Abstract
Objectives
Impaired cerebral autoregulation following neurological injury is a predictor of poor clinical outcome. We aimed to assess the relationship between body temperature and cerebral autoregulation in comatose patients.
Design
Retrospective analysis of prospectively collected data.
Setting
Neurocritical care unit of the Johns Hopkins Hospital.
Patients
Eighty-five acutely comatose patients (GCS ≤8) admitted between 2013 to 2017.
Interventions
None.
Measurement and Main Results
Cerebral autoregulation was monitored using multimodal monitoring with near infrared spectroscopy (NIRS) derived cerebral oximetry index (COx). COx was calculated as a Pearson correlation coefficient between low-frequency changes in regional cerebral oxygenation saturation and mean arterial pressure (MAP). Patients were initially analysed together, then stratified by temperature pattern over the monitoring period: no change (<1°C difference between highest and lowest temperatures, n=11), increasing (≥1°C, n=9), decreasing (≥1°C, n=9), and fluctuating (≥1°C difference but no sustained direction of change, n=56). Mixed random effects models with random intercept and multivariable logistic regression analysis were used to assess the association between hourly temperature and COx, as well as between temperature and clinical outcomes. COx showed a positive linear relationship with temperature (β=0.04±0.10, p=0.29). In patients where a continual increase or decrease in temperature was seen during the monitoring period, every 1°C change in temperature resulted in a COx change in the same direction by 0.04±0.01 (p<0.001) and 0.02±0.01 (p=0.12), respectively, after adjusting for PaCO2, haemoglobin, MAP, vasopressor and sedation use, and temperature probe location. There was no significant difference in mortality or poor outcome (mRS 4-6) between temperature pattern groups at discharge, 3, or 6 months.
Conclusion
In acute coma patients, increasing body temperature is associated with worsening cerebral autoregulation as measured by COx. More studies are needed to clarify the impact of increasing temperature on cerebral autoregulation in patients with acute brain injury.
Keywords: autoregulation, temperature, hypothermia, coma, neurocritical care
1. Introduction
Cerebral blood flow (CBF) is closely maintained by myogenic, neurogenic, endothelial, and metabolic mechanisms, which act in response to changes in cerebral perfusion pressure (CPP)[1, 2]. Cerebral autoregulatory dysfunction has been reported in the setting of acute neurological injury such as traumatic brain injury (TBI), subarachnoid haemorrhage (SAH), intracranial haemorrhage (ICH), and acute ischaemic stroke (AIS), as well as post-cardiac arrest and in sepsis[3–8]. In these instances, CBF becomes passive to systemic pressure changes, rendering the brain vulnerable to secondary insults such as ischaemia, infarction or haemorrhages[1]. Impaired cerebral autoregulation has indeed been established as a strong predictor of clinical outcome across these conditions[9–12]. Identifying factors which contribute to worsening autoregulation may therefore aid in the optimisation of clinical recovery in such patients.
Hypothermia has been suggested to offer some form of autoregulatory protection in patients with acute brain injury[13, 14]. Several studies have demonstrated preservation of vascular reactivity to various stimuli during hypothermia following TBI and cardiac arrest[13, 15]. Amongst these, Lee et al. reported a marked decrease in the lower limits of autoregulation with hypothermia in piglets following cardiac arrest compared to those who remained normothermic[14]. Further, Lavinio et al. have previously reported impairment of autoregulation, as measured by pressure reactivity index, when brain temperature exceeds 37°C following moderate hypothermia[16], and Cremer et al. noted impaired static autoregulation at temperatures above 40°C[17]. Despite these studies, the effect of body temperature on autoregulatory status in the initial few days following coma onset remains unclear.
This study aimed to assess the relationship between patterns of body temperature and CBF autoregulation. We used the near infrared spectroscopy (NIRS) derived cerebral oximetry index (COx) that has been validated in animals and humans as a non-invasive method for the continuous, clinical monitoring of CBF autoregulation[18–20]. Monitoring CBF autoregulation using NIRS relies on the use of regional cerebral oxygen saturation (rSO2) as a surrogate marker of CBF. Cerebral oxygenation is positively associated with CBF and tissue oxygen diffusivity, and negatively related to cerebral metabolic rate; the assumption that these remain stable throughout monitoring underpins the determination of CBF autoregulation using NIRS[21]. The comparison of NIRS derived rSO2 with functional MRI derived CBF by Alderliesten et al. demonstrated good correlation (Spearman 0.85, p=0.00001)[22]. We hypothesised that CBF autoregulation, as measured by COx, would be impaired at higher temperatures, and improve at lower body temperatures.
2. Methods
This is a retrospective analysis of data from an ongoing prospective study evaluating multimodal monitoring using NIRS in neurocritically ill patients. Data collection was approved by the Johns Hopkins School of Medicine Institutional Review Board. This study was subject to waiver of written consent due to minimal risk. Patients were identified from the period 2013 to 2017, and were eligible if acutely comatose (Glasgow Coma Score (GCS) ≤8) due to any aetiology, and being monitored with an arterial line. Exclusion criteria included non-comatose patients with GCS≤8, unavailability of autoregulation monitoring equipment, and change in temperature probe location during monitoring period. Aetiologies included were ICH, SAH, AIS, intraventricular haemorrhage, status epilepticus, meningitis, ventriculitis, encephalopathy, TBI, subdural haemorrhage, and post-cardiac arrest.
2.1 Autoregulation monitoring
Patients were monitored at the bedside after admission to the Neurocritical Care Unit (NCCU) of the Johns Hopkins Hospital. All patients had an acute neurological injury leading to coma; multimodal monitoring was initiated in the first 12-48 hours after coma onset for each patient, and was continued for up to three days. Monitoring was stopped if the patient emerged from coma or died during this period, and was halted or stopped if the patient was sent for a medical procedure. Continuous measurements of rSO2 of the left and right frontal lobe were made using NIRS INVOS™ 5100 (cerebral/somatic oximetry monitor, Covidien, Boulder, CO), as previously described[18, 19]. Mean arterial pressure (MAP) was continuously measured from an arterial catheter in the radial or femoral artery placed for clinical indications and connected to the analog outlet of the clinical haemodynamic monitor (Solar 8000i; General Electric, Boston, MA). The arterial transducer was zeroed and levelled at the right atrium after placement, and as required per nursing protocol. Both rSO2 and MAP signals were processed (MAP signals were sampled with an analogue-to-digital converter at 60 Hz) using ICM+ software (University of Cambridge, Cambridge, UK) to generate COx[20]. COx represents a continuous, moving Pearson correlation coefficient between rSO2 and MAP, where rSO2 serves as a surrogate for CBF[20]. The moving average of this coefficient was calculated over 10 second intervals in a five-minute window. Separate right and left COx values were generated; these were then averaged to provide the mean COx for each patient. Autoregulation improves as COx values approach 0, and is impaired as values exceed 0.1[23].
2.2 Temperature measurements
Temperature measurements were recorded between one and four-hourly, as determined by the NCCU managing team. Location of temperature measurement varied between patients as directed by the managing team, and included oesophageal, rectal, oral, axillary, forehead, and bladder probes. The method of monitoring in an individual patient remained constant during the period of autoregulation monitoring.
2.3 Temperature pattern group allocation
Patients were grouped by temperature pattern seen over the monitoring period as follows: no change (n=11), increasing (n=9), decreasing (n=9), or fluctuating (n=56). To determine the overall temperature change throughout the monitoring period, we first identified the minimum (Tmin) and maximum (Tmax) temperatures achieved in each patient. Patients were categorised as having no temperature change when Tmax – Tmin was <1°C. Where Tmax – Tmin was ≥1°C, we plotted absolute temperature against time during monitoring period to assess the trajectory of temperature change. Patients were categorised as having increasing or decreasing temperature when Tmax – Tmin was uniformly increased or decreased by ≥1°C, respectively. Patients in the fluctuating group had Tmax – Tmin ≥1°C but there was no sustained direction of change.
2.4 Patient outcome
Neurological outcome was assessed using the modified Rankin Scale (mRS); assessments were made at hospital discharge, and 3, and 6 months after discharge. These assessments were made from hospital charts and phone calls to patients using the validated telephonic mRS questionnaire[24].
2.5 Data analysis
Statistical analysis of data was performed using Stata 14 (StataCorp, College Station, TX). The Shapiro-Wilk test was used to assess normality of the continuous baseline variables; none of the variables were normally distributed. Baseline characteristics for each group were therefore compared using linear regression for continuous variables and logistic regression for categorical variables. The relationship between COx and temperature was initially assessed across all patients, before stratifying them into different temperature pattern groups. For each temperature pattern group, scatterplots were used to assess the relationship between COx and temperature prior to statistical analysis. Mixed random effects models with random intercept were then used to assess the association between hourly temperature and COx, adjusting for carbon dioxide (PaCO2), haemoglobin (Hb), MAP, vasopressor and sedation use, and temperature probe location, as vasomotor tone is influenced by the former three. To assess the association between temperature and outcomes, we performed multivariable logistic regression. The model was adjusted for age, GCS at coma onset, mRS on admission, maximum intracranial pressure (ICP), midline shift at pineal, and presence of thalamic lesion, herniation or infection. Significance level was accepted when p<0.05.
3. Results
A total of eighty-five patients were eligible and included in this study. All eighty-five patients were monitored using COx and had temperature recordings during the monitoring period. Patient demographics, comorbidities and clinical characteristics are given in Table 1 and Supplemental Table 1. The only difference between the temperature groups was Marshall Score. Although probe location varied between patients, there was consistency for each patient during monitoring. There was no difference in the number of patients on each sedative type, however sedative combinations and any alterations to this were not standardised.
Table 1.
Demographics, comorbidities and clinical characteristics of patients at baseline in each temperature group
| Demographic variables | No change (n=11) |
Increasing (n = 9) |
Decreasing (n=9) |
Fluctuating (n=56) |
P Value |
|---|---|---|---|---|---|
| Age in Years, median [IQR] | 54 [31] | 59 [23] | 61 [21] | 60 [18] | 0.38 |
| Sex, n (%) | 0.40 | ||||
| Female | 3 (27) | 4 (44) | 6 (67) | 27 (48) | |
| Race, n (%) | 0.08 | ||||
| Caucasian | 4 (40) | 4 (44) | 7 (78) | 18 (32) | |
| African American | 3 (30) | 4 (44) | 2 (22) | 32 (59) | |
| Other | 3 (30) | 1 (11) | 0 (0) | 5 (8) | |
| Diagnosis on Admission, n (%) | 0.89 | ||||
| ICH | 3 (27) | 4 (44) | 4 (44) | 15 (27) | |
| ICH Score, median [IQR] | 3.5 [1] | 2 [1] | 3 [1] | 3 [2] | 0.92 |
| Aneurysmal SAH | 2 (18) | 2 (22) | 1 (11) | 12 (21) | |
| Grade (modified Fischer scale), median [IQR] | 2 [2] | 4 [2] | 4 [0] | 4 [0] | 0.16 |
| TBI | 3 (27) | 3 (33) | 1 (11) | 9 (16) | |
| Marshall Score, median [IQR] | 6 [0] | 5 [2] | 3 [0] | 3 [1] | 0.02 |
| Status Epilepticus | 1 (9) | 0 (0) | 1 (11) | 7 (13) | |
| Ischaemic Stroke | 1 (9) | 0 (0) | 2 (22) | 8 (14) | |
| NIHSS, median [IQR] | 25 [0] | – | 25.5 [7] | 20.5 [5] | 0.44 |
| Other (Meningitis, Encephalopathy, Post-Cardiac Arrest) | 1 (9) | 0 (0) | 0 (0) | 5 (9) | |
| GCS at Coma Onset, median [IQR] | 3 [3] | 7 [2] | 7 [2] | 7 [4] | 0.14 |
| Midline Shift at Pineal in mm*, median [IQR] | 0.5 [5.6] | 2 [6] | 0 [3] | 0 [3] | 0.06 |
| Brain HerniationT, n (%) | 4 (36) | 2 (22) | 3 (33) | 15 (27) | 0.85 |
| HbŦ, median [IQR] | 11 [4.2] | 9.9 [1.3] | 11.4 [2.3] | 10 [2] | 0.63 |
| PaCO2Ŧ, median [IQR] | 37.5 [13] | 34.5 [6.2] | 37.5 [9.8] | 37.5 [8] | 0.63 |
| Infection presentT, n (%) | 1 (9) | 2 (22) | 3 (33) | 10 (18) | 0.57 |
| Mean COxŦ, median [IQR] | 0.08 [0.14] | 0.02 [0.11] | 0.09 [0.14] | 0.05 [0.10] | 0.79 |
| Mean rSO2Ŧ, median [IQR] | 66 [12] | 57 [16] | 63 [8] | 60 [20] | 0.13 |
| ICP Max, median [IQR] | 16.7 [8] | 18.3 [18.5] | 31 [23] | 17 [21] | 0.54 |
| Use of cooling or warming device | 3 (27) | 2 (22) | 3 (33) | 20 (36) | 0.89 |
| PressorsT | 5 (45) | 4 (44) | 7 (78) | 26 (46) | 0.38 |
| SedationT | |||||
| Propofol | 1 (9) | 3 (33) | 0 (0) | 11 (20) | 0.27 |
| Fentanyl | 3 (27) | 5 (56) | 5 (56) | 23 (41) | 0.51 |
| Midazolam | 0 (0) | 0 (0) | 0 (0) | 3 (5) | 1.00 |
| Dexmedetomidine | 3 (27) | 0 (0) | 1 (11) | 7 (13) | 0.38 |
No change group: <1°C difference between highest and lowest temperature during monitoring period; Increasing temperature group: ≥1°C increase in temperature throughout monitoring period; Decreasing temperature group: ≥1°C decrease in temperature throughout monitoring period; Fluctuating temperature group: ≥1°C difference between highest and lowest temperature during monitoring period but no consistent direction of change.
Abbreviations: IQR: interquartile range; ICH: Intracranial Haemorrhage; SAH: Subarachnoid Haemorrhage; TBI: Traumatic Brain Injury; NIHSS: National Institutes of Health Stroke Scale
maximum recorded throughout monitoring period, T at any time during the monitoring period, Ŧ mean recording from entire duration of monitoring period
3.1 Effect of temperature on autoregulation
The temperature changes for each group throughout the monitoring period are shown in Figure 1. The temperature change for the duration of monitoring (mean±SD) and p value for this change was 0.32±0.38°C (p=0.57) for the no change group; 2.52±1.07°C (p<0.001) for the increasing group; 1.77±1.14°C (p<0.001) for the decreasing group; and 2.30±1.41°C (p=0.39) for the fluctuating temperature group. The duration (mean±SD) of COx monitoring was 21.2±26.6 hours for the no temperature change group; 50±21.8 hours for the increasing temperature group; 31.3±19.4 hours for the decreasing temperature group; and 46.7±23.7 hours for the fluctuating temperature group (p=0.002 between groups).
Fig. 1.
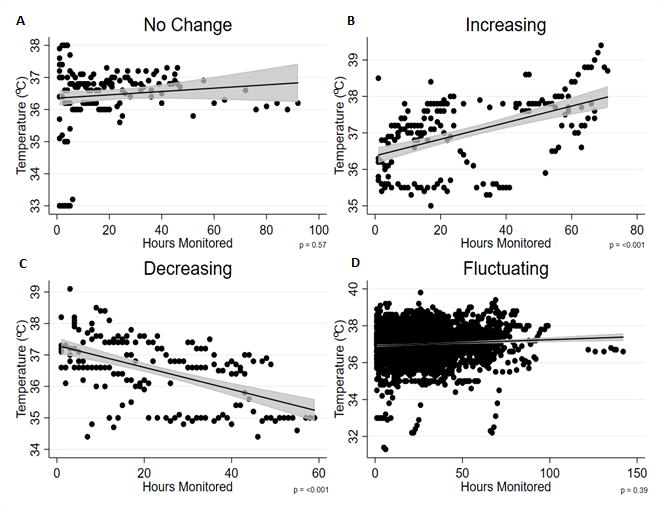
Scatter plot, regression line, 95% CI and p values for temperature versus hours monitored for each group
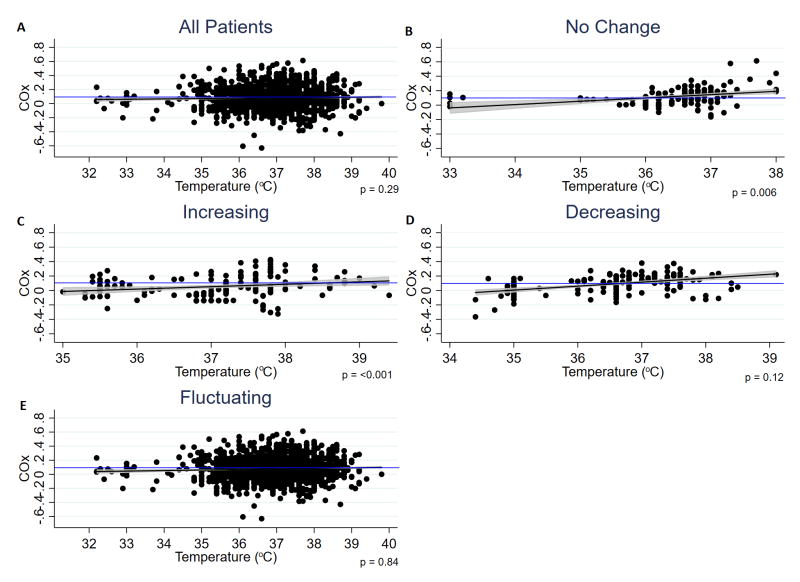
A linear relationship between autoregulation and body temperature was observed for all groups (Figure 2). COx increases (worsens) with increasing temperature, and decreases (improves) as temperature decreases. Autoregulatory impairment was seen above 38.6°C (Figure 2c), and improvement below 36°C (Figure 2b) and 36.6°C (Figure 2d).
Fig. 2.
Scatter plot, regression line and 95% CI for COx versus temperature. p values shown from multivariable analysis, which adjusted for PaCO2, Hb, MAP, vasopressor and sedation use, and temperature probe location. Blue line represents cut-off for autoregulatory impairment (COx=0.1)
Mixed random effects analysis of the relationship between COx and temperature change are listed in Table 2. Where all patients were analysed together, a positive linear relationship was seen; for each 1°C change in temperature, COx changed in the same direction by 0.04±0.10 (p=0.29). This relationship was significant in the univariate analysis of the no change (p=0.04) and increasing temperature groups (p<0.001). Multivariable mixed random effects analysis also showed a significant relationship in these two groups (p=0.006 for the no change group; p<0.001 for the increasing temperature group) after adjusting for PaCO2, Hb, MAP, vasopressor and sedation use, and temperature probe location, indicating that increasing body temperature is independently associated with worsening of autoregulation in acute coma patients in the neurocritical care setting, and that even relatively small variations in temperature may influence autoregulation.
Table 2.
Mixed random effects analysis showing the relationship between temperature pattern and COx
| Univariate Analysis | Multivariable Analysis* | |||
|---|---|---|---|---|
|
| ||||
| Temperature Pattern | Coefficient± SD | P Value | Coefficient± SD | P Value |
| All Groups (n† =1857) | 0.02 ± 0.09 | 0.13 | 0.04 ± 0.10 | 0.29 |
| No Change (n† =131) | 0.04 ± 0.02 | 0.04 | 0.06 ± 0.02 | 0.006 |
| Increasing (n† =189) | 0.05 ± 0.01 | <0.001 | 0.04 ± 0.01 | <0.001 |
| Decreasing (n† =159) | 0.02 ± 0.01 | 0.20 | 0.02 ± 0.01 | 0.12 |
| Fluctuating (n† =1417) | 0.01 ± 0.01 | 0.90 | 0.02 ± 0.02 | 0.84 |
Abbreviations: SD: standard deviation
model adjusted for PaCO2, Hb, MAP, vasopressor and sedation use, and temperature probe location
total number of independent measurements analysed
3.2 Effect of temperature on rSO2
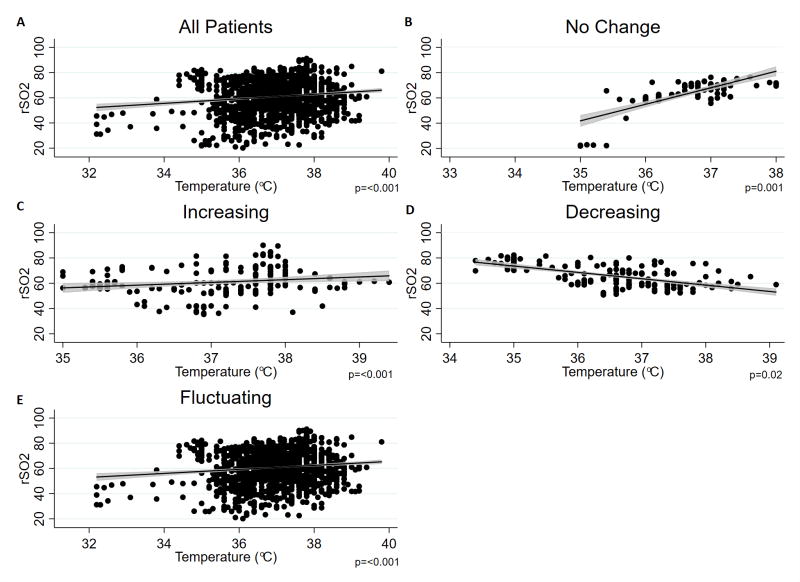
Changes in rSO2 with temperature are demonstrated in Figure 3. In all groups, apart from the decreasing temperature group, a positive relationship is seen between rSO2 and temperature; rSO2 increases with increasing temperature. In the decreasing temperature group, however, rSO2 is seen to decrease with increasing temperature. The beta coefficient±SD and p value is 1.6±0.2 (p<0.001) across all patients; 6.0±1.8 (p=0.001) for the no change group; 1.6±0.5 (p<0.001) for the increasing group; −1.1±0.4 (p=0.02) for the decreasing group; and 1.8±0.3 (p<0.001) for the fluctuating group.
Fig. 3.
Scatter plot, regression line and 95% CI for rSO2 versus temperature. p values shown from multivariable analysis, which adjusted for PaCO2, Hb, MAP, vasopressor and sedation use, and temperature probe location
3.3 Effect of temperature pattern on mortality and clinical outcomes
While our hypothesis and study design do not focus on clinical outcomes, we investigated the relationship of temperature effects and autoregulation on mortality and clinical outcomes. 99%, 82%, and 80% of patient outcome data was available at discharge, 3 and 6 months, respectively. Neither poor outcome, as defined by mRS score 4-6, nor mortality at discharge, 3 and 6 months were significantly different between each of the groups. The adjusted odds ratio (95% CI) for mortality at each interval assessed are: 11.5 (0.18-744, p=0.25), 9.6 (0.20-464, p=0.25) and 29.6 (0.57-1536, p=0.09) for the increasing, decreasing and fluctuating groups respectively. Multivariable logistic regression analysis was adjusted for age, GCS at coma onset, mRS on admission, maximum ICP, midline shift at pineal, and presence of thalamic lesion, herniation or infection.
4. Discussion
The main finding of this study is that CBF autoregulation, as measured by continuous COx monitoring was impaired with elevated temperature. Decreasing temperatures, however, showed some trend toward improvement in CBF autoregulation. Impairment in autoregulation appeared to occur at a temperature >38.6°C, and improvement below 36.0-36.6°C.
Fever is common in neurocritically ill patients, and is independently associated with poor outcomes following acute brain injury[25–28]. Our data thus highlight the potential role of impaired CBF autoregulation in contributing to these poor outcomes. Consequently, optimising cerebral perfusion based on CBF autoregulation monitoring in febrile critically ill patients might provide a means for improving outcomes.
Although a significant relationship between temperature and COx was also seen in the no change group, this may have been due to the large temperature range seen across all patients within this group (33-38°C; Figure 2b). Stratifying these patients further into groups based on mean temperature throughout the monitoring period i.e. normothermia (36-37.5°C, n=6), hypothermia (32-36°C, n=3) and hyperthermia (>37.5, n=2) would allow us to assess this. However, these sub-groups are too small to run regression analysis.
The Marshall Scores of patients in the no change and increasing temperature groups were significantly higher than those in the decreasing and fluctuating temperature groups. It has previously been established that autoregulatory disturbances occur more commonly, and to a greater degree, following severe TBI compared to moderate TBI[29–31]. Thus, it is possible that autoregulatory responsiveness to temperature is a function of severity of impairment, hence severity of brain injury. Alternatively, as temperature fluctuations are associated with more severe injury, change in temperature may represent an epiphenomenon rather than the cause of the autoregulatory changes seen here. Further prospective studies would be required to confirm these hypotheses.
COx measurements are generated using rSO2, with the assumption that cerebral metabolic rate remains constant throughout the period of monitoring. Cerebral metabolic rate, however, is temperature-dependent; for each degree centigrade rise in core body temperature, cerebral oxygen requirements increase by up to 13%[32]. Thus, the increase in COx with increasing temperatures seen here may simply be due to metabolism and/or cerebral blood flow associated changes in rSO2; the former may contribute to the effect on autoregulation itself. As these rSO2 measurements were obtained through forehead sensors, values of COx obtained reflect local CBF autoregulatory changes occurring in the frontal lobe compared to invasive ICP monitoring which provide a global measure[33]. Forehead skin blood flow and external carotid artery conductance also contribute to interference in these measurements[34]; changes in body temperature influence the magnitude of this contamination, however since the extent of this influence on rSO2 measurements is unclear, we were unable to correct for this[35]. Comparisons of rSO2 changes with temperature demonstrated a similar relationship to that seen between COx and temperature, except in the decreasing group, where the reverse was seen, and the fluctuating group, where a positive relationship was seen. Therefore, it is unlikely that changes in COx seen here are driven by changes in rSO2 alone.
Although we did not see a statistically significant improvement in autoregulation with drop in temperature, this may be because neuroprotective temperatures (<36°C)[35] were not sustained; the median temperature [IQR] in the decreasing temperature group was 36.6°C [1.8]. Previous animal studies have demonstrated significant improvement of autoregulation and vascular reactivity to acetylcholine and hypocapnia where hypothermic temperatures (32-33°C) were reached[13–15]. It would thus be beneficial to conduct prospective studies assessing autoregulatory changes in patients with temperatures at or lower than 36°C[36].
Additionally, the effects of neurological injury on autoregulatory status demonstrate temporal progression across different aetiologies of brain injury[37–39]. It is possible that secondary deterioration of autoregulation occurred in some patients during the monitoring period, which prevented us from seeing statistical significance between temperature decrease and autoregulation as measured by COx. The significant variation in length of monitoring may also have contributed to this, as the monitoring period may not have been long enough to capture any such autoregulatory changes. Indications for cooling in this group of patients included refractory intracranial hypertension and refractory status epilepticus. The decreasing temperature group had the highest mean maximum ICP (31mmHg). Furthermore, this group of patients had a higher rate of infection (33%) and pressor requirement (78%); septic shock could have both contributed to hypothermia and worsening of autoregulatory status. Therefore, improvements in autoregulatory status with decreasing temperature may not have been seen due to this group being comprised of more seriously ill patients.
Although there was no statistical difference in the number of patients on each type of sedation between each group, some patients were on multiple sedatives or had a change in sedative during the monitoring period. Sedatives are associated with reductions in both cerebral metabolic rate and CBF, the extent of which is dependent on sedative type and dose[40]. The effects on cerebral metabolic rate would also have influenced rSO2 measurements. Any changes in dosing or sedative type during the monitoring period may therefore have contributed to the autoregulatory changes seen. Use of vasopressors also increases CBF[41]; changes in COx may therefore have coincided with vasopressor administration rather than occurring as a result of temperature changes.
Despite observing a linear relationship between autoregulation and temperature, this did not translate into differential outcomes between the groups. Although both fever and impaired autoregulation have been independently associated with long term outcomes, we did not expect to see a relationship between temperature pattern and outcome as this study was not powered to assess this hypothesis. Even though this study investigated a large cohort of acutely comatose patients, there were only a small number of patients in the no change (n=11), increasing (n=9), and decreasing (n=9) groups; the majority of patients were in the fluctuating group (n=56). The multiple fluctuations in this latter group prevented us from finding a linear relationship. Furthermore, the short observation period could not have been reflective of the patient’s overall status throughout the duration of their admission; temperatures outside of this monitoring period are unknown. For example, patients who were febrile by the end of the monitoring period may have had this corrected subsequently, thus restoring autoregulatory function and eliminating the adverse effect of high temperatures. Outcomes may also be associated with length of time at high or low temperatures.
While our COx data was collected prospectively, the retrospective collection of temperature data in this study and heterogeneity of coma aetiology precludes our ability to definitively identify a causal relationship between autoregulatory impairment and changes in temperature. The relationship between temperature and COx may vary between aetiologies. The small number of patients within each aetiology group in this study, however, prevented us from investigating this in more detail. Although cooling or warming devices were used in a similar proportion of patients within each group (Table 1), temperature management protocols were not standardised so may also have influenced the results. We also note the difference in location of temperature probes used between patients; bladder probes show a variation of 0.03±0.23°C from pulmonary artery catheter measurement, compared to axillary probes which show a greater variability of −0.68±0.57°C[42]. However, consistency for each patient was sufficient to allow us to assess the association between temperature and changes in CBF autoregulation. We also used rSO2 measurements as a surrogate of CBF; this can be confounded by changes in acid-base status and PaCO2[43]. Additionally, this study was limited to comatose patients; assessment of such changes in patients prior and subsequent to coma would be useful.
Whilst our data presents evidence of some temperature-mediated effects on autoregulation, future work would require prospective studies to investigate the relationship between temperature and autoregulation in more detail. Such studies should be conducted across a population of patients with a specific brain injury and include a larger sample size and monitoring of other metabolic indicators. Assessments of the temperature range within which autoregulation is optimal, and whether these changes are dynamic in response to temperature manipulation, should also be made.
5. Conclusion
In acute coma patients, increasing body temperature is associated with worsening cerebral autoregulation as measured by COx. Close regulation of temperature within the neurocritical care setting may therefore have a role in management, however further prospective studies are needed to clarify the impact of changing temperature on cerebral autoregulation in relation to outcomes in patients with acute brain injury. Assessment of temperature effects on neurological outcomes, in particular, are required to determine whether temperature interventions are warranted.
Supplementary Material
Acknowledgments
Dr. Ziai received funding from Headsense. Dr. Hogue received funding from Medtronic (advisory board; Medtronic makes NIRS monitors), and he disclosed off-label product use of autoregulation monitoring. Drs. Hogue and Brown received support for article research from the National Institutes of Health. Dr. Brown’s institution received funding from Medtronic. Dr. Rivera-Lara disclosed a grant from Covidien/Medtronic that provided equipment for monitoring.
Conflicts of Interest and Source of Funding: Author LR has received research grants from Covidien/Medtronic.
Footnotes
Copyright form disclosure: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Armstead WM. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol Clin. 2016;34:465–477. doi: 10.1016/j.anclin.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, et al. Cerebral Autoregulation-oriented Therapy at the Bedside A Comprehensive Review. Anesthesiology. 2017;126:1187–1199. doi: 10.1097/ALN.0000000000001625. [DOI] [PubMed] [Google Scholar]
- 3.Czosnyka M, Smielewski P, Piechnik S, et al. Cerebral autoregulation following head injury. J Neurosurg. 2001;95:756–763. doi: 10.3171/jns.2001.95.5.0756. [DOI] [PubMed] [Google Scholar]
- 4.Heilbrun M, Olesen J, Lassen NA. Regional cerebral blood flow studies in subarachnoid hemorrhage. J Neurosurg. 1972;37:36–44. doi: 10.3171/jns.1972.37.1.0036. [DOI] [PubMed] [Google Scholar]
- 5.Oeinck M, Neunhoeffer F, Buttler K-J, et al. Dynamic Cerebral Autoregulation in Acute Intracerebral Hemorrhage. Stroke. 2013;44:2722–8. doi: 10.1161/STROKEAHA.113.001913. [DOI] [PubMed] [Google Scholar]
- 6.Eames P, Blake M, Dawson S, et al. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundgreen C, Larsen F, Herzog T, et al. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;31:128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 8.Schramm P, Klein K, Falkenberg L, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16:181. doi: 10.1186/cc11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeger M, Dengl M, Meixensberger J, et al. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010;38:1343–1347. doi: 10.1097/CCM.0b013e3181d45530. [DOI] [PubMed] [Google Scholar]
- 10.Panerai RB, Kerins V, Fan L, et al. Association between dynamic cerebral autoregulation and mortality in severe head injury. Br J Neurosurg. 2004;18:471–479. doi: 10.1080/02688690400012343. [DOI] [PubMed] [Google Scholar]
- 11.Czosnyka M, Smielewski P, Kirkpatrick P, et al. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17. doi: 10.1097/00006123-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, et al. Predictors of Outcome With Cerebral Autoregulation Monitoring: A Systematic Review and Meta-Analysis. Crit Care Med. 2017;45:695–704. doi: 10.1097/CCM.0000000000002251. [DOI] [PubMed] [Google Scholar]
- 13.Fujita M, Wei EP, Povlishock JT. Effects of Hypothermia on Cerebral Autoregulatory Vascular Responses in Two Rodent Models of Traumatic Brain Injury. Journal of Neurotrauma. 2012;29:1491–1498. doi: 10.1089/neu.2011.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JK, Brady KM, Mytar JO, et al. Cerebral Blood Flow and Cerebrovascular Autoregulation in a Swine Model of Pediatric Cardiac Arrest and Hypothermia. Crit Care Med. 2011;39:2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda Y, Wei E, Kontos H, et al. Effects of delayed, prolonged hypothermia on the pial vascular response after traumatic brain injury in rats. J Neurosurg. 2003;99:899–906. doi: 10.3171/jns.2003.99.5.0899. [DOI] [PubMed] [Google Scholar]
- 16.Lavinio A, Timofeev I, Nortje J, et al. Cerebrovascular reactivity during hypothermia and rewarming. British Journal of Anaesthesia. 2007;99:237–244. doi: 10.1093/bja/aem118. [DOI] [PubMed] [Google Scholar]
- 17.Cremer OL, Diephuis J, van Soest H, et al. Cerebral oxygen extraction and autoregulation during extracorporeal whole body hyperthermia in humans. Anesthesiology. 2004;100:1101–1107. doi: 10.1097/00000542-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, et al. Validation of Near-Infrared Spectroscopy for Monitoring Cerebral Autoregulation in Comatose Patients. Neurocrit Care. 2017 doi: 10.1007/s12028-017-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41:1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono M, Zheng Y, Joshi B, et al. Validation of a stand-alone near-infrared spectroscopy system for monitoring cerebral autoregulation during cardiac surgery. Anesth Analg. 2010;116:198–204. doi: 10.1213/ANE.0b013e318271fb10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner L, Pfister D, Strebel S, et al. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10:122–128. doi: 10.1007/s12028-008-9140-5. [DOI] [PubMed] [Google Scholar]
- 22.Alderliesten T, de Vis JB, Lemmers PM, et al. Simultaneous quantitative assessment of cerebral physiology using respiratory-calibrated mri and near-infrared spectroscopy in healthy adults. NeuroImage. 2014;85(Pt 1):255–263. doi: 10.1016/j.neuroimage.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Budohoski KP, Czosnyka MS, Smielewski P, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- 24.Bruno A, Akinwuntan AE, Lin C, et al. Simplified modified rankin scale questionnaire: Reproducibility over the telephone and validation with quality of life. Stroke. 2011;42:2276–2279. doi: 10.1161/STROKEAHA.111.613273. [DOI] [PubMed] [Google Scholar]
- 25.Bohman LE, Levine JM. Fever and therapeutic normothermia in severe brain injury: an update. Curr Opin Crit Care. 2014;20:182–188. doi: 10.1097/MCC.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 26.Greer DM, Funk S, Reaven N, et al. Impact of fever on outcome in patients with stroke and neurologic injury: a comprehensive meta-analysis. Stroke. 2008;39:3029–3035. doi: 10.1161/STROKEAHA.108.521583. [DOI] [PubMed] [Google Scholar]
- 27.Naidech AM, Bendok B, Tamul P, et al. Medical complications drive length of stay after brain hemorrhage: a cohort study. Neurocrit Care. 2009;10:11–19. doi: 10.1007/s12028-008-9148-x. [DOI] [PubMed] [Google Scholar]
- 28.Diringer MN, Reaven N, Funk S, et al. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004;32:1489–1495. doi: 10.1097/01.ccm.0000129484.61912.84. [DOI] [PubMed] [Google Scholar]
- 29.Bouma GJ, Muizelaar JP, Bandoh K, et al. Blood pressure and intracranial pressure-volume dynamics in severe head injury: relationship with cerebral blood flow. Journal of Neurosurgery. 1992;77:15–19. doi: 10.3171/jns.1992.77.1.0015. [DOI] [PubMed] [Google Scholar]
- 30.Hlatky R, Valadka AB, Robertson CS. Intracranial Pressure Response to Induced Hypertension: Role of Dynamic Pressure Autoregulation. Neurosurgery. 2005;57:917–923. doi: 10.1227/01.neu.0000180025.43747.fc. [DOI] [PubMed] [Google Scholar]
- 31.Jünger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. Journal of Neurosurgery. 1997;86:425–432. doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich WD, Busto R, Valdes I, et al. Effects of normothermic versus mild hyperthermic forebrain ischemia in rats. Stroke. 1990;21:1318. doi: 10.1161/01.str.21.9.1318. [DOI] [PubMed] [Google Scholar]
- 33.Ogoh S, Sato K, Okazaki K, et al. A decrease in spatially resolved near-infrared spectroscopy-determined frontal lobe tissue oxygenation by phenylephrine reflects reduced skin blood flow. Anesth Analg. 2014;118:823–829. doi: 10.1213/ANE.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 34.Bein B, Cavus E, Stadlbauer K, et al. Monitoring of cerebral oxygenation with near infrared spectroscopy and tissue oxygen partial pressure during cardiopulmonary resuscitation in pigs. European Journal of Anaesthesiology. 2006;23:501–509. doi: 10.1017/S0265021506000366. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36 °C after cardiac arrest. New England Journal of Medicine. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 36.Geocadin RG, Wijdicks E, Armstrong MJ, et al. Practice guideline summary: reducing brain injury following cardiopulmonary resuscitation. Neurology. 2017;88:2141–2149. doi: 10.1212/WNL.0000000000003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White JR, Farukhi Z, Bull C, et al. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29:534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger M, Schuhmann M, Soehle M, et al. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38:981–986. doi: 10.1161/01.STR.0000257964.65743.99. [DOI] [PubMed] [Google Scholar]
- 39.Reinhard M, Neunhoeffer F, Gerds T, et al. Secondary decline of cerebral autoregulation is associated with worse outcome after intracerebral hemorrhage. Intensive Care Med. 2010;36:264–271. doi: 10.1007/s00134-009-1698-7. [DOI] [PubMed] [Google Scholar]
- 40.Oddo M, Crippa IA, Mehta S, et al. Optimizing sedation in patients with acute brain injury. Crit Care. 2016;20:128. doi: 10.1186/s13054-016-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfister D, Strebel SP, Steiner LA. Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur J Anaesthesiol Suppl. 2008;42:98–103. doi: 10.1017/S0265021507003407. [DOI] [PubMed] [Google Scholar]
- 42.Erickson RS, Kirklin S. Comparison of ear-based, bladder, oral, and axillary methods for core temperature measurement. American Journal of Critical Care. 2007;16:485–496. doi: 10.1097/00003246-199310000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Vretzakis G, Georgopoulou S, Stamoulis K, et al. Cerebral oximetry in cardiac anaesthesia. J Thorac Dis. 2014;6:S60–S69. doi: 10.3978/j.issn.2072-1439.2013.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.