicillin-resistant S. aureus and Staphylococcus pseudintermedius: An in vitro study. Vet. Microbiol. 2012, 155, 389–394. 10.1016/j.vetmic.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R.; Purpi D. P.; Woodliff J.; Yang M.; Wereley J. P. Development of gallium compounds for treatment of lymphoma: gallium maltolate, a novel hydroxypyrone gallium compound, induces apoptosis and circumvents lymphoma cell resistance to gallium nitrate. J. Pharmacol. Exp. Ther. 2007, 322, 1228–1236. 10.1124/jpet.107.126342. [DOI] [PubMed] [Google Scholar]
- Wu X.; Wang T. W.; Lessmann G. M.; Saleh J.; Liu X.; Chitambar C. R.; Hwang S. T. Gallium Maltolate Inhibits Human Cutaneous T-Cell Lymphoma Tumor Development in Mice. J. Invest. Dermatol. 2015, 135, 877–884. 10.1038/jid.2014.476. [DOI] [PubMed] [Google Scholar]
- Collery P.; Keppler B.; Madoulet C.; Desoize B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. 10.1016/s1040-8428(01)00225-6. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272. 10.4155/fmc.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduray K.; Odhav B. The in vitro photodynamic effect of laser activated gallium, indium and iron phthalocyanine chlorides on human lung adenocarcinoma cells. J. Photochem. Photobiol., B 2013, 128, 58–63. 10.1016/j.jphotobiol.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Wehrung D.; Bi L.; Geldenhuys W. J.; Oyewumi M. O. Antitumor efficacy and tolerability of systemically administered gallium acetylacetonate-loaded gelucire-stabilized nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1029–1040. 10.1166/jbn.2013.1598. [DOI] [PubMed] [Google Scholar]
- Enyedy É. A.; Dömötör O.; Varga E.; Kiss T.; Trondl R.; Hartinger C. G.; Keppler B. K. Comparative solution equilibrium studies of anticancer gallium(III) complexes of 8-hydroxyquinoline and hydroxy(thio)pyrone ligands. J. Inorg. Biochem. 2012, 117, 189–197. 10.1016/j.jinorgbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R.; Antholine W. E. Iron-targeting antitumor activity of gallium compounds and novel insights into triapine(®)-metal complexes. Antioxid. Redox Signaling 2013, 18, 956–972. 10.1089/ars.2012.4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum B.; Srivanas S.; Beck J.; Vesole D.; Largey M.; Valone F. H.; Sayre P. H. Phase I trial of oral gallium maltolate in refractory malignancies. Progr. Proc. Am. Soc. Clin. Oncol. 2003, 22, 943. [Google Scholar]
- Hortobágyi G. N. Anthracyclines in the treatment of cancer. An overview. Drugs 1997, 54, 1–7. 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- Abraham R.; Basser R. L.; Green M. D. A risk-benefit assessment of anthracycline antibiotics in antineoplastic therapy. Drug Saf. 1996, 15, 406–429. 10.2165/00002018-199615060-00005. [DOI] [PubMed] [Google Scholar]
- Shan K. Anthracycline-Induced Cardiotoxicity. Ann. Intern. Med. 1996, 125, 47–58. 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- Zuckerman K. S. Efficacy of intensive, high-dose anthracycline-based therapy in intermediate- and high-grade non-Hodgkin’s lymphomas. Semin. Oncol. 1994, 21, 59–64. [PubMed] [Google Scholar]
- Koeller J.; Eble M. Mitoxantrone: a novel anthracycline derivative. Clin. Pharmacol. 1988, 7, 574–581. [PubMed] [Google Scholar]
- Wong E.; Giandomenico C. M. Current status of platinum-based antitumor drugs. Chem. Rev. 1999, 99, 2451–2466. 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- Reedijk J. Improved understanding in platinum antitumour chemistry. Chem. Commun. 1996, 801–806. 10.1039/cc9960000801. [DOI] [Google Scholar]
- Bernstein L. R.; Tanner T.; Godfrey C.; Noll B.; Road W.; Park M. Chemistry and Pharmacokinetics of Gallium Maltolate, a Compound with High Oral Gallium Bioavailability. Met.-Based Drugs 2000, 7, 33–47. 10.1155/mbd.2000.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein L. R.; van der Hoeven J. J. M.; Boer R. O. Hepatocellular Carcinoma Detection by Gallium Scan and Subsequent Treatment by Gallium Maltolate: Rationale and Case Study. Anti Canc. Agents Med. Chem. 2011, 11, 585–590. [DOI] [PubMed] [Google Scholar]
- Chitambar C. R.; Purpi D. P. A novel gallium compound synergistically enhances bortezomib-induced apoptosis in mantle cell lymphoma cells. Leuk. Res. 2010, 34, 950–953. 10.1016/j.leukres.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F.; Bundy B. N.; Greer B. E.; Fowler J. M.; Clarke-Pearson D.; Burger R. A.; Mannel R. S.; DeGeest K.; Hartenbach E. M.; Baergen R.; et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J. Clin. Oncol. 2003, 21, 3194–3200. 10.1200/jco.2003.02.153. [DOI] [PubMed] [Google Scholar]
- Jalilian A.; Bineshmarvasti M.; Sardari S. Application of Radioisotopes in Inflammation. Curr. Med. Chem. 2006, 13, 959–965. 10.2174/092986706776361049. [DOI] [PubMed] [Google Scholar]
- Peters A. M.; Saverymuttu S. H.; Reavy H. J.; Danpure H. J.; Osman S. e.; Lavender J. P. Imaging of inflammation with indium-111 tropolonate labeled leukocytes. J. Nucl. Med. 1983, 24, 39–44. 10.1007/BF01944094. [DOI] [PubMed] [Google Scholar]
- Thakur M. L.; Coleman R. E.; Welch M. J. Indium-111-labeled leukocytes for the localization of abscesses: preparation, analysis, tissue distribution, and comparison with gallium-67 citrate in dogs. J. Lab. Clin. Med. 1977, 89, 217–228. [PubMed] [Google Scholar]
- Datz F. L. Indium-111-labeled leukocytes for the detection of infection: Current status. Semin. Nucl. Med. 1994, 24, 92–109. 10.1016/s0001-2998(05)80225-9. [DOI] [PubMed] [Google Scholar]
- Fisher B.; Packard B. S.; Read E. J.; Carrasquillo J. A.; Carter C. S.; Topalian S. L.; Yang J. C.; Yolles P.; Larson S. M.; Rosenberg S. A. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J. Clin. Oncol. 1989, 7, 250–261. 10.1200/jco.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Divgi C. R.; Welt S.; Kris M.; Real F. X.; Yeh S. D. J.; Gralla R.; Merchant B.; Schweighart S.; Unger M.; Larson S. M.; et al. Phase I and Imaging Trial of Indium 111-Labeled Anti-Epidermal Growth Factor Receptor Monoclonal Antibody 225 in Patients With Squamous Cell Lung Carcinoma. JNCI, J. Natl. Cancer Inst. 1991, 83, 97–104. 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- Griffith K. D.; Read E. J.; Carrasquillo J. A.; Carter C. S.; Yang J. C.; Fisher B.; Aebersold P.; Packard B. S.; Yu M. Y.; Rosenberg S. A. In Vivo Distribution of Adoptively Transferred Indium- 111- Labeled Tumor Infiltrating Lymphocytes and Peripheral Blood Lymphocytes in Patients With Metastatic Melanoma. JNCI, J. Natl. Cancer Inst. 1989, 81, 1709–1717. 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- Head J. F.; Wang F.; Elliott R. L. Antineoplastic Drugs that Interfere with Iron Metabolism in Cancer Cells. Adv. Enzyme Regul. 1997, 37, 147–169. 10.1016/s0065-2571(96)00010-6. [DOI] [PubMed] [Google Scholar]
- Fazaeli Y.; Jalilian A. R.; Rahiminejad A.; Bolourinovin F. Indium-111 maltolate complex as a cell labeling agent for SPECT imaging. Iran. J. Nucl. Med. 2012, 20, 8–13. [Google Scholar]
- Clevette D. J.; Lyster D. M.; Nelson W. O.; Rihela T.; Webb G. A.; Orvig C. Solution Chemistry of Gallium and Indium 3-Hydroxy-4-pyridinone Complexes in Vitro and in Vivo. Inorg. Chem. 1990, 29, 667–672. 10.1021/ic00329a021. [DOI] [Google Scholar]
- Brother P. J.; Ruggiero C. E.. Coordination and solution chemistry of the metals: biological, medical and environmental relevance. In The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities; Aldridge S., Downs A. J., Eds.; Wiley: John Wiley & Sons: Chichester, West Sussex, United Kingdom, 2011; p 586. [Google Scholar]
- Neve R. M.; Chin K.; Fridlyand J.; Yeh J.; Baehner F. L.; Fevr T.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merli D.; Pivi F.; Profumo A.; Quadrelli P.; Milanese C.; Risi G.; Visai L. Carboxymethylinuline-chitosan nanoparticles for the delivery of the antineoplastic mitoxantrone. ChemMedChem 2016, 11, 2436–2444. 10.1002/cmdc.201600385. [DOI] [PubMed] [Google Scholar]
- Yasumoto E.; Nakano K.; Nakayachi T.; Morshed S. R. M.; Hashimoto K.; Kikuchi H.; Nishikawa H.; Kawase M.; Sakagami H. Cytotoxic Activity of Deferiprone, Maltol and Related Hydroxyketones against Human Tumor Cell Lines. Anticancer Res. 2004, 24, 755–762. [PubMed] [Google Scholar]
- Del Monte U. Does the cell number 10(9) still really fit one gram of tumor tissue?. Cell Cycle 2009, 8, 505–506. 10.4161/cc.8.3.7608. [DOI] [PubMed] [Google Scholar]
- Malarkey D. E.; Hoenerhoff M.; Maronpot R. R.. Carcinogenesis: Mechanisms and Manifestations. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3th ed.; Haschek W. M., Rousseaux C. G., Wallig M. A., Eds.; Elsevier, Academic Press: Amsterdam, 2013; Vol. 1, pp 107–144. [Google Scholar]
- Torti S. V.; Torti F. M. Review Ironing out cancer. Cancer Res. 2011, 71, 1511–1514. 10.1158/0008-5472.can-10-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal D. K.; Garrison J. C.. Pharmacodynamics: Molecular Mechanisms of Drug Action. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th ed.; Brunton L., Chabner B. A., Eds.; McGraw-Hill: New York, 2011; pp 41–72. [Google Scholar]
- Pollina G. F.; Pepe M.; Dean A.; Di Marco V.; Marton D. Reduction in absorption of gallium maltolate in adult horses following oral administration with food: chemistry and pharmacokinetics. J. Vet. Pharmacol. Therapeut. 2013, 36, 456–461. 10.1111/jvp.12022. [DOI] [PubMed] [Google Scholar]
- Tuck D. G.; Yang M. K. Co-ordination Compounds of Indium. Part XI The Stepwise One electron Reduction of Some Neutral Indium (III) Complexes. J. Chem. Soc. A 1971, 3100–3102. 10.1039/j19710003100. [DOI] [Google Scholar]
- Matsuba C. A.; Nelson W. O.; Rettig S. J.; Orvig C. Neutral Water-Soluble Indium Complexes of 3-Hydroxy-4-pyrones and 3-hydroxy-4-pyridinones. Inorg. Chem. 1988, 27, 3935–3939. 10.1021/ic00295a012. [DOI] [Google Scholar]
- Cailleau R.; Young R.; Olivé M.; Reeves W. J. Breast Tumor Cell Lines From Pleural Effusions. J. Natl. Cancer Inst. 1974, 53, 661–674. 10.1093/jnci/53.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Kersigo J.; D’Angelo A.; Gray B. D.; Soukup G. A.; Fritzsch B. The role of sensory organs and the forebrain for the development of the craniofacial shape as revealed by Foxg1-cre-mediated microRNA loss. Genesis 2011, 49, 326–341. 10.1002/dvg.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.