Abstract

This article reports a novel fabrication of branched cum cross-linked poly(lactic acid) (PLA) with nanosilk fibroin with graft chain topology by reactive extrusion process. It could be possible by the addition of a small amount of radical initiator (dicumyl peroxide (DCP)). Grafting of silk nanocrystals (SNCs) on PLA macromolecules that provides remarkable improvement in the rheological and thermal properties of the latter are confirmed by 1H NMR and Fourier transform infrared investigation. Significant improvement is observed in zero shear viscosities, and the crossover point shifts to lower frequencies as compared to the branched and cross-linked PLA system. Along with SNC grafting, the crystallization process is also enhanced and stable crystals appeared during cooling, which results in a single melting peak. The rate of crystallization of PLA has been improved although the percentage crystallinity reduces with DCP content, as higher grafting and cross-linking restricts the chain segmental motion, which is critical for crystallization process. Furthermore, SNC grafting increases the reprocessability performance of PLA and provides higher rheological properties as compared to the branched and cross-linked PLA at all reprocessing cycles.
1. Introduction
Petroleum-based polymers have served as an essential material for betterment of humans’ lifestyle but with a lot of undeniable limitations to the environment.1,2 Due to the concern of waste disposal and depletion of world oil resources, the interest of substituting conventional polymers with sustainable and biodegradable polymers has been the central part of research for the last two decades.3−7 In this prospect, poly(lactic acid) (PLA) is the leading biodegradable aliphatic polyester, which has emerged as an attractive industrial scale processable thermoplastic polymer with comparable mechanical properties to poly(ethylene terephthalate) (PET) and polystyrene (PS).6,8−10 However, because of its poor melt strength, it is difficult to process it for blown film and foaming to produce confectionary commodity articles with degradable characteristics. Furthermore, the application span is limited because of its high brittleness, poor barrier properties, and low heat distortion temperature.11,12
It is well known that processing of PLA with fillers (organic or inorganic) and plasticizers and by blending with toughened polymers can enhance its barrier and mechanical properties.5,13 Controlled polymerization of PLA with a mixture of l- and d-lactic acid or lactide leads to the formation of a stereocomplex, which in turn increased the heat distortion and melting temperatures to approximately 120–140 and 220–240 °C, respectively.14−16 PLA undergoes thermal degradation at high temperature and shear, which results in its low melt strength. Investigation of the macromolecular chain modification of PLA is a bit complex process due to the existence of different simultaneous reactions. Random chain scission, which is known to be a major cause of thermal degradation within the processing window of PLA (180–220 °C), is the reason for the drastic reduction in molecular weight.17−19 In addition to this, the reduction in molecular weight becomes more critical when trace amounts of water molecules, short oligomeric chains, residual catalysts, and monomeric units are present in the polymer matrix.
Reactive extrusion in the presence of multifunctional epoxy, highly reactive peroxides, such as dicumyl peroxide (DCP) and bezolperoxide, has been practiced to enhance the melt strength of polypropylene, poly(ethylene terephthalate) (PET), poly(butylene terephthalate), and the likes.20−23 Similar techniques have been adapted to improve the melt strength of PLA, which leads to enhancing the processing performance and other physical properties (mechanical, barrier, and thermal properties).24 Zhou et al. successfully prepared a chain-extended PLA–epoxy resin copolymer through reactive extrusion using diglycidyl ether of bisphenol A as a chain extender.25 Cailloux et al. also showed a one-step reactive calendering process to improve the thermal sensitivity of PLA with styrene acrylic multifunctional oligomer, which leads to improvement in melt rheology due to the predominance of long-chain branching.26 A significant macromolecular modification of PLA/poly(butylene adipate-co-terephtalate) blend at various proportions is observed due to reactive extrusion in the presence of Joncryl (glycidyl methacrylate), which further increases the mechanical and rheological properties.27 Radical initiator dicumyl peroxide (DCP) is also an effective chain extender, which is proven to initiate the grafting of PLA chains on the surface of cellulose nanocrystals, resulting in an improvement in melt strength and mechanical properties (Young’s modulus by ∼40% and tensile strength by ∼490%) with improved recycling performance.28,29 The same kind of result was also reported by Bian et al., in which the tensile strength and modulus of the poly(lactic acid)/poly(3-hydroxybutyrate-co-4-hydroxybutyrate) blend significantly increased with the addition of DCP.30
From our previous investigation, thermally stable silk nanocrystals (SNCs) are observed to stabilize the thermal properties of PLA even under repetitive extrusion.12 The hydrophobicity of SNCs along with their highly structured β-sheet network (arising because of strong hydrogen bonding between C=O and N–H groups) helps to reinforce the PLA matrix with good dispersion. The crystalline part of silk fibroin is proved to have amino acids repeating order of glycine–alanine–glycine–alanine–glycine–serine. Similar to cellulose, serine group of SNCs contains −CH2–, which is ideal for the formation of radicals using radical initiators for grafting of PLA chains, which may help to modify the matrix during reactive extrusion. If this approach is successful, then it may increase the performance of SNCs remarkably as a filler and may enhance the melt strength, which is the major drawback of PLA.
To have better understanding of the macromolecular level, proper characterization techniques and analysis procedures need to be followed. In PLA-reactive extrusion process, degradation and chain coupling occur simultaneously and the chain modification undergoes through long- and short-chain branching, cross-linking, and grafting on the surface of fillers. Estimation of the gel content and the molecular weight analysis of the system can provide general information about the chain modification, and it needs to be supported by rheological investigations, which is a powerful tool to have a clear understanding about the changes occurring at the macromolecular level.28,31 Zero shear viscosity, storage and loss modulus characteristics at terminal region, and crossover frequency can be used to quantify the macromolecular chain modification. Furthermore, the weight relaxation spectrum (λH(λ)) can verify different microstructural topologies, such as chain branching and cross-linking.13,26 Along with melt rheology, changes in the thermal stability (melting and crystallization properties and thermal decomposition behavior) of the sample also need to be investigated.
The present study deals with the reactive modification of PLA/SNC nanocomposite with dicumyl peroxide (DCP) and relates the impact of chain modification to the melt rheology, crystallization properties, and thermal stabilities. The structural change and possible reaction mechanisms are also assessed with the help of gel permeation chromatography (GPC), Fourier transform infrared (FTIR), and nuclear magnetic resonance (NMR) spectroscopy studies. Additionally, the impact of reprocessing on the above-mentioned important properties is discussed in subsequent sections.
2. Results and Discussion
2.1. Impact of DCP on the Structural Modification of PLA/Silk Bionanocomposite
It is already discussed in various literature works that DCP decomposes into free radicals at high temperature with strong ability to abstract hydrogen and initiates radicals on PLA backbone, which is the result of propagation of cross-linking reaction between the PLA chains.32 This reaction takes place in extruder when subjected to high shear and temperature, which provides sufficient mixing that leads to the modification of chain topology by cross-linking or branching (long- and/or short-chain branching). This phenomenon is totally different when fillers are incorporated into the reactive extrusion system because the radical initiator can get the chance to abstract hydrogen from the weak sites of the filler’s bond linkage.28 Gel percentage, which is estimated (samples collected at a residence time of 5 min) using eq 1 (dividing the weight of dry gel (Wgel) by the sample weight before washing (Wi) it with chloroform to remove unmodified macromolecules), has been used as a primary analysis to understand the reactive modification of PLA. Due to the weak interaction of the cross-linked portion of the polymeric system with solvent, the probability of the formation of a gel-like structure is high.
| 1 |
Two possible modifications are expected during reactive extrusion of the current system: PLA–PLA (cross-linking and branching) and PLA–SNC (grafting). Reactive extrusion of neat PLA (NPLA) is used as a reference system to assess the impact of SNC on PLA chain modification. As indicated in Table 1, for reactive extrusion of NPLA at 1 and 1.5 wt % DCP loading, gel percentages of ∼10 and ∼30% are observed, which indicates the presence of cross-linking topology. However, at lower DCP loading (0.5 wt %), no gel formation is observed. Similar observation is also reported for poly(3-hydoxybutyrate-co-4-hydroxybutyrate), in which no gel formation was observed at DCP content less than 0.5 wt %.33 However, the gel percentage of SNC–PLA increases from 10% (0.5 wt % DCP) to 60% (1.5 wt % DCP). This result confirms that, apart from the polymer-to-polymer cross-linking interaction, which leads to the long-chain branching, there is also a significant interaction between SNC and PLA macromolecules. This result gave the fundamental information to predict the formation of radicals on the SNC macromolecular structure (possible on serine group of −CH2OH, which will be explained by 1H NMR analysis) that promotes grafting on PLA backbone chains. As indicated in Table 1, specific rotation and optical rotation of reactively extruded samples decrease with increasing weight percentage of DCP. This may be attributed to the hindrance of the specific rotation due to branching of long chains attached to the central chiral carbon of PLA. Incorporation of SNC slightly lowers the specific rotation as compared to NPLA, which is due to the additional effect of grafting on the chiral carbon.28
Table 1. Effect of Reactive Extrusion on Gel Percentage, Specific Rotation, and Optical Rotation.
| samples | gel (%) | specific rotation (deg) |
|---|---|---|
| NPLA (extruded) | 150.56 | |
| 0.5DCP–PLA | 141.91 | |
| 1DCP–PLA | 13 ± 2 | 139.28 |
| 1.5DCP–PLA | 31 ± 3 | 133.86 |
| SNC–PLA | 148.32 | |
| 0.5DCP–SNC–PLA | 10 ± 1 | 137.51 |
| 1DCP–SNC–PLA | 43 ± 2 | 138.46 |
| 1.5DCP–SCN–PLA | 60 ± 3 | 130.41 |
Macromolecular structural modification after reactive extrusion is also confirmed by FTIR spectroscopy, as described in Figure 1. Neat poly(lactic acid) (NPLA) shows main characteristic peaks at 2854, 2925, 1382, and 1455 cm–1 attributed to symmetric and asymmetric stretching and symmetric and asymmetric bending of CH3, respectively; 2996 and 1358 cm–1 due to C–H stretching and deformation vibration, respectively; 1076 and 1180 cm–1 due to symmetric and asymmetric valance vibration of C–O–C, respectively; transmission bands at 867 and 753 cm–1 due to C–C stretching; and 1747 cm–1 due to the C=O stretching band. The intensity of the peak ranging from 1230 to 1000 cm–1 attributed to the C–O–C and C–H deformation peak at 1358 cm–1 decreases with DCP in case of NPLA. However, it further diminishes for modified SNC–PLA. It is an indication of chain modification due to branching and cross-linking through the formation of a new C–C bond (observed at 799 cm–1) on the backbone of PLA, which affects the intensity of the ester linkage. The significant impact of SNC observed on the ester linkage intensity may also be due to the grafting.
Figure 1.
Chemical structural analysis of reactively modified NPLA and SNC–PLA samples using FTIR spectra in (a) full range, (b) 3010–2700 cm–1, (c) 1890–1280 cm–1, and (d) 1280–650 cm–1.
A new peak is observed at 2960 cm–1 for reactively modified samples, and peaks at 2854 cm–1 (symmetric stretching CH3) and 2925 cm–1 (asymmetric stretching CH3) are shifted to 2851 and 2920 cm–1, respectively. Due to high branching, the backbone C–C bond-stretching (753 cm–1) band intensity is decreased with increasing DCP weight loading. Due to high branching and cross-linking, van der Waals interaction arises between the C=O and hydrogens of CH3 groups of the branched chains and a shoulder-like peak is identified at 1710 cm–1. The disappearance of transition peak at 2996 cm–1, which corresponds to C–H stretching, and the diminishing of C–H vibration deformation peak at 1361 cm–1 support radical-initiated abstraction of hydrogen, which leads to chain modification possibly by cross-linking, branching, and grafting. NMR investigation of reactively modified SNC–PLA system can provide insight into the grafting of SNC on PLA chain.
1H NMR analyses are performed for NPLA and SNC–PLA at 1 wt % DCP to study the possibility of grafting. As indicated in Figure 2, new characteristic peaks are observed for SNC–PLA system in the ranges of 4.10–4.15 ppm (marked as “a”) and 2.0–2.5 ppm (marked as “b”), which were missing in reactively modified NPLA samples (1H NMR spectra in Figure S1), which may be attributed to the methine proton formed due to the grafting of PLA chains on the serine −CH2OH group of SNC. As already discussed above, DCP forms radicals at high temperature and shear, which have a strong tendency to abstract hydrogen and form radicals on PLA backbone and the serine (−CH2OH) group of silk fibroin, which is the reason for the initiation of grafting of PLA chains on the silk macromolecular structure. On the basis of the results obtained from gel percentage, FTIR, and NMR analyses, the reaction mechanism for the reactive modification can be summarized as shown in reaction Scheme 1.
Figure 2.

1H NMR spectra of SNC–PLA grafting sample at 1 wt % DCP (“a” represents a methine proton formed during grafting and “b” represents the presence of silk structure backbone).
Scheme 1. Summarized Reaction Pathway for the Modification of SNC–PLA Chain Topology by Cross-Linking and Grafting through Reactive Extrusion Process.
The significant change observed in the chemical structure due to grafting is further addressed by the molecular weight analysis using GPC. Furthermore, the molar mass distribution (Mn, Mw, and polydispersity index (PDI)) is used to monitor the reaction progress and modification efficiency of reactively extruded SNC–PLA at various compositions of DCP (0.5, 1, and 1.5 wt %). As compared to PLA granules (GPLA), molecular weight of extruded PLA (NPLA) is reduced (22% in Mw and 36% in Mn) due to thermomechanical degradation at processing time of 5 min with polydispersity index (PDI) of 2.6. The addition of SNC leads to the formation of lower-molecular-weight fraction (14% area) and 86% area representing higher-molar-mass fraction with 8 and 10% increment in Mn and Mw, respectively, as compared to NPLA. In this study, reactively extruded samples are collected at residence times of ∼3 and ∼5 min for molecular weight analysis. Figure 3 shows the molecular weight distribution curve of SNC–PLA (at all DCP wt %) for residence times of ∼3 and ∼5 min. Interestingly, the percentage area for large macromolecules of reactively extruded SNC–PLA samples is observed to increase from 48% (0.5DCP–SNC–PLA) to 90% (1DCP–SNC–PLA) and 100% (1.5DCP–SNC–PLA) with 108, 96, and 133 kDa of Mn and 295, 317, and 267 kDa of Mw, respectively, at residence time of ∼3 min (Figure 3a). It indicates that sufficient amount of radicals are generated (at 1.5 wt % of DCP) to give higher molecular weight with shorter residence time, which is in agreement with the lifetime of DCP radicals (190 s), which is reported by Takamura et al.32 As the residence time increased to 5 min, the molecular weight drastically reduced with increasing DCP composition. However, the lower-molecular-weight fraction observed at residence time of 3 min is merged with the higher-molecular-weight fraction, as indicated in Figure 3c. From Figure 3b,d it can be clearly observed that the new broad peak appears in the higher-molecular-weight population side because of cross-linking and grafting. Due to the fact that the hydrodynamic volume is highly affected by the molecular chain topology, estimation of molecular weight distribution using GPC alone may not be a suitable technique. Additionally, it is difficult to conclude about the change in chain topology only using the results obtained from GPC. Rheological investigation can provide deep insight into change related to long-chain branching, cross-linking, and grafting, which are already confirmed by spectroscopy techniques.
Figure 3.
Molecular weight distribution of reactively extruded SNC–PLA at residence time of (a) 3 min and (b) 5 min. (c) Effect of residence time on shifting of lower-molecular-weight chains into higher-molecular-weight chains. (d) Broadening of molecular weight distribution curve at higher-molecular-weight region due to reactive modification (cross-linking/long-chain branching).
2.2. Rheological Characteristics of Reactively Modified Neat and PLA/SNC Nanocomposites
The influence of grafting on viscoelastic shear dependency and relaxation spectra are assessed using dynamic rheological measurement performed at 190 °C in the frequency range of 0.1–500 rad/s within the viscoelastic region (5% strain is taken on the basis of the small-amplitude strain sweep analysis). The frequency (ω) dependency of complex viscosity (η*) is displayed in Figure 4 for all DCP-modified samples in comparison to NPLA and SNC–PLA samples extruded under the same conditions without DCP. It is clearly observed that the NPLA, SNC–PLA, and 0.5DCP–NPLA samples exhibited the same trend with the Newtonian behavior up to 10 rad/s. The 0.5DCP–NPLA sample shows higher viscosity due to the observed increment in molecular weight. With increasing the DCP amount, the Newtonian behavior shifts to lower-frequency region, and significant increment in viscosity and shear-thinning behavior are observed for both reactively extruded NPLA and SNC–PLA.
Figure 4.
Effect of macromolecular chain modification on the change of complex viscosity with respect to frequency and the impact of SNC grafting on the change in viscosity at 0.1 rad/s frequency.
This pronounced improvement on the rheological characteristic can be related to long-chain branching and cross-linking. As observed from the gel percentage and 1H NMR spectroscopy studies, the incorporation of SNC into PLA matrix gives additional reinforcement, which leads to increase in the melt strength. More interestingly, complex viscosity of SNC–PLA at the frequency of 0.1 rad/s was observed to be higher than that of NPLA by 273, 3155, 10 875, and 12871 Pa s at DCP amounts of 0, 0.5, 1, and 1.5 wt %, respectively. This result gives a strong evidence of SNC grafting on PLA backbone with the formation of long-chain branching and cross-linking, which restrict the mobility of macromolecules by forming more entangled networks.
Figure 5 displays the frequency dependency of storage modulus (G′) of all analyzed samples and the increment observed at lower frequency (0.1 rad/s), which is probably due to grafting of SNC. The result related to the loss modulus is shown in Figure S2, from which it is clear that the storage modulus exhibits more plateau-like behavior at lower-frequency region with increasing weight percentage of DCP. Similarly to the complex viscosity, the storage modulus value of SNC–PLA increased by 3.8, 109, 789, and 1265 Pa as compared to the NPLA system at the respective DCP amounts of 0, 0.5, 1, and 1.5 wt %, which further supports the grafting phenomenon that increases the solidlike behavior of the matrix. The percolation effect of SNC is also enhanced because of grafting and provides a thermally stable entangled network, which increases the dominance of storage modulus over loss modulus.
Figure 5.
Effect of angular frequency and DCP content on the storage and loss moduli of NPLA and SNC–PLA.
Reactive extrusion-induced macromolecular chain modification can be further explained by using the crossover frequencies of storage and loss moduli, terminal region properties (slope, storage and loss moduli), and the slope of storage versus loss modulus graph (Han plot). As already discussed in the literature, this transition point is strongly affected by the macromolecular topology of polymer melt. The presence of long-chain branching, linear chain extension, and cross-linking or any kind of network formation (network formation due to grafting) provides the ability to absorb energy and relax slowly at higher relaxation time. As illustrated in Table 2, it can be noted that the crossover frequencies decrease with increasing DCP wt % for both NPLA and SNC–PLA. Reactive extrusion in the presence of SNC lowers the crossover frequency values from 123 to 116, 119 to 58, 21 to 1.4, and 0.11 to <0.1 Hz (NPLA to SNC–PLA, respectively) against DCP amounts of 0, 0.5, 1, and 1.5 wt %.
Table 2. Effect of DCP Amount on Various Properties of NPLA and SNC–PLA.
| crossover
properties |
terminal
region |
Han
plot |
|||||
|---|---|---|---|---|---|---|---|
| samples | ωc (rad/s) | G′ = G″ (kPa) | η* (Pa s) | G′−ωx | G″−ωy | slope | R2 |
| NPLA | 123 | 22.7 | 262 | 1.76 | 0.95 | 1.83 | 0.999 |
| 0.5DCP–PLA | 119 | 28.6 | 340 | 1.19 | 0.76 | 1.40 | 0.999 |
| 1DCP–PLA | 21 | 14.2 | 955 | 0.76 | 0.59 | 1.28 | 0.999 |
| 1.5DCP–PLA | 0.1 | 1.4 | 17 509 | 0.50 | 0.41 | 1.20 | 0.998 |
| SNC–PLA | 116 | 28.6 | 296 | 1.40 | 0.95 | 1.65 | 0.998 |
| 0.5DCP–SNC–PLA | 58 | 22.8 | 561 | 0.88 | 0.68 | 1.35 | 0.998 |
| 1DCP–SNC–PLA | 1.4 | 5.3 | 5462 | 0.57 | 0.46 | 1.24 | 0.998 |
| 1.5DCP–SNC–PLA | <0.1 | 0.39 | 0.36 | 1.10 | 0.996 | ||
The impact of SNC grafting can be easily observed from the significant reduction of crossover points that define the transition of solidlike behavior of the melt to the liquidlike behavior. This improvement is more pronouncing at higher DCP loading, and it is due to the increment in the degree of grafting or grafting efficiency with DCP content. Similar conclusion can also be drawn from the complex viscosity values extracted at the crossover points, which shows drastic increase with increasing DCP loading and grafting of SNC.
Interestingly, no change in storage and loss modulus values is observed at higher frequencies, which indicates the short-range dynamics of the polymeric melt, and hence are not affected by the chain modifications. However, at low frequencies, both properties decrease monotonically, and the terminal characteristics are observed to deviate from the regular linear polymer power law relationship of G′(ω) α ω2 and G″(ω) α ω for both reactively modified NPLA and SNC–PLA. It has been found that for polymeric melts in which the solidlike behavior is becoming more and more predominant, the G′ and G″ modulus dependency on power law at lower frequencies will significantly diminish from 1 and 2 to lower values, respectively. Reactive modification of PLA in the presence of DCP, which can form active radicals, will lead to the formation of a more networked melt having solidlike behavior, which can be related to the polymer-to-polymer interaction due to long-chain branching and cross-linking. We note in Table 2 that the terminal region G′ and G″ dependency on frequencies further diminishes in the presence of SNCs as compared to NPLA at all DCP contents, which is an indication of intense grafting characteristics. Similar observation can also be drawn from the Han plot shown in Figure 6 (slope of log G′ vs log G″), which provides information about the dependency of elasticity on loss properties. As illustrated in Table 2, the slopes of the reactively extruded NPLA samples are observed to decrease from 1.83 of NPLA (without DCP) to 1.40, 1.28, and 1.20 with 0.5, 1, and 1.5 wt % DCP, respectively. As expected, the introduction of SNC further decreases the slope at the respective DCP amount. This result strongly supports the SNC grafting on PLA, which results in the melt having more solidlike behavior at higher temperature.
Figure 6.

Han plot of NPLA and SNC–PLA at various DCP contents.
Similar observation can also be generated using Cole–Cole plot, which can be related to their molecular weight and distribution. As shown in Figure 7, the semicircle curve becomes larger and wider with increasing DCP weight fraction and with the addition of SNC. This clearly indicates long-chain branching, cross-linking, and grafting, which are already observed through molecular weight analysis using GPC. All of these important observations are an indication of the improvement of the melt strength of PLA due to the reactive modification. SNC plays a major role to give remarkable rheological property improvement due to grafting.
Figure 7.

Cole–Cole plots of NPLA and SNC–PLA at various DCP contents.
van Gurp (vGP) plot (phase angle vs log G*) has been used as a tool to understand the impact of long-chain branching on the mobility of polymers under melt condition. Figure 8 shows shifting of phase angle to lower values for modified samples with increasing DCP weight fractions. The grafting phenomenon observed in reactively modified SNC–PLA reduces the phase angle as compared to reactively modified NPLA. Because more solidlike property is introduced into the PLA matrix, the grafting of thermally stable and highly crystalline SNC limits the melt macromolecular movement that in turn leads to the reduction of phase angle.
Figure 8.

vGP plots of NPLA and SNC–PLA at various DCP contents.
Investigation of the rheological properties of modified samples is very complicated and it needs to be correlated with other characterization techniques. Predicting the macromolecular structural change is a big task because many simultaneous reactions take place in parallel, which give different macromolecular melt topologies. Due to this fact, limitation arises to fit the rheological data to different rheological models to have insight into the relaxation process. Taking this into consideration, the Carreau–Yasuda model (eq 2) is used to fit the melt rheology data and provide macromolecular chain relaxation phenomena through estimation of characteristic relaxation time (λ), zero shear viscosity (η0), Newtonian transition factor (a), and power law index (n).
| 2 |
As illustrated in Figure 9, with the values listed in Table 3, the zero shear viscosity (η0) significantly increases from 730 Pa s (NPLA) by factors of 2, 25, and 225 for DCP weight fractions of 0.5, 1, and 1.5, respectively. As compared to NPLA, the change further increased by factors of 1.6, 15, 40, and 1012 at DCP weight fractions of 0, 0.5, 1, and 1.5, respectively, for reactively modified SNC–PLA samples. As already discussed above, enhancement in the molecular weight through chain extension, long-chain branching, and cross-linking with significant grafting can be mentioned as a reason for the observed substantial increase in zero shear viscosity. Due to the same reason, the modified samples took relatively longer relaxation time and characteristic relaxation time (λ) of NPLA increased from 0.032 to 94 and 35 s for modified NPLA (1.5% DCP) and SNC–PLA (1.5% DCP).
Figure 9.

Carreau–Yasuda model fitting for the complex viscosity data of NPLA and SNC–PLA at various weight fractions of DCP.
Table 3. Carreau–Yasuda Model Fitting Parameters for NPLA and SNC–PLA at Various Weight Fractions of DCP.
| samples | η0 | λ | a | n |
|---|---|---|---|---|
| NPLA | 730.66 | 0.032013 | 0.94451 | 0.36897 |
| 0.5DCP–PLA | 1468.1 | 0.015565 | 0.58261 | 0.0001 |
| 1DCP–PLA | 18 530 | 0.086437 | 0.26076 | 0.0001 |
| 1.5DCP–SCN–PLA | 164 320 | 94.908 | 0.35 | 0.0001 |
| SNC–PLA | 1232.4 | 0.03952 | 1.3711 | 0.37667 |
| 0.5DCP–SNC–PLA | 11 009 | 0.018595 | 0.23499 | 0.0001 |
| 1DCP–SNC–PLA | 29 636 | 0.64697 | 0.17207 | 0.0001 |
| 1.5DCP–SNC–PLA | 739 440 | 35.671 | 0.14237 | 0.0001 |
The weighted relaxation spectrum (λH(λ)), which is estimated from linear relaxation spectrum, as proposed by Honerkamp and Weese, can be used to understand the time distribution of the chain relaxation mechanism. The relaxation processes of unmodified and modified NPLA and SNC–PLA samples are presented in Figure 10a–d, and for better understanding, the chain relaxation processes are further presented at various ranges of relaxation time (0.001–1 and 0.001–100). As indicated in Figure 10a, NPLA is observed to have one relaxation peak at λ ∼ 0.05 s, which indicates the linear polymer chain relaxation. Due to the possible long-chain grafting of PLA chains on the active surface of SNCs, a second melt relaxation is observed at λ ∼ 230 s other than the linear polymer chain relaxation peak at λ ∼ 0.05 s. In Figure 10b–d, the polymer melt macromolecular chain structure is completely modified after the addition of DCP. Long-chain branching can be confirmed from the longer-time relaxation peak, which is observed to increase with increasing DCP weight fraction. The polymeric melt becomes more entangled, and partial cross-linking can also be mentioned as one of the reasons for the observed significant change in the relaxation process.
Figure 10.
Relaxation spectra of (a) NPLA and SNC–PLA and (b) reactively modified NPLA and SNC–PLA at different weight fractions of DCP. Impact of reactive modification on relaxation spectrum ranging from lower relaxation time to (c) 1 s and (d) 100 s.
2.3. Crystallization, Melting Behavior, and Thermal Stability
Poly(lactic acid) is known to have a slow crystallization behavior and thus it is difficult to crystallize it from liquid phase even at low cooling rate. The melting and crystallization characteristics of polymer matrix are strongly influenced by the chain topology. Because of this, before giving explanation on the thermal characteristics of reactively modified samples, it is required to summarize and categorize the chain topology induced by the reactive extrusion. On the basis of the above obtained results, macromolecular chain modifications are grouped into three categories to simplify the explanation: scenario 1, short- and long-chain branching (0.5DCP–PLA); scenario 2, cross-linking and branching (1DCP–PLA and 1.5DCP–PLA); and scenario 3, grafting (in all DCP–SNC–PLA). The effect of chain topology modification on the differential scanning calorimetry (DSC) thermographs of cooling and second heating is displayed in Figure 11, and the values of glass-transition temperature (Tg), onset and maximum cold crystallization temperatures (Tc,onset, Tc,max), onset and maximum melting temperatures (Tm,onset, Tm,max), and enthalpies of crystallization and melting are listed in Table 4. In all scenarios, no significant change is observed on Tg values. Melt-extruded PLA at high temperature and shear is known for melt recrystallization process and produces defect or imperfect crystals, which melt in two stages. If lower-molecular-weight fraction is increased, the melt recrystallization phenomenon can occur relatively at lower temperature because the short chains can easily rearrange to recrystallize at lower temperature. On the other hand, when a nucleating agent is present in the matrix, the melt crystallization process can also occur at lower temperature. However, reduction observed in cold crystallization temperature (Tcc) by 3 °C (scenario 1) as compared to NPLA (Tcc ∼ 115 °C) can be related to the increment in the free volume due to short-chain branching, which leads to the formation of unstable crystals that increase the chain mobility and gives a double-melting peak, which is shifted to lower temperature as compared to NPLA. This phenomenon was briefly explained by Bian et al. for reactively extruded short-chain branched poly(3-hydoxybutyrate-co-4-hydroxybutyrate).33 The melt crystallization phenomenon of NPLA was not affected by the reactive modification at lower DCP content (0.5 wt %), and no crystallization peak was observed during cooling, which indicates the slow crystallization process, in which the cooling rate (5 °C/min) is too high for the nucleation and growth of crystals (Figure 11).
Figure 11.
DSC thermographs of (a) first heating, (b) cooling, and (c) second heating of NPLA and SNC–PLA at various weight fractions of DCP (a: NPLA, b: 0.5DCP–PLA, c: 1DCP–PLA, d: 1.5DCP–PLA, e: SNC–PLA, f: 0.5DCP–SNC–PLA, g: 1DCP–SNC–PLA, and h: 1.5DCP–SNC–PLA).
Table 4. Calorimetric Values of NPLA and SNC–PLA at Various Weight Fractions of DCP (First Heating, Cooling, and Second Heating).
| sample | Tg (°C) | Tc,onset (°C) | Tc,max (°C) | Hc (J/g) | Tm,onset (°C) | Tm,min (°C) | Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|---|
| NPLA | ||||||||
| cooling | ||||||||
| second heating | 58 | 104 | 115 | 23 | 146 | 151 | 24 | 1 |
| 0.5DCP–PLA | ||||||||
| cooling | ||||||||
| second heating | 57 | 103 | 112 | 22 | 145 | 149 | 23 | 1 |
| 1DCP–PLA | ||||||||
| cooling | 95 | 115 | 19 | |||||
| second heating | 58 | 88 | 103 | 3 | 144 | 151 | 23 | 22 |
| 1.5DCP–PLA | ||||||||
| cooling | 95 | 112 | 16 | |||||
| second heating | 57 | 86 | 103 | 4 | 140 | 151 | 20 | 17 |
| SNC–PLA | ||||||||
| cooling | ||||||||
| second heating | 58 | 102 | 110 | 23 | 146 | 149 | 24 | 1 |
| 0.5DCP–SNC–PLA | ||||||||
| cooling | 92 | 103 | 20 | |||||
| second heating | 58 | 85 | 98 | 3 | 144 | 150 | 24 | 23 |
| 1DCP–SNC–PLA | ||||||||
| cooling | 92 | 116 | 17 | |||||
| second heating | 58 | 86 | 100 | 3 | 143 | 150 | 21 | 19 |
| 1.5DCP–SNC–PLA | ||||||||
| cooling | 91 | 107 | 13 | |||||
| second heating | 58 | 88 | 101 | 4 | 140 | 148 | 18 | 15 |
On the other hand, the slow melt crystallization process of NPLA is significantly enhanced for scenario 2 (1DCP–PLA and 1.5DCP–PLA) with a small fraction of melt recrystallization, which can be strongly related to the cross-linking and branching, as shown by gel percentage and other rheology results. Interestingly, percentage crystallinity increased from 1% (0.5DCP–PLA) to 22% (1DCP–PLA) and decreased to 17% (1.5DCP–PLA). This crystallization behavior can be explained as follows: the rate of crystallization increased drastically as the macromolecular chain topology is changed from short-chain branching to cross-linking with lower gel percentage (low cross-linking fraction), and further increasing in cross-linking fraction hinders the chain rearrangement due to intense entanglement, which results in reduction of crystallization rate. As clearly shown in Figure 11c, the double-melting peaks observed for NPLA and 0.5DCP–PLA merged into one for cross-linked and branched samples (1DCP–PLA and 1.5DCP–PLA), due to the formation of more stable crystals.
As reported in Table 4, lower Tcc (by 5 °C) and distinguished double-melting peaks are observed for SNC–PLA compared to NPLA, which is attributed to early-stage formation of crystals due to the nucleating effect of SNC. However, in scenario 3 (unlike 0.5DCP–PLA), the reactive extrusion at lower DCP content (0.5 wt %) and 1% SNC changes the melting and crystallization processes. The rates of crystallization are enhanced, and melt crystallization is observed at the given cooling rate, which is missing in 0.5DCP–PLA. These interesting characteristics can be correlated with the grafting of SNC on the PLA chain, which provides nucleation site for the melt to arrange in an ordered form. The increment in interfacial adhesion between highly ordered crystalline SNC and amorphous PLA chains due to the formation of C–C cross-links leads to diminution of free volume, which improves the polymer chain folding guided by SNC, and crystallization upon cooling is enhanced. This can also be correlated with the reduction of activation energy required for chain folding by overcoming the barrier energy through SNC grafting. The crystallization percentage decreases from 23% (0.5DCP–SNC–PLA) to 19% (1DCP–SNC–PLA) and 15% (1.5DCP–SNC–PLA) due to the increase in grafting and cross-linking fractions that leads to the restriction of macromolecular movement in melt (increase in entanglement). The double-melting peak observed at 0.5DCP–SNC–PLA is merged into a single peak at 1 and 1.5 wt % of DCP. As already discussed above, the double-melting peak occurs because of the melt recrystallization due to the short-chain branching, which further improves and forms stable crystals with increasing grafting efficiency and cross-linking.
Before X-ray diffraction (XRD) analysis, reactively extruded samples are kept in an vacuum oven at 95 °C for 2 h. The onset temperature for the cold crystallization (∼95 °C) process of PLA obtained from the first heating cycle is taken as the reference point. The representative peaks observed at 16.5 and 18.8° on the XRD diffractogram represent the α-form of PLA crystals having crystalline planes of (011) and (110)/(200), respectively.28 As clearly indicated in Figure 12, crystallinity of NPLA is observed to be improved by the addition of SNC and further improved by the reactive extrusion. No intense peak is observed for neat PLA, whereas the addition of DCP improves the crystallization process, which can be observed by the intense peak at 16.5°. Maximum intensity is observed at 1 wt % DCP for the case of NPLA, which decreases with increasing the DCP content to 1.5 wt %, indicating that the fraction of cross-linking sites up to some extent can be used as a nucleation site to rearrange the macromolecules, in which further cross-linking may lead to restriction of the macromolecular chain rearrangement and tends to hinder the crystallization process. However, the intensity at 16.5° is observed to increase with increasing DCP content for the case of the SNC–PLA system. This shows that the crystallization process is highly influenced by grafting of SNCs, in which the macromolecular arrangement of PLA chains is directed by highly ordered SNC β-sheets.
Figure 12.

XRD spectra (a: NPLA, b: 0.5DCP–PLA, c: 1DCP–PLA, d: 1.5DCP–PLA, e: SNC–PLA, f: 0.5DCP–SNC–PLA, g: 1DCP–SNC–PLA, and h: 1.5DCP–SNC–PLA).
Figure 13 illustrates the effect of reactive modification on the thermal stability of NPLA and SNC–PLA nanocomposites, and the values are listed in Table 5. SNC–PLA (without the addition of DCP) exhibits the highest thermal stability, in which T5% ∼ 334 °C is observed to be 10 °C higher than that in NPLA. It confirms that the incorporation of SNC alone can enhance the thermal stability of PLA. Stabilizing effect of SNC is already studied in our previous investigation for PLA system under multiple extrusion cycles.12 A slight reduction in T5% is observed with the addition of DCP, and it reaches ∼322 °C for 1.5DCP–PLA. Relatively higher values of T5% are obtained for SNC–PLA compared to NPLA at all DCP fractions. No significant change is observed for T95%. However, Tmax reduced from ∼371 °C (NPLA and SNC–PLA) to ∼363 °C (1.5DCP–PLA and 1.5DCP–SNC–PLA).
Figure 13.
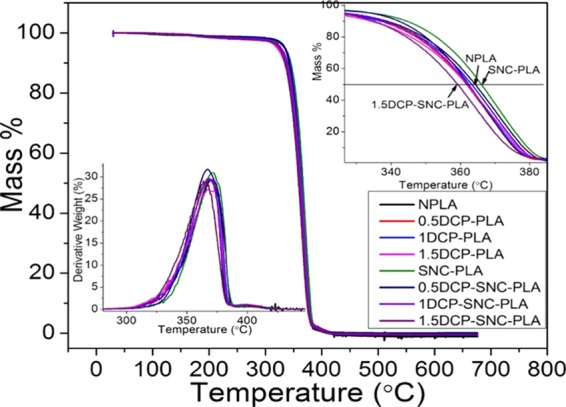
Thermal gravimetric analysis (TGA) of NPLA and SNC–PLA at various weight fractions of DCP.
Table 5. Impact of Reactive Modification on the Thermal Stability of NPLA and SNC–PLA.
| samples | T5% (°C) | T95% (°C) | Tmax (°C) |
|---|---|---|---|
| NPLA | 326 | 381 | 371 |
| 0.5DCP–PLA | 325 | 379 | 368 |
| 1DCP–PLA | 325 | 380 | 369 |
| 1.5DCP–PLA | 322 | 380 | 363 |
| SNC–PLA | 335 | 382 | 371 |
| 0.5DCP–SNC–PLA | 328 | 379 | 368 |
| 1DCP–SNC–PLA | 327 | 380 | 369 |
| 1.5DCP–SNC–PLA | 325 | 378 | 363 |
Decomposition process of the polymeric system is facilitated when the number of shorter chains increases. The observed broadening of molecular weight distribution (increasing in PDI) with increasing DCP weight fraction cloud be the reason for the lowering of thermal stability at the decomposing temperature.
The presence of silk nanocrystals is confirmed from the higher-magnification field emission scanning electron microscopy (FESEM) images, as clearly shown in Figure 14a,b. Due to the hydrophobic nature of SNCs, proper dispersion with minimum agglomeration is achieved in the hydrophobic PLA matrix. SNC is encapsulated inside the PLA matrix due to grafting, and microgel structure is observed in the morphology of the reactively extruded SNC–PLA (Figure 14d). From this, it can be concluded that the interfacial compatibility between PLA and SNC is improved and the grafting structure increases the dispersion and forms more cross-linked networks. The physical appearances of neat PLA, SNC–PLA, and SNC-grafted PLA samples at different DCP contents are displayed in Figure 14c; the transparency of the film is observed to decrease with increasing amount of DCP loading. As discussed above, the reactive extrusion in the presence of SNC provides significant grafting opportunity for the PLA macromolecules on the surface of SNC, which is an instantaneous process that improves the crystallinity. Transparency of the polymer film tends to decrease with increasing crystallinity and cross-linking efficiency.
Figure 14.
(a, b) FESEM images of SNC–PLA strips at different magnifications. (c) Melt-extruded samples and (d) FESEM image of reactively modified SNC–PLA at 1 wt % DCP.
2.4. Effect of Reactive Modification on Recyclability Performance of PLA
The influence of reactive extrusion on the reprocessability performance (up to three cycles) of NPLA, 1DCP–PLA, SNC–PLA, and 1DCP–SNC–PLA is studied. Zero shear viscosity, crossover points, and ratio of zero shear viscosity of Ri (“i” represents reprocessing cycles) to pristine PLA of R0 and R3 are used to evaluate the macromolecular change induced by the reprocessing cycles. As observed in Table 6, the zero shear viscosity of reactively modified samples drastically reduced after recycling. However, referring to the zero shear viscosity of R3-NPLA (433 Pa s), it is clearly observed that the reprocessed reactive extrusion samples have higher melt strength. Furthermore, the grafting of SNC increases the zero shear viscosity and shifts the crossover frequency to lower values.
Table 6. Impact of SNC Grafting on the Rheological Properties during Reprocessing.
| zero
shear viscosity |
crossover
frequency |
η0R/η0V | ||||
|---|---|---|---|---|---|---|
| R0 | R3 | R0 | R3 | R0 | R3 | |
| GPLA | 1767 | 79 | 1 | |||
| NPLA (ext) | 744 | 433 | 262 | >500 | 0.78 | 0.61 |
| SNC–PLA | 1232 | 702 | 116 | 338 | 0.9 | 0.76 |
| 1DCP–PLA | 7310 | 2397 | 21.1 | 67 | 1.52 | 1.09 |
| 1DCP–SNC–PLA | 18 186 | 4698 | 1.4 | 15.2 | 1.98 | 1.33 |
2.5. Conclusions
Reactively extruded NPLA and SNC–PLA with possible branching/cross-linking and grafting chain topologies have been demonstrated successfully, and their chemical structure, molar mass distribution, rheological characteristics, and thermal stability are investigated in detail. Three different scenarios are observed, including branching (0.5DCP–PLA), cross-linking and branching (1DCP–PLA and 1.5DCP–SNC–PLA), and grafting (DCP–SNC–PLA at 0.5, 1, and 1.5 wt % DCP). These three scenarios provide different levels of impact on important characteristic properties, such as structural and rheological behaviors, of the polymer. Bond formation between SNC (serine −CH2OH) and PLA main backbone carbon identified by 1H NMR spectroscopy confirms the grafting of SNC. Furthermore, all of the rheological properties (i.e., zero shear viscosity, storage modulus, crossover point, etc.) significantly improved with the grafting, which increases the reprocessability performance of PLA. The melting and crystallization phenomenon of PLA also completely changed with cross-linking and SNC grafting. Crystallinity percentage improved and PLA is observed to crystallize during cooling with cross-linking and SNC grafting. From this study, it can be concluded that the reactive extrusion of PLA in the presence of SNC will lead to the grafting topology with improved melt strength and other essential properties.
3. Experimental Section
3.1. Materials
In this study, NatureWorks’s poly(lactic acid) (PLA) (grade 2003D) with melt flow index of 6.0 g/10 min at 210 °C is used. It has number-average molecular weight (Mn), weight-average molecular weight (Mw), and polydispersity index (PDI) of ∼96 kDa, ∼207 kDa, and 2.15, respectively, which are estimated by gel permeation chromatography (GPC). Silk nanocrystals (SNCs) were prepared in lab as reported34,35 and dried for 24 h at 60 °C under vacuum. Dicumyl peroxide (DCP) was purchased from Sigma-Aldrich, India, to be used as radical initiator in the reactive extrusion. Muga silk cocoons (Antheraea assama) are delivered from Regional Muga Silk Station, Boko, Assam, India.
3.2. Preparation of Silk Nanocrystals (SNCs)
Impurities (eggs and plant debris) were removed from the cocoons and degummed at 98 °C for 30 min with 0.5% (w/w) sodium carbonate (Na2CO3), followed by washing multiple times with deionized water to remove salts. Prior to acid hydrolysis, the degummed fibroin was kept overnight in a hot air oven at 60 °C to remove moisture. Hydrolysis of dried muga fibroin was performed in aqueous sulfuric acid (64 wt %) for 2 h at 45 °C, followed by washing the hydrolysate multiple times with deionized water and centrifuging three times (10 000 rpm for 15 min each time) to isolate silk nanocrystals (SNCs). Finally, the pH of SNC suspension was maintained around 7 through continuous dialysis for ∼48 h and sonication was performed to homogenize the dispersion, followed by freeze drying after quench freezing with liquid nitrogen to obtain dried SNC powder for the current work.
3.3. Reactive Extrusion
Prior to the reactive extrusion process, PLA granules and silk nanocrystals were dried overnight at 60 °C under vacuum. Dicumyl peroxide (DCP) at three different percentage weight fractions (0.5, 1, and 1.5 wt %) was dissolved in acetone and mixed with PLA granules and freeze-dried SNCs (1 wt %), followed by removal of acetone to have proper dispersion of DCP on the PLA and SNC surface. The reactive extrusions were performed using HAAKE MiniLab corotating twin-screw extruder at a screw speed of 40 rpm, 185 °C, and residence time of ∼5 min. The samples were collected and kept under vacuum at 40 °C for further characterizations. The same procedures were followed for neat PLA in the presence of DCP to analyze the effect of SNC alone. Molecular weight analysis was performed for the samples collected at ∼3 and ∼5 min intervals.
The processing temperature for the reactive extrusion is selected on the basis of the processing temperature of PLA and thermal decomposition temperature of dicumyl peroxide (DCP). Reports showed that DCP decomposes into free radicals in the temperature range of 180–190 °C with a lifetime of 190 s.32,36 In our previous investigation, the thermal decomposition behavior of silk nanocrystals (SNCs) was studied thoroughly with the help of thermogravimetry-coupled Fourier transform infrared spectroscopy (TG-FTIR). From this study, we observe that decomposition of SNC starts at 280 °C, which is much higher than the processing temperature selected for the reactive extrusion. The degradation of SNCs occurs in a wide range of temperature (280–570 °C). From the Gram–Schmidt curve, we observed that SNC decomposes in two major stages: In the first stage, 30% decomposition occurs in the temperature range of 310–404 °C, which is the same range for 100% decomposition of neat poly(lactic acid) (NPLA) and SNC–PLA; In the 2nd stage, 60% decomposition occurs at a temperature range of 404–606 °C, which confirms thermal stability of SNCs.12,37
3.4. Reprocessing
In this study, NPLA and SNC–PLA with 1 wt % DCP were melt-reprocessed three times (temperature, 200 °C; screw speed, 100 rpm; and 1 min recycling), and rheological investigation is performed to understand the change. Zero shear viscosity and crossover frequencies are selected to assess the effect of melt-reprocessing cycles on reactively modified PLA. Under dynamic frequency sweep analysis, zero shear viscosity provides reliable information about the molecular-level structural change and has been used to predict the molecular weight as the frequency reaches zero. Crossover frequency is also another important parameter to evaluate the solidlike and liquidlike dominances of the melt.
The samples, neat PLA and PLA/silk nanocrystal biocomposites, which are extruded in the presence of different radical initiator (DCP) contents of 0, 0.5, 1, and 1.5 wt % are reported as NPLA, 0.5DCP–NPLA, 1DCP–NPLA, 1.5DCP–NPLA, SNC–PLA, 0.5DCP–SNC–PLA, 1DCP–SNC–PLA, and 1.5DCP–SNC–PLA.
3.5. Characterization
3.5.1. Molecular Weight Analysis
Number-average and weight-average molecular weights of reactively modified samples are estimated using gel permeation chromatography (GPC) with refractive index detector (RID-10A), at 1 mL/min eluent flow rate and 40 μL sample injection volume. The samples (30 mg) are dissolved in 1.5 mL of high-performance liquid chromatography-grade chloroform for 3 days and filtered using 0.25 μm filters. The instrument was calibrated with polystyrene standards.
3.5.2. Fourier Transform Infrared (FTIR) Spectroscopy
The effect of DCP on the structural modification of PLA/SNC nanocomposite was monitored using FTIR (PerkinElmer) attenuated total reflectance (ATR) mode in the range of 4000–650 cm–1 with 4 cm–1 resolution and 64 scan rate.
3.5.3. NMR Analysis
The macromolecular structural change of NPLA and SNC–PLA composite occurs due to the reactive extrusion process, which is investigated using a 600 MHz nuclear magnetic resonance (NMR) spectrometer. The samples were dissolved in deutrated chloroform (CDCl3) for 3 days and filtered with a 0.25 μm filter prior to the analysis.
3.5.4. Optical Polarity
The specific and optical rotations of reactively extruded NPLA and SNC–PLA with various amounts of DCP are estimated using AUTOPOL II polarimeter (Rudolph Research Laboratory) at a wavelength of 589 nm using a self-calibrated mechanism. The sample (200 mg) was dissolved in 20 mL of chloroform and filtered with a 0.25 μm filter prior to analysis.
3.5.5. XRD
The impact of reactive extrusion in the presence of SNC on the crystallographic behavior of PLA was investigated by wide-angle X-ray diffraction analysis using D8 Advance diffractometer (Bruker, Germany), with an X-ray source (40 kV, 40 mA) of Cu Kα radiation (λ = 0.1541 nm). Each sample was conditioned at 95 °C for 2 h before the analysis, and the test was performed at a scan rate of 0.05°/0.5 s with 2θ values ranging from 5 to 50°.
3.5.6. Differential Scanning Calorimetry (DSC)
The thermal characteristics of reactively extruded NPLA and SNC–PLA samples were analyzed using a differential scanning calorimeter (Netzsch, Germany). Samples weighing ∼6 mg were placed in a platinum crucible and scanned for a cycle of heat/cool/heat at a rate of 5 °C/min in the temperature range of 25–200 °C. Isothermal conditions were maintained at 200 °C for 3 min to erase the processing and thermal history after the first heating cycle. Data from the cooling and second heating cycles were considered for further analysis. Glass-transition temperature (Tg), cold crystallization (Tcc), melting temperature (Tm), heat of crystallization (Hcc), and heat of fusion (Hm) were estimated.
3.5.7. Thermal Degradation Analysis
Thermal degradation analyses of reactively modified NPLA and SNC–PLA were performed by PerkinElmer TGA4000. Each sample (6–10 mg) was scanned from 30 to 700 °C at a heating rate of 10 °C/min in the presence of inert nitrogen atmosphere.
3.5.8. Rheological Investigation
Rheological properties of each sample were investigated using interfacial rheometer (Anton-Paar model: MCR 301) at a temperature of 190 °C. Before the dynamic frequency sweep test analysis, amplitude sweep test was performed to estimate the linear viscoelastic region, and 5% strain is selected. Dynamic frequency sweep test analysis was performed in the frequency range of 0.1–600 Hz.
Acknowledgments
The authors acknowledge the Centre of Excellence for Sustainable Polymers (CoE-SusPol) funded by Department of Chemicals and Petrochemicals (DCPC) and Central Instruments Facility (CIF) at Indian Institute of Technology Guwahati (IIT Guwahati), India, for providing research and analytical facilities.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01005.
1H NMR spectra of NPLA sample at 1 wt % DCP (Figure S1); effect of angular frequency and DCP content on the loss modulus of NPLA and SNC–PLA (Figure S2) (DOCX)
The authors declare no competing financial interest.
Supplementary Material
References
- Imre B.; Pukánszky B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. 10.1016/j.eurpolymj.2013.01.019. [DOI] [Google Scholar]
- Scott G. ‘Green’ polymers. Polym. Degrad. Stab. 2000, 68, 1–7. 10.1016/S0141-3910(99)00182-2. [DOI] [Google Scholar]
- Eling B.; Gogolewski S.; Pennings A. J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 1982, 23, 1587–1593. 10.1016/0032-3861(82)90176-8. [DOI] [Google Scholar]
- Gupta B.; Revagade N.; Hilborn J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. 10.1016/j.progpolymsci.2007.01.005. [DOI] [Google Scholar]
- Anderson K. S.; Lim S. H.; Hillmyer M. A. Toughening of polylactide by melt blending with linear low-density polyethylene. J. Appl. Polym. Sci. 2003, 89, 3757–3768. 10.1002/app.12462. [DOI] [Google Scholar]
- Lim L. T.; Auras R.; Rubino M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. 10.1016/j.progpolymsci.2008.05.004. [DOI] [Google Scholar]
- Auras R.; Harte B.; Selke S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. 10.1002/mabi.200400043. [DOI] [PubMed] [Google Scholar]
- Gupta A. P.; Kumar V. New emerging trends in synthetic biodegradable polymers – Polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. 10.1016/j.eurpolymj.2007.06.045. [DOI] [Google Scholar]
- Auras R. A.; Singh S. P.; Singh J. J. Evaluation of oriented poly(lactide) polymers vs. existing PET and oriented PS for fresh food service containers. Packag. Technol. Sci. 2005, 18, 207–216. 10.1002/pts.692. [DOI] [Google Scholar]
- Anderson K. S.; Schreck K. M.; Hillmyer M. A. Toughening polylactide. Polym. Rev. 2008, 48, 85–108. 10.1080/15583720701834216. [DOI] [Google Scholar]
- Lehermeier H. J.; Dorgan J. R.; Way J. D. Gas permeation properties of poly(lactic acid). J. Membr. Sci. 2001, 190, 243–251. 10.1016/S0376-7388(01)00446-X. [DOI] [Google Scholar]
- Tesfaye M.; Patwa R.; Kommadath R.; Kotecha P.; Katiyar V. Silk nanocrystals stabilized melt extruded poly (lactic acid) nanocomposite films: Effect of recycling on thermal degradation kinetics and optimization studies. Thermochim. Acta 2016, 643, 41–52. 10.1016/j.tca.2016.09.008. [DOI] [Google Scholar]
- Gu S.-Y.; Zhang K.; Ren J.; Zhan H. Melt rheology of polylactide/poly(butylene adipate-co-terephthalate) blends. Carbohydr. Polym. 2008, 74, 79–85. 10.1016/j.carbpol.2008.01.017. [DOI] [Google Scholar]
- Tsuji H.; Ikada Y. Stereocomplex formation between enantiomeric poly(lactic acid)s. XI. Mechanical properties and morphology of solution-cast films. Polymer 1999, 40, 6699–6708. 10.1016/S0032-3861(99)00004-X. [DOI] [Google Scholar]
- Brizzolara D.; Cantow H. J.; Diederichs K.; Keller E.; Domb A. J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. 10.1021/ma951144e. [DOI] [Google Scholar]
- Kister G.; Cassanas G.; Vert M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. 10.1016/S0032-3861(97)00229-2. [DOI] [Google Scholar]
- Gupta M. C.; Deshmukh V. G. Thermal oxidative degradation of poly-lactic acid - Part I: Activation energy of thermal degradation in air. Colloid Polym. Sci. 1982, 260, 308–311. 10.1007/BF01447969. [DOI] [Google Scholar]
- Hyon S. H.; Jamshidi K.; Ikada Y. Effects of Residual Monomer on the Degradation of dl-Lactide Polymer. Polym. Int. 1998, 46, 196–202. . [DOI] [Google Scholar]
- Gogolewski S.; Jovanovic M.; Perren S. M.; Dillon J. G.; Hughes M. K. The effect of melt-processing on the degradation of selected polyhydroxyacids: polylactides, polyhydroxybutyrate, and polyhydroxybutyrate-co-valerates. Polym. Degrad. Stab. 1993, 40, 313–322. 10.1016/0141-3910(93)90137-8. [DOI] [Google Scholar]
- Lee S. H.; Kim J. I.; Park C. S.; Lee S. B.; Kim J. H.; Kim M. S. Preparation of intercross-linked poly(L-lactide) and epoxy resin using N-benzyl pyrazine hexafluoroantimonate. J. Polym. Res. 2013, 20, 264. 10.1007/s10965-013-0264-8. [DOI] [Google Scholar]
- Japon S.; Boogh L.; Leterrier Y.; Månson J. A. E. Reactive processing of poly(ethylene terephthalate) modified with multifunctional epoxy-based additives. Polymer 2000, 41, 5809–5818. 10.1016/S0032-3861(99)00768-5. [DOI] [Google Scholar]
- Incarnato L.; Scarfato P.; Di Maio L.; Acierno D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. 10.1016/S0032-3861(00)00032-X. [DOI] [Google Scholar]
- Lamnawar K.; Maazouz A. Rheological study of multilayer functionalized polymers: characterization of interdiffusion and reaction at polymer/polymer interface. Rheol. Acta 2006, 45, 411–424. 10.1007/s00397-005-0062-2. [DOI] [Google Scholar]
- Corre Y.-M.; Duchet J.; Reignier J.; Maazouz A. Melt strengthening of poly (lactic acid) through reactive extrusion with epoxy-functionalized chains. Rheol. Acta 2011, 50, 613–629. 10.1007/s00397-011-0538-1. [DOI] [Google Scholar]
- Zhou Z. F.; Huang G. Q.; Xu W. B.; Ren F. M. Chain extension and branching of poly(L-lactic acid) produced by reaction with a DGEBA-based epoxy resin. eXPRESS Polym. Lett. 2007, 1, 734–739. 10.3144/expresspolymlett.2007.101. [DOI] [Google Scholar]
- Cailloux J.; Santana O. O.; Franco-Urquiza E.; Bou J. J.; Carrasco F.; Maspoch M. L. Sheets of branched poly(lactic acid) obtained by one-step reactive extrusion–calendering process: physical aging and fracture behavior. J. Mater. Sci. 2014, 49, 4093–4107. 10.1007/s10853-014-8101-y. [DOI] [Google Scholar]
- Al-Itry R.; Lamnawar K.; Maazouz A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. 10.1016/j.polymdegradstab.2012.06.028. [DOI] [Google Scholar]
- Dhar P.; Tarafder D.; Kumar A.; Katiyar V. Thermally recyclable polylactic acid/cellulose nanocrystal films through reactive extrusion process. Polymer 2016, 87, 268–282. 10.1016/j.polymer.2016.02.004. [DOI] [Google Scholar]
- de Paula E. L.; Roig F.; Mas A.; Habas J.-P.; Mano V.; Pereira F. V.; Robin J.-J. Effect of surface-grafted cellulose nanocrystals on the thermal and mechanical properties of PLLA based nanocomposites. Eur. Polym. J. 2016, 84, 173–187. 10.1016/j.eurpolymj.2016.09.019. [DOI] [Google Scholar]
- Bian Y.; Han C.; Han L.; Lin H.; Zhang H.; Bian J.; Dong L. Toughening mechanism behind intriguing stress-strain curves in tensile tests of highly enhanced compatibilization of biodegradable poly(lactic acid)/poly(3-hydroxybutyrate-co-4-hydroxybutyrate) blends. RSC Adv. 2014, 4, 41722–41733. 10.1039/C4RA06199C. [DOI] [Google Scholar]
- Hiljanen-Vainio M.; Karjalainen T.; Seppälä J. Biodegradable lactone copolymers. I. Characterization and mechanical behavior of ε-caprolactone and lactide copolymers. J. Appl. Polym. Sci. 1996, 59, 1281–1288. . [DOI] [Google Scholar]
- Takamura M.; Nakamura T.; Takahashi T.; Koyama K. Effect of type of peroxide on cross-linking of poly(l-lactide). Polym. Degrad. Stab. 2008, 93, 1909–1916. 10.1016/j.polymdegradstab.2008.07.001. [DOI] [Google Scholar]
- Bian Y.; Han L.; Han C.; Lin H.; Zhang H.; Bian J.; Dong L. Intriguing crystallization behavior and rheological properties of radical-based crosslinked biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). CrystEngComm 2014, 16, 2702–2714. 10.1039/c3ce42498g. [DOI] [Google Scholar]
- Ling S.; Qi Z.; Knight D. P.; Huang Y.; Huang L.; Zhou H.; Shao Z.; Chen X. Insight into the structure of single Antheraea pernyi silkworm fibers using synchrotron FTIR microspectroscopy. Biomacromolecules 2013, 14, 1885–1892. 10.1021/bm400267m. [DOI] [PubMed] [Google Scholar]
- Tao Y.; Xu W.; Yan Y.; Cao Y. Preparation and characterization of silk fibroin nanocrystals. Polym. Int. 2012, 61, 760–767. 10.1002/pi.4136. [DOI] [Google Scholar]
- Coltelli M.-B.; Bronco S.; Chinea C. The effect of free radical reactions on structure and properties of poly(lactic acid) (PLA) based blends. Polym. Degrad. Stab. 2010, 95, 332–341. 10.1016/j.polymdegradstab.2009.11.015. [DOI] [Google Scholar]
- Tesfaye M.; Patwa R.; Gupta A.; Kashyap M. J.; Katiyar V. Recycling of poly (lactic acid)/silk based bionanocomposites films and its influence on thermal stability, crystallization kinetics, solution and melt rheology. Int. J. Biol. Macromol. 2017, 101, 580–594. 10.1016/j.ijbiomac.2017.03.085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










