Abstract
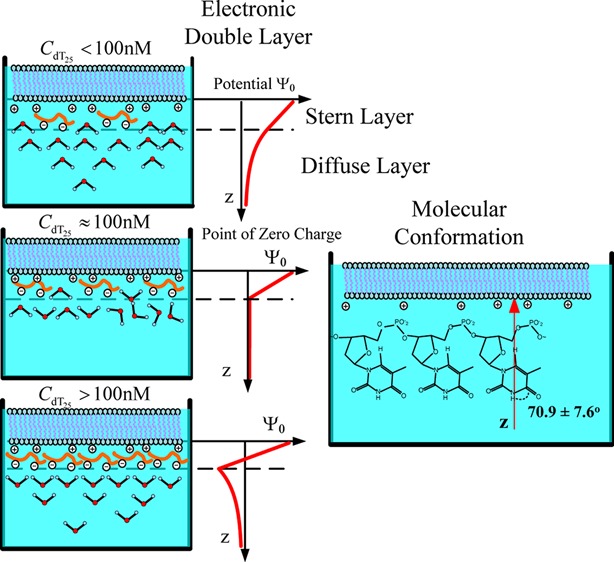
The molecular interaction between the oligonucleotides and lipid membranes is the key to the functions of virus, aptamer, and various oligonucleotide-based materials. In this study, the conformational changes of oligonucleotides (dT25) on lamellar cationic 1,2-dimyristoyl-3-trimethylammonium-propane (DMTAP) bilayer were investigated by polarization-resolved sum frequency generation vibrational spectroscopy (SFG-VS) in situ. The SFG-VS spectra within different wavenumber ranges were analyzed to give conformation details of thymine groups, phosphate groups, and OD/OH groups and to provide a comprehensive and fundamental understanding of the oligonucleotide adsorption on a model bilayer. It is shown that the adsorption of dT25 on DMTAP bilayer reaches maximum at CdT ≈ 500 nM. And the conformation of dT25 molecules change significantly when surface charge of DMTAP bilayer reaches the point of zero charge (PZC) at CdT ≈ 100 nM. Combined spectroscopic evidences also indicate that the formation of electric double layer at the DMTAP/dT25 surface follows the Gouy–Chapman–Stern model. The analysis results also show that the symmetric PO2– stretching mode of oligonucleotide molecules can serve as a sensitive vibration molecular probe for quantifying the oligonucleotide/lipid charge ratio and determine the point of zero charge (PZC) of lipid bilayer surface, which may help researchers to control the layer-by-layer assembly of oligonucleotide–lipid complexes and to improve the efficiency genetic therapy against cancer and viral infections.
1. Introduction
The complexes formed by cationic lipid membrane and oligonucleotides have attracted extensive interest because of their applications in gene delivery as nonviral carriers of genetic material into living cells.1,2 The molecular interactions (forces) which assemble the oligonucleotides and lipid bilayers into complex but ordered lipoplexes are also the key to the functions of virus, aptamer, and various oligonucleotide-based materials.2−6 The understanding of molecular interactions between lipid membrane and oligonucleotides will help researchers perform “bottom up” assemblies and build lipoplexes with equilibrium structures, which can ensure the safety and effectiveness of oligonucleotide-based materials during the gene therapy against the cancer and viral infections.
A number of experimental and theoretical studies have been conducted to comprehend the mechanism of formation of lipid bilayer–oligonucleotide complexes.1,7−9 Small-angle X-ray scattering (SAXS),1,9 stopped-flow spectrofluorometry,10−12 dynamic laser scattering (DLS),13,14 fluorescence resonance energy transfer,15−17 surface plasmon resonance,18,19 atomic force microscopy,20 cryotransmission electron microscopy,21,22 Fourier transform infrared spectroscopy, and Raman vibrational spectroscopy23−25 were applied to investigate the lipoplex structures and kinetics of lipoplex formation. Theoretical modeling and simulation were also applied to understand the molecular driving force during the DNA adsorption.26,27 In general, the oligonucleotide–lipid bilayer complex formation can be described in three steps: the accumulation of anionic oligonucleotides on the cationic bilayer, the reverse/rupture of lipid vesicles, and the formation of aggregated multilamellar structure. It has been well established that the formation of lipoplex is driven by electrostatic attraction between the oppositely charged nucleic acid and lipids. Results of DLS and SAXS experiments also show that the size and ζ potential of liposomes can be tuned by lipid composition and the cationic lipid/DNA charge ratio, which may improve the transfection efficiency of the lipoplexes.28−31 Nevertheless, due to the limitations of characterizing methods, the interface/surface specific information at the molecular level during the lipid–oligonucleotide interaction and lipoplex formation was hard to be obtained. The insights of accumulation/adsorption of oligonucleotide at molecular level are required to control the successive events of charge reversing, vesicle rupturing, and multilamellar lipoplex formation.
Sum frequency generation vibrational spectroscopy (SFG-VS) has been successfully applied as an interface-specific probe to investigate the molecular structure and kinetics at biointerfaces.32−39 Recent progresses prove that SFG-VS is also very useful for abstracting the structural and conformational information from the interfacial anchored oligonucleotides.40−47 The structure of single-stranded DNA monolayers on platinum substrate was investigated by Sartenaer et al. using SFG-VS.40 The divalent metal ion-induced deformation of DNA monolayer was also investigated by Asanuma et al. using SFG-VS and X-ray photoelectron spectroscopy.41 Ion–oligonucleotide interactions were also investigated extensively by Geiger’s group using multiple nonlinear optical techniques, such as SFG-VS and second harmonic generation.42,43 The structural differences between oligonucleotide films in air and under various aqueous situations were also characterized by Howell et al.44,45 SFG-VS was also applied to investigate the interactions between oligonucleotides and model lipid membranes. A pioneer work, which investigated the interaction between the cationic lipid monolayer and λ-phage DNA molecules by monitoring the OD signals within the wavenumber range of 2000–2700 cm–1, was carried out by Wurpel et al.46 By focusing on the SFG spectra in the wavenumber range of 1200–1800 cm–1, the interfacial conformation and G-quadruplex forming kinetics of G-rich oligonucleotides on 1,2-dimyristoyl-3-trimethylammonium-propane (DMTAP) lipid bilayers were also elucidated by Wei et al.47 In spite of these previous investigations, the conformational and kinetic properties of oligonucleotides during the adsorption/interaction remain elusive. For instance, Wurpel et al.’s results indicate significant reconstruction of surface water molecules during the DNA–lipid interaction, which can account for the Langmuir adsorption of DNA molecules.46 However, the conformation changes of oligonucleotide molecules during such adsorption are still unclear.
In the current study, the spectra of dT25–DMTAP bilayer complexes were collected by the polarization-resolved SFG-VS system in situ. The SFG spectra detected within different wavenumber regions can provide conformational details of different molecular groups, such as phosphate, thymine, and deoxyribose groups, thus facilitating the elucidation of the molecular mechanism of dT25 molecule–membrane interaction.
2. Results and Discussion
2.1. SFG Spectra in H2O and D2O and Peak Assignments
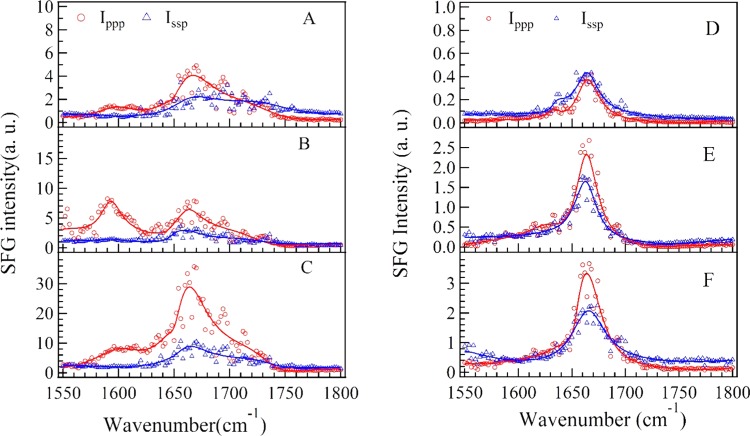
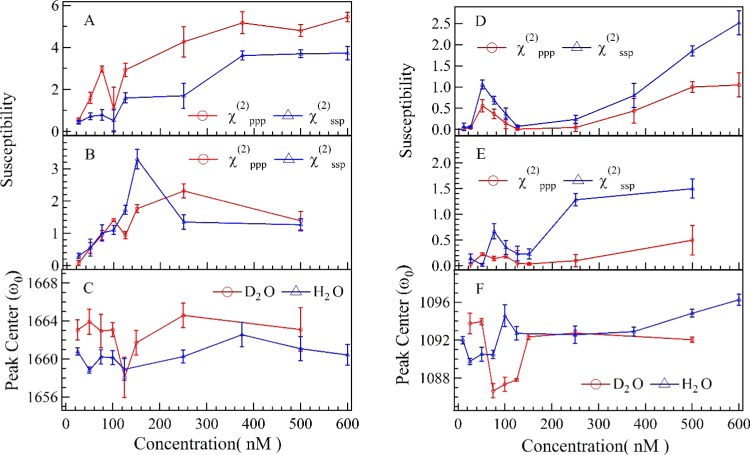
Figure 1 shows the ppp and ssp spectra in the wavenumber range of 1550–1800 cm–1 at various concentration of dT25 after the dT25–DMTAP bilayer interaction. Several characteristic peaks at ∼1600 cm–1 (NH2 bending mode of tris buffer), ∼1650 cm–1 (thymine C4=O and C5=C6 out-of-phase 180° stretching mode), ∼1660 cm–1 (thymine C4=O and C5=C6 in-phase 0° stretching mode), and ∼1730 cm–1 (thymine C2=O stretching mode) can be observed in Figure 1A–C (see Table 1 for details).48,49 As shown in Figure 3A–C, the peak intensities of C4=O and C5=C6 in-phase 0° stretching mode increase gradually as CdT increases to 250 nM due to the adsorption of dT25 molecules on DMTAP bilayers. The peak position of NH2 bending mode is significantly red-shifted to 1592 cm–1 at CdT = 100 nM (fitting parameters are listed in Table 2), which indicates that the hydrogen-bonding strength is significantly weakened.53 On the other hand, the peak width of C4=O and C5=C6 in-phase 0° stretching mode is significantly narrowed (from 22 to 12 cm–1) at CdT = 100 nM, which indicates that thymine group of dT25 molecules forms a more compacted conformation at such concentration.
Figure 1.
SFG spectra collected in the wavenumber range of 1550–1800 cm–1 at different concentrations of dT25(CdT). (A) CdT = 50 nM in H2O; (B) CdT = 100 nM in H2O; (C) CdT = 250 nM in H2O; (D) CdT = 50 nM in D2O; (E) CdT = 100 nM in D2O; (F) CdT = 100 nM in D2O.
Table 1. Spectra Assignments of dT25 Molecules.
| peaks (cm–1) | assignmentsa |
|---|---|
| ∼1060 | ribose C–C stretching |
| ∼1090 | PO2– symmetric stretching |
| ∼1150 | ribose C–O–C stretching |
| ∼1600 | tris δ-NH2 bending |
| ∼1650 | dT C4=O and C=C out-of phase |
| ∼1660 | dT C4=O and C=C in-phase |
| ∼1730 | C2=O stretching |
| ∼2350 | OD stretching of D2O |
| ∼2550 | |
| ∼2720 | |
| ∼3060 | dT C6–H stretching |
| ∼3140 | dT N3–H stretching |
| ∼3200 | OH stretching of H2O, dT, and tris molecules |
| ∼3400 | |
| ∼3600 |
Check Figure S6 for the corresponding atoms of thymidine molecule.
Figure 3.
Concentration dependence of SFG susceptibilities (|χ(2)| = |Aq/Γq) and peak center wavenumbers (ω0) of thymine groups (A–C) and PO2– groups (D–F). (A) χppp(2) and χssp of C4=O and C5=C6 in-phase 0° stretching mode in H2O solutions; (B) χppp(2) and χssp of C4=O and C5=C6 in-phase 0° stretching mode in D2O solutions; (C) ω0 of C4=O and C5=C6 in-phase 0° stretching mode in H2O and D2O solutions; (D) χppp(2) and χssp of symmetric PO2– stretching mode in H2O solutions; (E) χppp(2) and χssp of symmetric PO2– stretching mode in D2O solutions; (F) ω0 of symmetric PO2– stretching mode.
Table 2. Fitting Parameters Calculated from Figure 1A–C (H2O) and Figure 1D–F (D2O).
| 50 nM |
100 nM |
250 nM |
||||
|---|---|---|---|---|---|---|
| Ippp | Issp | Ippp | Issp | Ippp | Issp | |
| H2O | ||||||
| A0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| B0 | 0.48 | 0.84 | 1.06 | 0.73 | 1.41 | 1.42 |
| χ1 | 0.6 | –0.1 | 1.3 | 0.2 | 1.3 | –0.2 |
| ω1 | 1599.0 | 1592.2 | 1602.1 | |||
| τ1 | 22.0 | 11.6 | 25.9 | |||
| χ2 | –0.2 | 0.0 | –0.8 | 0.5 | –0.8 | –0.1 |
| ω2 | 1648.8 | 1650.8 | 1650.3 | |||
| T2 | 6.6 | 8.6 | 6.6 | |||
| χ3 | 1.6 | 0.7 | 4.3 | 0.5 | 4.3 | 1.7 |
| ω3 | 1661.1 | 1660.3 | 1660.2 | |||
| τ3 | 22.9 | 12.9 | 19.9 | |||
| χ4 | –1.4 | –2.2 | –3.3 | –1.7 | –3.3 | –3.3 |
| ω3 | 1735.6 | 1728.8 | 1734.7 | |||
| τ3 | 35.3 | 31.8 | 24.7 | |||
| χNR | –4.46 | 1.87 | 2.63 | 3.93 | 5.63 | 8.02 |
| D2O | ||||||
| A0 | –0.08 | 0.03 | –0.07 | –0.01 | 0.00 | –0.04 |
| B0 | 0.19 | 0.27 | 0.43 | 0.43 | –0.40 | –0.20 |
| χ1 | –0.2 | –3.1 | 7.5 | 0.3 | –20.3 | 0.0 |
| ω1 | 1635.0 | 1630.8 | 1653.1 | |||
| τ1 | 7.4 | 22.1 | 8.5 | |||
| χ2 | 1.0 | 0.8 | 1.7 | 1.7 | 0.9 | |
| ω2 | 1663.9 | 1663.1 | 1664.6 | |||
| T2 | 12.8 | 12.6 | 18.5 | |||
| χ3 | 1.0 | 0.2 | 1.4 | –3.8 | 1.4 | 3.3 |
| ω3 | 1759.7 | 1750.0 | 1804.3 | |||
| τ3 | 113.7 | 50.6 | 54.2 | |||
| χNR | 0.00 | 0.04 | –0.23 | 0.05 | 0.08 | 0.32 |
As to the dT25 molecules in D2O solution, the intensities of SFG spectra shown in Figure 1D,F are only 1/10 of those shown in Figure 1A,C. The peak intensities of thymine C2=O stretching mode may decrease significantly due to the weaker hydrogen-bonding strength in D2O solution.49 The peak intensity of tris buffer NH2 bending mode and thymine C2=O stretching mode are not clearly shown in Figure 1D–F. The frequency of NH2 bending is usually red-shifted (out of this wavenumber range) because the NH2 group was replaced by the ND2 group in D2O solution.50 The peak widths (9–18 cm–1) of C4=O and C5=C6 in-phase 0° stretching mode shown in Figure 3D–F are much lower than the peak widths (19–27 cm–1) shown in Figure 3A–C (fitting parameters are listed in Table 3). Such spectra characteristics indicate that thymine groups of dT25 oligonucleotide chain in D2O solution may adopt a more compacted conformation comparing to the thymine groups in H2O solution. Such difference in DNA conformations may be induced by the weaker hydrogen-bonding strength in D2O solution comparing to that in H2O solution due to the isotope effect. It has been reported that hydrogen-bonding environment is very essential for maintaining the oligonucleotide conformations: the stronger hydrogen-bonding environment will enhance the ordering of the nucleotide groups, and the weakening of hydrogen-bonding environment will lead to a collapsed conformation of oligonucleotides.50,51 Previous electron microscopy experiments also indicate that the thermal denaturation temperature of DNA molecules in H2O solution is slightly lower than that in D2O solution.52
Table 3. Fitting Parameters Calculated from Figure 2A–C (H2O) and Figure 2D–F (D2O).
| 50 nM |
100 nM |
250 nM |
||||
|---|---|---|---|---|---|---|
| Ippp | Issp | Ippp | Issp | Ippp | Issp | |
| H2O | ||||||
| A0 | –0.26 | 0.22 | 0.92 | –0.11 | –0.21 | 0.20 |
| B0 | 0.14 | –0.31 | –1.11 | –0.05 | 0.27 | 0.36 |
| χ1 | –0.38 | 0.35 | 0.52 | 0.15 | 0.19 | 0.00 |
| ω1 | 1069.1 | 1057.1 | 1055.9 | |||
| τ1 | 4.8 | 28.0 | 19.4 | |||
| χ2 | –0.56 | 1.06 | 0.15 | –0.36 | –0.05 | 0.24 |
| ω2 | 1090.5 | 1094.6 | 1092.6 | |||
| T2 | 9.1 | 9.1 | 9.1 | |||
| χ3 | 0.62 | –0.11 | ||||
| ω3 | 1156.1 | |||||
| τ3 | 54.1 | |||||
| χNR | –0.56 | 0.00 | –0.08 | 0.02 | –0.10 | –0.03 |
| D2O | ||||||
| A0 | 0.13 | 0.19 | –0.10 | –0.10 | –0.03 | –0.18 |
| B0 | 0.01 | –0.10 | –0.05 | –0.35 | 0.00 | –0.03 |
| χ1 | 0.22 | –0.02 | 0.31 | 1.04 | 0.46 | 0.64 |
| ω1 | 1045.1 | 1059.8 | 1057.5 | |||
| τ1 | 6.7 | 6.9 | 10.1 | |||
| χ2 | 0.23 | 0.61 | –0.19 | –0.36 | 0.10 | –1.28 |
| ω2 | 1094.0 | 1087.4 | 1092.8 | |||
| T2 | 12.6 | 10.2 | 10.7 | |||
| χ3 | 0.10 | 0.17 | 0.04 | –0.04 | –0.04 | –0.13 |
| ω3 | 1154.9 | 1134.2 | 1152.1 | |||
| τ3 | 6.0 | 11.3 | 6.7 | |||
| χNR | 0.02 | 0.00 | –0.01 | –0.12 | 0.04 | –0.01 |
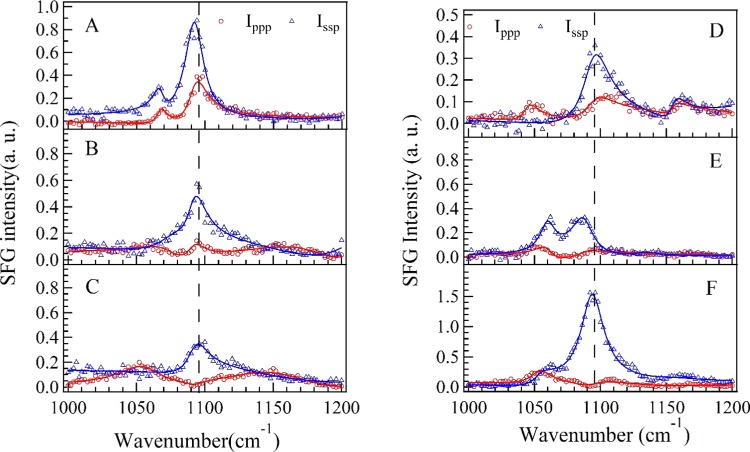
Figure 2 shows the Ippp and Issp spectra in the wavenumber range of 1000–1200 cm–1 at various concentrations of dT25 after dT25–DMTAP bilayers interaction. As seen in Figure 2, several characteristic peaks at ∼1060 cm–1 (deoxyribose C–C stretching), ∼1090 cm–1 (symmetric PO2– stretching), and ∼1150 cm–1 (deoxyribose C–O–C stretching) are shown in Figure 2A–F.53
Figure 2.
SFG spectra of DMTAP bilayers in the wavenumber range of 1000–1200 cm–1 at different CdT. (A) CdT = 50 nM in H2O; (B) CdT = 100 nM in H2O; (C) CdT = 250 nM in H2O; (D) CdT = 50 nM in D2O; (E) CdT = 100 nM in D2O; (F) CdT = 250 nM in D2O.
The peak position of symmetric PO2– stretching mode in H2O solution is significantly red-shifted at CdT = 50 nM, and the peak position of symmetric PO2– stretching mode in D2O solution is significantly red-shifted at CdT = 100 nM. The peak width of symmetric PO2– stretching mode in H2O solution is slightly smaller than that in D2O solution. However, the changes of the peak amplitudes of deoxyribose C–C stretching and deoxyribose C–O–C stretching seem unpatented (shown in Figures S2 and S4).
Figure 3 shows the susceptibilities (|χ(2)| = |Aq/Γq|) and center wavenumbers (ω0,q) fitted from the SFG spectra in different wavenumber ranges (Figures S1–S4). As shown in Figure 3A,B, the susceptibilities (χppp(2) and χssp) of thymine groups increase as CdT increases and reach a maximum value at CdT = 600 nM in H2O solution and CdT = 500 nM in D2O solution. As to the PO2– groups (Figure 3D,E), the curves of susceptibilities in H2O solution and D2O solution show a significant increase at CdT ≈ 100 nM, which indicates that ordering or surface abundance of PO2– groups is improved at such concentration. However, the values of χppp,PO2–(2) and χssp,PO2– are showing ongoing increases at CdT = 600 and 500 nM. On the other hand, it is also easy to note that the peaks of thymine C4=O and C5=C6 in-phase 0° stretching mode and symmetric PO2– stretching mode at CdT ≈ 100 nM (Figure 3C,F) in both D2O solution and H2O solution are significantly red-shifted comparing to those observed at other concentrations. The fitted parameters indicate that the peak position of thymine C4=O and C5=C6 in-phase 0° stretching (ω0Thymine) is red-shifted ∼4 cm–1 in H2O and ∼7 cm–1 in D2O at CdT = 100 nM. And the peak position of symmetric PO2– stretching (ω0) is red-shifted ∼3 cm–1 in H2O and ∼6 cm–1 in D2O at CdT = 75 nM. Such red shifts of both peaks indicate that local environments around the PO2– groups and thymine groups at CdT ≈ 100 nM are very different from the local environments at other concentrations.53
2.2. Adsorption Model for dT25 Molecules
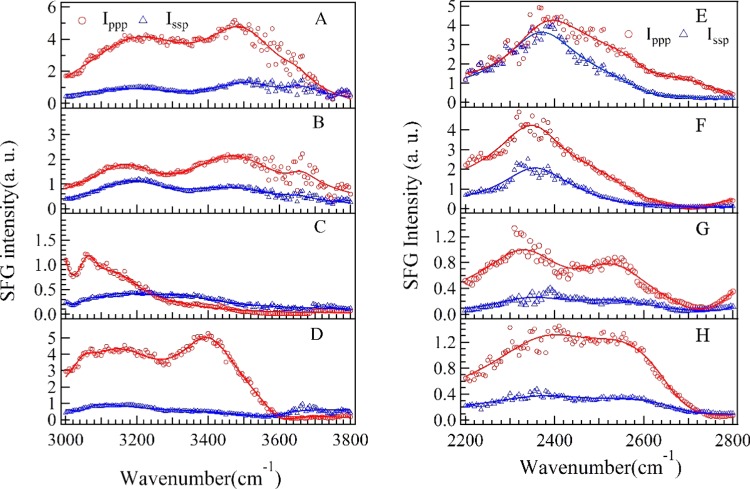
To understand the influence of local environment changes at different CdT values and to clarify the IR assignment, the SFG spectra of both OH stretching modes and OD stretching modes were collected (shown in Figure 4). The concentration dependence of effective polarizabilities of OD stretching (|POD(2)| = |Assp,OD/Γ|) is shown in Figure 6. The peaks at ∼3200, ∼3400, and ∼3650 cm–1, originated from the OH stretching modes of surface H2O molecules,54,55 are shown in the wavenumber range of 3000–3800 cm–1 (Figure 4A). The peaks at ∼2350, ∼2550, and ∼2720 cm–1, originated from the OD stretching modes of surface D2O molecules,56−58 are shown in the wavenumber range of 2200–2800 cm–1 (Figure 4B). SFG-VS intensities of OH stretching modes and OD stretching modes can be used as indicators of surface charge density and hydrogen-bonding strength (function S1 in the Supporting Information). The spectra intensities of OH stretching modes show a drop-then-rise trend with a minimum value at CdT = 100 nM, and the spectra intensities of OD stretching modes show a minimum value at CdT = 75 nM (Figure 5B). As shown in Figure 5A, the peak width of OD stretching mode is slightly increased at CdT = 75 nM. The SFG-VS signals generated from the charged surface have contributions from both the second-order nonlinear susceptibilities χeff (interface contribution) and third-order nonlinear susceptibilities χeff(3) (bulk contribution).59,60 It should be noted that the surface charge can increase not only the molecular ordering of H2O/D2O molecules, which will increase the interface contribution, but also the surface potential, which will increase the bulk contribution. And the signs of both terms in function S1 are determined by the sign of surface charge (σSum = σ0,DMTAP + σdT25). Thus, despite the difficulties in quantifying the contribution form the interface and the bulk, combined spectroscopic evidences in OD spectra and OH spectra indicate that DMTAP bilayer surface reaches a point of zero charge (PZC) at CdT ≈ 100 nM.61−63
Figure 4.
Left: SFG spectra in the wavenumber range of 2800–3800 cm–1 at different concentrations of dT25/H2O solution; (A) CdT = 0 nM (bilayer only); (B) CdT = 25 nM; (C) CdT = 100 nM; (D) CdT = 250 nM. Right: SFG spectra in the wavenumber range of 2000–2800 cm–1 at different concentrations of dT25/D2O solution; (E) CdT = 0 nM (bilayer only); (F) CdT = 25 nM; (G) CdT = 75 nM; (H) CdT = 250 nM.
Figure 6.
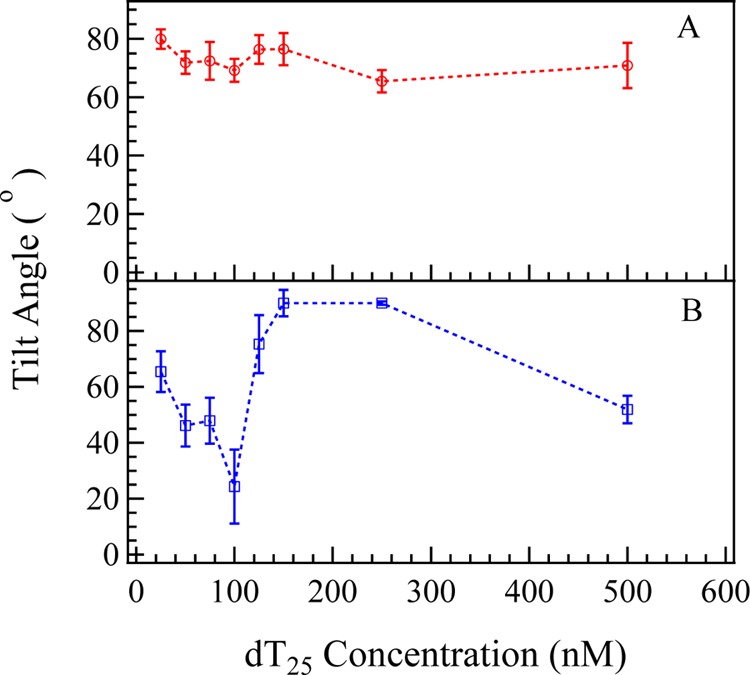
Concentration dependence of calculated tilt angle of (A) in-phase C4=O and C5=C6 stretching mode at ∼1660 cm–1 (θD2OThymine) and (B) symmetric PO2– stretching at ∼1090 cm–1 (θD2O) in D2O solution.
Figure 5.
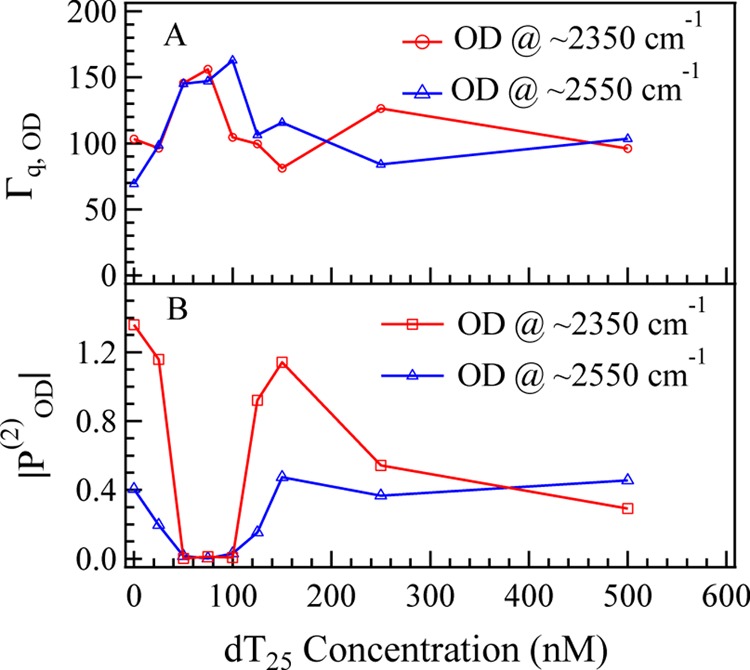
Concentration dependence of (A) peak widths (Γq) and (B) effective polarizabilities of OD stretching (|POD(2)| = |Assp,OD/Γ|).
It is also interesting to see that the adsorption of dT25 molecules continues after the surface reaches PZC at CdT ≈ 100 nM. A condensed layer of negatively charged electrolytes can be formed by overcoming repulsive electrostatic forces during the adsorption of dT25 molecules at high concentration (CdT > 100 nM), which is the typical formation of electric double layer of the Gouy–Chapman–Stern model.64,65 These newest experimental results indicate that even with the oligonucleotides without conjugated hydrophobic molecular groups, the electrostatic force between the negatively charged oligonucleotide and the positively charged DMTAP bilayer is not the only driven force during the oligonucleotide adsorption. Such conclusion coincides with experimental results of the alkyl chain conjugated oligonucleotides in previous literature works.47
2.3. Conformation of dT25 Molecules
Orientation analyses were also performed based on the Raman polarizabilities reported in the literature.66,67Figure 6A,B shows the calculated tilt angle of in-phase C4=O and C5=C6 stretching mode at ∼1660 cm–1 and symmetric PO2– at ∼1090 cm–1 at various concentrations. As seen in Figure 6A, the tilt angle of thymine groups in D2O (θD2OThymine) fluctuates slightly and stays around 70° when CdT increases from 25 to 500 nM. The final average tilt angle of thymine groups is 70.9 ± 7.6° at CdT = 100 nM. Such trend of θD2O indicates that the tilt angle of thymine groups are insensitive to the changes of surface charge density and surface electrostatic force field. The tilt angle of the symmetric PO2– stretching mode in D2O solution (θD2OPO2–) decreases significantly at CdT = 50 and 100 nM. The decrease of tilt angle and the significant increase of χppp and χssp(2) (shown in Figure 3E) indicate that the molecular ordering of PO2– groups is greatly improved at CdT ≈ 100 nM.
Both red shifts of symmetric PO2– stretching
mode and enhanced ordering of PO2– groups
indicate that the binding affinity between DMTAP and dT25 molecules is greatly improved at PZC. Such improved binding affinity
may be induced by the significant decrease of electrolyte concentration
and ion screening effects around the PO2– groups (in dT25 molecules) and NH3+ groups (in DMTAP molecules) at PZC.68,69 As estimated
by Levinson et
al.,53 the PO2– symmetric stretching frequency can be tuned by the interaction strength
(of various chemical environments) along the C2 axis with a rate of 0.40 cm–1 (MV/cm) via
linear Strak effects. The changes of interaction strength ΔF during the dT25–DMTAP bilayer interaction
in H2O and D2O are estimated to be 7.5 ±
1.9 and 15.1 ± 2.4 MV/cm, respectively, 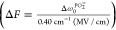 . Thus, the lowest point of ω0PO2– curve can be a mark
of PZC point of the DMTAP bilayer surface due to the strongest intermolecular
interaction at PZC, where the oligonucleotide/lipid charge ratio =
1:1. And the PO2– groups of oligonucleotide
molecule can act as a sensitive vibration molecular probe68,69 for quantifying the oligonucleotide/lipid charge ratio and determining
the point of zero charge (PZC) of lipid bilayer surface.
. Thus, the lowest point of ω0PO2– curve can be a mark
of PZC point of the DMTAP bilayer surface due to the strongest intermolecular
interaction at PZC, where the oligonucleotide/lipid charge ratio =
1:1. And the PO2– groups of oligonucleotide
molecule can act as a sensitive vibration molecular probe68,69 for quantifying the oligonucleotide/lipid charge ratio and determining
the point of zero charge (PZC) of lipid bilayer surface.
3. Summary
In this study, adsorption structure and conformation of oligonucleotide (dT25) molecules were investigated by SFG-VS in situ. The SFG-VS spectra within different wavenumber ranges were analyzed to provide conformation details of thymine groups, phosphate groups, and OD/OH groups and to provide a comprehensive and fundamental understanding of the oligonucleotide adsorption on a model bilayer. Combined spectroscopic evidences also indicate that the formation of an electric double layer on the DMTAP/dT25 surface follows the Gouy–Chapman–Stern model. And the conformation of dT25 molecules changed significantly when the DMTAP bilayer reaches a point of zero charge (PZC) at CdT ≈ 100 nM. On the basis of the orientation analysis, the final average tilt angle of thymine groups is 70.9 ± 7.6°. These results demonstrated that the vibrational spectra collected by in situ label-free polarization-resolved SFG-VS detections can provide new molecular insights into the mechanisms of oligonucleotide–membrane interaction.
Our analysis results also show that the symmetric PO2– stretching mode of oligonucleotide molecules can serve as a sensitive vibration molecular probe to quantify the oligonucleotide/lipid charge ratio and determine the point of zero charge (PZC) of lipid bilayer surface, which may help the researcher to control the layer-by-layer assembly of oligonucleotide–lipid complexes and improve the efficiency of genetic therapy against cancer and viral infections.70,71
4. Experimental Section
Cationic lipid 1,2-dimyristoyl-3-trimethylammonium-propane (DMTAP, purity > 99%, purchased from Avanti Polar Lipids, Inc.) was used to build solid-supported lamellar lipid bilayers. The DMTAP monolayers were deposited on CaF2 prisms at a surface pressure of 33 mN/m (∼50 Å2/mol) via the Langmuir–Blodgett method. The CaF2 prisms with DMTAP monolayer were slowly dehydrated overnight to remove the residue water and improve the bilayer stability. The second DMTAP monolayer was also deposited on CaF2 prisms at a similar surface pressure (30–33 mN/m) via the Langmuir–Schaefer method to form prism-supported lamellar DMTAP bilayers. The detailed preparation processes can be found in previous papers.72−74 The lamellar DMTAP bilayers prepared via the Langmuir–Blodgett and Langmuir–Schaefer methods are highly controllable and repeatable, which can be hardly achieved by the liposomes prepared in solutions. Such bilayer can be used as an ideal model system to investigate the oligonucleotide–membrane interaction.
After the preparation, the lipid bilayers were immersed in a small reservoir with a volume of 2.5 mL. The dT25 oligonucleotide molecules (purity > 98% by HPLC, Takara Bio, Inc., Dalian) were dissolved in 10 mM tris buffer solution (pH ≈ 7.4) with a concentration of 10 nmol/mL (μM). The dT25–tris solution was added to the reservoir with a microsyringe to interact with the DMTAP bilayers.
All of the SFG-VS measurements were performed on a picosecond sum frequency generation system purchased from EKSPLA, Lithuania. The optical arrangements and programs were custom-modified to perform polarization-resolved detections (see Scheme 1 for details).75,76 Briefly, the polarization angle of visible beam (Ωvis) was switched between 0 and 90° by a rotational motorized half-wave plate (HWP-M) at each wavenumber point during the IR scanning SFG spectra collection. The SFG signals (the ssp and psp signals at Ωvis = 0°, the ppp and spp signals at Ωvis = 90°, where the first letter s/p stands for the polarization state of SFG signal, the second letter stands for the polarization state of visible light, and the third letter stands for the polarization state of IR light) were separated by a polarization splitter (PS). The separated SFG signals passed through Glan polarizers (GP1, GP2), half-wave plates (HWP1, HWP2), notch filters (F1, F2), and focusing lenses (L1, L2) separately and then entered two sets of identical detection systems (Monochromator 3501/3504, SOLAR TII and PMT R7899, Hamamatsu). The SFG-VS spectra within the wavenumber ranges of 1000–1200, 1550–1800, and 3000–3800 cm–1 (2200–2800 cm–1 for D2O solutions) were collected after the sample reached equilibrium. The incident angles of visible beam and IR beam are 63° and 52°, respectively. Over 150 pulses were averaged for each wavelength point in the SFG-VS spectra. All of the SFG intensities shown in the figures below were normalized by the energy of visible beam Ivis and IR beam IIR. Half of the path of IR beam was purged by N2 gas to decrease the absorbing effects of atmospheric H2O and CO2 molecules. The spectra within the wavenumber range of 2200–2800 cm–1 were also normalized to the SFG spectra of z-cut quartz to remove the IR absorbance of atmospheric CO2.
Scheme 1. Experimental Setup of Polarization-Resolved SFG-VS Detection.
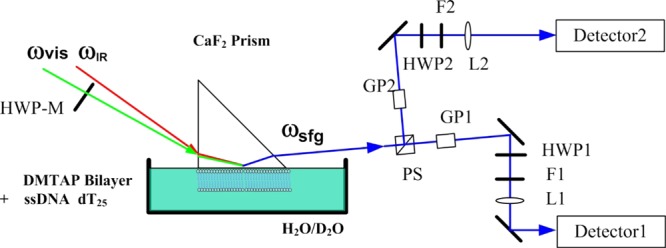
PS: polarization splitter, GP: Glan laser polarizer, HWP: half-wave plate, HWP-M: motorized half-wave plate; F: filter, L: lens.
Acknowledgments
This work was supported by the China National Natural Science Foundation (NSF) (Nos. 21503093 and 61575085) and the Doctorial Program of Jianghan University (No. 1019-0610001). Dr. W.L. acknowledges the support from NSF of China (No. 21606103). Dr. Cao acknowledges the support from China NSF (No. 51703081), 4th Yellow Crane Talent Programme of Wuhan City (No. 08010004), and National Science and Technology Major Project (No. 2016YFB0401505). The Guassian calculations were carried out at the supercomputing center of the University of Science and Technology of China.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01214.
Details about the molecular structure of thymidine and tilt angle analysis procedures of SFG data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rädler J. O.; et al. Structure of DNA-Cationic Liposome Complexes: DNA Intercalation in Multilamellar Membranes in Distinct Interhelical Packing Regimes. Science 1997, 275, 810–814. 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- Braun C. S.; Jas G. S.; Choosakoonkriang S.; Koe G. S.; Smith J. G.; Middaugh C. R. The structure of DNA within cationic lipid/DNA complexes. Biophys. J. 2003, 84, 1114–1123. 10.1016/S0006-3495(03)74927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montis C.; Sostegni S.; Milani S.; Baglioni P.; Berti D. Biocompatible cationic lipids for the formulation of liposomal DNA vectors. Soft Matter 2014, 10, 4287–4297. 10.1039/C4SM00142G. [DOI] [PubMed] [Google Scholar]
- Ahmed S.; Savarala S.; Chen Y.; Bothun G.; Wunder S. L. Formation of lipid sheaths around nanoparticle-supported lipid bilayers. Small 2012, 8, 1740–1751. 10.1002/smll.201101833. [DOI] [PubMed] [Google Scholar]
- Tu R. S.; Tirrell M. Bottom-up design of biomimetic assemblies. Adv. Drug Delivery Rev. 2004, 56, 1537–1563. 10.1016/j.addr.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Unzueta U.; Saccardo P.; Domingo-Espín J.; Cedano J.; Conchillo-Solé O.; García-Fruitós E.; Céspedes M. V.; Corchero J. L.; Daura X.; Mangues R.; Ferrer-Miralles N.; Villaverde A.; Vázquez E. Sheltering DNA in self-organizing, protein-only nano-shells as artificial viruses for gene delivery. Nanomedicine 2014, 10, 535–541. 10.1016/j.nano.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Montis C.; Milani S.; Berti D.; Baglioni P. Complexes of nucleolipid liposomes with single-stranded and double-stranded nucleic acids. J. Colloid Interface Sci. 2012, 373, 57–68. 10.1016/j.jcis.2011.10.058. [DOI] [PubMed] [Google Scholar]
- Dan N.; Danino D. Structure and kinetics of lipid–nucleic acid complexes. Adv. Colloid Interface Sci. 2014, 205, 230–239. 10.1016/j.cis.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Koynova R.; Wang L.; Macdonald R. C. An Intracellular Lamellar-nonlamellar Phase Transition Rationalizes the Superior Performance of some Cationic Lipid Transfection Agents. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 14373–14378. 10.1073/pnas.0603085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreleiro P. C. A.; May R. P.; Lindman B. Mechanism of formation of DNA–cationic vesicle complexes. Faraday Discuss. 2003, 122, 191–201. 10.1039/B200796G. [DOI] [PubMed] [Google Scholar]
- Barreleiro P. C. A.; Lindman B. The Kinetics of DNA–Cationic Vesicle Complex Formation. J. Phys. Chem. B 2003, 107, 6208–6213. 10.1021/jp0277107. [DOI] [Google Scholar]
- Zhang Y.; Garzon-Rodriguez W.; Manning M. C.; Anchordoquy T. J. The use of fluorescence resonance energy transfer to monitor dynamic changes of lipid–DNA interactions during lipoplex formation. Biochim. Biophys. Acta, Biomembr. 2003, 1614, 182–192. 10.1016/S0005-2736(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Lai E.; van Zanten J. H. Real time monitoring of lipoplex molar mass, size and density. J. Controlled Release 2002, 82, 149–158. 10.1016/S0168-3659(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Lai E.; van Zanten J. H. Monitoring DNA/Poly-l-Lysine Polyplex Formation with Time-Resolved Multiangle Laser Light Scattering. Biophys. J. 2001, 80, 864–873. 10.1016/S0006-3495(01)76065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Garzon-Rodriguez W.; Manning M. C.; Anchordoquy T. J. The use of fluorescence resonance energy transfer to monitor dynamic changes of lipid–DNA interactions during lipoplex formation. Biochim. Biophys. Acta, Biomembr. 2003, 1614, 182–192. 10.1016/S0005-2736(03)00177-9. [DOI] [PubMed] [Google Scholar]
- Madeira C.; Loura L. M.; Aires-Barros M. R.; Fedorov A.; Prieto M. Characterization of DNA/lipid complexes by fluorescence resonance energy transfer. Biophys. J. 2003, 85, 3106–3119. 10.1016/S0006-3495(03)74729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozharski E. Fluorescence Resonance Energy Transfer-based Analysis of Lipoplexes. Methods Mol. Biol. 2010, 606, 393–398. 10.1007/978-1-60761-447-0_27. [DOI] [PubMed] [Google Scholar]
- Li A.; Sun Z.; Ma Y.; Yang F.; Ouyang H.; Yang X. The Interaction of Recombinant Pseudomonas Aeruginosa Exotoxin A with DNA and Mimic Biomembrane Investigated by Surface Plasmon Resonance and Electrochemical Methods. Talanta 2006, 70, 330–335. 10.1016/j.talanta.2006.02.056. [DOI] [PubMed] [Google Scholar]
- Zhdanov R. I.; Strazhevskaya N. B.; Jdanov A. R.; Bischoff G. A Spectroscopic and Surface Plasmon Resonance Study of Oleic Acid/DNA Complexes. J. Biomol. Struct. Dyn. 2002, 20, 231–242. 10.1080/07391102.2002.10506839. [DOI] [PubMed] [Google Scholar]
- Huebner S.; Battersby B. J.; Grimm R.; Cevc G. Lipid-DNA Complex Formation: Reorganization and Rupture of Lipid Vesicles in the Presence of DNA As Observed by Cryoelectron Microscopy. Biophys. J. 1999, 76, 3158–3166. 10.1016/S0006-3495(99)77467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby B. J.; Grimm R.; Huebner S.; Cevc G. Evidence for three-dimensional interlayer correlations in cationic lipid-DNA complexes as observed by cryo-electron microscopy. Biochim. Biophys. Acta, Biomembr. 1998, 1372, 379–383. 10.1016/S0005-2736(98)00062-5. [DOI] [PubMed] [Google Scholar]
- Choosakoonkriang S.; Wiethoff C. M.; Anchordoquy T. J.; Koe G. S.; Smith J. G.; Middaugh C. R. Infrared Spectroscopic Characterization of the Interaction of Cationic Lipids with Plasmid DNA. J. Biol. Chem. 2001, 276, 8037–8043. 10.1074/jbc.M010592200. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Kang X.; Li R.; Du X.; Shang Y.; Liu H.; Hu Y. Structure of the Complex Monolayer of Gemini Surfactant and DNA at the Air/Water Interface. Langmuir 2012, 28, 3429–3438. 10.1021/la204089u. [DOI] [PubMed] [Google Scholar]
- Benevides J. M.; Overman S. A.; Thomas G. J. Raman, Polarized Raman and Ultraviolet Resonance Raman Spectroscopy of Nucleic Acids and Their Complexes. J. Raman Spectrosc. 2005, 36, 279–299. 10.1002/jrs.1324. [DOI] [Google Scholar]
- Oberle V.; Bakowsky U.; Zuhorn I. S.; Hoekstra D. Lipoplex formation under equilibrium conditions reveals a three-step mechanism. Biophys. J. 2000, 79, 1447–1454. 10.1016/S0006-3495(00)76396-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antipina A. Y.; Gurtovenko A. A. Molecular Mechanism of Calcium–Induced Adsorption of DNA on Zwitterionic Phospholipid Membranes. J. Phys. Chem. B 2015, 119, 6638–6645. 10.1021/acs.jpcb.5b01256. [DOI] [PubMed] [Google Scholar]
- Sushko M. L.; Shluger A. L. DLVO theory for like-charged polyelectrolyte and surface interactions. Mater. Sci. Eng., C 2007, 27, 1090–1095. 10.1016/j.msec.2006.09.017. [DOI] [Google Scholar]
- Reimer D. L.; Kong S.; Bally M. B. Analysis of Cationic Liposome-mediated Interactions of Plasmid DNA with Murine and Human Melanoma Cells in Vitro. J. Biol. Chem. 1997, 272, 19480–19487. 10.1074/jbc.272.31.19480. [DOI] [PubMed] [Google Scholar]
- Pires P.; Simões S.; Nir S.; Gaspar R.; Düzgünes N.; Pedroso de Lima M. C. Interaction of cationic liposomes and their DNA complexes with monocytic leukemia cells. Biochim. Biophys. Acta, Biomembr. 1999, 1418, 71–84. 10.1016/S0005-2736(99)00023-1. [DOI] [PubMed] [Google Scholar]
- Pozzi D.; Marchini C.; Cardarelli F.; Amenitsch H.; Garulli C.; Bifone A.; Caracciolo G. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim. Biophys. Acta, Biomembr. 2012, 1818, 2335–2343. 10.1016/j.bbamem.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Henriques A. M.; Madeira C.; Fevereiro M.; Prazeres D. M. F.; Aires-Barros M. R.; Monteiro G. A. Effect of cationic liposomes/DNA charge ratio on gene expression and antibody response of a candidate DNA vaccine against Maedi Visna virus. Int. J. Pharm. 2009, 377, 92–98. 10.1016/j.ijpharm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Shen Y. R.The Principles of Nonlinear Optics, 1st ed.; Wiley: New York, 1984. [Google Scholar]
- Ye S.; Wei F.; Li H.; Tian K.; Luo Y. Structure and Orientation of Interfacial Proteins Determined by Sum Frequency Generation Vibrational Spectroscopy: Method and Application. Adv. Protein Chem. Struct. Biol. 2013, 93, 213–255. 10.1016/B978-0-12-416596-0.00007-5. [DOI] [PubMed] [Google Scholar]
- Liu J.; Conboy J. C. Direct Measurement of the Transbilayer Movement of Phospholipids by Sum Frequency Vibrational Spectroscopy. J. Am. Chem. Soc. 2004, 126, 8376–8377. 10.1021/ja048245p. [DOI] [PubMed] [Google Scholar]
- Wang H. F.; Gan W.; Lu R.; Rao Y.; Wu B. H. Quantitative Spectral and Orientational Analysis in Surface Sum Frequency Generation Vibrational Spectroscopy (SFG-VS). Int. Rev. Phys. Chem. 2005, 24, 191–256. 10.1080/01442350500225894. [DOI] [Google Scholar]
- Castellana E. T.; Cremer P. S. Solid Supported Lipid Bilayers: From Biophysical Studies to Sensor Design. Surf. Sci. Rep. 2006, 61, 429–444. 10.1016/j.surfrep.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. G.; Yang P.; Zhang C.; Li B.; Han X.; Song M.; Chen Z. Molecular interactions between amantadine and model cell membranes. Langmuir 2014, 30, 8491–4899. 10.1021/la501718n. [DOI] [PubMed] [Google Scholar]
- Wu F. G.; Yang P.; Zhang C.; Han X.; Song M.; Chen Z. Investigation of Drug–Model Cell Membrane Interactions Using Sum Frequency Generation Vibrational Spectroscopy: A Case Study of Chlorpromazine. J. Phys. Chem. C 2014, 118, 17538–17548. 10.1021/jp503038m. [DOI] [Google Scholar]
- Li B.; Wang H.-Y.; Feng P.; Han X.; Chen Z.; Lu X.; Wu F. G. Qualitative and Quantitative Analyses of the Molecular-Level Interaction between Memantine and Model Cell Membranes. J. Phys. Chem. C 2015, 119, 17074–17083. 10.1021/acs.jpcc.5b05747. [DOI] [Google Scholar]
- Sartenaer Y.; Tourillon G.; Dreesen L.; Lis D.; Mani A. A.; Thiry P. A.; Peremans A. Sum-Frequency Generation Spectroscopy of DNA Monolayers. Biosens. Bioelectron. 2007, 22, 2179–2183. 10.1016/j.bios.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Asanuma H.; Noguchi H.; Uosalki K.; Yu H. Z. Metal Cation-induced Deformation of DNA Self-assembled Monolayers on Silicon: Vibrational Sum Frequency Generation Spectroscopy. J. Am. Chem. Soc. 2008, 130, 8016–8022. 10.1021/ja801023r. [DOI] [PubMed] [Google Scholar]
- Chen E. H.; Walter S. R.; Nguyen S. T.; Geiger F. M. Arylsilanated SiOx Surfaces for Mild and Simple Two-Step Click Functionalization with Small Molecules and Oligonucleotides. J. Phys. Chem. C 2012, 116, 19886–19892. 10.1021/jp306437b. [DOI] [Google Scholar]
- Walter S. R.; Geiger F. M. DNA on Stage: Showcasing Oligonucleotides at Surfaces and Interfaces with Second Harmonic and Vibrational Sum Frequency Generation. J. Phys. Chem. Lett. 2010, 1, 9–15. 10.1021/jz9001086. [DOI] [Google Scholar]
- Howell C.; Schmidt R.; Kurz V.; Koelsch P. Sum-Frequency-Generation Spectroscopy of DNA Films in Air and Aqueous Environments. Biointerphases 2008, 3, FC47–FC51. 10.1116/1.3064107. [DOI] [PubMed] [Google Scholar]
- Howell C.; Zhao J.; Koelsch P.; Zharnikov M. Hybridization in SSDNA Films—A Multi-Technique Spectroscopy Study. Phys. Chem. Chem. Phys. 2011, 13, 15512–15522. 10.1039/c1cp20374f. [DOI] [PubMed] [Google Scholar]
- Wurpel G. W. H.; Sovago M.; Bonn M. Sensitive Probing of DNA Binding to a Cationic Lipid Monolayer. J. Am. Chem. Soc. 2007, 129, 8420–8421. 10.1021/ja072552o. [DOI] [PubMed] [Google Scholar]
- Wei F.; Tian K.; Zheng W. Interfacial Structure and Transformation of Guanine-Rich Oligonucleotides on Solid Supported Lipid Bilayer Investigated by Sum Frequency Generation Vibrational Spectroscopy. J. Phys. Chem. C 2015, 119, 27038–27044. 10.1021/acs.jpcc.5b08747. [DOI] [Google Scholar]
- Zhang S. L.; Michaelian K. H.; Loppnow G. R. Vibrational Spectra and Experimental Assignments of Thymine and Nine of Its Isotopomers. J. Phys. Chem. A 1998, 102, 461–470. 10.1021/jp972385m. [DOI] [Google Scholar]
- Weng G.; Chen C. X.; Balogh-Nair V.; Callender R.; Manor D. Hydrogen bond interactions of G proteins with the guanine ring moiety of guanine nucleotides. Protein Sci. 1994, 3, 22–29. 10.1002/pro.5560030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner S.; Čuma M. Relative Stability of Hydrogen and Deuterium Bonds. J. Am. Chem. Soc. 1996, 118, 1511–1521. 10.1021/ja9530376. [DOI] [Google Scholar]
- Lucazeau G.; Guemas L.; Novak A. Vibrational spectra and structure of K2Zn(NH2)4 and Rb2(NH2)4 amides. Inorg. Chim. Acta 1976, 20, 11–18. 10.1016/S0020-1693(00)94083-0. [DOI] [Google Scholar]
- Izzo V.; Fornili S. L.; Cordone L. Thermal denaturation of B. subtilis DNA in H2O and D2O observed by electron microscopy. Nucleic Acids Res. 1975, 2, 1805–1810. 10.1093/nar/2.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson N. M.; Bolte E. E.; Miller C. S.; Corcelli S. A.; Boxer S. G. Phosphate vibrations probe local electric fields and hydration in biomolecules. J. Am. Chem. Soc. 2011, 133, 13236–13239. 10.1021/ja2042589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W.; Wu D.; Zhang Z.; Feng R. R.; Wang H. F. Polarization and experimental configuration analyses of sum frequency generation vibrational spectra, structure, and orientational motion of the air/water interface. J. Chem. Phys. 2006, 124, 114705 10.1063/1.2179794. [DOI] [PubMed] [Google Scholar]
- Gan W.; Wu D.; Zhang Z.; Guo Y.; Wang H. F. Orientation and motion of water molecules at air/water interface. Chin. J. Chem. Phys. 2006, 19, 20–24. 10.1360/cjcp2006.19(1).20.5. [DOI] [Google Scholar]
- Mondal J. A.; Nihonyanagi S.; Yamaguchi S.; Tahara T. J. Am. Chem. Soc. 2010, 132, 10656–10657. 10.1021/ja104327t. [DOI] [PubMed] [Google Scholar]
- Mondal J. A.; Nihonyanagi S.; Yamaguchi S.; Tahara T. J. Am. Chem. Soc. 2012, 134, 7842–7850. 10.1021/ja300658h. [DOI] [PubMed] [Google Scholar]
- Sheu S.-Y.; Schlag E. W.; Selzle H. L.; Yang D.-Y. Molecular Dynamics of Hydrogen Bonds in Protein–D2O: The Solvent Isotope Effect. J. Phys. Chem. A 2008, 112, 797–802. 10.1021/jp0771668. [DOI] [PubMed] [Google Scholar]
- Sovago M.; Vartiainen E.; Bonn M. Observation of buried water molecules in phospholipid membranes by surface sum-frequency generation spectroscopy. J. Chem. Phys. 2009, 131, 161107 10.1063/1.3257600. [DOI] [PubMed] [Google Scholar]
- Gonella G.; Lütgebaucks C.; de Beer A. G. F.; Roke S. Second Harmonic and Sum-Frequency Generation from Aqueous Interfaces Is Modulated by Interference. J. Phys. Chem. C 2016, 120, 9165–9173. 10.1021/acs.jpcc.5b12453. [DOI] [Google Scholar]
- Wang H. F. Sum frequency generation vibrational spectroscopy (SFG-VS) for complex molecular surfaces and interfaces: Spectral lineshape measurement and analysis plus some controversial issues. Prog. Surf. Sci. 2016, 91, 155–182. 10.1016/j.progsurf.2016.10.001. [DOI] [Google Scholar]
- Ong S.; Zhao X.; Eisenthal K. B. Polarization of water molecules at a charged interface: second harmonic studies of the silica/water interface. Chem. Phys. Lett. 1992, 191, 327–335. 10.1016/0009-2614(92)85309-X. [DOI] [Google Scholar]
- Eftekhari-Bafrooei A.; Borguet E. Effect of Electric Fields on the Ultrafast Vibrational Relaxation of Water at a Charged Solid–Liquid Interface as Probed by Vibrational Sum Frequency Generation. J. Phys. Chem. Lett. 2011, 2, 1353–1358. 10.1021/jz200194e. [DOI] [Google Scholar]
- Lis D.; Backus E. H.; Hunger J.; Parekh S. H.; Bonn M. Liquid flow along a solid surface reversibly alters interfacial chemistry. Science 2014, 344, 1138–1142. 10.1126/science.1253793. [DOI] [PubMed] [Google Scholar]
- Oldham K. B. A Gouy–Chapman–Stern model of the double layer at a (metal)/(ionic liquid) interface. J. Electroanal. Chem. 2008, 613, 131–138. 10.1016/j.jelechem.2007.10.017. [DOI] [Google Scholar]
- Grahame D. C. The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441–501. 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- Tsuboi M.; Benevides J. M.; Thomas J. G. J. Raman Tensors and their application in structural studies of biological systems. Proc. Jpn. Acad., Ser. B 2009, 85, 83–97. 10.2183/pjab.85.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumakura A.; Tsuboi M.; Ushizawa K.; Ueda T. Polarized Raman spectrum of a single crystal of AZT. Biospectroscopy 1996, 2, 233–242. . [DOI] [Google Scholar]
- Fried S. D.; Boxer S. G. Measuring electric fields and noncovalent interactions using the vibrational stark effect. Acc. Chem. Res. 2015, 48, 998–1006. 10.1021/ar500464j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Pazos I. M.; Zhang W.; Culik R. M.; Gai F. Site-Specific Infrared Probes of Proteins. Annu. Rev. Phys. Chem. 2015, 66, 357–377. 10.1146/annurev-physchem-040214-121802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltover I.; Salditt T.; Safinya C. R. Phase Diagram, Stability, and Overcharging of Lamellar Cationic Lipid–DNA Self-Assembled Complexes. Biophys. J. 1999, 77, 915–924. 10.1016/S0006-3495(99)76942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhakamy N. A.; Elandaloussi I.; Ghazvini S.; Berkland C. J.; Dhar P. Effect of lipid headgroup charge and pH on the stability and membrane insertion potential of calcium condensed gene complexes. Langmuir 2015, 31, 4232–4245. 10.1021/la504970n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F.; Ye S.; Li H.; Luo Y. Phosphate Ions Promoting Association between Peptide and Modeling Cell Membrane Revealed by Sum Frequency Generation Vibrational Spectroscopy. J. Phys. Chem. C 2013, 117, 11095–11103. 10.1021/jp400378d. [DOI] [Google Scholar]
- Wei F.; Li H.; Ye S. Specific Ion Interaction Dominates over Hydrophobic Matching Effects in Peptide–Lipid Bilayer Interactions: the Case of Short Peptide. J. Phys. Chem. C 2013, 117, 26190–26196. 10.1021/jp411413u. [DOI] [Google Scholar]
- Wei F.; Xiong W.; Li W.; Lu W.; Allen H. C.; Zheng W. Assembly and relaxation behaviours of phosphatidylethanolamine monolayers investigated by polarization and frequency resolved SFG-VS. Phys. Chem. Chem. Phys. 2015, 17, 25114–25122. 10.1039/C5CP03977K. [DOI] [PubMed] [Google Scholar]
- Wei F.; Xia W. X.; Hu Z. J.; Li W. H.; Zhang J. Y.; Zheng W. Q. Laser Linewidth and Spectral Resolution in Infrared Scanning Sum Frequency Generation Vibrational Spectroscopy System. Chin. J. Chem. Phys. 2016, 29, 171–178. 10.1063/1674-0068/29/cjcp1601001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.