Abstract
In this study, two kinds of novel carbazole-based ethynylpyridine salts: 3,6-bis[2-(1-methylpyridinium)ethynyl]-9-pentyl-carbazole diiodide (BMEPC) and 3,6-bis[2-(1-methylpyridinium)ethynyl]-9-methyl-carbazole diiodide (BMEMC) have been employed as photosensitizers owing to their excellent antibacterial activity. These molecules possess symmetric A−π–D−π–A-type structures, which would bring in the unique optical properties. The inhibition zone measurement of a gradient concentration from 0 to 100 μM showed BMEPC and BMEMC photoinduced antibacterial activity against Escherichia coli. Diameters of zone of inhibition were up to 15 and 14 mm under laser irradiations. Under the exposure of the laser of 442 nm with a power density of 20 mW/cm2, the minimum inhibitory concentrations (MICs) of BMEPC on E. coli were between 3.5 and 6.9 μM and that of BMEMC were between 9.4 and 18.8 μM, respectively. In the dark experiments as a control, the MIC value is between 6.9 and 13.8 μM for BMEPC, whereas it is between 187.5 and 225.0 μM for BMEMC. By the comparison of the MIC values of BMEPC and BMEMC with laser irradiation and in dark, the laser-induced toxicity on bacteria is more evident, though both of the derivatives have dark toxicity. With the laser irradiation duration of 30 s and 10 min for BMEPC and BMEMC, respectively, the survival rate of E. coli approximates zero. An antibacterial mechanism has been proposed based on the electron paramagnetic resonance characterization, which indicates that a nitride radical is generated under laser irradiation. The carbazole-based ethynylpyridine photosensitizers would provide high potential for further applications in photodynamic therapy.
1. Introduction
In recent years, the excessive use of antibiotics has caused the spread of multiresistant bacterial strains as one of the most threatening issues.1,2 Therefore, the research for new approaches that can kill bacteria without inducing the appearance of undesired drug-resistant strains is imperative. Antimicrobial photodynamic therapy (PDT)3 is a new promising strategy to kill bacteria including Gram-positive and Gram-negative bacteria. Gram-negative bacteria such as Escherichia coli (E. coli) have an impermeable outer cell membrane that contains endotoxins and serves as a shelter, blocking antibiotics and drugs and protecting the sensitive inner membrane and cell wall.4,5 PDT has advantages over conventional antibiotic therapy because it eradicates bacteria by producing free radicals in the presence of a photosensitizer and the irradiation of visible light or laser will not induce drug resistance.4
It is worth noting that the PDT process requires the penetration of photosensitizers into the cell walls of bacteria and finally the cytoplasm.6,7 However, the outer membrane barrier of Gram-negative bacteria can effectively prevent the uptake of anionic and neutral photosensitizers. Therefore, Gram-negative bacteria are far more difficult to be inactivated by PDT.8 To solve this problem, one way is to involve hydrophobic interaction by engineering the photosensitizers with hydrophobic segments.9 Another effective way is to employ some cationic photosensitizers.10 A pronounced cationic charge induced by a macromolecule with a large alkyl chain can bind to negatively-charged bacteria and alter the permeability of the outer membrane.11 In the past decades, multiple kinds of photosensitizers including phthalocyanines,12 chlorins,13 porphyrins,14 chlorophyll,15 and bacteriochlorophyll16 have displayed a fairly appreciable antimicrobial activity as photodynamic agents in the presence of oxygen because these derivatives generate potent oxygen species with suitable wavelengths.
Furthermore, photosensitizers are expected to have low dark toxicity and absorption in the optical window (600–900 nm) for light penetration through tissues to achieve ideal antibacterial PDT performance. Besides, antibacterial photosensitizers should be able to kill multiple microbial cells at a relatively low intensity of light, which indicates that photosensitizers should have a relatively large photon absorption cross section. Compared to the reported photosensitizers, carbazole motif is a more naturally occurring heterocycle. It has attracted increasing attention in the modifications of natural compounds and synthesis of new derivatives.17 The carbazole compound families have been known to have multifunctions including anticancer, antibacterial, anti-HIV, and anti-inflammatory properties.18 Their affinity toward DNA19 enables drugs more easily to bind with bacterial DNA and display their potency against bacteria. Previously reported carbazole-based compounds are primarily used to characterize the interaction with DNA in vitro, whereas the carbazole scaffold in this study further focuses on the antibacterial activity of such kind of compounds in vivo. Carbazole scaffolds are one of the most important organic molecular skeletons for optical functional molecule design.20−22 On one side, compared to the reported photosensitizers, carbazole-based derivatives, as an organic chromophore, have a large conjugate system that exhibits a large absorption cross section and contributes to large intramolecular electron transfer17 and thus helps stabilize the formed cation. On the other side, according to the literature, carbazole derivatives have high binding affinity. Cations formed by carbazole-based derivatives have high photochemical stability,23 unique biologically activity,24 and photoelectronic25 properties, which are expected to display effective inhibition on Gram-negative bacteria.
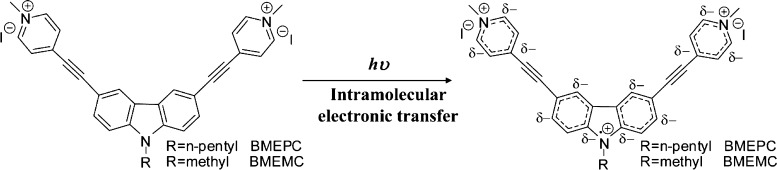
In this study, we have employed two kinds of novel carbazole-based ethynylpyridine salts: 3,6-bis[2-(1-methylpyridinium)ethynyl]-9-pentyl-carbazole diiodide (BMEPC) and 3,6-bis[2-(1-methylpyridinium)ethynyl]-9-methyl-carbazole diiodide (BMEMC) in antibacterial application (Figure 1a). The chemical structures are shown in Figure 1b, which have been reported in our previous work.26 The advantages of symmetric A−π–D−π–A-type structures, different affinities, and water solubility result in the unique biological activity and optical properties. Inhibition zone test and the antibacterial activity of BMEPC and BMEMC photoinduced antibacterial activity against E. coli under laser irradiation of 442 nm have been investigated. The minimum inhibitory concentrations (MICs) of BMEPC and BMEMC on E. coli have been characterized, respectively. The MIC of BMEPC on E. coli was achieved between 3.5 and 6.9 μM, which is effective in inhibiting the growth of E. coli. An antibacterial mechanism is proposed based on the electron paramagnetic resonance (EPR) characterization. This study would provide high potential for the carbazole-based ethynylpyridine molecules to be applied as photosensitizers in PDT.
Figure 1.
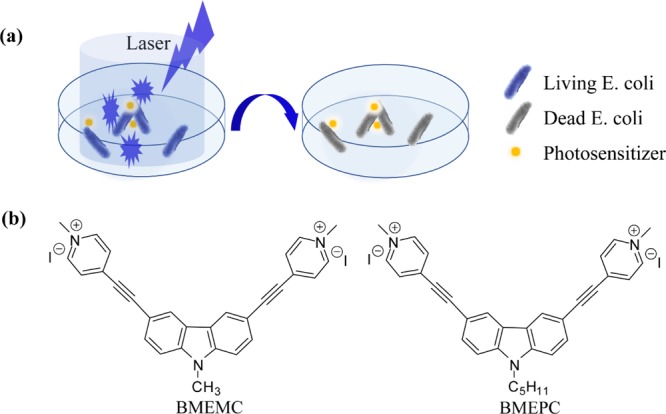
(a) Process of photodynamic inactivation of E. coli. (b) Chemical structure of carbazole-based ethynylpyridine salts.
2. Results and Discussion
2.1. UV–Vis Absorption and Fluorescence Spectra
The normalized one-photon absorption and fluorescence spectra of BMEPC and BMEMC in water are shown in Figure 2. The absorption spectra of BMEPC and BMEMC are extremely similar but different in absorbance intensity. There are two obvious absorption peaks, where the absorption peak around 330 nm is corresponding to the π–π* electron transfer and the absorption peak around 420 nm is due to the intramolecular charge transfer from the ground state to excited state. The fluorescence emission spectra were measured with an excitation wavelength of 425 nm for BMEPC and BMEMC in phosphate-buffered saline (PBS) solution. Figure 2 shows that the compounds exhibit fluorescence emission peaks at 576 and 592 nm for BMEPC and BMEMC, respectively. The fluorescence quantum yield was calculated using fluorescein in 0.1 N aqueous NaOH solution (Φ = 0.9) as a reference standard.16 The quantum yields of BMEPC and BMEMC in PBS buffer solution were measured to be 2.0 × 10–4 and 6.0 × 10–5, respectively. It is so weak because of the effect of a heavy atom and fluorescence quenching caused by intramolecular D–A electron transfer.27,28
Figure 2.
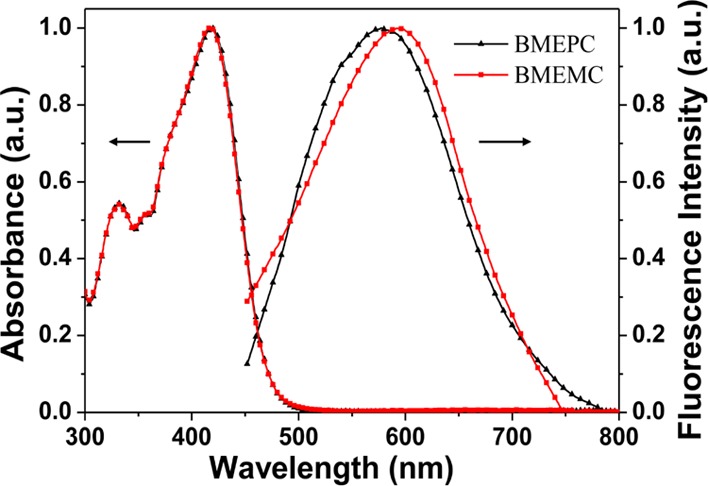
Absorption and normalized one-photon-induced fluorescence spectra of BMEPC and BMEMC in water.
2.2. Zone of Inhibition Measurement
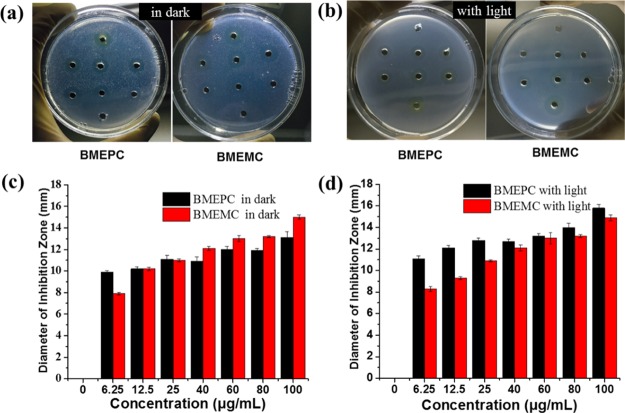
Inhibitory zone characterization is an effective way to visualize the antibacterial activity directly. The inhibitory zone was determined by different concentrations of photosensitizers ranging from 6.3, 12.5, and 25 to 40, 60, 80, and 100 (mg/mL) against E. coli. Large inhibition zone was observed around BMEPC and BMEMC samples either in dark or under laser irradiation of 442 nm (Figure 3a,b), whereas no inhibition zone was seen around the hole without BMEPC and BMEMC (not circled area in Figure 3a,b). It was revealed that all of the BMEPC and BMEMC samples with the total concentration range showed antibacterial effect. Results of statistical analysis showed that the mean diameters of inhibitory zone formed around BMEPC and BMEMC samples were significantly different (P < 0.05) (Figure 3c,d). Under the same concentration, both BMEPC and BMEMC exhibit an improved antibacterial property with light compared to the group in dark. Compared with BMEMC, the data of BMEPC in dark are lower but in reverse in dark. These comparisons indicate the crucial effect of irradiation on the inhibition of bacteria. Also, BMEPC has a larger diameter of inhibition zone than BMEMC in light, as can be explained by different lengths of alkyl chain they carried. With a longer chain of alkyl, BMEPC displays a strong hydrophobic property, which enables a highly enhanced affinity to the lipid layer of microbial cells.29 Note that even in dark, BMEMC displayed antibacterial activity, which may also account for the electrostatic interaction between the ionic bacterial membrane and the cationic photosensitizer. According to Quang et al.,30 inhibitory zone measurements proved that BMEPC and BMEMC were effective against Gram-negative E. coli.
Figure 3.
Measurement of diameters of photosensitizers BMEPC and BMEMC against E. coli (a) in dark & (b) under irradiation of laser of 442 nm inhibition after 24 h incubation at 37 °C. The statistical analysis shows the diameter of prohibition zone as a function of the concentration of BMEPC and BMEMC in dark (c) and with light (d).
2.3. Antimicrobial Properties
We have carried out the MIC characterization to determine the MIC. Under the laser irradiation with a power density of 20 mW/cm2, the MIC value of BMEPC falls in the interval of 3.5 and 6.9 μM, whereas that of BMEMC is between 9.4 and 18.8 μM (Figure S2a,c). In dark, the MIC value interval for BMEPC is from 6.9 to 13.8 and that for BMEMC is from 187.5 to 225.0 μM (Figure S1b,d). All of the concentrations are referred to the final effective concentration. The MIC value with a laser of 442 nm irradiation is much lower than that in dark, which indicates that the antibacterial activity is much stronger with laser irradiation. This photodynamic toxicity may be caused by the free radicals generated with laser. In addition, the compounds also display appreciable toxicity in dark, which can be explained by the electrostatic interaction between the microbial cell membrane and the photosensitizers.10,31 According to Zhang et al.,32 carbazole derivatives can permeate the membrane of E. coli cells and accumulate inside. We have performed an experiment by confocal scanning laser microscopy (CSLM) to study the viability membrane disruption of E. coli bacteria treated with BMEPC and BMEMC (Figure S2). Furthermore, propidium iodide (PI) was used to identify the dead bacteria. The result indicates that the carbazole derivatives BMEPC and BMEMC can penetrate the membrane of E. coli and accumulate inside. The images stained by PI verified the antibacterial activity by carbazole derivatives BMEPC and BMEMC. Compared with the previously reported photosensitizers,33,34 the MIC value of BMEPC is relatively lower under irradiation, indicating a more prominent effect on bacterial inhibition. These results suggest that photoinduced active species are the dominant factors to kill bacteria.
2.4. Photoinduced Bacterial Inactivation
The antibacterial activities of BMEPC & BMEMC have been evaluated by 442 nm laser-induced disinfection of E. coli. The number of E. coli cells remains almost unchanged in control experiments without photosensitizers (Figure S3a), indicating the negligible effect of the visible light to the bacterial cells. Figure 4 shows the colonies of E. coli inoculated on the agar plate and indicates that the survival rate varied as the time of interaction between E. coli and BMEPC and BMEMC varied, corresponding to the value in Table S1. It is illustrated that the growth of E. coli on the plate reflects the survival rate of E. coli after interacting with reagents after a certain time interval.
Figure 4.
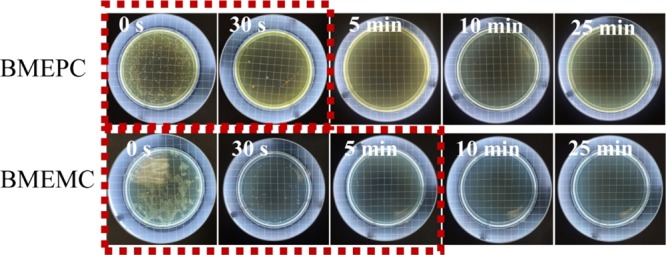
Photos of agar plates with the initial concentration of E. coli and after being treated with BMEPC and BMEMC under irradiation of a 442 nm laser for different times.
For BMEPC, it shows a very strong antibacterial ability after 30 s laser irradiation. For BMEMC, the antibacterial rate could achieve 95% after 30 s laser irradiation. When the laser irradiation was extended for about 10 min, all E. coli cells are killed. These results suggest that both BMEPC and BMEMC have strong photoinduced toxicity against bacteria. Note that the BMEPC photosensitizer exhibits a stronger antibacterial capability than BMEMC because of the higher affinity of the longer alkyl chain to the cellular structure,29 in accordance with the former results. As shown in the Figure S3a, the agar plate was covered with colonies when the E. coli suspension was irradiated by laser even for 25 min. However, compared with the results shown in Figure 4, there were fewer colonies of 105 cfu/mL E. coli suspension with BMEPC or BMEMC inoculated on the agar plates across certain time intervals for 442 nm laser irradiation, which can indicate the successful inhibition of the growth of E. coli. Compared with the result in dark (Figure S3b and Table S2), the inhibitory efficiency is obviously higher under laser irradiation of 442 nm with a relatively lower E. coli survival rate in a shorter time interval. Although it has less bactericidal effect in dark in a relatively short time compared to the group with laser irradiation, a prominent effect was observed after the irradiation time is extended to 25 min, which reflects the crucial role of laser irradiation in bacterial inactivation.
2.5. EPR Measurement
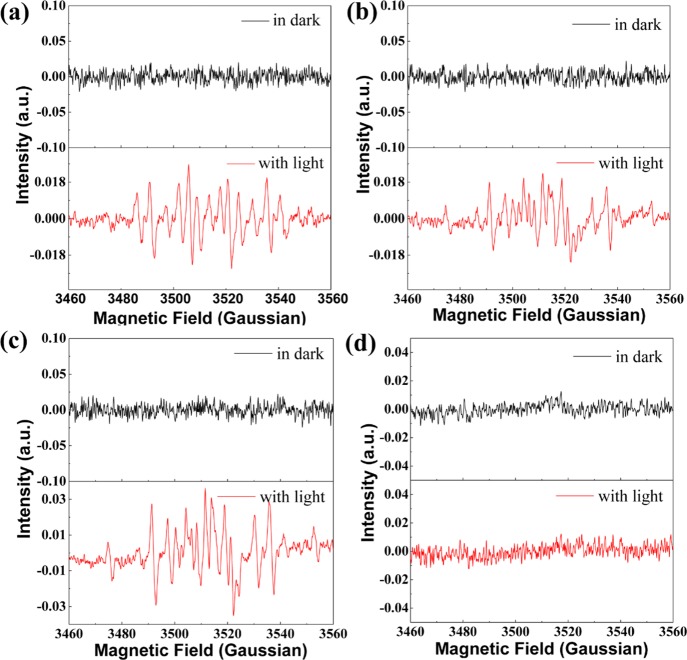
The experimental results prompt us to figure out the PDT mechanism. We use EPR characterization to detect the free radicals with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as the spin-trapping agent. Both BMEMC and BMEPC yield a similar spectrum under irradiation of a mercury lamp (Figure 5a,b). BMEMC alone and BMEMC with E. coli dissolved in PBS solution were exposed to the irradiation of the mercury lamp, yielding a similar signal (Figure 5a,c), which is in consistence with the previous report of nitride radicals in the literature.35 There is barely no signal for BMEPC with the E. coli suspension (Figure 5d), though, there is a strong signal under irradiation for BMEPC alone (Figure 5b). A reasonable assumption could be that the nitride radicals generated under irradiation have been consumed completely by E. coli. We fitted the EPR spectra by spin-fitting function in Xepr software (Bruker) considering carbon, nitride, and hydroxyl radicals, as shown in Figure S4, which is in good agreement with the experimental result. From the fitted spectra, we can get the integral area of three kinds of radicals, which is in proportion to the percentage of the total. However, there is a difference of the percentage of different radicals between BMEMC with and without E. coli, calculated from the integral area of the spectrum, as listed in Table 1. The percentage of the respective radical is an important indicator that reflects a far more remarkable bacterial inactivation ability. From Table 1, we find that the signal of the nitride radical for BMEMC with E. coli is much smaller than that without E. coli, whereas there is no signal for BMEPC with E. coli. Therefore, it indicates that BMEPC and BMEMC under the light irradiation can form an N-centered nitride ionic radical, which can interact with the negative Gram bacteria E. coli and further inactivate E. coli (Figure 6). Thus, it is reasonable that the nitride ionic radical was consumed and the intensity of the peak related to the nitride radical decreased, which is in good agreement with the experimental result. The understanding of the mechanism would be helpful in prompting the application of carbazole-based molecules in PDT.
Figure 5.
(a) EPR spectra of BMEMC in PBS (1.5 mM) (nitrogen saturated, spin parameters: g = 2.0062, aNα = 15.9119 G, and aHβ = 22.8817 G). (b) EPR spectra of BMEPC in PBS (1.5 mM) (nitrogen saturated, spin parameters: g = 2.0062, aNα = 16.2328 G, and aHβ = 22.1877 G). (c) EPR spectra of BMEMC (1.5 mM) with 105 cfu/mL E. coli suspension, PBS as a solvent (nitrogen saturated, spin parameters: g = 2.0062, aNα = 16.5305 G, and aHβ = 22.7553 G). (d) EPR spectra of BMEPC (1.5 mM) with 105 cfu/mL E. coli suspension, PBS as a solvent (nitrogen saturated).
Table 1. Percentage of Respective Radicals Obtained from the Fitting Value of the EPR Spectra.
| E. coli | BMEMC | BMEMC with E. coli | BMEPC | |
|---|---|---|---|---|
| carbon (%) | 9.48 | 18.13 | 41.36 | 40.13 |
| nitride (%) | 30.23 | 39.46 | 6.90 | 10.14 |
| hydroxyl (%) | 60.29 | 42.44 | 51.78 | 49.74 |
Figure 6.
Mechanism of formation of the nitride radical.
3. Conclusions
In summary, the carbazole-based photosensitizers display excellent antibacterial capability under the laser irradiation of 442 nm. In the inhibition zone test, the diameters of zone of inhibition for Gram-negative bacteria with laser irradiation were up to 15 and 14 mm compared to 12.5 and 15 mm for BMEPC and BMEMC, respectively, indicating the antibacterial activity. The MIC value intervals of BMEPC and BMEMC on E. coli were 2.8–3.5 and 9.4–18.7 μM, respectively, under laser irradiation. In dark control experiments, the MIC value intervals of BMEPC and BMEMC were 3.5–6.9 and 112.5–131.3 μM, respectively. For BMEMC, with the laser irradiation within 10 min, almost no E. coli cells can survive. For BMEPC, E. coli was inhibited after 30 s irradiation. The improved photoinduced antibacterial activities are mainly attributed to strong one-photon absorption and cationic radicals formed under the laser irradiation. The photoinduced nitride radicals and the consumption in the interaction with E. coli are detected by EPR spectroscopy under laser irradiation of 442 nm, which is effective to kill Gram-negative bacteria. Compared with the previously reported photosensitizers, BMEPC and BMEMC can be photoactivated in a broader wavelength range according to the UV–vis absorption spectrum and exhibits an enhanced biocompatibility. These results indicate that BMEPC and BMEMC as the typical carbazole-based photosensitizers are potential antibacterial agents that can be used for disinfection and PDT.
4. Experimental Section
4.1. Materials
Sodium chloride, disodium hydrogen phosphate, potassium chloride, and potassium phosphate were obtained from AMRESCO Company. Resazurin was obtained from Shanghai MaiKun Chemical Industry Co., Ltd. Nutrient Broth was obtained from Beijing HongHu Combined Chemical Products Co., Ltd. Agarose was obtained from Biowest Reagents Company.
4.2. Preparation of the E. coli Suspension
E. coli was prepared by the Laboratory of Controllable Preparation and Application of Nanomaterials. The strains of E. coli was inoculated to 15 mL of Luria Bertani broth as a nutrient source and incubated at 37 °C with a 100 rpm shaking rate overnight. Then, the E. coli was gently cooled and stored at 4 °C before use. The concentration of E. coli suspension was estimated by counting the clone on the coating plate. For MIC and photoinduced bacterial inactivation experiments, the concentration of E. coli suspension was diluted to 105 cfu/mL with PBS solution.
4.3. Measurements
1H NMR spectra were recorded on a Varian Gemini-300/Bruker AV 400 spectrometer using DMSO-d6 as a solvent, and all shifts are referred to tetramethylsilane. The chemical shift (s = singlet, d = doublet, t = triplet, and m = multiplet) is shown in Figure S5. The high-resolution electrospray ionization mass spectrometry (HR-ESI MS) spectra have been verified in our previous study.26 Both 1H NMR and HR-ESI MS measurements have successfully verified the molecule structures. The UV–vis absorption and fluorescence emission spectra of BMEPC and BMEMC were recorded on a Shimadzu UV-2500 spectrophotometer and a Hitachi FL-4500 spectrophotometer, respectively. According to the absorption spectra, we choose a continuous-wave He–Cd laser system (IK57511-G, Kimmon, 442 nm) for experimental investigation. CSLM (Nikon, A1R MP) is used to study the viability membrane disruption of E. coli bacteria treated with BMEPC and BMEMC. EPR spectra were recorded by an EPR spectrometer (Bruker E500) with a mercury lamp. The solution of BMEPC and BMEMC in PBS for the measurement is 100 μL with the concentration of 1.5 mM. For E. coli suspension alone, 1 μL of 105 cfu/mL E. coli suspension was added to 100 μL of PBS solution. For BMEPC solution with the E. coli suspension, 1 μL of 105 cfu/mL E. coli suspension was added to 100 μL of BMEPC solution. For BMEMC solution with the E. coli suspension, 1 μL of 105 cfu/mL E. coli suspension was added to 100 μL of BMEMC solution. The same preparation method is applied for BMEPC. DMPO (20 μL) as a spin-trap reagent was added into the above solutions. EPR spectra were collected with a microwave frequency of 9.75 GHz and a modulation frequency of 150 Hz at room temperature. Samples were loaded in 0.6 mm inner diameter quartz capillary tubes in an N2 atmosphere for 10 min to get rid of oxygen and exposed to irradiation from a mercury lamp (λ > 400 nm). All of the measurements were carried out in room temperature.
4.4. Zone of Inhibition Measurement
First, we prepared molten agar (50 °C) and inoculated E. coli (1010 cfu/mL) into the prepared agar. Then, we poured the molten agar (50 °C) into sterilized Petri dishes and allowed them to solidify. The solid agar was completely inoculated with bacteria (108 cfu/mL). Once the agar had been aseptically dried, seven 6 mm wells were punched into the agar with a sterile Pasteur pipette. Ten microliters of varied concentrations was placed in the wells over solidified agar gel.36 The plates were incubated at 37 °C for 24 h. Finally, the diameter of the growth inhibition zones around the well was measured by a digital caliper. All measurements were conducted in triplicate.
4.5. Photoinduced Bacterial Inactivation
To determine the activity of the photosensitizers on bacterial inactivation, the survival rate of E. coli was determined by using the ratio of Nt/N0, where N0 is the initial number of cfu and Nt is that at different time intervals. The time intervals were set to be 0 s, 30 s, 5, 10, and 25 min. For each time interval, 100 μL of the suspension was injected to the surface of the solid agar in 60 mm × 15 mm Petri dishes. The plates were then incubated in a 37 °C incubator for 24 h.
4.6. Study on the Minimum Inhibition Concentration
The MIC of the compound was determined using the resazurin dye reduction assay. The blue resazurin was changed to resorufin by microbial cells inside oxidoreductase. The blue color of the dye indicates the dead microbial cells, whereas the pink color indicates the activity of viable cells.37 We added the sample compounds (BMEPC/BMEMC) to inhibit reproduction of cells during incubation (Figure S1). The antibacterial activity of the compounds can be evaluated by the color of the dye in wells. Dye remaining blue or purple indicates that microbial cells have been successfully inhibited. The pink color conversion indicates the viability of microbial cells. The least dilution of compounds that prevents dye turning pink can be taken as the MIC value for the corresponding compound. The synthesized compound was subjected to MIC studies against E. coli and the MIC values are given.
Acknowledgments
The authors thank the financial support of the National Natural Science Foundation of China (NSFC, grant nos. 61475164, 91323301, 31371014, and 61205194), the National Key Research and Development Program of China (grant nos. 2016YFC1100502 and 2016YFA0200501), Cooperative R&D Projects between Austria, FFG and China, CAS (GJHZ1720), and the Beijing science and Technology Commission Project (Z161100001516013).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00150.
The results of experiment for antimicrobial properties; bacterial membrane disruption; bacterial survival rate of E. coli only with laser irradiation and E. coli with BMEPC and BMEMC in dark; fitting result of the EPR measurement; and 1H NMR spectrum (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Salman A.; Sharaha U.; Rodriguez-Diaz E.; Shufan E.; Riesenberg K.; Bigio I.; Huleihel M. Detection Of Antibiotic Resistant Escherichia Coli Bacteria Using Infrared Microscopy And Advanced Multivariate Analysis. Analyst 2017, 142, 2136–2144. 10.1039/c7an00192d. [DOI] [PubMed] [Google Scholar]
- Wright G. D. Molecular Mechanisms Of Antibiotic Resistance. Chem. Commun. 2011, 47, 4055. 10.1039/c0cc05111j. [DOI] [PubMed] [Google Scholar]
- Maisch T. Resistance In Antimicrobial Photodynamic Inactivation Of Bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. 10.1039/c5pp00037h. [DOI] [PubMed] [Google Scholar]
- Steinbuch K. B.; Fridman M. Mechanisms of Resistance to Membrane-Disrupting Antibiotics in Gram-Positive And Gram-Negative Bacteria. Med. Chem. Commun. 2016, 7, 86–102. 10.1039/c5md00389j. [DOI] [Google Scholar]
- Sperandio F.; Huang Y.-Y.; Hamblin M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Anti-Infect. Drug Discovery 2013, 8, 108–120. 10.2174/1574891x113089990012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M. R. Antimicrobial Photodynamic Inactivation: A Bright New Technique to Kill Resistant Microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. 10.1016/j.mib.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourré L.; Giuntini F.; Eggleston I. M.; Mosse C. A.; MacRobert A. J.; Wilson M. Effective Photoinactivation Of Gram-Positive And Gram-Negative Bacterial Strains Using An HIV-1 Tat Peptide–Porphyrin Conjugate. Photochem. Photobiol. Sci. 2010, 9, 1613. 10.1039/c0pp00146e. [DOI] [PubMed] [Google Scholar]
- Malik Z.; Ladan H.; Nitzan Y. Photodynamic Inactivation of Gram-Negative Bacteria: Problems And Possible Solutions. J. Photochem. Photobiol., B 1992, 14, 262–266. 10.1016/1011-1344(92)85104-3. [DOI] [PubMed] [Google Scholar]
- Jia H.-R.; Zhu Y.-X.; Chen Z.; Wu F.-G. Cholesterol-Assisted Bacterial Cell Surface Engineering For Photodynamic Inactivation Of Gram-Positive And Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 15943–15951. 10.1021/acsami.7b02562. [DOI] [PubMed] [Google Scholar]
- Moura N. M. M.; Ramos C. I. V.; Linhares I.; Santos S. M.; Faustino M. A. F.; Almeida A.; Cavaleiro J. A. S.; Amado F. M. L.; Lodeiro C.; Neves M. G. P. M. S. Synthesis, Characterization And Biological Evaluation Of Cationic Porphyrin–Terpyridine Derivatives. RSC Adv. 2016, 6, 110674–110685. 10.1039/c6ra25373c. [DOI] [Google Scholar]
- George S.; Hamblin M. R.; Kishen A. Uptake Pathways of Anionic and Cationic Photosensitizers Into Bacteria. Photochem. Photobiol. Sci. 2009, 8, 788. 10.1039/b809624d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.; Mai B.; Wang A.; Gao Y.; Wang X.; Liu X.; Song S.; Liu Q.; Wei S.; Wang P. Photodynamic Antimicrobial Chemotherapy With Cationic Phthalocyanines Against Escherichia Coli Planktonic And Biofilm Cultures. RSC Adv. 2017, 7, 40734–40744. 10.1039/c7ra06073d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guern F.; Ouk T.-S.; Grenier K.; Joly N.; Lequart V.; Sol V. Enhancement of Photobactericidal Activity of Chlorin-E6-Cellulose Nanocrystals By Covalent Attachment Of Polymyxin B. J. Mater. Chem. B 2017, 5, 6953–6962. 10.1039/c7tb01274h. [DOI] [PubMed] [Google Scholar]
- Malik Z.; Hanania J.; Nitzan Y. New Trends in Photobiology Bactericidal Effects of Photoactivated Porphyrins—an Alternative Approach To Antimicrobial Drugs. J. Photochem. Photobiol., B 1990, 5, 281–293. 10.1016/1011-1344(90)85044-w. [DOI] [PubMed] [Google Scholar]
- Taniguchi M.; Mass O.; Boyle P. D.; Tang Q.; Diers J. R.; Bocian D. F.; Holten D.; Lindsey J. S. Structural Studies Of Sparsely Substituted Synthetic Chlorins And Phorbines Establish Benchmarks For Changes In The Ligand Core And Framework Of Chlorophyll Macrocycles. J. Mol. Struct. 2010, 979, 27–45. 10.1016/j.molstruc.2010.05.035. [DOI] [Google Scholar]
- Wasielewski M. R.; Svec W. A. Synthesis Of Covalently Linked Dimeric Derivatives Of Chlorophyll A, Pyrochlorophyll A, Chlorophyll B, And Bacteriochlorophyll A. J. Org. Chem. 1980, 45, 1969–1974. 10.1021/jo01298a043. [DOI] [Google Scholar]
- Knölker H.-J.; Reddy K. R. Isolation and Synthesis Of Biologically Active Carbazole Alkaloids. Chem. Rev. 2002, 102, 4303–4428. 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- Głuszyńska A. Biological Potential Of Carbazole Derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. 10.1016/j.ejmech.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Zheng Y.-C.; Zheng M.-L.; Chen S.; Zhao Z.-S.; Duan X.-M. Biscarbazolylmethane-Based Cyanine: A Two-Photon Excited Fluorescent Probe For DNA And Selective Cell Imaging. J. Mater. Chem. B 2014, 2, 2301–2310. 10.1039/c3tb21860k. [DOI] [PubMed] [Google Scholar]
- Zheng M.-L.; Fujita K.; Chen W.-Q.; Smith N. I.; Duan X.-M.; Kawata S. Comparison Of Staining Selectivity For Subcellular Structures By Carbazole-Based Cyanine Probes In Nonlinear Optical Microscopy. ChemBioChem 2010, 12, 52–55. 10.1002/cbic.201000593. [DOI] [PubMed] [Google Scholar]
- Chen S.; Zheng Y.-C.; Zheng M.-L.; Dong X.-Z.; Jin F.; Zhao Z.-S.; Duan X.-M. Nondegenerate Two-Photon Absorption Properties Of A Newly Synthesized Carbazole Derivative. J. Mater. Chem. C 2017, 5, 470–475. 10.1039/c6tc04676b. [DOI] [Google Scholar]
- Al Mousawi A.; Dumur F.; Garra P.; Toufaily J.; Hamieh T.; Graff B.; Gigmes D.; Fouassier J. P.; Lalevée J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems And Application In LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758. 10.1021/acs.macromol.7b00210. [DOI] [Google Scholar]
- Chen Y.; Yamamura T.; Igarashi K. Photosensitization Of Carbazole Derivatives In Cationic Polymerization With A Novel Sensitivity To Near-UV Light. J. Polym. Sci., Part A: Polym. Chem. 2000, 38, 90–100. . [DOI] [Google Scholar]
- Głuszyńska A. Biological Potential Of Carbazole Derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. 10.1016/j.ejmech.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Grigalevicius S. 3,6(2,7),9-Substituted Carbazoles As Electroactive Amorphous Materials For Optoelectronics. Synth. Met. 2006, 156, 1–12. 10.1016/j.synthmet.2005.10.004. [DOI] [Google Scholar]
- Zheng Y.-C.; Zheng M.-L.; Li K.; Chen S.; Zhao Z.-S.; Wang X.-S.; Duan X.-M. Novel Carbazole-Based Two-Photon Photosensitizer For Efficient DNA Photocleavage In Anaerobic Condition Using Near-Infrared Light. RSC Adv. 2015, 5, 770–774. 10.1039/c4ra11133h. [DOI] [Google Scholar]
- Lower S. K.; El-Sayed M. A. The Triplet State And Molecular Electronic Processes In Organic Molecules. Chem. Rev. 1966, 66, 199–241. 10.1021/cr60240a004. [DOI] [Google Scholar]
- Susumu K.; Fisher J. A. N.; Zheng J.; Beratan D. N.; Yodh A. G.; Therien M. J. Two-Photon Absorption Properties Of Proquinoidal D-A-D And A-D-A Quadrupolar Chromophores. J. Phys. Chem. A 2011, 115, 5525–5539. 10.1021/jp2000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss É.; Heine E. T.; Hill K.; He Y. C.; Keusgen N.; Pénzes C. B.; Schnöller D.; Gyulai G.; Mendrek A.; Keul H.; et al. Membrane Affinity And Antibacterial Properties Of Cationic Polyelectrolytes With Different Hydrophobicity. Macromol. Biosci. 2012, 12, 1181–1189. 10.1002/mabi.201200078. [DOI] [PubMed] [Google Scholar]
- Quang D. V.; Sarawade P. B.; Hilonga A.; Kim J.-K.; Chai Y. G.; Kim S. H.; Ryu J.-Y.; Kim H. T. Preparation Of Amino Functionalized Silica Micro Beads By Dry Method For Supporting Silver Nanoparticles With Antibacterial Properties. Colloids Surf., A 2011, 389, 118–126. 10.1016/j.colsurfa.2011.08.042. [DOI] [Google Scholar]
- Moreira L. M.; dos Santos F. V.; Lyon J. P.; Maftoum-Costa M.; Pacheco-Soares C.; da Silva N. S. Photodynamic Therapy: Porphyrins And Phthalocyanines As Photosensitizers. Aust. J. Chem. 2008, 61, 741. 10.1071/ch08145. [DOI] [Google Scholar]
- Zhang Y.; Tangadanchu V. K. R.; Cheng Y.; Yang R.-G.; Lin J.-M.; Zhou C.-H. Potential Antimicrobial Isopropanol-Conjugated Carbazole Azoles As Dual Targeting Inhibitors of Enterococcus Faecalis. ACS Med. Chem. Lett. 2018, 9, 244. 10.1021/acsmedchemlett.7b00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai B.; Gao Y.; Li M.; Wang X.; Zhang K.; Liu Q.; Xu C.; Wang P. Photodynamic Antimicrobial Chemotherapy For Staphylococcus Aureus And Multidrug-Resistant Bacterial Burn Infection In Vitro And In. Int. J. Nanomed. 2017, 12, 5915–5931. 10.2147/ijn.s138185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Gao Y.; Meng S.; Yang B.; Pang L.; Wang C.; Liu T. Mechanism And In Vivo Evaluation: Photodynamic Antibacterial Chemotherapy Of Lysine-Porphyrin Conjugate. Front. Microbiol. 2016, 7, 242. 10.3389/fmicb.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan R.; Lin T.-S.; Karwa A. S.; Poreddy A. R.; Asmelash B.; Dorshow R. B. Type 1 Phototherapeutic Agents. 2. Cancer Cell Viability And ESR Studies Of Tricyclic Diarylamines. ACS Med. Chem. Lett. 2012, 3, 284–288. 10.1021/ml200266v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I.; Rosenman M.; Harwig S. S. S. L.; Jackson R.; Eisenhauer P. Ultrasensitive Assays For Endogenous Antimicrobial Polypeptides. J. Immunol. Methods 1991, 137, 167–173. 10.1016/0022-1759(91)90021-7. [DOI] [PubMed] [Google Scholar]
- Elavarasan T.; Chhina S. K.; Parameswaran M.; Sankaran K. Resazurin Reduction Based Colorimetric Antibiogram In Microfluidic Plastic Chip. Sens. Sens. Actuators, B 2013, 176, 174–180. 10.1016/j.snb.2012.10.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.