Abstract
The increasing complexity of environmental pollution nowadays poses a severe threat to the public health, which attracts considerable attentions in searching for nanomaterials of multiproperty. In this study, mesoporous silica of KIT-6-encapsulated bismuth oxychloride (BiOCl), an intrinsically multifunctional material exhibiting bunched structure in the composites, are facilely prepared under hydrothermal conditions. Subsequently, the produced materials of multifunctionality were applied for photocatalysis, antibacterial test, and simultaneous determination of heavy metals including lead and cadmium. A combination of physiochemical characterizations have revealed that the BiOCl–KIT-6 composites exhibit enlarged yet refined surface morphology contributing to the improved photocatalytic ability with a band gap of 3.06 eV at a molecular ratio of 8Bi–Si. Moreover, the antibacterial activities of our BiOCl–KIT-6 composites were explored, and possible antimicrobial mechanism related to the production of reactive oxygen species was discussed. Furthermore, a sensitive electrochemical determination of heavy metals of lead and cadmium using square-wave anodic stripping voltammetry was also achieved. The composites-modified glassy carbon electrode displays a linear range of calibration curve from 0.2 to 300 μg/L with a detection limit of 0.05 μg/L (Pb2+) and 0.06 μg/L (Cd2+), respectively.
1. Introduction
Nowadays, due to its increasing complexity of origins, environmental pollution such as industrial discharge of heavy metals or dyes resistant to degradation or infectious microbes remains a severe concern for public health.1−4 The available methods such as chlorination and UV irradiation, devoid of microbial contamination, used in water disinfection are limited because of the harmful disinfection byproducts5,6 or the cautious operating conditions.
Recently, multifunctional nanomaterials have attracted considerable concerns because of their enormous potentials in diverse applications in industry and biomedicine, such as degradation and detection of environmental contaminants and targeted drug delivery.7−10 To confer the multifunctionality of materials such as optical, electronic, magnetic, and thermal properties,11−14 many efforts have been made on tuning the microstructure of the constituent or constructing hybrid nanomaterials, which usually involves relatively delicate chemical conjugations and thus may be difficult to achieve.15 To tackle environmental problems, a search for economical, efficient, and biosafe material with intrinsic multifunctionality is demanding.16
Bismuth materials have long been regarded as green materials because of their eco-friendliness. In particular, owing to its large surface area, extraordinary electronic transport properties, and high electrocatalytic activities, layered bismuth oxychloride (BiOCl) has been extensively studied especially for industrial purpose.17,18 Notably, due to its nontoxic nature, BiOCl has also been involved in biomedical practice.19,20 It was reported that bismuth subsalicylate (BSS), the active ingredient of an antacid drug named Pepto-Bismol that was approved to sell for over a century in the USA, is hydrolyzed into BiOCl in the human body to effectively treat diarrhea and stomach upsets.21,22 However, the layered microstructure of BiOCl is not ideal for a batch production that may preclude its further practical applications.
Porous silica materials such as KIT-6 (Korea Advanced Institute of Science and Technology-6), SBA-15 (Santa Barbara Amorphous-15), and MCM-41 (Mobil Composition of Matter-41) have demonstrated an ordered mesostructure, which is ideal for applications such as the construction of matrixes for drug delivery, molecular probes, and catalyst.23−26 In addition, due to the structural composition of regular mesopores, mesoporous silica materials have been used as templates to fabricate nanomaterials.27 Among the mesoporous silica materials, KIT-6 shows advantageous features for the structural modifications28 because of large tunable pores with thick pore walls, high hydrothermal stability, high specific surface area, and large pore volume. Therefore, we set out to integrate the intrinsically multifunctional BiOCl on modular KIT-6 support aimed at producing BiOCl of defined morphology and test the potential photocatalytic/electrochemical as well as antibacterial activity of composite.
In this study, a multifunctional BiOCl–KIT-6 composite with defined mesostructure was successfully synthesized by a facile hydrothermal method. Because of the intrinsic multiproperty of BiOCl, the obtained BiOCl/mesoporous silica composites were physiochemically characterized and applied for electrochemical stripping analysis of heavy metals of human blood samples, photocatalytic degradation of rhodamine B, and antibacterial assay. In the antibacterial test, two typical kinds of bacteria including the Gram-positive (G+) and the Gram-negative (G–) ones were used, and possible antimicrobial mechanism was discussed.
2. Results and Discussions
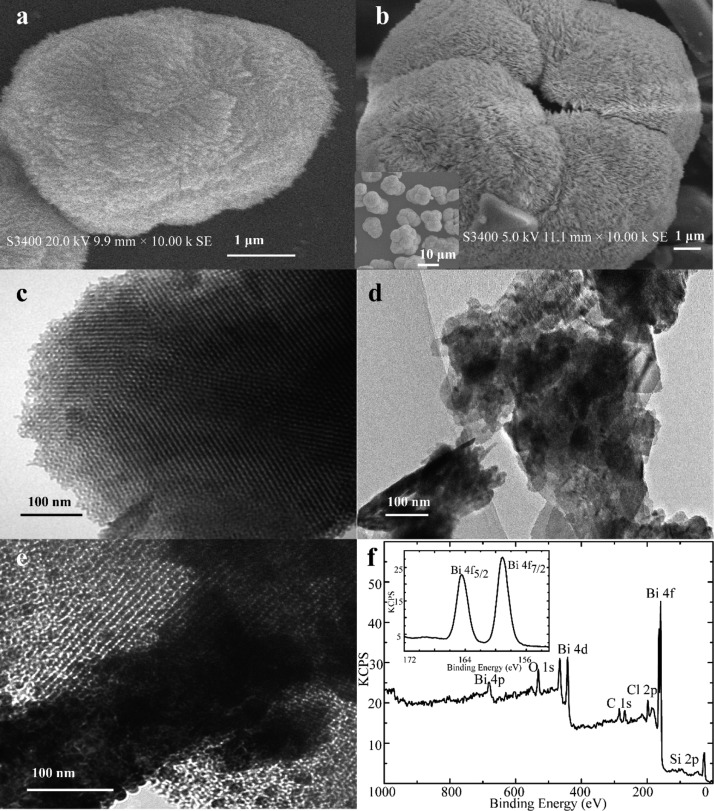
The surface morphology of the prepared materials is illustrated in Figures 1a–e and S1. Scanning electron microscopy (SEM) images revealed that the obtained BiOCl on siliceous support is composed of microspheres with a diameter of about 4 μm (Figure 1a). The BiOCl–KIT-6 composites are relatively large with size distributions about tens of micrometers (Figure 1b), which display bunched structure in comparison with the layered assembly of BiOCl. This observation may suggest a confining role of amorphous KIT-6 (Figure S1) during the formation of bunched structured BiOCl contributing to its enlarged surface area, which is favorable for the incorporation and transfer of substances. Transmission electron microscopy (TEM) provides additional structural information about the in situ growth of the BiOCl nanoplates on the supporting KIT-6. Because of the phase contrast, at the edge of KIT-6 supports, the visible distribution of BiOCl particles in the composites were observed (Figure 1c–e), demonstrating the well-formation of BiOCl particles on the KIT-6 mesopores.
Figure 1.
SEM images of (a) BiOCl and (b) BiOCl–KIT-6. TEM images of the pure (c) KIT-6, (d) BiOCl, and (e) BiOCl–KIT-6. (f) XPS spectra of BiOCl–KIT-6 and (inset) high-resolution Bi 4f region.
To estimate the surface elemental configurations of materials, an X-ray photoelectron spectroscopy (XPS) analysis was performed. The resulting XPS spectra of BiOCl–KIT-6 showed that the surface elements of composites mainly contain Bi, Cl, O, and negligible amount of absorbed C from the ambient atmosphere (Figure 1f). The O 1s core peak locates at 528.3 eV, which is attributed to O2– from a bismuth–oxygen bond in BiOCl as well as the Cl 2p peak with the binding energy at about 197.0 eV.29 The Bi 4f region (inset) of the composite was composed of two well-separated peaks located at 158.2 and 163.5 eV corresponding to Bi 4f7/2 and Bi 4f5/2 spin–orbit components, respectively, which is similar to that of pure BiOCl.29 This phenomenon suggests that the composite formation may avoid strong chemical bonding between silica matrix and BiOCl. The calculated atom percentage of surface Si in the composites was 8.67%, and the corresponding atomic ratio of Bi–Si was 4.1:1 that is close to the theoretical ratio of 6:1, which suggested that some amount of BiOCl were encapsulated into the siliceous mesopores.30
The X-ray diffraction (XRD) patterns of BiOCl, KIT-6, and composites were recorded. The wide-angle XRD spectra of composites pointed out the presence of well-crystallized BiOCl phase with characteristic diffraction peaks of the tetragonal matlockite phase of BiOCl (JCPDS 06-0249) (Figure S2A), of which the peak intensities are decreasing in the presence of KIT-6. The Raman scattering spectrum of BiOCl–KIT-6 recorded with a laser wavelength of 633 nm is shown in Figure S2B. Three dominant peaks at 201, 145, and 62 cm–1 were observed, of which the bands at 62 and 145 cm–1 are assigned to the A1g internal Bi–Cl stretching mode and the band at 201 cm–1 is assigned to the Eg internal Bi–Cl stretching mode. Whereas Eg external Bi–Cl stretching was not identified likely because of the strong scattering at 145 cm–1.31,32
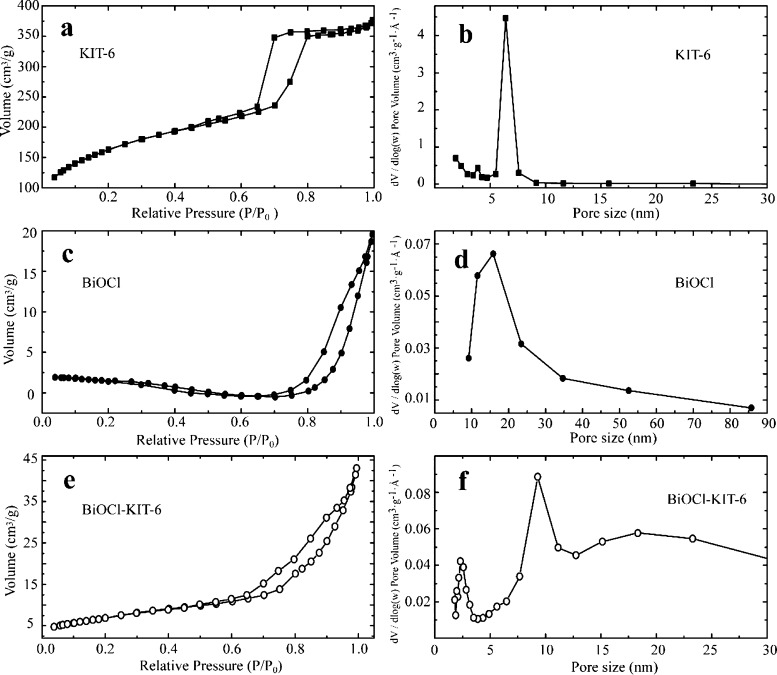
The N2 adsorption–desorption isotherms for pure KIT-6, BiOCl, and composites are depicted in Figure 2. The KIT-6 isotherms exhibited well-defined type IV isotherm characteristics of mesoporous silica materials.28 The isotherms of BiOCl show steady type IV isotherms with H3-type hysteresis loops reflecting the sheet structure. The composite showed a steep step of N2 adsorption–desorption at a relative pressure of 0.6–1.0 indicating the partial retaining of the mesoporous structure. The resulting textural properties of the samples (BET surface area, pore volume, and pore size) are outlined in Table S1. The presence of KIT-6 in the composites induced a marked increase of surface area and pore volume with an evident decrease of pore size, which accord well with the SEM results. BJH pore size distributions were also obtained (as shown in Figure 2b,d,f). The narrow and sharp pore size distribution of KIT-6 was observed indicating a regularity of mesopores. The pore size of BiOCl was distributed widely in the range of 10–30 nm arising from its composition of sheet structure. As expected, the maximum pore size of the composite was increased, verifying its incorporation of BiOCl.
Figure 2.
N2 adsorption–desorption isotherms (a,c,e) and Barrett–Joyner–Halenda (BJH) pore size distribution (b,d,f) of produced materials. The specific surface areas and pore size distribution of samples were calculated using Brunauer–Emmett–Teller (BET) and BJH methods, respectively.
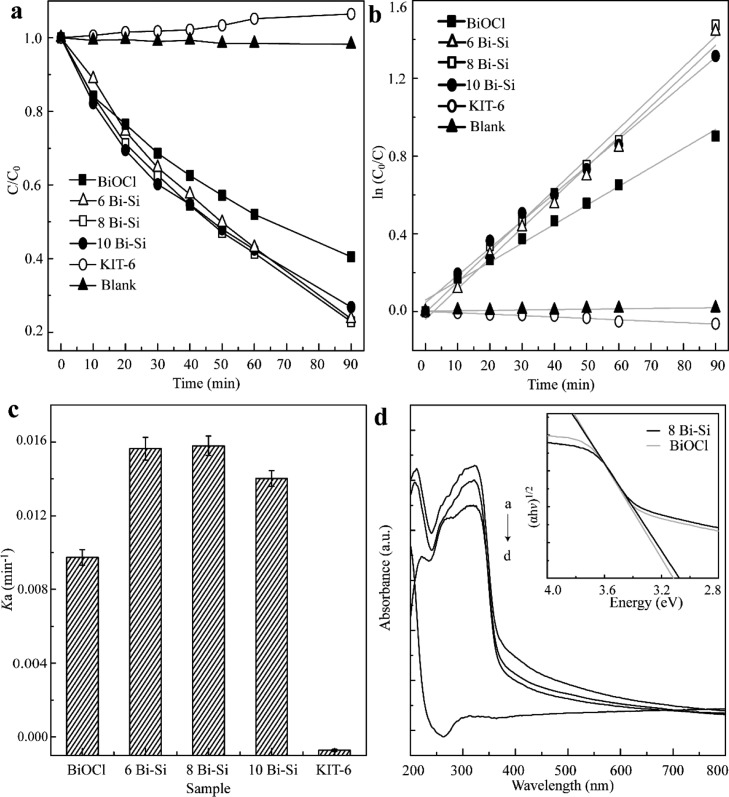
We first tested the photocatalytic performance of composites by the decomposition of RhB under UV light irradiation.33 The ratio of RhB concentration C relative to initial concentration C0 (C/C0) versus the degradation time is plotted in Figure 3a. There are two factors involved in the photodegradation of RhB including the adsorption of RhB onto the surface of photocatalyst and photoassisted reaction.34 As shown in Figure 3a, few dyes were degraded within 90 min in cases of KIT-6 and controls. When BiOCl was incorporated in the composites, the concentration of RhB decreased significantly under light exposure attributing to the high photoactivity of xBiOCl–KIT-6 (the adsorption spectral changes of RhB over 8Bi–Si are shown in Figure S3). Considering the excessive absorbent in the solution, the photodegradation kinetics of RhB can be fitted with a pseudo-first-order function35 as described below.
| 1 |
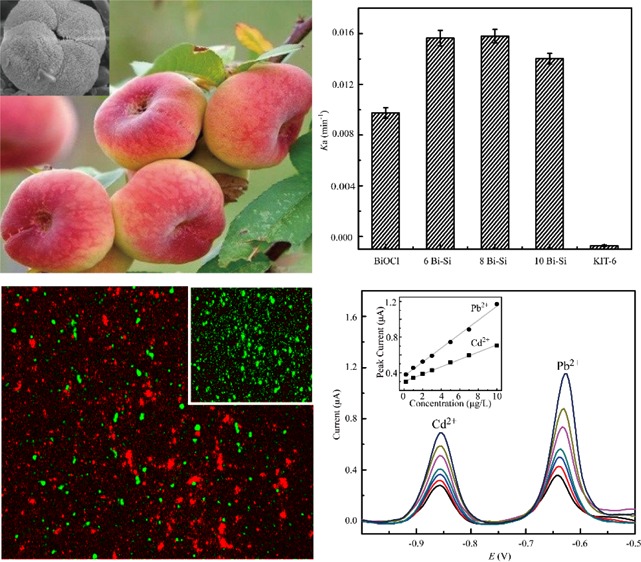
where C is the concentration of RhB remaining in the solution after irradiation and C0 is the initial concentration of RhB. Figure 3b presented the plots of ln(C0/C) versus irradiation time for the degradations of RhB. The calculated apparent rate constants (ka) for the degradations of RhB are outlined in Figure 3c. As shown in Table S2, the apparent rate constants of RhB over BiOCl, 6Bi–Si, 8Bi–Si, and 10Bi–Si were 0.0156, 0.0158, 0.0140, and 0.0097 min–1, respectively. From these results, we concluded that 8Bi–Si sample exhibited the maximized photocatalytic activity for the degradation of RhB with the calculated ka of ∼1.6 times of that over BiOCl indicating that the bunched structured of BiOCl on the mesopore support may facilitate the catalyzing of photons of RhB molecules. Additionally, 8Bi–Si sample with enlarged surface area favored both absorption and photodegradation of RhB.
Figure 3.
(a) Photodegradation curves of variation in RhB concentration (C/C0) with UV light irradiation time over xBi–Si (x = 6, 8, and 10), BiOCl, KIT-6, and blank control. (b) Kinetic curves of ln(C0/C) vs time for RhB photodegradation over xBi–Si (x = 6, 8, and 10), BiOCl, KIT-6, and blank control. (c) Histograms of reaction rate constants (ka) of RhB over xBi–Si (x = 6, 8, and 10), BiOCl, and KIT-6 under UV light irradiation (λ ≤ 420 nm). (d) Ultraviolet–visible (UV–vis) diffuse reflectance spectra of the obtained materials ((i) 10Bi–Si; (ii) BiOCl; (iii) 8Bi–Si; and (iv) KIT-6) and (inset) plot of (αhν)1/2 vs photon energy for 8Bi–Si and BiOCl.
To study the electronic state of the obtained samples, we further performed UV–vis diffuse reflectance spectroscopy (Figure 3d). The UV–vis spectrum of KIT-6 showed visible absorption bands in the range of 200–300 nm. Compared to pure KIT-6, all BiOCl and composites exhibit strong light adsorption (<370 nm) as previously reported,36 confirming the presence of BiOCl. The spectra were also used to calculate the band gap according to Kubelka–Munk transformation.31 A plot of [αhν]1/2 (α denotes the absorption coefficient) versus the photon energy of 8Bi–Si resulted in a band gap of 3.06 eV lower than that of BiOCl (3.12 eV), which is correlated to its improved photocatalytic performance.
As mentioned above, BiOCl is a major decomposed product of BSS drug in the gastrointestinal tract that treats dyspepsia and diarrhea effectively.21 We reasoned that BiOCl–KIT-6 may exhibit some antibiosis properties. To this end, we investigated the antibacterial activities of BiOCl–KIT-6 against Gram-positive bacteria, including Staphylococcus aureus and Enterococcus faecalis, and Gram-negative bacteria, including Escherichia coli and Pseudomonas aeruginosa. According to our study, the antibacterial activity of BiOCl–KIT-6 composites against Gram-negative bacteria (E. coli and P. aeruginosa) is not effective. However, the antibacterial effect of BiOCl–KIT-6 composites against Gram-positive ones (S. aureus and E. faecalis) was pronounced (Figure S4). Notably, 6Bi–Si composite has the maximized antibacterial activity against Gram-positive S. aureus, and the inhibition rate is up to 96.6% much higher than 10Bi–Si (77.7%), 8Bi–Si (91.5%), BiOCl (78.3%), and KIT-6 (52.5%), which is attributed to the mesoporous KIT-6 support favoring the antibiosis effect of BiOCl. The minimum inhibitory concentrations (MICs) of 6Bi–Si toward S. aureus and E. faecalis were generally observed to be 32 and 40 μg/mL, respectively, which corroborates the antibacterial activities of 6Bi–Si.
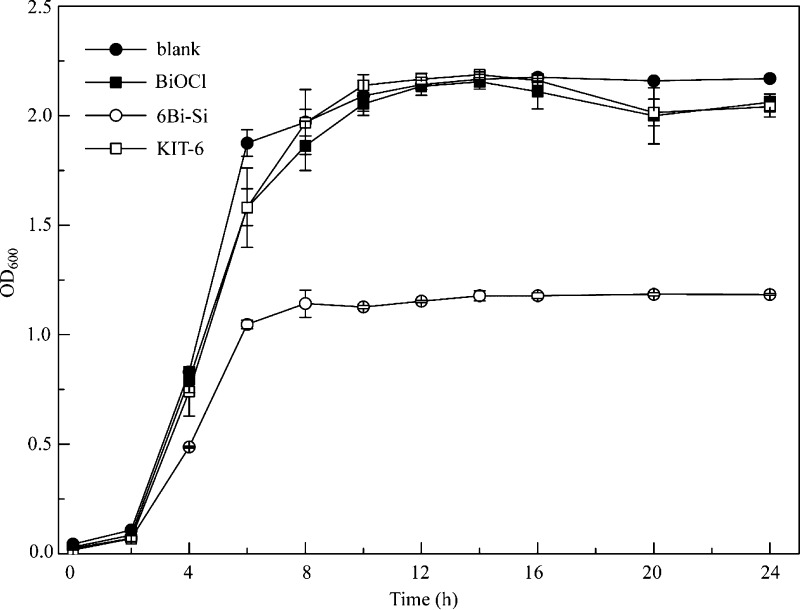
To further study the antibacterial properties of bismuth-based mesoporous silica materials, three types of materials named BiOCl, 6Bi–Si, and KIT-6 were added individually into LB liquid media of S. aureus, and the growth curves achieved are shown in Figure 4. In the presence of 20 μg/mL BiOCl or KIT-6 the growth curve of S. aureus cells is similar to those in just LB media, suggesting that neither BiOCl nor KIT-6 has observable antibacterial activity under the tested condition. An apparent decrease in the growth curve was observed when adding 6Bi–Si, which agreed well with the results of MIC experiment.
Figure 4.
Growth curves of S. aureus cultured in Luria Bertani (LB) media containing 20 μg/mL BiOCl, 6Bi–Si, and KIT-6 suspensions. Each data point is the average of three independent assays with the standard error of the mean.
The ability of the xBi–Si composites to prevent viable bacteria colonization was also verified by fluorescence staining as shown in Figure 5. After incubation at 37 °C for 16 h, there were large amounts of viable bacteria in blank, KIT-6, and BiOCl suspensions and relatively small amount of living bacteria in those xBi–Si suspensions (Figure 5d–f). The ratios of dead cells (ethidium bromide labeled) to total cells are counted as 19.5% (KIT-6), 50.2% (BiOCl), 67.1% (6Bi–Si), 53.2% (8Bi–Si), and 51.9% (10Bi–Si), of which 6Bi–Si suspension exhibited the most cytocidal effect (Figure 5d).
Figure 5.
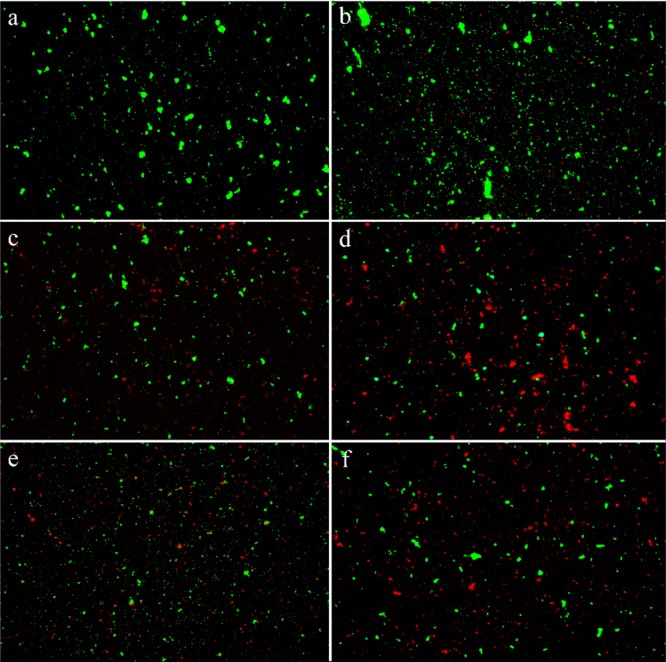
Representative images showing viability of the S. aureus bacteria in blank (a), KIT-6 (b), BiOCl (c), 6Bi–Si (d), 8Bi–Si (e), and 10Bi–Si (f) suspensions of 20 μg/mL after incubation at 37 °C for 16 h with the fluorescent dye of acridine orange and ethidium bromide. The live bacteria appear green, whereas the dead ones turn orange. The ratio of fluorescent cells of living (green) and dead (orange) status was calculated by manual counting under a microscope.
The documented literature illustrated that the toxicity of nanomaterials on bacteria may be related to the production of reactive oxygen species (ROS). ROS accumulation was detected in S. aureus cells using the fluorogenic dye 2′,7′-dichlorofluorescein diacetate (DCFDA) based on the oxidation of the nonfluorescent 2′,7′-dichlorodihydrofluorescein (DCFH) into the green highly fluorescent dichlorofluorescein (DCF). This reaction is considered to give a general indication of ROS levels, since DCFH reacts with H2O2, O2–, and ONOO–. As shown in Figure S5, S. aureus incubated with xBi–Si, BiOCl, and KIT-6 showed more green fluorescence compared with the untreated group (negative control), indicating that ROS were formed and may induce the cell death. We also found that the bacteria treated by 6Bi–Si, 8Bi–Si, 10Bi–Si, BiOCl, and KIT-6 resulted in the formation of DCF+ with a conversion ratio of 92.6, 76.9, 53.4, 68.2, and 38.6%, respectively, which was consistent with the antibacterial assay results (Figures 4 and 5). Similarly, the cells treated with 25 mM H2O2 as the positive control also exhibited green fluorescence (Figure S5). These results may point out a molecular mechanism of intracellular ROS formation involved with the bacterial S. aureus growth, that is, oxidative stress response, in the presence of BiOCl–KIT-6. Nevertheless, compared with G–E. coli consisting of a thin membrane of peptidoglycan and an outer membrane, G+S. aureus does not possess the outer membrane that is more vulnerable for the uptake of foreign chemical substances. A possible membrane-disruption process in the presence of mesocomposites may also occur to induce the cell death.
For the simultaneous anodic stripping voltammetry (ASV) analysis of lead and cadmium using BiOCl–KIT-6/GCE, we optimized three key operational parameters including pH of acetate buffer, deposition potential, and assay time. The electrochemical stripping voltammetric responses of BiOCl–KIT-6/GCE are recorded in Figure S6. The optimal analytical parameters for ASV in the simultaneous determination of Pb2+ and Cd2+ obtained by BiOCl–KIT-6/GCE are outlined in Table 1. Compared with bare glassy carbon electrode (GCE) and BiOCl/GCE, BiOCl–KIT-6/GCE achieved higher stripping peak currents of 100 μg/L Pb2+ and 100 μg/L Cd2+ (Figure S7), which was probably attributed to the better formation of bismuth–lead–cadmium alloys on mesoporous KIT-6 supports.37
Table 1. Experimental Parameters of ASV in the Simultaneous Determination of Pb2+ and Cd2+ Using BiOCl–KIT-6/GCE.
| electrode | pH | deposition potential (V) | deposition time (s) |
|---|---|---|---|
| BiOCl–KIT-6/GCE | 4.0 | –1.1 | 120 |
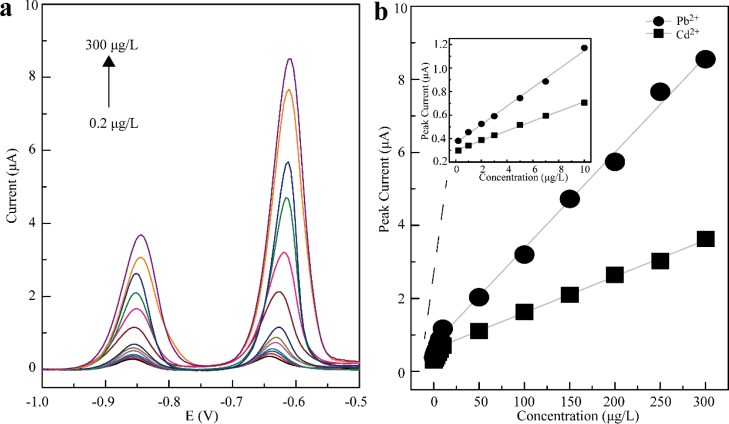
Under the optimized conditions given in Table 1, the obtained stripping curves of Pb2+ and Cd2+ using BiOCl–KIT-6/GCE are shown in Figure 6a. The calibration curves of the simultaneous determination of Pb2+ and Cd2+ displayed two linear ranges from 0.2 to 10 and 10 to 300 μg/L with regression equations of y = 0.0263x + 0.7491 (R2 = 0.9936, [Pb2+] > 10 μg/L), y = 0.0785x + 0.3624 (R2 = 0.9955, [Pb2+] ≤ 10 μg/L), y = 0.00995x + 0.6132 (R2 = 0.9983, [Cd2+] > 10 μg/L), and y = 0.0412x + 0.3010 (R2 = 0.9986, [Cd2+] ≤ 10 μg/L), where y and x are the peak currents (μA) and heavy metal concentrations (μg/L), respectively (Figure 6b). The resulting limit of detection (LOD) is 0.05 μg/L (Pb2+) and 0.06 μg/L (Cd2+), respectively, calculated by the equation of LOD = 3sy/x/a, where sy/x is the standard error of the regression curve and a is the slope of the regression curve.38 The analytical performance of our constructed sensor in comparison with the previous reports based on bismuth film-modified electrodes for the simultaneous determination of lead and cadmium is summarized in Table 2. Therefore, by using BiOCl–KIT-6/GCE, a relatively wide range of linear calibration curves and low LODs were obtained for the simultaneous determination of lead and cadmium compared with those in the earlier reports.39,40
Figure 6.
(a) Stripping voltammograms of simultaneous determination of Pb2+ and Cd2+ by using BiOCl–KIT-6/GCE over the concentration range of 0.2–300 μg/L in 0.1 M acetate buffer solution (pH 4.0) and (b) calibration curves of Pb2+ and Cd2+. Deposition potential: −1.1 V and deposition time: 120 s.
Table 2. Comparison of Analytical Performance of Bi Film-Modified Electrodes for Simultaneous Determination of Pb(II) and Cd(II) by ASVa.
| linear range (μg/L) |
LOD (μg/L) |
|||||||
|---|---|---|---|---|---|---|---|---|
| sensor | method | deposit. potent. (V) | Deposit time (s) | Pb | Cd | Pb | Cd | refs |
| BiOCl/MWCNT-GCE | SWASV | –1.2 | 120 | 5–50 | 5–50 | 0.57 | 1.2 | (39) |
| Bi-CNT SPE | SWASV | –1.4 | 300 | 2–100 | 2–100 | 1.3 | 0.7 | (40) |
| NCBFE | DPASV | –1.4 | 180 | 4–36 | 4–36 | 0.17 | 0.17 | (41) |
| Bi/GCE | SWASV | –1.2 | 600 | 5–60 | 5–60 | 0.8 | 0.4 | (42) |
| BispSPE | DPASV | –1.3 | 360 | 0.5–20 | 0.3–12 | 0.16 | 0.10 | (43) |
| BiOCl–KIT-6/GCE | SWASV | –1.1 | 120 | 0.2–300 | 0.2–300 | 0.05 | 0.06 | this work |
MWCNT, multiwalled carbon nanotube; Bi-CNT SPE, bismuth-modified carbon nanotube-modified screen-printed electrode; NCBFE, Nafion-coated bismuth film electrode; Bi/GCE: bismuth nanoparticles-modified GCE; BispSPE, sputtered bismuth screen-printed electrode; and DPASV, differential pulse anodic stripping voltammetry.
We further used the standard addition approach on BiOCl–KIT-6/GCE for the simultaneous determination of lead and cadmium in blood samples. The blood samples were collected from the local hospital following the guidelines. Under the same optimal stripping parameters on BiOCl–KIT-6/GCEs, the reasonable recovery of simultaneous determination of Pb2+ (96.8–104.4%) and Cd2+ (95.2–103.6%) in blood samples by ASV was achieved, validating the accuracy of our Bi-based sensor for the simultaneous determination of lead and cadmium in blood samples. The interference study was performed by adding some interfering heavy metal ions including Zn2+, Cu2+, Ca2+, Co2+, Mg2+, Fe3+, Ni2+, and Sn2+ in 20-fold excess with standard solution of analytes containing 100 μg/L Pb2+ and 100 μg/L Cd2+.
Under the optimal assay conditions, the obtained square-wave anodic stripping voltammetry (SWASV) response currents of Pb2+ and Cd2+ in the absence (I0) and presence (Ii) of interfering metal ions and relative signal changes (Ii/I0 – 1) are shown in Table 3. It was found that the peak currents of Pb2+ and Cd2+ in the presence of interfering metal ions changed slightly with the relative signal value from −6.7 to +4.1%, suggesting a satisfying selectivity for the simultaneous determination of lead and cadmium. Moreover, as we tested, the BiOCl–KIT-6/GCE was capable of the repeated usage for at least 2 weeks. Therefore, the constructed BiOCl-based sensor provides a practical, easy, and accurate way for the simultaneous determination of lead and cadmium in blood samples.
Table 3. Influence of Interference Ions on the Simultaneous Detection of 100 μg/L Pb2+ and 100 μg/L Cd2+.
| peak current
(μA) |
relative signal change
(%) |
|||
|---|---|---|---|---|
| interference ions | Pb2+ | Cd2+ | Pb2+ | Cd2+ |
| no interference ions | 1.63 | 3.20 | ||
| Zn2+ | 1.67 | 3.03 | +2.5 | –5.3 |
| Cu2+ | 1.58 | 3.02 | –3.1 | –5.6 |
| Ca2+ | 1.56 | 3.10 | –4.3 | –3.1 |
| Co2+ | 1.64 | 3.06 | +0.6 | –4.4 |
| Mg2+ | 1.59 | 3.33 | –2.5 | +4.1 |
| Fe3+ | 1.52 | 3.14 | –6.7 | –1.9 |
| Ni2+ | 1.59 | 3.02 | –2.5 | –5.6 |
| Sn2+ | 1.58 | 3.21 | –3.1 | +0.3 |
3. Conclusions
In this study, we have successfully synthesized the BiOCl–KIT-6 composites by a facile, in situ hydrothermal method. In particular, the prepared BiOCl–KIT-6 composites demonstrated the multifunctional character in photocatalysis, antibacterial activity, and simultaneous determination of lead and cadmium by ASV. The obtained composites exhibited an improved photocatalytic performance in degrading rhodamine B and pronounced antibacterial activities against Gram-positive S. aureus and E. faecalis. Moreover, the BiOCl–KIT-6 composite-modified GCE was applied for the anodic stripping analysis of the simultaneous detection of lead and cadmium with the linear range of 0.2–300 μg/L and a detection limit of 0.05 μg/L (Pb2+) and 0.06 μg/L (Cd2+), respectively, which is applicable for the simultaneous detection of lead and cadmium in real blood samples. It should be noted that the overall photocatalytic performance of composites was not remarkable compared with other bismuth-based materials.44 Therefore, it is anticipated that the BiOCl–KIT-6 composites upon further structural modification will lead to the broad use for environmental and analytical purposes.
4. Experimental Section
4.1. Materials and Preparation of BiOCl–KIT-6 Composites
All chemicals were used as received. Perfluorinated sulfonic acid ester (Nafion) and ethylene glycol (EG) were purchased from Sinopharm Chemical Reagent Co., Ltd. A triblock copolymer of Pluronic P123 (EO20PO70EO20) was obtained from Sigma-Aldrich (China). GCE (Φ = 3 mm) was purchased from Tianjin Incole Union Technology Co., Ltd, China. The standard solutions (Pb, Cd, Zn, Cu, Ca, Co, Mg, Fe, Ni, and Sn) were purchased from NACIS, China. The preparation of KIT-6 was synthesized as reported.28,45 The BiOCl–KIT-6 composites were obtained by a facile and in situ hydrothermal method. Briefly, 1.5 mmol of Bi(NO3)3·5H2O was fully dissolved in 30 mL EG at 25 °C before adding 0.2 mmol KIT-6. The mixture was further stirred for 30 min, and 10 mmol NaCl was introduced. Then, the mixture was heated at 170 °C for 6 h in a Teflon-lined stainless steel autoclave and cooled down to collect the product. The product was centrifuged and washed with distilled water for several times and then dried at 60 °C for 5 h. The composite materials of xBiOCl/KIT-6 (the molar ratio of Bi–Si of x = 6, 8, and 10) were labeled as 6Bi–Si, 8Bi–Si, and 10Bi–Si, respectively, in the text.
4.2. Characterization
The XRD patterns were obtained from SmartLab TM 9 kW diffractometer using Cu Kα radiation (λ = 0.154 nm). The SEM images were taken on a Hitachi SU-1510 microscope using an accelerating voltage of 15 kV. The TEM images were collected on a JEOL-1010 microscope operated at 200 kV. The X-ray photoelectron spectra were obtained on a PHI 5000 VersaProbe XPS system. The contaminant carbon (C 1s = 284.6 eV) was used as the calibration reference for all binding energies. The nitrogen adsorption–desorption isotherms were performed on an ASAP-2020 Micromeritics volumetric adsorption analyzer. The Raman spectra were recorded on a DRX spectrometer. The UV–vis diffuse reflectance spectra of samples were obtained on a LAMBDA 950 spectrophotometer (PerkinElmer). The electrochemical experiment was run on a CHI760D electrochemical analyzer with a three-electrode system including GCE modified with BiOCl–KIT-6 as a working electrode.
4.3. Photocatalytic Degradation Reactions
Photocatalytic activities of the samples were evaluated by the photocatalytic decomposition of rhodamine B (RhB). Typically, 30 mg of powder (10Bi–Si, 8Bi–Si, 6Bi–Si, BiOCl, and KIT-6) was added stepwise into the RhB solution (30 mL, 10 mg/L), which was irradiated with a 500 W Xe arc lamp equipped with a UV light (λ ≤ 420 nm). The suspension was stirred to reach the sorption equilibrium of dye molecules on the surface of the photocatalyst. At certain time intervals, 2 mL aliquot was pipetted out for the measurement.
4.4. Antibacterial Assay and ROS Detection
Before the antibacterial test, all of the materials and reagents in the experiments were sterilized at 120 °C for 20 min. The bacteria including S. aureus (S. aureus ATCC 6538), E. faecalis (E. faecalis FA2-2), E. coli (E. coli ATCC 25922), and P. aeruginosa (P. aeruginosa PAO1) were cultured in the LB liquid medium at 37 °C for 16 h. The antibacterial activities of BiOCl–KIT-6 toward E. coli and S. aureus were evaluated by the colony-counting method. The colony-counting test was performed by mixing 2 mL of 105 CFU/mL diluted bacterial suspension with 2 mL of BiOCl–KIT-6 (32 μg/mL) dissolved in LB liquid medium followed by a 16 h-incubation at 37 °C with shaking at 220 rpm. Then, 10 μL of the mixture was plated onto LB agar plates, and the number of the colonies was counted after 24 h. Plates containing only medium or bacterial suspension were also prepared as controls. The inhibition rate of composites was calculated by (N0 – NS)/N0, where N0 and NS represent the number of the colonies on LB agar plates in the absence and presence of produced materials samples (6Bi–Si, 8Bi–Si, 10Bi–Si, BiOCl, or KIT-6), respectively. The MIC value was determined by mixing 2 mL of 105 CFU/mL bacterial suspensions with 2 mL of 6Bi–Si of different concentrations in the range of 1–128 μg/mL into the tubes and incubated for 16 h. Control tubes containing only 6Bi–Si suspension, LB liquid media, and diluted bacterial suspension without materials were also prepared. To investigate the growth experiments, we chose S. aureus as a model. BiOCl (20 μg/mL), 6Bi–Si, and KIT-6 were added to the LB liquid media and then 105 CFU/mL bacterial suspensions were added to the culture at 37 °C with shaking at 220 rpm. The bacterial growth rates were determined over 24 h by measuring the optical density at 600 nm by a UV–vis spectrophotometer. To further look at the viability of bacteria when different materials were induced, the bacteria were cultured as mentioned above. The culture medium was then removed and the 20 μg/mL materials were rinsed with PBS, stained using acridine orange and ethidium bromide, and observed by a fluorescence microscope. Ethidium bromide stained only the dead cells, whereas acridine orange can penetrate the membrane and thus stain the viable and dead cells. Therefore, the living cells appeared green, whereas the dead ones were orange under the fluorescent microscope.46−48
The cellular toxicity of BiOCl–KIT-6 related to reactive oxygen species (ROS) formation has been evaluated using a conventional DCFDA assay,49 in which S. aureus cells were treated with the composites of altered molar ratio of Bi–Si, and the resulting fluorescence of dye DCFDA were recorded using a fluorescence microscope (Zeiss MTB2004, Germany). The ratios of DCF+-labeled cells in different treated groups were calculated by manually counting under a microscope.
4.5. Electrochemical Measurement
SWASV was performed for the simultaneous analysis of lead and cadmium using BiOCl–KIT-6/GCE as the working electrode. To construct the BiOCl–KIT-6/GCE, the composite was first dispersed in the permselective membrane of the Nafion solution (1%, v/v), which was capable of reducing the surface contamination of electrode. Aliquots (10 μL) were dropped on the GCE and dried at room temperature. Then, the test buffer was made to stand for 20 s before the stripping curve was recorded at the frequency of 20 Hz and amplitude of 25 mV under room temperature.
4.6. Pretreatment of Real Blood Samples
To minimize the matrix effect of samples,42 the whole blood samples of humans were treated based on the U.S. EPA Method 3050B with some modifications. Typically, 1 mL of the whole blood was digested and heated for 5 h close to dryness with concentrated HNO3. Subsequently, 1 mL of 0.1 M acetate buffer (pH 4.0) was added for the analysis. For the calibration, standard Pb and Cd solutions were spiked into blood samples.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (U1703118), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Shuangchuang Program, Open Funds of the State Key Laboratory for Chemo/Biosensing and Chemometrics (2016015), the National Laboratory of Biomacromolecules (2017kf05), and Jiangsu Specially-Appointed Professor project, China.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01590.
Mesostructural parameters; apparent reaction rate constant of RhB over composites; SEM results of KIT-6; XRD and Raman spectrum results; absorption spectra of photodegradation of RhB; inhibition rate of obtained materials against S. aureus; ROS detection results; optimization results of SWASV measurement; and SWASV curves of Pb2+ and Cd2+ recorded by different electrodes (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ashbolt N. J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- Ranganathan K.; Jeyapaul S.; Sharma D. C. Assessment of water pollution in different bleaching based paper manufacturing and textile dyeing industries in India. Environ. Monit. Assess. 2007, 134, 363–372. 10.1007/s10661-007-9628-z. [DOI] [PubMed] [Google Scholar]
- Li X.; Poon C.-S.; Liu P. S. Heavy metal contamination of urban soils and street dusts in Hong Kong. Appl. Geochem. 2001, 16, 1361–1368. 10.1016/s0883-2927(01)00045-2. [DOI] [Google Scholar]
- Ashbolt N. J. Risk analysis of drinking water microbial contamination versus disinfection by-products (DBPs). Toxicology 2004, 198, 255–262. 10.1016/j.tox.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Richardson S. Disinfection by-products and other emerging contaminants in drinking water. TrAC, Trends Anal. Chem. 2003, 22, 666–684. 10.1016/s0165-9936(03)01003-3. [DOI] [Google Scholar]
- Jia F.; Liu X.; Li L.; Mallapragada S.; Narasimhan B.; Wang Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J. Controlled Release 2013, 172, 1020–1034. 10.1016/j.jconrel.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Gong Y.-K.; Winnik F. M. Strategies in biomimetic surface engineering of nanoparticles for biomedical applications. Nanoscale 2012, 4, 360–368. 10.1039/c1nr11297j. [DOI] [PubMed] [Google Scholar]
- Govindhan M.; Adhikari B.-R.; Chen A. Nanomaterials-based electrochemical detection of chemical contaminants. RSC Adv. 2014, 4, 63741–63760. 10.1039/c4ra10399h. [DOI] [Google Scholar]
- Liu Q.-C.; Ma D.-K.; Hu Y.-Y.; Zeng Y.-W.; Huang S.-M. Various bismuth oxyiodide hierarchical architectures: alcohothermal-controlled synthesis, photocatalytic activities, and adsorption capabilities for phosphate in water. ACS Appl. Mater. Interfaces 2013, 5, 11927–11934. 10.1021/am4036702. [DOI] [PubMed] [Google Scholar]
- Bigall N. C.; Parak W. J.; Dorfs D. Fluorescent, magnetic and plasmonic—Hybrid multifunctional colloidal nano objects. Nano Today 2012, 7, 282–296. 10.1016/j.nantod.2012.06.007. [DOI] [Google Scholar]
- Qu L.; Vaia R. A.; Dai L. Multilevel, Multicomponent Microarchitectures of Vertically-Aligned Carbon Nanotubes for Diverse Applications. ACS Nano 2011, 5, 994–1002. 10.1021/nn102411s. [DOI] [PubMed] [Google Scholar]
- Frey N. A.; Peng S.; Cheng K.; Sun S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. 10.1039/b815548h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F.; Zhao H.; Li G.; Yang H.; Li J.; Wang R.; Liu Y.; Hu J.; Sun H.; Chen R. Size-tunable fabrication of multifunctional Bi2O3 porous nanospheres for photocatalysis, bacteria inactivation and template-synthesis. Nanoscale 2014, 6, 5402–5409. 10.1039/c3nr06870f. [DOI] [PubMed] [Google Scholar]
- Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. 10.1039/c3cs60273g. [DOI] [PubMed] [Google Scholar]
- Liu W.; Bertrand M.; Chaneac C.; Achouak W. TiO2 nanoparticles alter iron homeostasis in Pseudomonas brassicacearum as revealed by PrrF sRNA modulation. Environ. Sci.: Nano 2016, 3, 1473–1482. 10.1039/c6en00316h. [DOI] [Google Scholar]
- Guo C. F.; Zhang J.; Tian Y.; Liu Q. A general strategy to superstructured networks and nested self-similar networks of bismuth compounds. ACS Nano 2012, 6, 8746–8752. 10.1021/nn303467r. [DOI] [PubMed] [Google Scholar]
- Qu X.; Alvarez P. J. J.; Li Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- Rivera E. J.; Tran L. A.; Hernández-Rivera M.; Yoon D.; Mikos A. G.; Rusakova I. A.; Cheong B. Y.; Cabreira-Hansen M. d. G.; Willerson J. T.; Perin E. C.; Wilson L. J. Bismuth@US-tubes as a potential contrast agent for X-ray imaging applications. J. Mater. Chem. B 2013, 1, 4792–4800. 10.1039/c3tb20742k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Yang G.; Li J.; Gai S.; He F.; Yang P. NIR-driven water splitting by layered bismuth oxyhalide sheets for effective photodynamic therapy. J. Mater. Chem. B 2017, 5, 4152–4161. 10.1039/c7tb00688h. [DOI] [PubMed] [Google Scholar]
- Bierer D. W. Bismuth subsalicylate: history, chemistry, and safety. Clin. Infect. Dis. 1990, 12, S3–S8. 10.1093/clinids/12.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L. Bismuth therapy in gastrointestinal diseases. Gastroenterology 1990, 99, 863–875. 10.1016/0016-5085(90)90983-8. [DOI] [PubMed] [Google Scholar]
- Slowing I.; Trewyn B. G.; Lin V. S.-Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. 10.1021/ja0645943. [DOI] [PubMed] [Google Scholar]
- Trewyn B. G.; Giri S.; Slowing I. I.; Lin V. S.-Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007, 3236–3245. 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- Subhan F.; Aslam S.; Yan Z.; Ikram M.; Rehman S. Enhanced desulfurization characteristics of Cu-KIT-6 for thiophene. Microporous Mesoporous Mater. 2014, 199, 108–116. 10.1016/j.micromeso.2014.08.018. [DOI] [Google Scholar]
- Walcarius A. Mesoporous materials and electrochemistry. Chem. Soc. Rev. 2013, 42, 4098–4140. 10.1039/c2cs35322a. [DOI] [PubMed] [Google Scholar]
- Jones M. R.; Osberg K. D.; Macfarlane R. J.; Langille M. R.; Mirkin C. A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. 10.1021/cr1004452. [DOI] [PubMed] [Google Scholar]
- Suzuki N.; Kiba S.; Yamauchi Y. Fabrication of mesoporous silica KIT-6/polymer composite and its low thermal expansion property. Mater. Lett. 2011, 65, 544–547. 10.1016/j.matlet.2010.10.027. [DOI] [Google Scholar]
- Zhang X.; Wang X.-B.; Wang L.-W.; Wang W.-K.; Long L.-L.; Li W.-W.; Yu H.-Q. Synthesis of a highly efficient BiOCl single-crystal nanodisk photocatalyst with exposing {001} facets. ACS Appl. Mater. Interfaces 2014, 6, 7766–7772. 10.1021/am5010392. [DOI] [PubMed] [Google Scholar]
- Tsoncheva T.; Ivanova L.; Rosenholm J.; Linden M. Cobalt oxide species supported on SBA-15, KIT-5 and KIT-6 mesoporous silicas for ethyl acetate total oxidation. Appl. Catal., B 2009, 89, 365–374. 10.1016/j.apcatb.2008.12.015. [DOI] [Google Scholar]
- Myung Y.; Wu F.; Banerjee S.; Park J.; Banerjee P. Electrical conductivity of p-type BiOCl nanosheets. Chem. Commun. 2015, 51, 2629–2632. 10.1039/c4cc09295c. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Liang J.; Wang S.; Liu J.; Ren K.; Zheng X.; Luo H.; Peng Y.; Zou X.; Bo X.; Li J.; Yu X. BiOCl Sub-Microcrystals Induced by Citric Acid and Their High Photocatalytic Activities. Cryst. Growth Des. 2012, 12, 793–803. 10.1021/cg201112j. [DOI] [Google Scholar]
- Shamaila S.; Sajjad A. K. L.; Chen F.; Zhang J. WO3/BiOCl, a novel heterojunction as visible light photocatalyst. J. Colloid Interface Sci. 2011, 356, 465–472. 10.1016/j.jcis.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Li M.; Yuan J.; Wang X. Synthesis and characterizations of metastable Bi2SiO5 powders with a synergistic effect of adsorption and photocatalysis. Appl. Phys. A: Mater. Sci. Process. 2017, 123, 543. 10.1007/s00339-017-1144-6. [DOI] [Google Scholar]
- Zhang W.; Li Y.; Wang C.; Wang P. Kinetics of heterogeneous photocatalytic degradation of rhodamine B by TiO2-coated activated carbon: Roles of TiO2 content and light intensity. Desalination 2011, 266, 40–45. 10.1016/j.desal.2010.07.066. [DOI] [Google Scholar]
- Ye L.; Jin X.; Leng Y.; Su Y.; Xie H.; Liu C. Synthesis of black ultrathin BiOCl nanosheets for efficient photocatalytic H2 production under visible light irradiation. J. Power Sources 2015, 293, 409–415. 10.1016/j.jpowsour.2015.05.101. [DOI] [Google Scholar]
- Shen H.; Qin D.; Li Y.; Li S.; Yang C.; Yuan Q.; Wagberg T.; Hu G. In situ Magnesiothermal Synthesis of Mesoporous MgO/OMC Composite for Sensitive Detection of Lead Ions. Electroanalysis 2016, 28, 2939–2946. 10.1002/elan.201600348. [DOI] [Google Scholar]
- Desimoni E.; Brunetti B. Presenting Analytical Performances of Electrochemical Sensors. Some Suggestions. Electroanalysis 2013, 25, 1645–1651. 10.1002/elan.201300150. [DOI] [Google Scholar]
- Cerovac S.; Guzsvány V.; Kónya Z.; Ashrafi A. M.; Švancara I.; Rončević S.; Kukovecz Á.; Dalmacija B.; Vytřas K. Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrode. Talanta 2015, 134, 640–649. 10.1016/j.talanta.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Hwang G.; Han W.; Park J.; Kang S. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. 10.1016/j.talanta.2008.02.039. [DOI] [PubMed] [Google Scholar]
- Xu H.; Zeng L.; Huang D.; Xian Y.; Jin L. A Nafion-coated bismuth film electrode for the determination of heavy metals in vegetable using differential pulse anodic stripping voltammetry: An alternative to mercury-based electrodes. Food Chem. 2008, 109, 834–839. 10.1016/j.foodchem.2007.12.065. [DOI] [PubMed] [Google Scholar]
- Yang D.; Wang L.; Chen Z.; Megharaj M.; Naidu R. Anodic stripping voltammetric determination of traces of Pb(II) and Cd(II) using a glassy carbon electrode modified with bismuth nanoparticles. Microchim. Acta 2014, 181, 1199–1206. 10.1007/s00604-014-1235-4. [DOI] [Google Scholar]
- Sosa V.; Serrano N.; Ariño C.; Díaz-Cruz J. M.; Esteban M. Sputtered bismuth screen-printed electrode: a promising alternative to other bismuth modifications in the voltammetric determination of Cd(II) and Pb(II) ions in groundwater. Talanta 2014, 119, 348–352. 10.1016/j.talanta.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Xie J.; Cao Y.; Jia D.; Li Y. Dahlia-shaped BiOClxI1-x structures prepared by a facile solid-state method: Evidence and mechanism of improved photocatalytic degradation of rhodamine B dye. J. Colloid Interface Sci. 2017, 503, 115–123. 10.1016/j.jcis.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Song Y.; Xu Z.; Yu X.; Shi X.; Jiang H.; Li X.; Kong Y.; Xu Q.; Chen J. Raspberry-Like Bismuth Oxychloride on Mesoporous Siliceous Support for Sensitive Electrochemical Stripping Analysis of Cadmium. Molecules 2017, 22, 797. 10.3390/molecules22050797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela D.; Stanton M. M.; Parmar J.; Sánchez S. Microbots decorated with silver nanoparticles kill bacteria in aqueous media. ACS Appl. Mater. Interfaces 2017, 9, 22093–22100. 10.1021/acsami.7b03006. [DOI] [PubMed] [Google Scholar]
- Radzig M. A.; Nadtochenko V. A.; Koksharova O. A.; Kiwi J.; Lipasova V. A.; Khmel I. A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: influence on the growth and biofilms formation, mechanisms of action. Colloids Surf., B 2013, 102, 300–306. 10.1016/j.colsurfb.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Yu B.; Fan L.-H.; Wang M.; Zhu Y.; Ding X.; Xu F.-J. Multiple types of hydroxyl-rich cationic derivatives of PGMA for broad-spectrum antibacterial and antifouling coatings. Polym. Chem. 2016, 7, 5709–5718. 10.1039/c6py01242f. [DOI] [Google Scholar]
- Schenk S.; Laddaga R. A. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 94, 133–138. 10.1111/j.1574-6968.1992.tb05302.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.