Abstract
Cancer survivorship rates have drastically increased due to improved efficacy of oncologic treatments. Consequently, clinical concerns have shifted from solely focusing on survival to quality of life, with fertility preservation as an important consideration. Among fertility preservation strategies for female patients, ovarian tissue cryopreservation and subsequent re-implantation has been the only clinical option available to cancer survivors with cryopreserved tissue. However, follicle atresia post-transplantation and risk of re-introducing malignant cells have prevented this procedure from becoming widely adopted in clinics. Herein, we investigated the encapsulation of ovarian follicles in alginate hydrogels that isolate the graft from the host, yet allows for maturation following transplantation at a heterotopic (i.e. subcutaneous) site, a process we termed in vivo follicle maturation. Survival of multiple follicle populations was confirmed via histology, with notable development of the antral follicles. Collected oocytes (63%) exhibited polar body extrusion and were fertilized by intracytoplasmic sperm injection (ICSI) and standard IVF procedures. Successfully fertilized oocytes developed to the pro-nucleus (14%), 2-cell (36%), and 4-cell (7%) stages. Furthermore, ovarian follicles co-transplanted with metastatic breast cancer cells within the hydrogels allowed for retrieval of the follicles, and no mice developed tumors post-removal of the implant, supporting that the hydrogel prevented seeding of disease within the host. Collectively, these findings demonstrate a viable option for safe use of potentially cancer-laden ovarian donor tissue for in vivo follicle maturation within a retrievable hydrogel and subsequent oocyte collection. Ultimately, this technology may provide novel options to preserve fertility for young female cancer patients.
Introduction
Advances in chemo- and radiotherapy have significantly improved cancer survivorship rates worldwide. As of 2016, the American Cancer Society (ACS) estimates that over 15 million individuals in the United States are currently in remission. By 2024, ACS projects this number will increase to over 19 million, of which 9 million will be female (American Cancer Society, 2014; American Cancer Society, 2017). In particular, the five-year survival rate for pediatric patients (0–14-year age group) has improved to 87% during the 2008–2012 period (Wallace et al., 2016). Given these estimates, clinical concerns have shifted to include fertility preservation post-treatment.
Alkylating chemotherapy or irradiation to the pelvis or abdomen in female patients can be gonadotoxic and cause irreversible damage to the ovaries, reducing the patient’s ability to conceive successfully by 50% (Salama and Woodruff, 2015; Shea et al., 2014). In cases where doses of abdominal radiation of 20–30 Gy are used, the risk of adverse pregnancy outcomes can be as high as 90% (Kim et al., 2016; Wallace et al., 1989; Wo and Viswanathan, 2009). Currently available options to preserve fertility prior to treatment include embryo cryopreservation, oocyte cryopreservation, and ovarian tissue cryopreservation, though embryo and oocyte cryopreservation may not be applicable to pediatric patients.
The transplantation of cryopreserved ovarian tissue is the only clinical option available to restore fertility using cryopreserved tissue, and has resulted in 60 live births reported to date (Donnez and Dolmans, 2015). This procedure does not require hormonal stimulation or a sperm donor, preserves ovarian follicles at all stages of maturation including primary and primordial, and can be applied to pre-pubertal girls (Kim et al., 2016; Kondapalli, LA, Ginsberg, 2012; Meirow et al., 2014). However, this method is still considered experimental and is associated with several challenges upon re-implantation that prevent it from becoming the gold standard for fertility preservation. These challenges include ischemic injury early post-transplantation due to insufficient tissue re-vascularization, which significantly reduces the ovarian follicle pool; follicular atresia, and risk of re-seeding malignant cancer cells (Demeestere et al., 2009; Donnez et al., 2009; Salama and Woodruff, 2015). In particular, reintroduction of malignant disease remains a primary concern as 12.4% of patients died due to recurrence after re-implantation of cryopreserved ovarian tissue according to a 12-year study (Imbert et al., 2014). Thus the presence of cancer cells in cryopreserved ovarian tissue (Abir et al., 2014; Dolmans et al., 2013; Rosendahl et al., 2013) has motivated strategies such as follicle isolation and transplantation as a means to reduce or remove the cancer cell population (Kniazeva et al., 2015).
Hydrogels have been employed for the transplantation of ovarian tissue or isolated follicles as a means to enhance efficacy. The three-dimensional architecture of the hydrogel physically supports the follicles, maintains oocyte-somatic cell connections, and permits expansion of early-stage follicles (Shea et al., 2014). In particular, fibrin hydrogels have been employed for ovarian follicle transplantation to facilitate the interaction of the transplant with the host. Transplantation of fibrin-encapsulated follicles has promoted their survival post-transplantation, enabled growth and maturation in vivo, and restored endocrine function in the ovariectomized mice (Kniazeva et al., 2015; Luyckx et al., 2014; Smith et al., 2014a). Restoration of endocrine function is particularly important and necessary for successful clinical pregnancies (Donnez et al., 2013; Oktay et al., 2001). Fibrin hydrogels modified with vascular endothelial growth factor (VEGF) and transplanted into the orthotropic site of ovarian bursa have improved encapsulated murine follicle function and resulted in live births via natural pregnancy (Kniazeva et al., 2015; Shikanov et al., 2011a; Smith et al., 2014b). There are at least two significant challenges with these approaches, however: i) the live births required a relatively large number of transplanted follicles that may be difficult to obtain in a clinical setting from human tissue, and ii) the degradation of the fibrin hydrogel and integration of the graft with the host tissue could allow for dissemination of residual cancer cells.
In this report, we investigated a strategy for transplantation of ovarian follicles within non-degradable alginate hydrogels to allow in vivo follicle maturation, with subsequent retrieval of the graft to obtain mature oocytes for in vitro fertilization (IVF). Alginate hydrogels have been used for encapsulation to provide support for culture and maturation of mouse follicles (Filatov et al., 2014; Hornick et al., 2012; Hornick et al., 2013; Telfer and Zelinski, 2013; Xiao et al., 2015a; Xu et al., 2009) and human follicles (Laronda et al., 2014; Xiao et al., 2015b) in vitro. Oocytes can be retrieved from alginate-encapsulated murine follicles for in vitro fertilization and subsequently implanted to produce healthy offspring (Xu et al., 2006). Here, we proposed to apply the alginate hydrogels for in vivo maturation at a subcutaneous site, with the hydrogels providing support for follicle growth, while also presenting an effective barrier that limits integration with the host tissue and potentially facilitates implant retrieval. Following encapsulation and transplantation, hydrogels were extracted and carefully dissected to collect oocytes for in vitro maturation (IVM) and subsequent fertilization by intracytoplasmic injection (ICSI) and standard IVF. Furthermore, we assessed the ability of cancer cells encapsulated together with the follicles to initiate tumor development within the transplant recipient. We were able to demonstrate that retrievable hydrogels used for follicle maturation provide a unique opportunity to maintain follicle architecture and allow maturation within the in vivo environment, while eliminating the risks associated with potentially cancer-laden ovarian tissue.
Materials and Methods
Follicle isolation and hydrogel fabrication
Ovaries were extracted from 12-day old C57BL/6j x CBA/Ca female mice (Harlan Laboratories, USA) and mechanically dissected using insulin syringe needles (1cc, 28G1/2) (BD Biosciences, USA). The tissue is dissected as a means to collect follicles primarily, and reduce the population of stromal cells. While stromal cell are present, their quantity has been reduced. The resulting ovarian fragments, approximately 300 μm in diameter, were allowed to aggregate in a 0.6 mL Eppendorf tube in Leibovitz’s L-15 media (Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA) and 1% PenStrep (Sigma-Aldrich, St. Louis, MO, USA). Follicle isolation was performed on a heated stage at 37°C in a sterilized hood to prevent contamination. Either 3, 2 or 1 ovary were dissected and incorporated into each of the gels, which correspond to a mixture of primordial, primary and secondary follicles with approximately 1100, 730, or 360 follicles respectively, based on follicle quantification from native 12-day old ovaries. To form alginate hydrogels, supernatant was removed until only the follicle aggregate remained. Next, 7 μL of 0.5% alginate (NovaMatrix, Sandvika, Norway) mixed with sterile PBS (−/−), were slowly added to the tube without disturbing the follicles, followed by 50 μL of thrombin/Ca2+ (Sigma-Aldrich, St. Louis, MO, USA) and the resulting mixture allowed to crosslink for 3 minutes. The thrombin/Ca2+solution was created by combining 50 IU/mL thrombin with 40 mM CaCl2. Hydrogels were approximately 3 mm in diameter and kept in L-15 media prior to transplantation.
Ovariectomy and transplantation
Hydrogels were transplanted into C57BL/6j x CBA/Ca adult 6–7-week old female mice following ovariectomy, either into the ovarian bursa or subcutaneously in the dorsal region. Mice were anesthetized with an intraperitoneal injection of 100 mg/kg of ketamine and 15 mg/kg of xylazine. Hydrogels were implanted into the bursa according to a previous report (Kniazeva et al., 2015). For subcutaneous implants, a small incision was made on the back to form a pocket, hydrogels were inserted, and then the incision was closed using a 5-0 vicryl suture (Ethicon, USA). Sterile surgical procedures, post-operative procedures, and daily care were performed according to the Northwestern University Institutional Animal Care and Use Committee (IACUC).
Retrieval and analysis of hydrogel transplants
After 7 days, mice were euthanized and ovarian bursae or subcutaneous implants were retrieved for histological analysis. Upon removal, samples were fixed in 4% paraformaldehyde, dehydrated in 70% ethanol, paraffin-embedded, serial-sectioned, and stained with hematoxylin and eosin (H&E). Follicle counts were performed by an experienced researcher blinded to experimental conditions. Follicles were quantified according to the following classification scheme: primordial follicles contained 4–6 squamous granulosa cells, primary follicles were enclosed by and contained a mix of squamous and cuboidal cells, secondary follicles contained two layers of cuboidal granulosa cells, multi-layered secondary follicles contained more than two layers of cuboidal layers with no presence of a cavity (i.e. corpus luteum), and antral follicles were identified by the presence of an oocyte surrounded by several layers of cuboidal cells and a defined corpus luteum.
Oocyte collection, in vitro maturation assay (IVM), and immunofluorescence
Hydrogels were explanted after 7 days post-transplant in the bursa or subcutaneous site and placed in L-15 medium. Hydrogel samples were mechanically dissected on a heated-stage microscope using insulin syringe needles (1cc, 28G1/2) to obtain antral follicles. Antral follicles were carefully punctured to release oocytes into the surrounding media. Only oocytes with a visible germinal vesicle, and thus arrested at prophase I, were transferred to IVM media (α-MEM) with 10% fetal bovine serum (FBS), 1.5 IU/mL human chorionic gonadotropin (hCG), and 10 ng/mL epidermal growth factor (EGF) (BD Biosciences, USA). Oocytes were incubated for 16 hours at 37°C in 5% CO2 and then imaged to confirm MII status, denoted by polar body extrusion. A subset of MII oocytes derived from hydrogel samples from the bursa or subcutaneous site were immunofluorescently-stained and imaged with a confocal microscope (Leica Microsystem, US) using previously cited methods (Xiao et al., 2015b) to assess the morphology of the meitotic spindle, a marker of egg quality.
In vitro fertilization and embryo development
Oocytes derived from subcutaneously-transplanted hydrogel samples containing 1,100 follicles were used for IVF and embryo development studies. Following confirmation of MII status via polar body extrusion post-IVM, oocytes were fertilized with sperm collected from the epididymis of 8–10 week old C57BL/6j x CBA/Ca male mice. Isolated epididymi were placed in a 1.5 mL Eppendorf tube with 1 mL of human tubular fluid (HTF) medium supplemented with 0.4% of bovine serum albumin (BSA). Sperm were incubated for 30 minutes at 37°C and the supernatant layer containing healthy and motile sperm was collected. Sperm was either injected directly into oocytes using intracytoplasmic injection or placed in HTF medium with oocytes using standard IVF procedures. For ICSI, MII oocytes were placed in 50 μL drops of EmbryoMax KSOM Medium (1X with ½ amino acids) (Millipore, USA) on a heated microscope stage (Nikon Eclipse TE300) and sperm was microinjected using a Piezo-drill tip (Eppendorf, USA) containing Fluorinert FC770 (Sigma-Aldrich, USA) to generate a pulse sufficient to penetrate the zona pellucida. Following sperm injection, MII oocytes were left in the 50 μL of KSOM medium submersed in Embryo Culture Oil (Irvine Scientific, USA) and monitored for up to 96 hours to evaluate embryo outcomes. Standard IVF was also used to fertilize MII oocytes. In brief, the zona pellucida was denuded using acetic acid and transferred to HTF medium. A low sperm concentration of 5,000 sperm total was added to 1 mL of human tubular fluid with MII oocytes for 24 hours and then transferred to a fresh 50 μL of KSOM without sperm. MII oocytes were then cultured for another 72 hours for a total of 96 hours to assess embryo outcomes post-fertilization.
Cancer-cell laden hydrogel transplants, in vivo luminescence imaging, and organ histology
Alginate hydrogel (7 μL, 0.5%) containing approximately 200 MDA-MB-231 BR cells expressing luciferase and ~360 ovarian follicles from a CD-1 female mouse donor were transplanted into the dorsal subcutaneous site of NOD SCID gamma (NSG) mice. At Day 7, mice were injected (150 mg/kg) with D-luciferin firefly (20 mg/mL in sterile PBS) (Perkin Elmer, Waltham, MA) and imaged 10 minutes post-injection on an in vivo imaging system (IVIS) (Perkin Elmer Xenogen IVIS Spectrum Bioluminescence System) to confirm the presence of cancer cells in the gel. Hydrogel implants were removed after imaging and mice were monitored for 3 weeks post-removal of the gel, at which time the mice were imaged again to determine if cancer cells were present at the subcutaneous transplant site. Liver and lungs were excised from all recipients within a week after the last imaging time point, fixed in 4% PFA, paraffin-embedded, and stained with H&E to assess if metastasis occurred from the initial transplant site.
Results
In vivo follicle transplantation within hydrogels and histological analysis
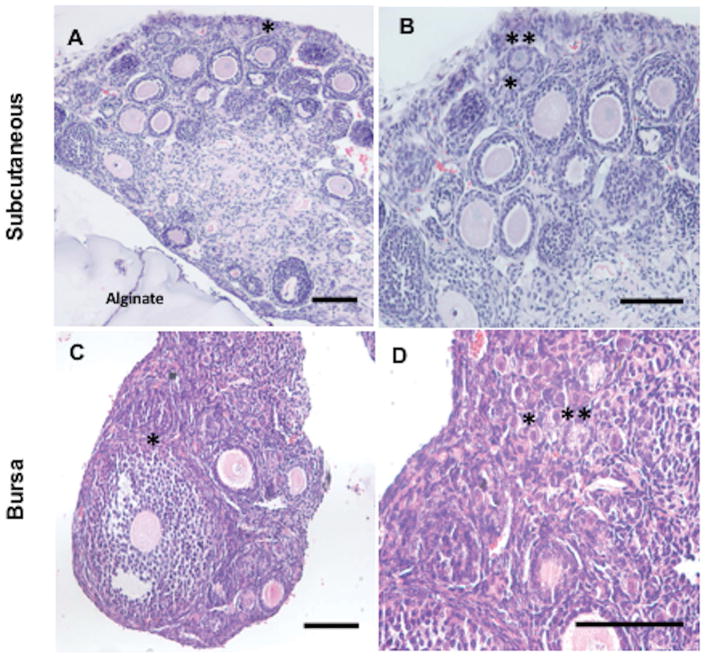
Hydrogel encapsulation was evaluated for its ability to support follicle survival and development in vivo. Specifically, alginate was chosen to encapsulate mechanically-isolated follicle aggregates and subsequently transplanted subcutaneously in the dorsal region (heterotopic site) of ovariectomized female recipient mice, with transplantation into the ovarian bursa (orthotopic site) employed as a control based on previous reports of follicle maturation and consequent successful live births. A range of follicle quantities (1100, 730, or 360) was encapsulated within hydrogels and surviving populations of follicles at all developmental stages were observed in grafts extracted from both the subcutaneous and bursa transplant sites (Figure 1A–D). Notably, antral follicles containing oocytes were identified within the hydrogel explants (Figure 1A, 1C), which were not initially present within the encapsulated follicle populations and thus developed from this original pool (Supplemental Figure 1A–C).
Figure 1. Follicle Survival and Growth in Hydrogel Explants 7 Days Post-Transplantation in Subcutaneous and Bursa Sites.
Antral, multi-layered secondary, and secondary follicles were observed in extracted ovarian grafts from the subcutaneous and bursa sites (A–B, C–D). In panel A, surrounding alginate material served to maintain separation of host tissue from follicles transplanted subcutaneously. A representative antral follicle is denoted with an asterisk (*) in panels A and C. Large numbers of primordial and primary follicles were identified in all extracted hydrogels and denoted with a single (*) or double asterisk (**) respectively in panels B and D. Hydrogel implant in panel A–B and C–D contained approximately 360 and 1,100 follicles, respectively. Similar results were observed for alginate hydrogels with follicle populations of ~730 ovarian follicles at both sites. Scale bar: 100 μm.
Quantification of follicle populations from removed grafts implanted subcutaneously indicated that a majority of the follicles were primordial, with antral follicles observed in all conditions (Figure 2A–B). For the subcutaneous grafts, the greatest number of transplanted follicles resulted in the smallest percentage of surviving follicles (Figure 2C). Transplantation of the smallest initial follicle numbers resulted in the greatest survival percentage (≈76%). Relative to the transplanted population, the number and percentage of primordial follicles had decreased, with a corresponding increase in the number and percentage of primary and secondary follicles suggesting follicle maturation within the bead during the time of transplantation. The number of recovered follicles from the bursa significantly decreased and recovery from this site was inefficient relative to the subcutaneous site. Transplantation of the smallest initial numbers of follicles (i.e. 360 follicles) in the bursa had the greatest survival percentage of 20%. As with the subcutaneous site, the largest follicle populations were in primordial and secondary stages. Finally, despite the lower resulting number of primordial and secondary follicles, the number of antral stage follicles was similar to the subcutaneous site.
Figure 2. Percentage of Follicle Populations Recovered from Alginate Hydrogel Explants 7 Days Post-Transplant.
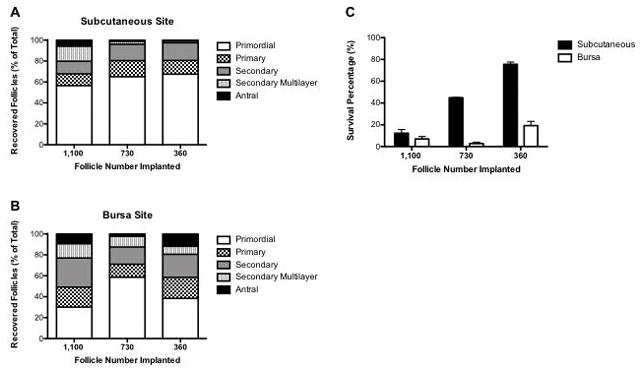
Follicle populations (primordial, primary, secondary, multi-layered secondary, and antral) were quantified and displayed as percent of recovered follicles for the 1100, 730, and 360 follicle implant conditions for the (A) subcutaneous (n=3/follicle condition) and (B) bursa transplant sites (n=4 for 1,100 follicle and 730 follicle condition, n=3 for 360 follicle condition). (C) Percentage of surviving follicles for the subcutaneous and bursa transplant sites (±SEM).
Hydrogel retrieval, oocyte collection, and in vitro maturation studies
Upon retrieval, the subcutaneously transplanted hydrogels were used to obtain antral follicles for investigation of oocyte quality. Alginate hydrogels retained their integrity upon extraction and allowed for easy follicle dissection (Figure 3A). For transplantation of the largest initial follicle numbers, a total of 54 oocytes in the germinal vesicle stage were retrieved, transferred to IVM media, and imaged after a 16-hour incubation period. Polar body extrusion was evident in 34 oocytes (MII stage), which corresponds to a 63% MII rate (Figure 3B–C). For transplants with the reduced number of follicles transplanted, the oocytes had an MII rate of 55% (730 follicles transplanted) and 46% (360 follicles transplanted). Importantly, the MII rate was comparable between all 3 conditions, suggesting that MII oocytes can be achieved with modest numbers of follicles transplanted. Finally, normal spindle morphology was confirmed in MII oocytes derived from alginate hydrogels initially loaded with 1,100 follicles (Figure 3D).
Figure 3. Egg Retrieval from Encapsulated Follicles in Alginate Hydrogels in Subcutaneous Site 7 Days Post-Transplant and Their Meiotic Maturation In Vitro.
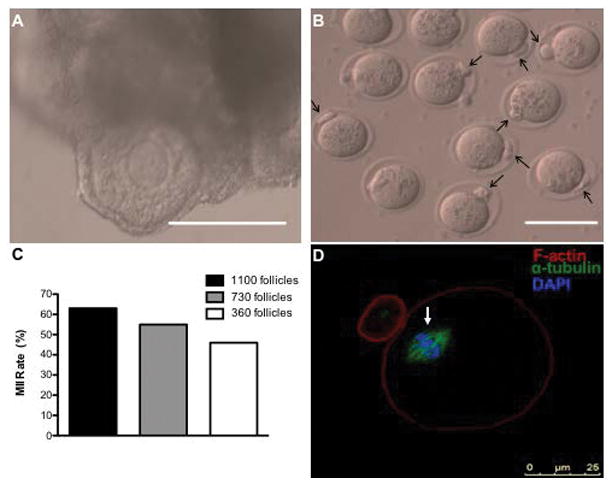
(A) Follicles were easily identified in explanted hydrogels. (B) MII oocytes were confirmed via polar body extrusion (indicated with black arrows). (C) Egg retrieval and MII status from the subcutaneous site. Of the GV oocytes collected from antral follicles in Day 7 explants with 1,100 ovarian follicles, 34/54 oocytes were MII post-in vitro maturation (63% MII rate) from 3 trials. Oocytes collected from Day 7 explants with 730 or 360 ovarian follicles resulted in an MII rate of 55% (6 MII oocytes/11 GV oocytes) and 46% (10 MII oocytes/22 GV oocytes collected), respectively (D) Normal spindle morphology (indicated by an arrow) was also confirmed in subcutaneously-matured oocytes. Image (D) depicts an oocyte obtained from an explant containing ovarian follicles from 3 ovaries. Scale bar: 100 μm (A, B).
Germinal vesicle oocytes isolated from antral follicles transplanted into the bursa (Figure 4A) underwent IVM to produce a MII rate of 20% (Figure 4B–C). Normal spindle morphology indicative of oocyte quality was confirmed in MII eggs post-IVM (Figure 4D). Intact hydrogel retrieval from the bursa was more challenging than from the subcutaneous space, which resulted in a lower yield. Subsequent studies thus focused only on the subcutaneous site.
Figure 4. Egg Retrieval from Encapsulated Follicles in Alginate Hydrogels in Bursa Site 7 Day Post-Transplant and Their Meiotic Maturation In Vitro.
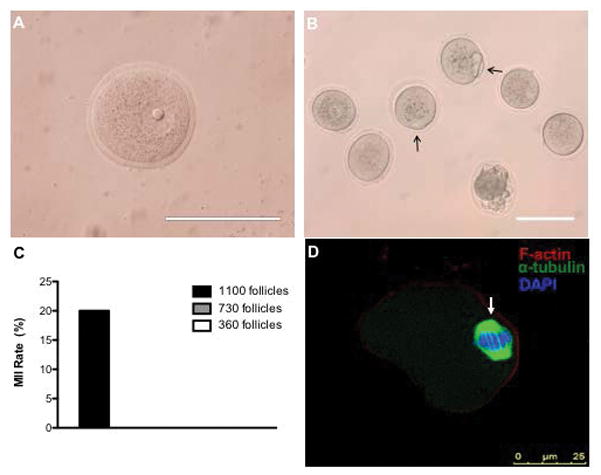
(A) Germinal vesicle (GV) oocytes were retrieved from antral follicles (B) MII oocytes were confirmed via polar body extrusion (indicated with black arrows) (C) Egg retrieval and MII status from the bursa site. Of the 15 GV oocytes collected at Day 7 from 1 trial, 3 were MII post-IVM (20% MII rate). Note: MII follicles were only observed with the 1,100 follicle condition and not for the 730 or 360 follicle condition. (D) Normal spindle morphology (indicated by an arrow) was also confirmed in MII oocytes. Image (D) depicts an oocyte obtained from an explant containing ovarian follicles from 3 ovaries. Scale bar: 100 μm (A, B).
Fertilization and embryonic development
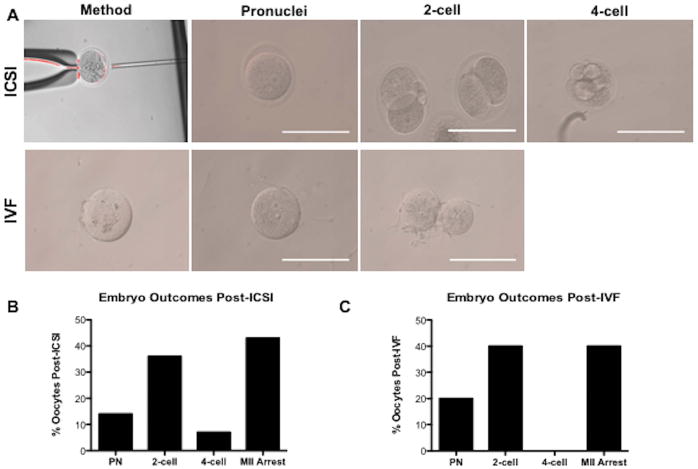
The fertilization competency of the MII stage oocytes that were obtained from the subcutaneously transplanted follicles were subsequently investigated (Figure 5A). ICSI was employed to fertilize eggs and embryo development was monitored for 96 hours post-ICSI. Of the 14 MII eggs that were successfully injected with sperm, oocytes progressed to the pronucleus (14%), 2-cell (36%), and 4-cell (7%) stages, while 40% of oocytes remained in MII arrest (Figure 5B). Embryos fertilized via standard IVF progressed to the pronucleus (20%) and 2-cell (40%) stages, while 40% of oocytes remained in MII arrest (Figure 5C).
Figure 5. Fertilization Competency of MII Oocytes Matured in Subcutaneous Site is Assessed via Intracytoplasmic Sperm Injection (ICSI) and In Vitro Fertilization (IVF).
(A) Post-ICSI, MII oocytes progressed to the pronucleus, 2-cell, and 4-cell embryonic stages. Post-IVF, denuded eggs progressed to the pronucleus and 2-cell stages. Scale bar: 100 μm (B) After 14 MII oocytes were injected with sperm via ICSI, embryos progressed to the pronucleus (14%), 2-cell (36%), and 4-cell (7%) stages, while 43% of oocytes remained in MII arrest (C) Embryos resulting from 10 MII oocytes denuded and placed in an IVF dish with sperm, progressed to the pronucleus (20%) and 2-cell (40%) stages, while 40% of oocytes remained in MII arrest. Results obtained from 2 ICSI trials and 1 IVF trial.
Cancer-laden hydrogel implants and in vivo imaging
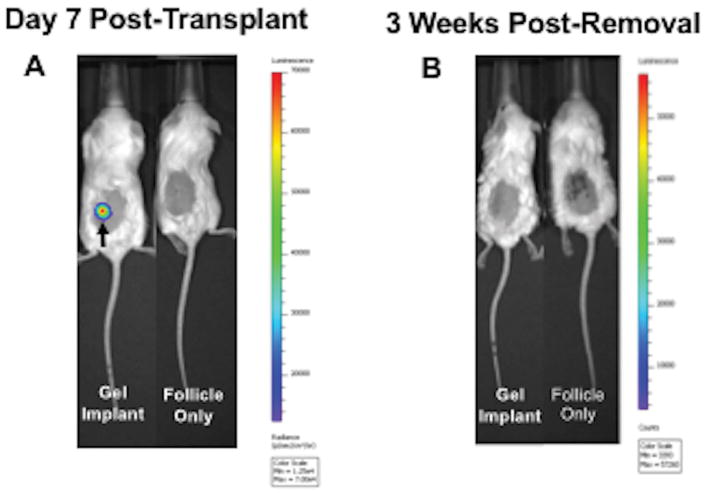
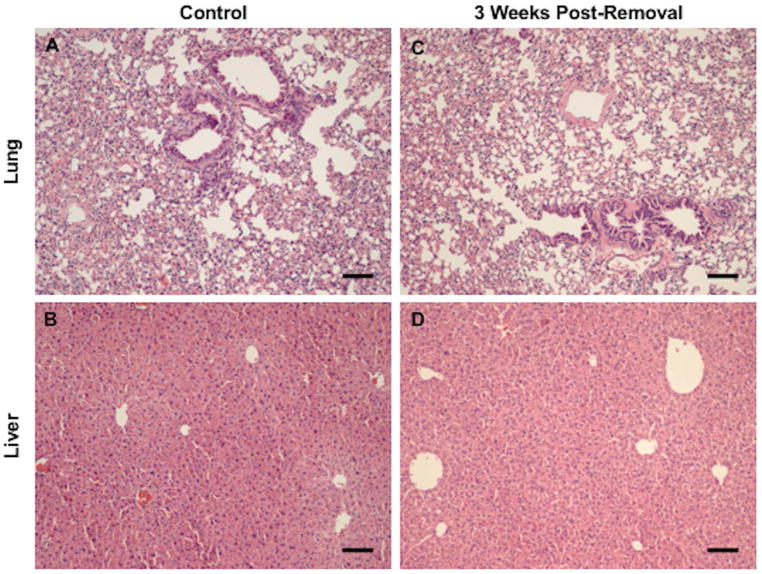
We next investigated the safety of this strategy by addition of cancer cells to the isolated follicles followed by their encapsulation and transplantation. We hypothesized that the alginate hydrogel, which prevents direct contact between the host and transplanted tissue, would prevent the escape of cancer cells into the host tissue following transplantation and during retrieval. Approximately 200 MDA-MD-231 BR cells expressing luciferase were encapsulated with ~360 ovarian follicles into alginate hydrogels and implanted subcutaneously into NSG mice. The delivery of 20 cells or more of the MDA-MD-231 BR cells can lead to tumor formation in this model (Supplemental Figure 2A–D). In vivo bioluminescence imaging of the follicle/cancer cell transplants at Day 7 confirmed the presence of cancer cells within the implant (n=4), whereas a luminescence signal was not detected in the follicle transplant only control group (n=4) (Figure 6A). Hydrogels were then removed and recipient mice were imaged again at 3 weeks post-removal of the gel, with no detection of a bioluminescence signal (Figure 6B). The lung and liver were removed within 1 week post-imaging and analyzed for metastases. Histology confirmed no metastatic lesions in the lung or liver compared to control organs in all recipient mice, supporting the safety of this strategy (Figure 7A–D).
Figure 6. In Vivo Imaging of NSG Mice Pre- and Post-Removal of Cancer-Laden Alginate Hydrogels.
(A) Representative image of NSG mouse with subcutaneously-transplanted alginate hydrogel containing 200 MDA-MB-231 cells expressing luciferase (imaged Day 7 post-transplant). The presence of cancer cells in the hydrogels was confirmed by the positive luminescent signal (signal detection is 600–60,000 counts). The hydrogel implant at Day 7 is denoted with a black arrow. For the control group, mice transplanted with only ovarian follicles were imaged. Cancer cells were present only in the gel implant. (B) Mice were imaged 3 weeks after hydrogels removal to assess cancer cell presence. Cancer cells were not detected in experimental mice that had cancer-laden hydrogels removed at Day 7. Cancer cells were not detected in negative controls as well. 360 ovarian follicles were also incorporated into transplanted alginate hydrogels. n=4 per group.
Figure 7. Absence of Metastatic Lesions in Liver and Lungs of Recipient Mice 3 Weeks Post-Removal of Hydrogel.
Representative image of (A) liver from naïve NSG control (n=3), (B) lung from naïve NSG control (n=3), (C) liver from recipient mouse with 200 cancer cells in hydrogel (n=4), and (D) lung from recipient mouse with 200 cancer cells in hydrogel (n=4). Lung and liver tissue removed at 3 weeks did not contain any cellular abnormalities or metastatic growths according to staining with hematoxylin and eosin (H&E). Scale bar: 100 μm.
Discussion
This report presents a strategy for in vivo maturation of ovarian follicles, which involved the isolation and encapsulation of follicles into alginate hydrogels, transplantation into the subcutaneous space, and their subsequent retrieval for recovery of antral follicles containing mature oocytes. Alginate hydrogels have been widely used for in vitro follicle culture (Brito et al., 2014; Camboni et al., 2013; Hornick et al., 2012; Kniazeva et al., 2015; Kreeger et al., 2006; Laronda et al., 2014; West et al., 2007; Xiao et al., 2015a; Xiao et al., 2015b; Xu et al., 2009), as well as for cell transplantation, such as islets as a therapy for type 1 diabetes (Köllmer et al., 2015; Ludwig et al., 2013; Qi, 2014; Scharp and Marchetti, 2013) and also ovarian follicles (David et al., 2017; Vanacker et al., 2014). Islets are encapsulated within the hydrogel for isolation from the host over several months, yet the islets are able to survive, sense blood glucose levels, and secrete insulin that can distribute systemically to normalize blood glucose levels. Herein, we used alginate hydrogels for the transplantation of ovarian follicles with the objectives of sensing the hormonal milieu that can drive follicle development and maturation, yet also isolation of the follicles from the host to facilitate their recovery upon maturation and prevent the re-seeding of cancer cells that may be present within the donor tissue. For the studies herein, the hydrogel was loaded primarily with early stage follicles (i.e. primordial and primary follicles), the extracted hydrogel grafts contained follicles at all developmental stages, with a notable number of antral follicles. Of the MII oocytes identified post-IVM from matured antral follicles, more than a third progressed to the 2-cell embryo stage after either ICSI or standard IVF, which is consistent with results found in previous fertilization studies (Ellenbogen et al., 2014; Jin et al., 2010; Walls et al., 2018). These findings confirm the feasibility for use and utility of alginate hydrogels for in vivo maturation of ovarian follicles to obtain meiotically competent oocytes.
Subcutaneous implantation of the hydrogel grafts was initially investigated based on the potential for relatively easy implantation and retrieval, yet the number of follicles recovered was significantly enhanced relative to implantation in the bursa. The bursa site was employed as a control for comparison at the subcutaneous site based on previous reports of live births achieved with transplantation of ovarian tissue and ovarian follicles (Kniazeva et al., 2015; Shikanov et al., 2011c). While antral follicles could be recovered from alginate hydrogels implanted into the bursa, and oocyte maturation produced MII stage oocytes, the hydrogel retrieval was challenging and reduced the yield of follicles. The retrieval process likely contributed to the relatively low number of retrieved follicles from the bursa relative to subcutaneous site. An additional advantage of subcutaneous implantation is that follicle growth can easily be monitored via ultrasound (Oktay et al., 2001) and human antral follicles can be removed when they mature (typically >15 mm) for oocyte collection. Previous studies by Oktay et al. demonstrated that while a 4-cell human embryo could be obtained from a subcutaneous transplant of ovarian tissue, it failed to implant (Oktay et al., 2004). To date, only 2 live human births have been achieved worldwide for transplantation of ovarian tissue at this location (Salama and Woodruff, 2015). The transplantation of ovarian follicles, rather than ovarian tissue, may enhance the development of the follicles to improve oocyte quality.
Murine oocytes from extracted alginate hydrogels in the subcutaneous site matured to MII, could be fertilized, and developed to the 4-cell embryo stage. The duration of in vivo implantation (7 days) suggests that the antral follicles developed from secondary follicles. Longer transplantation times, approximately 3–7 weeks, are necessary for primordial follicles to develop to the antral stage (Zheng et al., 2014). Collectively, the primordial and primary follicles constitute the most abundant populations in the ovarian reserve. The ability to mature these populations for oocyte collection would ultimately provide options for fertility preservation (Laronda et al., 2014). Future studies may explore the use of exogenous gonadotropins to promote ovarian follicle maturation and increase the number of oocytes retrieved for fertilization (Drummond, 2006; Yang et al., 2006). For either ICSI or standard IVF, 40% of MII oocytes remained in MII arrest (i.e. did not progress). This result may suggest oocyte quality can be improved to facilitate progression to a blastocyst stage for embryo transfer. Oocyte quality may be improved by longer maturation periods (> 7 days), or modulation of the hydrogel (e.g. alginate, fibrin) (Papavasiliou et al., 2012; Shikanov et al., 2011b) or transplant environment. Heterotopic sites offer advantages regarding ease of access, yet may lack the natural cues present at orthotopic sites. Localized delivery of exogenous growth factors, hormones, antioxidants, or pharmacological agents (e.g. PTEN inhibitors) provide a means to locally modulate the transplant environment to improve follicle viability and oocyte output post-transplant (Demeestere et al., 2009). Gonadotropin treatment pre-transplant has been shown to improve follicle survival post-transplant (Imthurn et al., 2000). Such modulation may enhance new blood vessel formation around the bead and can be triggered mechanically (Demeestere et al., 2006) or through delivery of angiogenic factors (e.g. VEGF) (Kniazeva et al., 2015; Shikanov et al., 2011c). Gonadotropin delivery can also upregulate VEGF to induce vessel formation (Demeestere et al., 2009). Taken together, modification to the hydrogel or subcutaneous site may ultimately improve oocyte quality and thus clinical outcomes.
Cryopreserved ovarian tissue from young cancer patients can contain tens to hundreds of thousands of follicles depending on the age of the patient, and advances in the cryopreservation approach have supported long-term storage of ovarian tissue. One report has confirmed normal tissue morphology for up to 18 years following cryopreservation (Fabbri et al., 2016). Ovarian tissue is typically dissected into relatively small pieces that can be more readily cryopreserved relative to large ovarian tissue chunks. The dissection of ovarian follicles from the tissue can be challenging, owing to the relative dense extracellular matrix of the ovary. Dissecting the ovarian pieces results in retrieval of modest numbers of ovarian follicles (Telfer and Zelinski, 2013), and thus the fertility preservation strategy must efficiently support survival and maturation given the low numbers of available follicles. The average number of follicles transplanted herein ranged from 360 follicles to 1100 follicles, with the lowest number of follicles transplanted producing the greatest efficiency for maturating to antral stage. Additional reductions in follicle number may be necessary for translation.
We demonstrated that the in vivo maturation strategy involving an alginate hydrogel, which does not permit integration of host tissue with the graft, can prevent the escape of cancer cells to vital organs such as the liver and lungs. The cryopreserved tissue may contain cancer cells, such as those present within the circulation (i.e., circulating tumor cells). In the particular case of leukemia, more than 50% of cryopreserved ovarian tissue can contain leukemic cells (Soares et al., 2015). The reseeding of disease has been observed clinically with ovarian tissue transplantation (Dolmans et al., 2013). Protocols are being developed to rid ovarian tissue of cancerous cells (Soares et al., 2015), but have not been widely adopted for clinical use, which underscores the need to develop technologies that prevent re-seeding of cancer cells. Isolation of transplanted follicles from host tissue has the potential to substantially reduce the number of cancer cells in the graft (Donnez et al., 2011; Kniazeva et al., 2015), yet follicle isolation may not be able to remove the presence of all tumor cells in every graft. Herein, the hydrogel provided an additional safety precaution by preventing direct contact of the host tissue with the graft, and did not allow the escape of the tumor cells to colonize alternative tissues. Previous studies with the transplantation of ovarian tissue indicated that alginate may lose integrity with the expansion of many follicles growing and maturing simultaneously (David et al., 2017). Herein, transplantation was performed with isolated ovarian follicles which did not allow the escape of cancer, yet the studies were performed for transplant times of 7 days, which was sufficient for the maturation of murine follicles and to confirm feasibility of the approach. The translation of this strategy to humans may require substantially longer periods of time and development of alternative materials with tunable degradation and mechanical properties to maximize oocyte maturation and recovery.
Conclusion
We present an alginate hydrogel as a retrievable technology to mature ovarian follicles subcutaneously and to prevent escape and subsequent metastasis of cancer cells. Early stage follicles were transplanted within alginate hydrogels, resulting in retrieval of antral follicles and subsequent collection of oocytes. Post-IVM, MII oocytes were fertilized and progressed to the 2-cell and 4-cell embryo stages. These findings collectively demonstrate retrievable hydrogels as a novel approach to mature ovarian follicles in order to obtain fertilizable oocyte, which also prevent direct contact with host tissue to alleviate concerns related to re-seeding disease from cryopreserved auto-transplanted ovarian tissue. This strategy may provide a method to enhance the safety and improve oocyte quality relative to ovarian tissue transplantation with the potential to improve clinical outcomes for female cancer patients aiming to preserve their fertility.
Supplementary Material
(A) Intact 12-day old whole ovary stained with H&E. (B) Magnified image displays follicles at all development stages (primary, primordial, secondary, multi-layered secondary, and antral) (C) Primordial follicles are the majority of the follicle pool (63±10%), while primary, secondary, multi-layered secondary, and antral follicles compose 18±1%, 16±3%, 2±0.3%, and 1±0.3% of the follicle population respectively (n=3, ±SEM). Scale bar: 100 μm
Mice were implanted with non-encapsulated luciferase-expressing tumor cells (syngeneic 4T1 in BALB/c) with either (A) 0, (B) 20, (C) 200, or (D) 2×106 cells then monitored for tumor growth as evaluated via bioluminescence over 3 weeks. Mice without tumor cells showed no signs of growth. Mice with 20, 200, 2×106 all showed bioluminescent signal from tumor cells and outgrowth from non-encapsulated tumor cells. Scale bar for bioluminescent radiance is normalized between all cohorts to range from 3.00×106 p/sec/cm2/sr to 6.00×108 p/sec/cm2/sr.
Acknowledgments
This work was supported by the Center for Reproductive Health After Disease (P50HD076188) from the National Institutes of Health National Center for Translational Research in Reproduction and Infertility (NCTRI). No conflict of interest to declare.
References
- Abir R, Aviram A, Feinmesser M, Stein J, Yaniv I, Parnes D, Ben-Haroush A, Meirow D, Rabizadeh E, Fisch B. Ovarian minimal residual disease in chronic myeloid leukaemia. Reprod Biomed Online. 2014;28:255–60. doi: 10.1016/j.rbmo.2013.10.011. http://www.ncbi.nlm.nih.gov/pubmed/24365024. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer treatment & survivorship facts & figures 2014–2015 2014 [Google Scholar]
- American Cancer Society. Cancer Facts and Figures. Am Cancer Soc. 2017:1–71. https://old.cancer.org/acs/groups/content/@editorial/documents/document/acspc-048738.pdf.
- Brito IR, Lima IMT, Xu M, Shea LD, Woodruff TK, Figueiredo JR. Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices. Reprod Fertil Dev. 2014;26:915–30. doi: 10.1071/RD12401. http://www.ncbi.nlm.nih.gov/pubmed/23866836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camboni a, Van Langendonckt a, Donnez J, Vanacker J, Dolmans MM, Amorim Ca. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology. 2013;67:64–9. doi: 10.1016/j.cryobiol.2013.05.002. http://www.ncbi.nlm.nih.gov/pubmed/23688636. [DOI] [PubMed] [Google Scholar]
- David A, Day JR, Cichon AL, Lefferts A, Cascalho M, Shikanov A. Restoring Ovarian Endocrine Function with Encapsulated Ovarian Allograft in Immune Competent Mice. Ann Biomed Eng. 2017;45:1685–1696. doi: 10.1007/s10439-016-1780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere a, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–65. doi: 10.1093/humupd/dmp021. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2759329&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, Delbaere A, Englert Y. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod. 2006;21:2010–4. doi: 10.1093/humrep/del092. http://www.ncbi.nlm.nih.gov/pubmed/16585122. [DOI] [PubMed] [Google Scholar]
- Dolmans M-M, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99:1514–22. doi: 10.1016/j.fertnstert.2013.03.027. http://www.ncbi.nlm.nih.gov/pubmed/23541406. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans M-M. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–70. doi: 10.1007/s10815-015-0544-9. http://www.ncbi.nlm.nih.gov/pubmed/26210678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans M-M, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. http://www.ncbi.nlm.nih.gov/pubmed/23635349. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Dolmans M-M. Frozen-thawed ovarian tissue retransplants. Semin Reprod Med. 2009;27:472–8. doi: 10.1055/s-0029-1241057. http://www.ncbi.nlm.nih.gov/pubmed/19806516. [DOI] [PubMed] [Google Scholar]
- Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, Dolmans MM. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011;95:36–39. doi: 10.1016/j.fertnstert.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16. doi: 10.1186/1477-7827-4-16. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1459164/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen A, Shavit T, Shalom-Paz E. IVM results are comparable and may have advantages over standard IVF. Facts, Views Vis ObGyn. 2014;6:77–80. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4086019/ [PMC free article] [PubMed] [Google Scholar]
- Fabbri R, Macciocca M, Vicenti R, Pasquinelli G, Caprara G, Valente S, Seracchioli R, Paradisi R. Long-term storage does not impact the quality of cryopreserved human ovarian tissue. J Ovarian Res. 2016;9:1–10. doi: 10.1186/s13048-016-0261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov MA, Khramova YV, Semenova ML. In Vitro Mouse Ovarian Follicle Growth and Maturation in Alginate Hydrogel: Current State of the Art. Acta Naturae. 2014;7:48–56. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4463412&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27:1801–10. doi: 10.1093/humrep/der468. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3357191&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145:19–32. doi: 10.1530/REP-12-0233. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3884596&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, Dechene J, Ferster A, Veys I, Fastrez M, Englert Y, Demeestere I. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod. 2014;29:1931–1940. doi: 10.1093/humrep/deu158. http://humrep.oxfordjournals.org/lookup/doi/10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163:141–6. doi: 10.1016/s0303-7207(00)00218-5. http://www.ncbi.nlm.nih.gov/pubmed/10963886. [DOI] [PubMed] [Google Scholar]
- Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633–9. doi: 10.1016/j.fertnstert.2009.10.027. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2873094&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim SK, Lee JR, Woodruff TK. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J Gynecol Oncol. 2016;27:e22. doi: 10.3802/jgo.2016.27.e22. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4717227&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS, Shea LD. Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model. Sci Rep. 2015;5:17709. doi: 10.1038/srep17709. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4668556&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köllmer M, Appel AA, Somo SI, Brey EM. Long-Term Function of Alginate-Encapsulated Islets. Tissue Eng Part B Rev. 2015 doi: 10.1089/ten.TEB.2015.0140. http://www.ncbi.nlm.nih.gov/pubmed/26414084. [DOI] [PubMed]
- Kondapalli LA, Ginsberg JP. Ovarian Tissue Cryopreservation and Transplantation. Oncofertility Med Pract Clin Issues Implement. 2012:63–75. http://link.springer.com/10.1007/978-1-4419-9425-7.
- Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–28. doi: 10.1007/s10815-014-0252-x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4130945&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E, Solimena M, Gendler Z, Rotem A, Barkai U, Bornstein SR. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A. 2013;110:1–5. doi: 10.1073/pnas.1317561110. http://www.ncbi.nlm.nih.gov/pubmed/24167261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyckx V, Dolmans M-M, Vanacker J, Legat C, Fortuño Moya C, Donnez J, Amorim CA. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril. 2014;101:1149–56. doi: 10.1016/j.fertnstert.2013.12.025. http://www.ncbi.nlm.nih.gov/pubmed/24462059. [DOI] [PubMed] [Google Scholar]
- Meirow D, Ra H, Biderman H. Human Fertility. 2014:1154. doi: 10.1007/978-1-4939-0659-8_21. http://link.springer.com/10.1007/978-1-4939-0659-8. [DOI] [PubMed]
- Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001;286:1490–3. doi: 10.1001/jama.286.12.1490. http://www.ncbi.nlm.nih.gov/pubmed/11572742. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet (London, England) 2004;363:837–40. doi: 10.1016/S0140-6736(04)15728-0. http://www.ncbi.nlm.nih.gov/pubmed/15031026. [DOI] [PubMed] [Google Scholar]
- Papavasiliou G, Sokic S, Turturro M. Synthetic PEG Hydrogels as Extracellular Matrix Mimics for Tissue Engineering Applications. Biotechnol - Mol Stud Nov Appl Improv Qual Hum Life. 2012:111–135. [Google Scholar]
- Qi M. Transplantation of Encapsulated Pancreatic Islets as a Treatment for Patients with Type 1 Diabetes Mellitus. Adv Med. 2014;2014:1–15. doi: 10.1155/2014/429710. http://www.hindawi.com/journals/amed/2014/429710/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30:11–24. doi: 10.1007/s10815-012-9912-x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3553351&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev. 2015;34:807–22. doi: 10.1007/s10555-015-9600-2. http://www.ncbi.nlm.nih.gov/pubmed/26589603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2013;67–68:35–73. doi: 10.1016/j.addr.2013.07.018. http://www.ncbi.nlm.nih.gov/pubmed/23916992. [DOI] [PubMed] [Google Scholar]
- Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Annu Rev Biomed Eng. 2014;16:29–52. doi: 10.1146/annurev-bioeng-071813-105131. http://www.ncbi.nlm.nih.gov/pubmed/24849592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Ph D, Zhang Z, Xu M, Smith RM, Rajan A, Sc M, Woodruff TK, Shea LD. Fibrin Encapsulation and Vascular Endothelial Growth Factor Delivery Promotes Ovarian Graft Survival in Mice. 2011a:17. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials. 2011b;32:2524–31. doi: 10.1016/j.biomaterials.2010.12.027. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3040241&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011c;17:3095–104. doi: 10.1089/ten.tea.2011.0204. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3226061&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-Mediated Delivery of an Ovarian Follicle Pool in a Mouse Model of Infertility. Tissue Eng Part A. 2014a;0:1–10. doi: 10.1089/ten.tea.2013.0675. http://www.ncbi.nlm.nih.gov/pubmed/24802617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK, Shea LD. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng Part A. 2014b;20:3021–30. doi: 10.1089/ten.tea.2013.0675. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4229702&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M, Sahrari K, Amorim CA, Saussoy P, Donnez J, Dolmans M-M. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil Steril. 2015;104:672–80. e2. doi: 10.1016/j.fertnstert.2015.05.021. http://www.ncbi.nlm.nih.gov/pubmed/26095134. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99:1523–33. doi: 10.1016/j.fertnstert.2013.03.043. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3929501&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker J, Dolmans M-M, Luyckx V, Donnez J, Amorim CA. First transplantation of isolated murine follicles in alginate. Regen Med. 2014;9:609–19. doi: 10.2217/rme.14.33. http://www.ncbi.nlm.nih.gov/pubmed/25372078. [DOI] [PubMed] [Google Scholar]
- Wallace WHB, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of ovarian tissue cryopreservation. Fertil Steril. 2016;105:6–12. doi: 10.1016/j.fertnstert.2015.11.041. http://www.ncbi.nlm.nih.gov/pubmed/26674557. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Shalet SM, Hendry JH, Morris-Jones PH, Gattamaneni HR. Ovarian failure following abdominal irradiation in childhood: the radiosensitivity of the human oocyte. Br J Radiol. 1989;62:995–8. doi: 10.1259/0007-1285-62-743-995. http://www.ncbi.nlm.nih.gov/pubmed/2510900. [DOI] [PubMed] [Google Scholar]
- Walls M, Junk S, Ryan JP, Hart R. IVF versus ICSI for the fertilization of in-vitro matured human oocytes. Reprod Biomed Online. 2018;25:603–607. doi: 10.1016/j.rbmo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–48. doi: 10.1016/j.biomaterials.2007.07.001. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2034204&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wo JY, Viswanathan AN. Impact of radiotherapy on fertility, pregnancy, and neonatal outcomes in female cancer patients. Int J Radiat Oncol Biol Phys. 2009;73:1304–12. doi: 10.1016/j.ijrobp.2008.12.016. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2865903&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction. 2015a;150:183–92. doi: 10.1530/REP-15-0175. http://www.ncbi.nlm.nih.gov/pubmed/26116002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep. 2015b;5:17323. doi: 10.1038/srep17323. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4661442&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378–86. doi: 10.1002/bit.22250. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2778231&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2648391&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HY, Cox S-L, Jenkin G, Findlay J, Trounson A, Shaw J. Graft site and gonadotrophin stimulation influences the number and quality of oocytes from murine ovarian tissue grafts. Reproduction. 2006;131:851–9. doi: 10.1530/rep.1.00916. http://www.ncbi.nlm.nih.gov/pubmed/16672350. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhang H, Gorre N, Risal S, Shen Y, Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum Mol Genet. 2014;23:920–928. doi: 10.1093/hmg/ddt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Intact 12-day old whole ovary stained with H&E. (B) Magnified image displays follicles at all development stages (primary, primordial, secondary, multi-layered secondary, and antral) (C) Primordial follicles are the majority of the follicle pool (63±10%), while primary, secondary, multi-layered secondary, and antral follicles compose 18±1%, 16±3%, 2±0.3%, and 1±0.3% of the follicle population respectively (n=3, ±SEM). Scale bar: 100 μm
Mice were implanted with non-encapsulated luciferase-expressing tumor cells (syngeneic 4T1 in BALB/c) with either (A) 0, (B) 20, (C) 200, or (D) 2×106 cells then monitored for tumor growth as evaluated via bioluminescence over 3 weeks. Mice without tumor cells showed no signs of growth. Mice with 20, 200, 2×106 all showed bioluminescent signal from tumor cells and outgrowth from non-encapsulated tumor cells. Scale bar for bioluminescent radiance is normalized between all cohorts to range from 3.00×106 p/sec/cm2/sr to 6.00×108 p/sec/cm2/sr.