Abstract
Early diagnosis of dopamine and serotonin metabolic defects is of importance notably because of the availability of therapeutic strategies able to prevent the associated progressive brain dysfunction. The diagnosis of these diseases relies on the determination of monoamine metabolites and pterins in cerebrospinal fluid (CSF). Current methods involve at least two high-performance liquid chromatography runs of CSF analysis. The first one is devoted to the quantification of dopamine and serotonin metabolites and the second one to the quantification of pterins. Here, we describe a single-step method to measure monoamine neurotransmitter metabolites and pterins of interest in less than 10 min by ultrahigh-performance liquid chromatography coupled to sequential coulometric oxidation and fluorescence detections. All target compounds were quantified in CSF with a small volume (50 μL) and a single filtration step for sample preparation and analysis. After validation, the proposed method was applied to the determination of age-related reference ranges in the CSF of target compounds from a series of 1372 samples collected in France from 2008 to 2014. In the same period, the results obtained for 19 CSF samples from patients with known neurotransmitter disorders and 115 CSF samples with known immune system activation confirmed the expected pattern of changes in monoamine metabolites and pterins.
Introduction
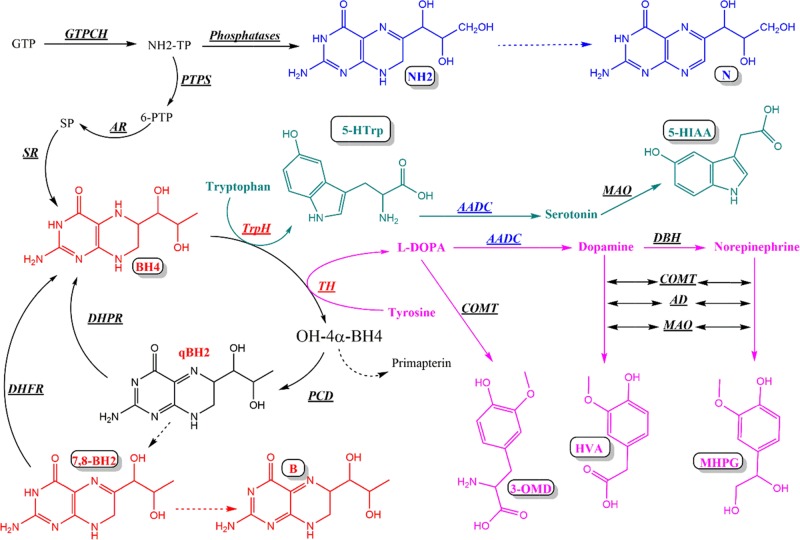
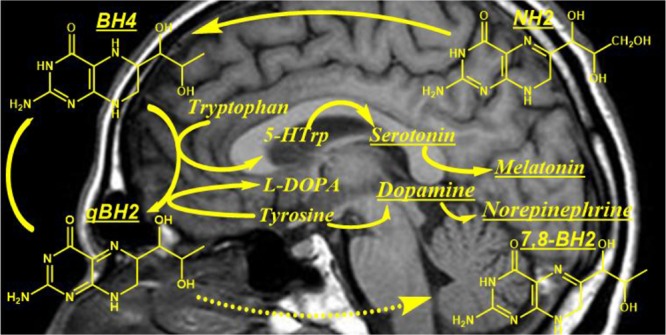
Since the description of the Segawa syndrome, also known as dihydroxy-phenyl-alanine (DOPA)-responsive dystonia, several neurotransmitter-inherited metabolic disorders including biosynthesis, breakdown, or transport defects of dopamine and serotonin have been reported.1−3 Inherited defects leading to these metabolic diseases have been described or predicted at the level of each enzyme involved in the metabolism of dopamine and serotonin (Figure 1).1−3
Figure 1.
Tetrahydrobiopterin (BH4) and monoamine neurotransmitter metabolism. GTP (guanosine triphosphate), GTPCH (guanosine triphosphate cyclohydrolase), NH2-TP (dihydroneopterin triphosphate), NH2 (dihydroneopterin), N (neopterin), PTPS (6-pyruvoyl-tetrahydropterin synthase), AR (aldose reductase), SR (sepiapterin reductase), BH4 (tetrahydrobiopterin), PCD (pterin-4α-carbinolamine dehydratase), qBH2 (quinonoid dihydrobiopterin), DHPR (dihydropteridin reductase), 7,8-BH2 (dihydrobiopterin), DHFR (dihydrofolate reductase), B (biopterin), TrpH (tryptophan hydroxylase), 5-HTrp (5-hydroxytryptophan), AADC (aromatic amino acid decarboxylase, cofactor: pyridoxal phosphate), MAO (monoamine oxidase), 5-HIAA (5-hydroxyindoleacetic acid), TH (tyrosine hydroxylase), l-DOPA (l-dihydroxyphenylalanine), DBH (dopamine β-hydroxylase), COMT (catechol-O-methyltransferase), AD (aldehyde dehydrogenase), 3-OMD (3-ortho-methyl DOPA), HVA (homovanillic acid), and MHPG (3-methoxy-4-hydroxyphenylglycol) (dashed arrows: nonenzymatic).
Tetrahydrobiopterin (BH4), the cofactor of aromatic amino acid hydroxylases, plays a pivotal role in the synthesis of dopamine and serotonin (Figure 1).4−6 Hence, defects in the biosynthesis or regeneration of BH4 result in several dopamine and serotonin metabolic disorders. Each of these conditions exhibits a characteristic cerebrospinal fluid (CSF) profile of dopamine and serotonin precursors and metabolites (Table 1).4−6
Table 1. Changes in CSF Neurotransmitter Metabolites and Pterins Concentrations in Disorders of Dopamine and Serotonin Metabolism (According to Refs (4)–6).
| pterins |
neurotransmitter
metabolites |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| enzymatic defect | Phea | BH4 | BH2 | NH2 | HVA | HIAA | HVA/HIAA | 3-OMD | 5-HTrp | MHPG |
| GTPCH AR | ↑ | ↓ | N | ↓ | ↓ | ↓ | N | N | N | ↓ |
| PTPS | ↑ | ↓ | N | ↑ | ↓ | ↓ | N | N | N | ↓ |
| PCD | ↑ | ↓ | N | N | N | N | N | N | N | N |
| DHPR | ↑ | ↓ | ↑ | N | ↓ | ↓ | N | N | N | ↓ |
| GTPCH AD | N | ↓ | N | ↓ | ↓ | N or ↓ | ↓ | N | N | ↓ |
| SR | N | ↓ | ↑ | N | ↓ | ↓ | N | N | N | ↓ |
| TH | N | N | N | N | ↓ | N | ↓ | N | N | ↓ |
| AADC | N | N | N | N | ↓ | ↓ | N | ↑ | ↑ | ↓ |
| DTDS | N | N | N | N | ↑ | N | ↑ | N | N | N |
Phe (phenylalanine as determined in plasma), N (normal), GTPCH AR (GTP-cyclohydrolase deficiency autosomal recessive), PTPS (6-pyruvoyl-tetrahydropterin synthase deficiency), PCD (pterin-4α-carbinolamine dehydratase deficiency), DHPR (dihydropteridine reductase deficiency), GTPCH AD (GTP-cyclohydrolase deficiency autosomal dominant also known as Segawa syndrome or DOPA-responsive dystonia), SR (sepiapterin reductase deficiency), TH (tyrosine hydroxylase deficiency), AADC (aromatic l-amino acid decarboxylase deficiency), and DTDS (dopamine transporter deficiency syndrome).
As BH4 is also the cofactor of phenylalanine hydroxylase (Figure 1), BH4 deficiencies can be detected at the time of newborn screening except for autosomal dominant GTPCH deficiencies, certain cases of heterozygote GTPCH deficiencies, and autosomal recessive SR deficiencies, which do not lead to hyperphenylalaninemia (Table 1).4−6
Early diagnosis of dopamine and serotonin metabolic disorders is of importance notably because some of these diseases can be well-treated.7−9 In addition to the determination of CSF neurotransmitter metabolites, the etiological diagnosis of these inherited disorders also requires the determination of pterins in CSF, notably dihydroneopterin (NH2), the precursor of BH4, and 7,8-dihyrobiopterin (BH2), the stable oxidized form of the latter (Figure 1, Table 1).1−6
To sum it up, the differential diagnosis of dopamine and serotonin metabolic disorders requires the determination of homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), 3-ortho-methyl-di-hydroxyphenylalanine (3-OMD), 5-hydroxytryptophan (5-HTrp), NH2, and BH2 in the CSF (Table 1). To this purpose, current methods require at least two chromatographic steps of CSF analysis. The first one is devoted to the quantification of dopamine and serotonin metabolites and the second one to the quantitation of pterins.10−19
As dopamine and serotonin metabolites are oxidizable compounds, high-performance liquid chromatography coupled to electrochemical detection (HPLC-ECD) is commonly used to quantify them in the CSF.10−17 More recently, some liquid chromatography–mass spectrometry (LC–MS) methods have also been implemented.19,20 On the other hand, the CSF pterin concentrations are in the low nM range at basal levels. Thus, their direct quantification does require an HPLC method coupled to fluorescence or MS detections.1,10,11,17,19
Here, we describe a single-step ultrahigh-performance liquid chromatography (UHPLC) method for the rapid diagnosis of dopamine and serotonin metabolic disorders. Neurotransmitter metabolites as well as pterins of interest are determined in a single step in less than 10 min by UHPLC coupled to sequential electrochemical and fluorescence detections.
After validation, the proposed method requiring only a single filtration step for sample preparation and analysis was applied to the analysis of a series of 1650 CSF samples including several cases of known neurotransmitter disorders and known immune system activation.
Results and Discussion
Method Development
Preliminary HPLC separations were achieved using the same polar-embedded C18 column (4.6 × 150 mm, 3 μm, and Atlantis T3) previously used for the separation of neurotransmitter metabolites17 and pterins.18 In contrast to classical Si-C18 columns, this stationary phase allows the separation of polar compounds including both neurotransmitter metabolites and pterins without using an ion-pairing reagent.18
Systematic investigation of the effects of pH, mobile phase composition, and temperature allowed us to obtain a separation with high efficiency and high resolution of all target compounds in less than 50 min (Figure S1A). High resolution is mandatory for the specific ECD and quantification of neurotransmitter metabolites because real CSF samples exhibit several unknown additional peaks (Figure S1C). The electrochemical detector also served as a postcolumn coulometric oxidation reactor for the oxidation of reduced pterins prior to sequential fluorescence detection (Figure S1B,D). Hence, this method allows the simultaneous separation of both neurotransmitter metabolites and pterins in a final run time of 50 min.
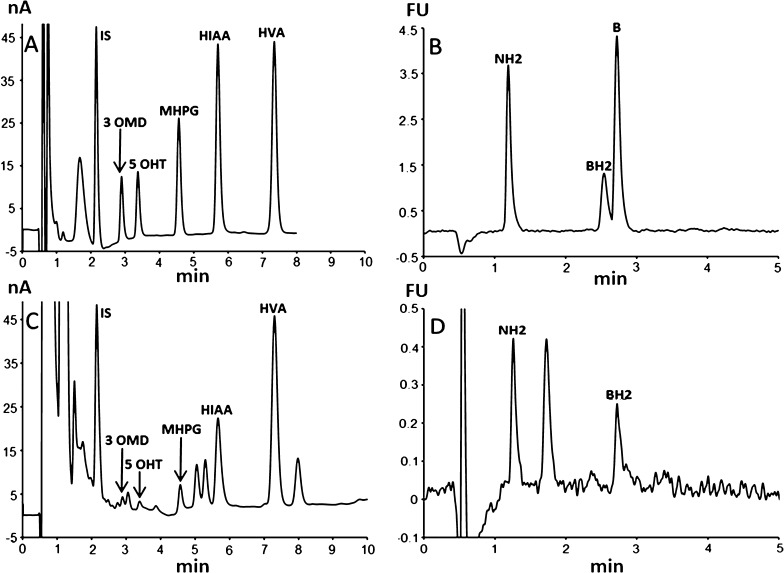
To speed up the separation, we converted the HPLC method to UHPLC. For this purpose, we used an ACQUITY UPLC HSS T3 column (2.1 × 100 mm and 1.8 μm) with the same mobile phase as for the HPLC separation, delivered at a flow rate of 0.4 mL/min at 30 °C. This conversion resulted in the separation of the whole of the analytes in less than 8 min (Figure 2) with a final run time of 10 min for real samples, thus allowing the complete elution of possible unknown peaks eluting after the HVA peak (Figure 2C).
Figure 2.
Chromatographic profiles obtained by the proposed method of (A,B) a standard mixture with (A) ECD followed by (B) sequential fluorescence detection and (C,D) a CSF sample of a patient from the control group with (C) ECD and (D) sequential fluorescence detection. Chromatographic conditions: stationary phase = Atlantis T3 (4.6 × 150 mm and 3 μm) column; mobile phase: pH 5.2, 0.05 M citrate buffer, and methanol (97/3, v/v). Flow rate was set at 0.5 mL/min at 30 °C. The metabolites present in the effluent are measured at the second electrode with a potential set at 400 mV, whereas the fluorescence of pterins is sequentially measured at 450 nm after column electrooxidation at the same potential and excitation at 350 nm (FU: arbitrary fluorescence units).
The best separation in terms of efficiency and resolution is obtained with a mobile phase consisting of a mixture of pH 5.2 and 0.05 M citrate buffer and methanol (97/3, v/v). The pH of the mobile phase should not fall below 5.2, otherwise the run time would become too long. Increasing the pH above 5.4 results in shortening the run time together with a loss of resolution. The concentration of methanol should not vary by more than 1%. Increasing the concentration of methanol above 4% results in shortening the run time with a concomitant loss of resolution. Decreasing the percentage of methanol below 2% results in a dramatic loss of efficiency and resolution. In the same way, the best efficiency and resolution were obtained at 30 °C.
We checked the absence of possible endogenous interferences including DOPA, dopamine, dihydroxy-phenylacetic acid, serotonin, and 3-methoxy-tyramine, which is the intermediate degradation product of dopamine leading to HVA. All of these metabolites are oxidizable compounds that could coelute with the targeted metabolites. Figure S2 shows that under our conditions, there is no coeluting peak with the used internal standard (IS). Dopamine elutes before the IS with a resolution higher than 1.3. Although serotonin and 3MT are not separated, these metabolites cannot interfere with 5-HIAA because their coeluting peaks are well-separated from the latter with a resolution higher than 1.4.
Proposed Method
Taken together, these analytical developments led us to adopt the following chromatographic conditions: separations were performed on an ACQUITY UPLC HSS T3 (2.1 × 100 mm and 1.8 μm) column protected by an ACQUITY UPLC HSS T3 VanGuard precolumn (2.1 mm × 5 mm and 1.8 μm), with a mobile phase consisting of pH 5.2 and 0.05 M sodium citrate/methanol (97/3, v/v) delivered at a flow rate of 0.5 mL/min at 30 °C. The ECD of the neurotransmitter metabolites is performed at +600 mV at the second electrode, and the sequential fluorescence detection of pterins was performed at λex 350 nm and λem 450 nm after coulometric oxidation at the same potential. The final run time was 10 min.
Method Evaluation
The identification of metabolites and pterins in authentic CSF samples was based on their retention factors and their electrochemical and fluorescence properties as well as proportional increases of corresponding peak areas after spiking with known amounts of each target compound (Table S2).
All unknown peaks are well-separated from the target metabolites under our chromatographic conditions. After more than 8 years of experience and more than 1500 handled CSF samples, we never observed any interfering peak at the level of the IS.
In some rare cases, probably because of the treatment, an interfering peak may coelute with 3-OMD. In these cases, this peak disappears just by decreasing the potential of the working electrode from 0.6 to 0.4 V. It is then necessary to calibrate the method at 0.4 V to determine the 3-OMD concentration. Nevertheless, such an interfering peak cannot influence the diagnosis because the diagnosis is not only based on the variation of a metabolite alone but rather based on the modification of the CSF metabolic profile, as shown in Table 1.
Table S1 summarizes the linearity data and LOQs for target analytes. The method was linear for all compounds over the calibration range with a correlation coefficient (R2) higher than 0.99 for all instances. However, the intercepts are negative in all instances. Hence, with this regression model, it will not be possible to make a prediction for a point that is outside the range of the data.
Within-run and between-run precision for standard solutions did not exceed 9.7% for all instances (Table S2). Within-run precision for authentic CSF samples spiked with known amounts of metabolites and pterins did not exceed 9.5% with a recovery higher than 91.8% for all instances. For each compound, the recovery was determined after analyzing a pool of authentic CSFs before (P0) and after (Ps) spiking it with a known amount of the corresponding analyte, by calculating the ratio: obtained amount (Ps) versus the theoretical amount (Th = P0 + added concentration) that gives [R = (Ps/Th) × 100]. Furthermore, there was no significant difference between within-run precision for spiked CSF samples and standard solutions as evidenced by p-values greater than 0.05, with recoveries higher than 91.8% for all instances (Table S2), thus indicating the absence of significant matrix effect. Hence, we used aqueous standard samples rather than authentic pooled CSF (p-CSF) samples for method calibration.
We checked the stability of the samples at 10 °C in the dark in the injector after several hours. We did not observe significant differences for 24 h. After 24 h, NH2 concentrations decreased by about 15%, whereas BH2 concentrations increased by about 10% of their respective initial values (Figure S3). Hence, using this method, the samples maintained at 10 °C in the dark should be analyzed within 24 h following defrosting.
In the same way, we checked the stability of the samples frozen at −80 °C immediately after collection. Compared with the initial values determined at day 4 after collection (and extemporaneous defrosting), the data obtained for the aliquots of the same samples defrosted after 12 months of storage at −80 °C showed no significant differences. Hence, we avoided adding a preservative agent to the samples, thus facilitating the collection procedure.
Age-Related Reference Ranges of Neurotransmitter Metabolites and Pterins in Cerebrospinal Fluid
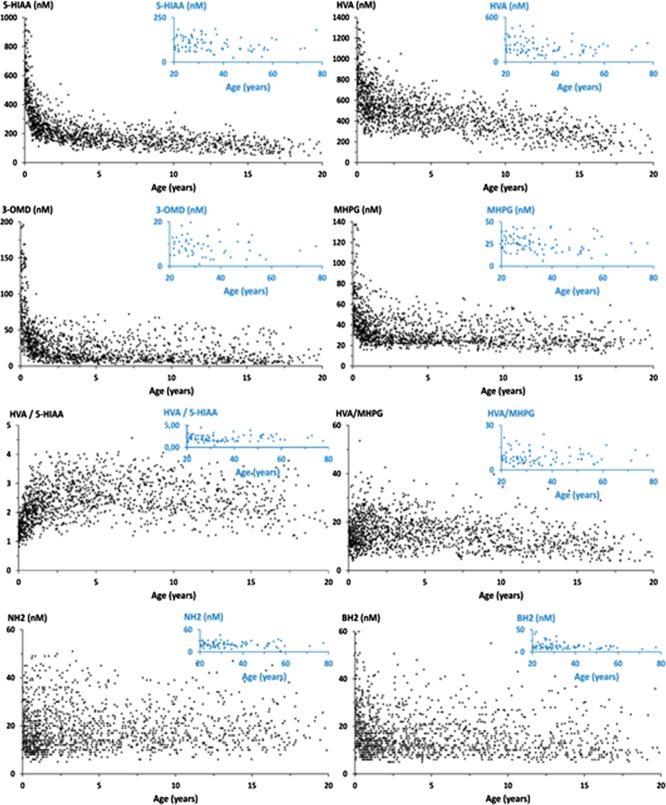
Figure 3 shows the distribution of the data obtained with the proposed method for 1516 CSF samples collected from 1386 infants and children aged 1 day to 16 years (median 3.34 years) and 130 patients aged 16.01–80 years (median 21 years) without known neurotransmitter disorder or 5-MTHF deficiency.
Figure 3.
Variation of metabolite concentrations in human CSF with patient age.
The first PCA model was built with HVA, 5-HIAA, MHPG, 3-OMD, 5-OTRP, BH2, and NH2 as variables and 1516 observations remaining after removing the outliers corresponding to the known 19 cases of neurotransmitter disorders and 115 cases of immune system activation. Figure 4A shows the score plot where observations are colored according to the patient’s age, from blue for the lowest values to red for the highest values. Principal components (CPs) 1 and 2 account for 37 and 17% of the total variance, respectively. There is an evolution as a function of the age; however, the overlap of the colored points indicates that it will be difficult to distinguish age intervals.
Figure 4.
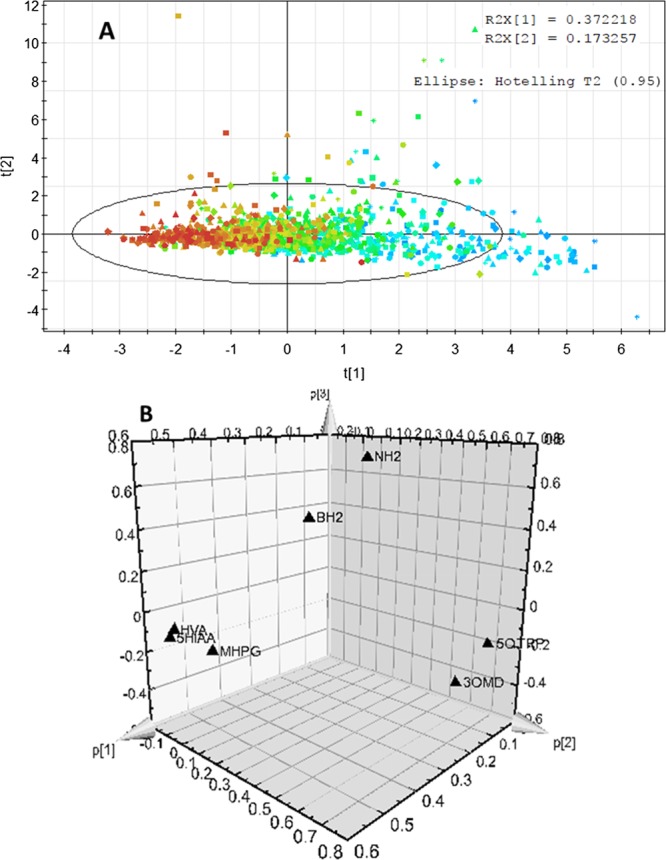
CP analysis with HVA, 5-HIAA, MHPG, 3-OMD, 5OH-Trp (5-HTrp), BH2, and NH2 as variables and 1516 observations. (A) Score plot and (B) loading plot.
In a second step, we considered only HVA, 5-HIAA, and MHPG, which are the main metabolites of dopamine, serotonin, and norepinephrine, respectively. The score plot still indicates that there is an obvious evolution with age, but it is still difficult to distinguish the age intervals (Figure S4A). Nevertheless, the loading plot (Figure S4B) clearly shows that HVA and 5-HIAA are the main contributors to CP 1 and that they are strongly correlated. MHPG also contributes to CP 1 but to a less extent. MHPG mainly contributes to CP 2 (Figure S4B). This makes sense once we consider the metabolic pathway of these metabolites (Figure 1). Whereas HVA and 5-HIAA are derived from dopamine and serotonin, which are the products of the same enzyme, namely, AADC, MHPG is derived from norepinephrine, which depends on the action of an additional enzyme, namely, DHB (Figure 1). This is also true for 3-OMD and 5-HTrp, which are the substrates of AADC but the products of two different hydroxylases, TH and TrpH (Figure 1). The loading plot (Figure S4C) obtained in the third step clearly shows that the latter mainly contributes to CP 2, which separates them.
In the fourth step, we added BH2, and we observed that this parameter is a major outlier, contributing to a third CP (Figure S4D). This also makes sense as BH2 results from the slow isomerization of qBH2, which is the oxidized intermediate of BH4, the common cofactor of TrpH and TH (Figure 1). Finally, in the last step, we added NH2, and we observed that it also contributes to the third component while it is slightly correlated with BH2. The latter is being separated by the axis of the third component (Figure 4B). This also makes sense once we consider that NH2 is the precursor of BH4 (Figure 1).
In conclusion, although the PCA model well-establishes the evolution of the main metabolites with the age of patients as well as the correlations between these parameters, it remains difficult to distinguish with this model the relevant age intervals and thus to establish the reference ranges.
Hence, we investigated the normality of the data by using both graphical representation (histograms and Q–Q plots) and Shapiro–Wilk or Shapiro–Francia tests depending on the kurtosis.21 In most cases, the data were not normally distributed, but they are log-normally distributed as shown by p-values less than 0.001 (Table S3). Nevertheless, we used Pearson correlation coefficient to study the correlation between metabolite concentrations and age.
As expected,16,18,22,23 a negative correlation was observed between most metabolites and age in the whole group of patients (HIAA: r = −0.5549, p < 0.0001; HVA: r = −0.5602, p < 0.0001; 3-OMD: r = −0.3929, p < 0.0001; MHPG: r = −0.3355, p < 0.0001; and BH2: r = −0.1459, p < 0.0001). Whereas the HVA/MHPG ratio showed a slight negative correlation with age (r = −0.1829 and p < 0.0001), HVA/HIAA and HVA/3-OMD ratios showed a positive correlation with age (r = 0.2365, p < 0.0001 and r = 0.0790, p = 0.0157, respectively). By contrast, NH2 did not exhibit any correlation with age (r = 0.0373 and p = 0.2613).
In a second step, we grouped the patients by age month by month for the first year and year by year for the patients older than one year. We also grouped the patients according to the previously published data.16 Considering a p-value less than 0.05 statistically significant, data were mostly log-normally distributed in all groups older than one month (Table S3).
Age groups were then compared by using Student’s t-test. For HVA and 5-HIAA concentrations, the differences between the groups older than 1 month were significant. However, in contrast to previous studies,16 HVA, MHPG, and BH2 concentrations in the group of newborns younger than one month were not statistically different from the group aged from 1 to 6 months. This discrepancy may be attributed to the lower number of patients included in previous studies.16 5-HTrp was found to be less than 12 nmol/L in every CSF sample (Table 2). Nevertheless, the correlation with age among the target metabolites was mostly similar to those previously published.16,22
Table 2. Reference Ranges for CSF Concentrations of Neurotransmitter Metabolites and Pterinsa.
| mean
(med.) |
|||||||
|---|---|---|---|---|---|---|---|
| groups | 5-HIAA | HVA | HVA/5-HIAA | 3-OMD | MHPG | NH2 | BH2 |
| A (n = 27) | 661 (620) | 868 (838) | 1.31 (1.37) | 109 (100) | 62 (56) | 19 (17) | 27 (26) |
| 0–1 month | 426–1186 | 405–1430 | 0.81–1.73 | 37–156 | 37–110 | 7–36 | 9–59 |
| A vs B | p = 0.002 | p = 0.111 | p = 0.122 | p < 0.001 | p = 0.172 | p = 0.543 | p = 0.030 |
| B (n = 166) | 468 (441) | 726 (700) | 2 (2) | 74 (61) | 54 (47) | 18 (15) | 21 (18) |
| 1 month to 0.5 year | 185–990 | 274–1487 | 0.8–3.7 | 11–250 | 18–138 | 7–65 | 6–69 |
| B vs C | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.704 | p < 0.001 |
| C (n = 457) | 249 (235) | 555 (539) | 2.33 (2.23) | 32 (26) | 35 (31) | 19 (16) | 16 (14) |
| 0.5–3 years | 111–631 | 174–1176 | 0.79–4.95 | 4–209 | 11–106 | 5–50 | 5–51 |
| C vs D | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.667 | p = 0.758 |
| D (n = 328) | 179 (170) | 473 (463) | 2.78 (2.71) | 21 (15) | 30 (26) | 19 (17) | 16 (113) |
| 3–7 years | 46–583 | 62–1185 | 1.29–5.96 | 3–179 | 8–67 | 6–69 | 5–48 |
| D vs E | p < 0.001 | p < 0.001 | p = 0.159 | p = 0.490 | p = 0.490 | p = 0.498 | p = 0.666 |
| E (n = 222) | 156 (149) | 402 (386) | 2.69 (2.63) | 20 (13) | 30 (27) | 19 (17) | 16 (14) |
| 7–11 years | 57–406 | 81–815 | 1.20–4.61 | 4–113 | 14–66 | 6–40 | 6–55 |
| E vs F | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.069 | p = 0.003 | p = 0.669 | p = 0.009 |
| F (n = 186) | 132 (129) | 309 (300) | 2.41 (2.34) | 17 (12) | 27 (10) | 19 (17) | 14 (12) |
| 11–16 years | 46–276 | 67–669 | 0.85–5.74 | 3–69 | 12–71 | 6–47 | 5–41 |
| F vs G | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.656 | p = 0.913 | p = 0.100 | p = 0.421 |
| G (n = 130) | 113 (112) | 241 (215) | 2.20 (2.07) | 18 (12) | 27 (25) | 21 (20) | 14 (11) |
| over 16 years | 40–186 | 65–488 | 1.04–4.90 | 3–55 | 12–59 | 7–51 | 5–45 |
| Known Metabolic Disorders (n = 18) and Elevated Levels of NH2 (n = 115) | |||||||
| Segawa (n = 2) 4.2 and 5.6 years | 10–17 | 15–20 | 1.50–1.18 | 2–3 | 4–6 | 2–4 | 1–3 |
| SR (n = 1) 27.88 years | 21 | 56 | 0.72 | 25 | 3 | 35 | 67 |
| TH (n = 11) 0.01–18.63 years | 212 (189) | 129 (117) | 0.62 (0.62) | 40 (22) | 35 (33) | 15 (12) | 28 (26) |
| 104–405 | 44–256 | 0.31–1.1 | 5–167 | 7–71 | 8–49 | 6–58 | |
| AADCb (n = 3) 0.46–2.40 years | 9 (3) | 15 (15) | 5.55 (7.50) | 1939 (2500) | 28 (25) | 15 (17) | 21 (21) |
| 2–23 | 3–27 | 0.15–9 | 252–3065 | 7–50 | 10–55 | 14–47 | |
| PTPS (n = 1) 24.46 years | 15 | 109 | 7.38 | 29 | 11 | 4 | 5 |
| DTDS (n = 1) 0.92 year | 302 | 2426 | 8.03 | 96 | 38 | 48 | 7 |
| elevated levels of NH2 (n = 115) | 258 (232) | 498 (490) | 2.09 (2.11) | 44 (29) | 34 (28) | 316 (184) | 38 (24) |
| 0.02–60.64 years | 26–732 | 23–1091 | 0.49–3.84 | 3–116 | 4–76 | 90–2500 | 21–91 |
Results are expressed in nanometers as average (median) and range.
For AADC, the main concentration of 5-HTrp is 505 nM, with a median of 316 nM (range: 300–900 nM).
Irrespective of the age or sex, strong positive correlations were observed between HVA and HIAA (r = 0.7742 and p < 0.0001), HVA and MHPG (r = 0.4446 and p < 0.0001), HVA and 3-OMD (r = 0.4483 and p < 0.0001), MHPG and 3-OMD (r = 0.5918 and p < 0.0001), HVA and BH2 (r = 0.2781 and p < 0.0001), and HIAA and BH2 (r = 0.2339 and p < 0.0001). BH2 and NH2 also exhibited a slightly positive correlation (r = 0.1241 and p = 0.0002). The correlations between HIAA, HVA, and BH2 confirm that the BH2 concentration well-reflects the BH4 availability in the CSF.
Table 2 summarizes the reference intervals obtained with the proposed method. Except for the patients aged less than one month, which are not different from those aged between 1 and 6 months, the obtained reference ranges are similar to those previously obtained in other countries by classical methods.16,22,23 These results thus strengthen the validation of the proposed method.
Analysis of the Known Cases of Dopamine and Serotonin Metabolism Disorders
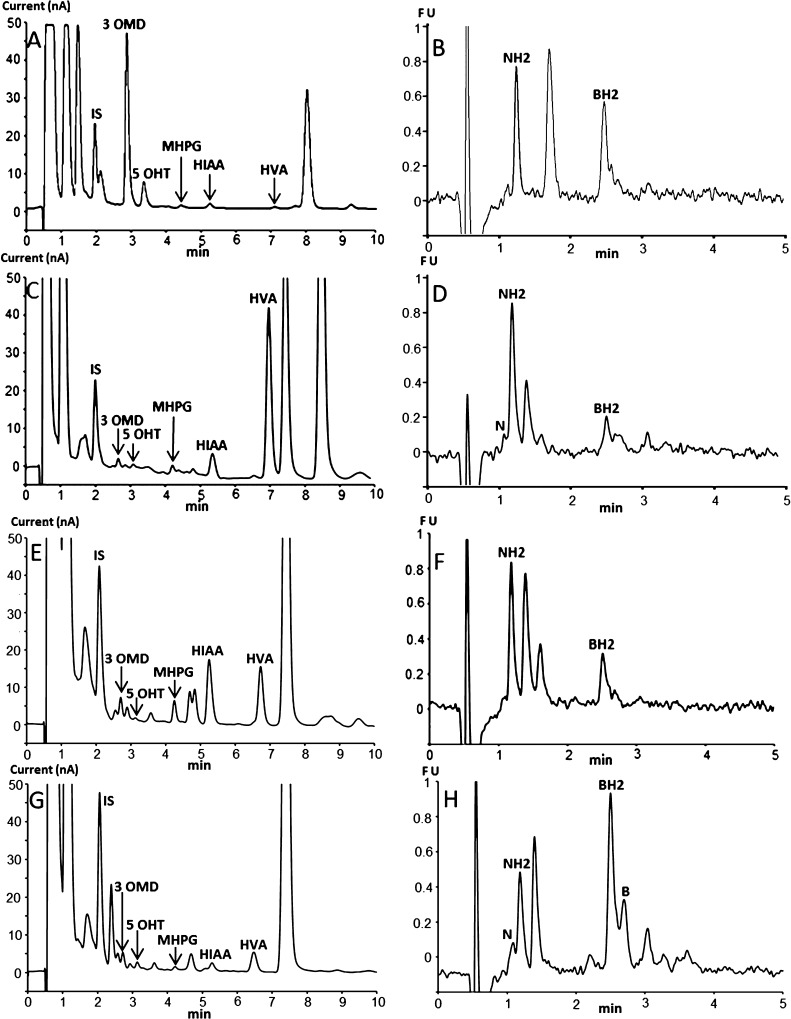
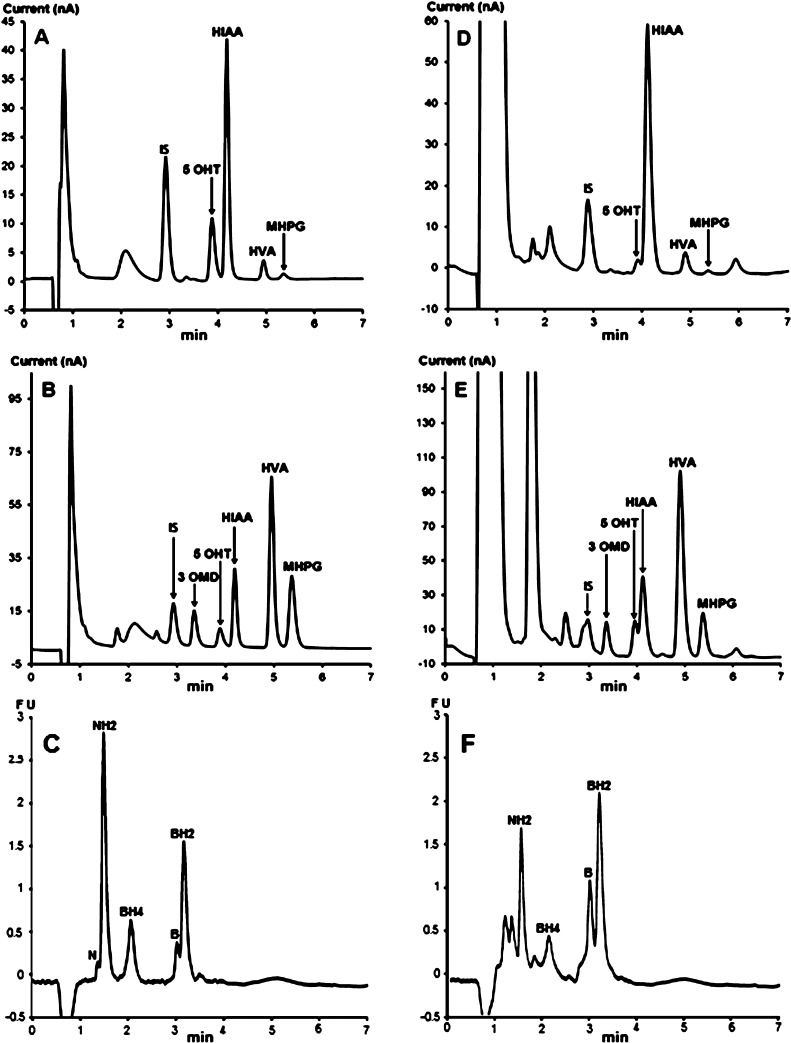
The proposed method was applied to the diagnosis of several known inborn errors of dopamine and serotonin metabolism including 2 cases of Segawa syndrome, 1 case of sepiapterin reductase deficiency, 3 cases of AADC deficiency, 1 case of dopamine transporter deficiency, 1 case of PTPS deficiency, 11 cases of tyrosine hydroxylase deficiency, as well as more than 100 cases of intracerebral immune activation with elevated levels of NH2 (Table 2, Figure 5). The obtained results (Table 2) confirmed the expected CSF pattern of changes in pterins and monoamine metabolites in patients.
Figure 5.
Chromatographic profiles obtained with the proposed method for some patients with known neurotransmitter disorders. (A,B) AADC deficiency, (C,D) DTDS, (E,F) TH deficiency, and (G,H) SR deficiency. The CSF sample (C) has been diluted twice before injection. The injection volume was 25 μL for (A,C) and 50 μL for (E,G).
Otherwise, the proposed method allows the unambiguous detection of NH2 increase, which is a known biomarker of immune system activation.24 It is worth noting that about 7% of the analyzed CSF samples exhibited elevated levels of NH2. Hence, the proposed method is a useful tool for the diagnosis of not only neurotransmitter disorders but also all cases of intracerebral immune system activation, which appear much more frequent than the former in the studied population.
Simultaneous Determination of BH4
Although BH4 determination is not mandatory for the etiologic diagnosis of neurotransmitter disorders, the determination of this cofactor may be interesting for metabolism and pharmacokinetics studies.18
As BH4 cannot be detected at pH 5.2 and can be detected at pH 7.4,18 we later developed a variant of the proposed method able to simultaneously determine BH4. In fact, this variant only differs in the pH of the mobile phase, that is, pH 7.4 instead of pH 5.2 as used in the proposed method.
Setting the pH of the mobile phase at 7.4 resulted in the simultaneous detection of BH4 in addition to all target compounds (Figure 6) with a good resolution but with different selectivities as reflected in the changes of the elution order. Under these conditions, the optimum sensitivity is obtained at an oxidation potential of +400 mV (first working electrode) for 5-HTrp and 5-HIAA (Figure 6A) and an oxidation potential of +600 mV (second working electrode) for 3-OMD, HVA, and MHPG (Figure 6B). The signal decrease for 5-HTrp and 5-HIAA at +600 mV is linked to the consumption of a large part of these metabolites at the first electrode set at +400 mV. To optimize the transformation of BH4 into its fluorescent counterpart, it is mandatory to use both electrodes for postcolumn oxidation of this pterin.
Figure 6.
Chromatographic profiles of a standard mixture and a CSF sample obtained with a pH 7.4 mobile phase. (A–C) Standard mixture with ECD at (A) +400 mV for the first electrode, (B) +600 mV for the second electrode, followed by (C) sequential fluorescence detection. (D–F) CSF sample with ECD at (D) +400 mV for the first electrode, (E) +600 mV for the second electrode, followed by (F) sequential fluorescence detection (see Figure 2 for the other chromatographic conditions).
Method evaluation showed that the analytical performances of this variant method operating at pH 7.4 are similar to those of the proposed method operating at pH 5.2 (Table S4). Also, the results obtained with the variant operating at pH 7.4 for real-CSF samples (n = 50) showed a good correlation with those obtained with the proposed method operating at pH 5.2 with a correlation coefficient higher than 0.99 for all target metabolites and pterins (Figure S5).
As the stability of the CSF samples at pH 7.4 is limited to 6 h in an autosampler at 10 °C18 instead of 24 h for the proposed method and because the stationary phase is less stable at pH 7.4 than under the conditions of the proposed method operating at pH 5.2, we recommend using the proposed method for the routine diagnosis of neurotransmitter disorders. Nevertheless, the derived method operating at pH 7.4 can be used for the simultaneous quantification of BH4 if needed as well as for checking the results of any CSF sample suspected to contain some potential interferences. In such cases, the difference in selectivity observed at pH 7.4 may help to detect the possible unexpected exogenous interfering compounds coeluting with the targeted compounds at pH 5.2 and vice versa.
After more than 6 years of experience, the only possible interfering peaks we observed are those present on the chromatograms of Figures 2, 5, and 6. As shown in these figures, all unknown peaks are well-separated from those of the targeted metabolites.
In fact, the only possible interferences that may occur are only unexpected exogenous interferences linked to unknown treatments. If there are some interferences coeluting with analytes and resulting in one peak, which is obviously possible for any method of separation of complex mixtures, it would be easy to suspect their presence. Indeed, as the diagnosis of neurotransmitter disorders is based on the characteristic CSF patterns (Table 1), such an interference would result in an unusual doubtful metabolic profile. Hence, an alternative method of separation with a different selectivity may be helpful to highlight such an interference.
Conclusions
An UHPLC method for a one-step rapid diagnosis of inborn errors of metabolism of dopamine and serotonin is presented. The use of an embedded-polar-group-bonded phase (ACQUITY HSS T3) and sequential coulometric and fluorescence detections allows the simultaneous quantification of neurotransmitter metabolites and pterins of interest. All target neurotransmitter metabolites and pterins were quantified in a small volume of CSF (50 μL) using a single filtration step for sample preparation and analysis.
As the samples are stable without adding any antioxidant agent for 24 h in an autosampler at 10 °C and as the run time is 10 min, the number of samples including calibrators and quality control that can be analyzed per run is 144.
The application of the proposed method to the analysis of 1516 human CSF samples without known neurotransmitter disorders allowed us to give the age-related reference ranges for key metabolites and pterins among French population. Furthermore, the application of the proposed method to several cases of known enzymatic defects confirmed the expected CSF pattern of changes in pterins and monoamine metabolites in patients.
Although previous methods using MS/MS detection were less sensitive than FD for pterin quantification,18 recent advances in MS/SM detection19 notably in terms of sensitivity show that the latter mode of detection is now able to replace the former. As MS/MS detection is less dependent on the resolution of the separation, the translation of the proposed method to UHPLC–MS/MS is one avenue to improve the overall throughput of this method. For the time being, the proposed method is quite appropriate for the laboratories not already equipped with the LC–MS/MS technology.
Methods
Chemicals and Reagents
All reagents were purchased from Sigma (Saint-Quentin Fallavier, France) and were used without further purification.
Patient Samples
The CSF samples were collected from 2008 to 2014 by lumbar puncture as previously described.18 Lumbar punctures were performed in several French hospitals covering the entire French territory as part of normal clinical management and research activities aiming to enhance the diagnosis of neurological disorders of unknown origin with the written informed consent of parents or legal representatives of each patient. This study was performed according to French public health regulations (Code de la santé publique—Article L1121-3, modified by Law no. 2011-2012, December 29 2011—Article 5). Samples were collected in five fractions of 0.3 mL each as previously described18 and were immediately frozen with liquid nitrogen and then stored at −80 °C until analysis. The exclusion criteria were traumatic punctures, inadequate collection and preservation of the samples, and l-DOPA and BH4 treatments. Determination of 5-methyl tetrahydrofolate by HPLC–FD17 was performed for all patients.
The collected CSF samples were divided into three groups. Group 1 includes CSF samples collected by lumbar puncture from 1386 infants and children aged 1 day to 16 years (median 3.34 years) and 130 patients aged 16.01 to 80 years (median 21 years). Group 1 was considered as a reference population with the same inclusion criteria as previously described (ref (18)). This group includes patients suffering from several neurological disorders with initially unknown etiology including movement disorders with or without encephalopathy, epileptic or neurodegenerative encephalopathy, and meningoencephalitis. Group 2 includes CSF samples with known neurotransmitter disorders collected from 16 infants and children (aged 1 day to 6.63 years, median 2.36 years) and 3 adults (aged 18.63 to 27.88 years, median 24.46 years). Group 3 includes CSF samples collected from 115 patients (aged 0.02 to 64.64 years, median 25.6 years) with known immune system activation.
Chromatographic Conditions and Sample Preparation
Preliminary HPLC separations were performed on a Dionex Summit HPLC system (Les Ulis, France). For the development of the method, the HPLC system was coupled to a model 5011 cell controlled by a Coulochem 5100A module (ESA, France) connected to a JASCO FP 920 detector equipped with a 16 μL standard cell. For simultaneous separation of neurotransmitter metabolites and pterins, we used an Atlantis T3 (4.6 × 150 mm and 3 μm) column (Waters, France). The mobile phase consisted of a mixture of pH 5.2 and 0.05 M citrate buffer and methanol (97/3, v/v). The flow rate was set at 0.5 mL/min at 30 °C. The metabolites present in the effluent were measured at the second electrode with a potential set at 400 mV, whereas the fluorescence of pterins was sequentially measured at 450 nm after column electrooxidation at the same potential and excitation at 350 nm.
For the UHPLC separations, we used a Waters ACQUITY UPLC system connected to an ESA Coulochem III electrochemical detector equipped with a 6011 model cell followed by an ACQUITY UPLC fluorescence detector. For the separation of neurotransmitter metabolites and pterins, we used an ACQUITY UPLC HSS T3 (2.1 × 100 mm and 1.8 μm) column (Waters, France). The mobile phase consisted of a mixture of pH 5.2 and 0.05 M citrate buffer and methanol (97/3, v/v). The flow rate was set at 0.5 mL/min at 30 °C. The metabolites and pterins present in the effluent were sequentially measured under the same conditions as for the HPLC separation but with a potential set at 600 mV. We changed the potential to 600 mV to reach the limiting current. Obviously, the potential shift is due to the used reference electrodes that are likely different between the two used Coulochem models.
For sample preparation, 50 μL of CSF or calibrator or QC sample was diluted (1/1, v/v) in a solution of 2,5-dihydroxybenzoic acid (250 nM) used as an IS prepared in the mobile phase, before filtration in a 5000 MWCO PES Vivaspin 500 filter (Sartorius, Aubagne, France) and centrifugation (10 min at 12 000g and 4 °C). The resulting filtrate (50 μL) was injected into the chromatograph. However, to respect the linearity of the method, the injected volume must be appropriately reduced for some CSF samples containing high levels of metabolites. Also, to avoid the dramatic reduction in the area of the IS peak, some samples containing high levels of metabolites must be appropriately diluted before addition of the IS and injection.
Calibrators and Quality Controls
Stock standard solutions were prepared by dissolving 400 μM of biopterin (B), neopterin (N), BH2, and NH2 in 0.1 M HCl or 1 mM of HVA, HIAA, 3-OMD, 5-HTrp, and MHPG in deionized water. After rapid preparation, aliquots of the standard solutions were immediately frozen at −80 °C until use.
To plot the calibration curves, the stock standard solutions of N, NH2, B, and BH2 were diluted to give final concentrations of 5, 12.5, 25, 50, and 100 nM/L of each pterin. HVA was diluted to give final concentrations of 15, 75, 187.5, 375, 750, and 1500 nM/L. HIAA was diluted to give final concentrations of 10, 50, 125, 250, 500, and 1000 nM/L. MHPG, 3-OMD, and 5-HTrp were diluted to give final concentrations of 5, 25, 62.5, 125, 250, and 500 nM.
Analytical recovery and precision studies were performed on the p-CSF samples spiked with 3-OMD, 5-HTrp, MHPG, HIAA, HVA, BH2, B, and NH2 at three concentration levels (low, medium, and high). Aliquots of 200 μL of p-CSF sample residues (n = 10) were spiked with 5, 12.5, and 50 nM/L of each pterin; 75, 187.5, and 750 nM/L of HVA; 50, 125, and 500 nM/L of HIAA, and 25, 62.5, and 250 nM/L of MHPG, 3-OMD, and 5-HTrp. The basal content of the p-CSF sample was determined with the standard addition method (Skoog, DA. 1996). As internal quality control, we used aliquots of p-CSF frozen at −80 °C until analysis. As external QC (EQC), we used aliquots of diluted water (1/100, v/v) “Special assays in urine” from ERNDIM (manufactured by the MCA Laboratory of the Queen Beatrix Hospital, Netherlands) stored at −80 °C until analysis. This EQC is used to control the 5-HIAA and HVA levels. For the control of 3-OMD, 5-HTrp, and MHPG, we used their response factors as compared to those of 5-HIAA and HVA.
Statistics
The Shapiro–Wilk or Shapiro–Francia tests were used to check the normality of the data distribution. Histogram and normal probability plots were also drawn to graphically assess the normality of the data. Comparisons were performed with a two-tailed Student’s t-test, after checking the homogeneity of variances using Fisher’s test. The p values less than 0.05 were considered statistically significant. Relationships between variables were examined using Pearson coefficient, linear regression, and analysis of variance. Statistical analysis was performed using MATLAB and excel.
Glossary
Abbreviations
- 3-OMD
3-ortho-methyl DOPA
- 5-HIAA
hydroxyl indole acetic acid
- 5-HTrp
5-hydroxytryptophan
- AADC
amino acid decarboxylase
- AD
autosomal dominant
- AR
autosomal recessive
- BbiopterinBH2
biopterinBH2dihydrobiopterin;
- BH4
tetrahydrobiopterin;
- CSF
cerebrospinal fluid
- DHPR
dihydropteridine reductase
- DTDS
dopamine transporter deficiency syndrome
- EQC
external quality control
- FD
fluorescence detection
- GTP-CH
guanosine triphosphate cyclohydrolase
- HPLC
high-performance liquid chromatography
- HVA
homovanillic acid
- IQC
internal quality control
- IS
internal standard
- LOD
limit of detection
- LOQ
limit of quantification
- MHPG
3-methoxy-4-hydroxyphenylglycol
- N
neopterin
- NH2
dihydroneopterin
- Phe
phenylalanine
- PCD
pterin-4α-carbinolamine dehydratase
- PTPS
6-pyruvoyl tetrahydropterin synthase
- SR
sepiapterin reductase
- TH
tyrosine hydroxylase
- UHPLC
ultrahigh-performance liquid chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01008.
Linearity, analytical recovery, and accuracy of the proposed method; Shapiro–Wilk normality test on population and log-transformed population; chromatographic profiles of a standard mixture and a CSF sample with the proposed method under HPLC conditions; and correlation between the results obtained with the proposed method at pH 5.2 and at pH 7.4 (PDF)
Author Contributions
A.L., P.G., J.-Y.H., and F.M. performed research and analyzed the data. D.D., E.R., D.R., and T.B.D.V. managed the children and collected the CSF samples. R.C. provided material support. F.M. designed research and analyzed the data. A.L., P.G., and F.M. wrote the paper. All authors discussed the results and comments on the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hyland K. Inherited disorders affecting dopamine and serotonin: critical neurotransmitters derived from aromatic amino acids. J. Nutr. 2007, 137, 1568S–1575S. [DOI] [PubMed] [Google Scholar]
- Longo N. Disorders of biopterin metabolism. J. Inherited Metab. Dis. 2009, 32, 333–342. 10.1007/s10545-009-1067-2. [DOI] [PubMed] [Google Scholar]
- Kurian M. A.; Gissen P.; Smith M.; Heales S. J. R.; Clayton P. T. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. 10.1016/s1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- Koshimura K.; Murakami Y.; Tanaka J.; Kato Y. The role of 6R-tetrahydrobiopterin in the nervous system. Prog. Neurobiol. 2000, 61, 415–438. 10.1016/s0301-0082(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Blau N.; Bonafé L.; Blaskovics M. E.. Disorders of phenylalanine and tetrahydrobiopterin metabolism. In Physician’s Guide to the Laboratory Diagnosis of Metabolic Diseases, 2nd ed.; Blau N., Duran M., Blaskovics M. E., Gibson K. M., Eds.; Springer: New York, 2003; pp 89–106. [Google Scholar]
- Roze E.; Blau N.. Biogenic monoamine disorders. In Inherited Metabolic Disease in Adults: A Clinical Guide, 1st ed.; Hollak C. E. M., Lachmann R., Eds.; OUP: Oxford, USA, 2016; pp 203–208. [Google Scholar]
- Hyland K. Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid. Clin. Chem. 2008, 54, 633–641. 10.1373/clinchem.2007.099986. [DOI] [PubMed] [Google Scholar]
- Allen G. F. G.; Land J. M.; Heales S. J. R. A new perspective on the treatment of aromatic L-amino acid decarboxylase deficiency. Mol. Genet. Metab. 2009, 97, 6–14. 10.1016/j.ymgme.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Leu-Semenescu S.; Arnulf I.; Decaix C.; Moussa F.; Clot F.; Boniol C.; Touitou Y.; Levy R.; Vidailhet M.; Roze E. Sleep and Rhythm Consequences of a Genetically Induced Loss of Serotonin. Sleep 2010, 33, 307–314. 10.1093/sleep/33.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T.; Nixon J. C. Analysis of reduced forms of biopterin in biological tissues and fluids. Anal. Biochem. 1980, 102, 176–188. 10.1016/0003-2697(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Howells D. W.; Smith I.; Hyland K. Estimation of tetrahydrobiopterin and other pterins in cerebrospinal fluid using reversed-phase high-performance liquid chromatography with electrochemical and fluorescence detection. J. Chromatogr. B: Biomed. Sci. Appl. 1986, 381, 285–294. 10.1016/s0378-4347(00)83594-x. [DOI] [PubMed] [Google Scholar]
- Koyama E.; Minegishi A.; Ishizaki T. Simultaneous determination of four monoamine metabolites and serotonin in cerebrospinal fluid by “High-Performance” Liquid Chromatography with electrochemical detection; application for patients with Alzheimer’s Disease. Clin. Chem. 1988, 34, 680–684. [PubMed] [Google Scholar]
- Tani Y.; Ohno T. Analysis of 6R- and 6S-tetrahydrobiopterin and other pterins by reversed-phase ion-pair liquid chromatography with fluorimetric detection by post-column sodium nitrite oxidation. J. Chromatogr. B: Biomed. Sci. Appl. 1993, 617, 249–255. 10.1016/0378-4347(93)80495-p. [DOI] [PubMed] [Google Scholar]
- Hubbard K. E.; Wells A.; Owens T. S.; Tagen M.; Fraga C. H.; Stewart C. F. Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection. Biomed. Chromatogr. 2010, 24, 626–631. 10.1002/bmc.1338. [DOI] [PubMed] [Google Scholar]
- Manini P.; Andreoli R.; Cavazzini S.; Bergamaschi E.; Mutti A.; Niessen W. M. A. Liquid chromatography–electrospray tandem mass spectrometry of acidic monoamine metabolites. J. Chromatogr. B: Biomed. Sci. Appl. 2000, 744, 423–431. 10.1016/s0378-4347(00)00285-1. [DOI] [PubMed] [Google Scholar]
- Ormazabal A.; García-Cazorla A.; Fernández Y.; Fernández-Álvarez E.; Campistol J.; Artuch R. HPLC with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins. J. Neurosci. Methods 2005, 142, 153–158. 10.1016/j.jneumeth.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Verbeek M. M.; Blom A. M.; Wevers R. A.; Lagerwerf A. J.; van de Geer J.; Willemsen M. A. A. P. Technical and biochemical factors affecting cerebrospinal fluid 5-MTHF, biopterin and neopterin concentrations. Mol. Genet. Metab. 2008, 95, 127–132. 10.1016/j.ymgme.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Guibal P.; Lévêque N.; Doummar D.; Giraud N.; Roze E.; Rodriguez D.; Couderc R.; de Villemeur T. B.; Moussa F. Simultaneous Determination of All Forms of Biopterin and Neopterin in Cerebrospinal Fluid. ACS Chem. Neurosci. 2014, 5, 533–541. 10.1021/cn4001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arning E.; Bottiglieri T.. LC-MS/MS analysis of cerebrospinal fluid metabolites in the pterin biosynthetic pathway. JIMD Reports; Springer, 2014; Vol. 29, pp 1–9. [DOI] [PMC free article] [PubMed]
- Bourcier S.; Benoist J.-F.; Clerc F.; Rigal O.; Taghi M.; Hoppilliard Y. Detection of 28 neurotransmitters and related compounds in biological fluids by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1405–1421. 10.1002/rcm.2459. [DOI] [PubMed] [Google Scholar]
- Razali N. M.; Wah Y. B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Hyland K.; Surtees R. A. H.; Heales S. J. R.; Bowron A.; Howells D. W.; Smith I. Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population. Pediatr. Res. 1993, 34, 10–14. 10.1203/00006450-199307000-00003. [DOI] [PubMed] [Google Scholar]
- Molero-Luis M.; Serrano M.; Ormazábal A.; Pérez-Dueñas B.; García-Cazorla À.; Pons R.; Artuch R.; The Neurotransmitter Working Group Homovanillic acid in cerebrospinal fluid of 1388 children with neurological disorders. Dev. Med. Child. Neurol. 2013, 55, 559–566. 10.1111/dmcn.12116. [DOI] [PubMed] [Google Scholar]
- Hagberg L.; Cinque P.; Gisslen M.; Brew B. J.; Spudich S.; Bestetti A.; Price R. W.; Fuchs D. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010, 7, 15. 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.