Abstract
There is increasing evidence from pre-clinical and human studies that nutrition in the late fetal and early neonatal period has a significant impact on neurodevelopment across the lifespan. Certain nutrients have particularly large effects in this time period, and their deficits cause greater long-term risk. The mechanisms by which nutrients influence early brain growth and the sensitive periods for when certain nutrients should be provided are being elucidated. Assessments of nutritional status that index brain growth and predict long-term development are important to assess the efficacy of early life nutritional therapies.
Conclusion
Optimizing nutrition during fetal and early postnatal life is a golden opportunity to impact neurodevelopment and brain function across the lifespan.
Keywords: Nutrition, Brain, Fetus, Neonate, Development
Overview
One of the most important relevant long-term health outcomes of early life experience is neurodevelopment. Much of brain development in early life is highly pre-programmed and is to a great extent experience-independent. Nevertheless, there are important and controllable environmental factors that profoundly influence early brain development including nutrition, reduction of toxic stress and environmental enrichment (1). Of these, nutrition falls in the direct purview of medical caregivers and can be optimized to promote neurodevelopment or can be ignored and thereby confer a risk to the developing nervous system. An important issue is to identify more precisely the sensitive time windows within which to provide nutritional interventions in order to promote optimal neurodevelopment in children (1). Recent studies suggest that the window for nutrition is in late fetal and early postnatal life with greater and greater recognition that postnatal nutritional success is highly dependent on optimizing fetal nutritional status prior to birth (1,2). Therefore, a timely question is whether appropriate assessments and interventions are being made during the period of greatest vulnerability to the developing brain: from fetal life through the first two years of postnatal life.
Milestones in Early Brain Development
The newborn human brain undergoes a remarkable transformation in form and function beginning at the age of extra-uterine viability at approximately 23-24 weeks post-conceptional age (PCA) through the first two years of life (3). Even during the first four months of that time span, from 24 weeks to 40 weeks PCA, the brain changes from a smooth, bi-lobed, and relatively non-complex organ into one characterized physically by the sulci and gyri more typical of the adult brain. The increased physical complexity reflects the rapid rate of neuronal and glial growth and development within the brain. As these cells become more complex and interconnected, brain functional capacity increases. Some fundamental behavioral functions are already present at birth, implying that structural connectivity of the relevant brain circuits have begun. Examples of these early developing circuits include primary sensory systems such as hearing, touch and pain sensation, taste and, to a lesser extent, vision (3,4). More complex behaviors such as recognition memory, dependent in large part on hippocampal development, can even be demonstrated in the term and preterm neonate (3,4,5). Other brain functions that are being supported through this burst of circuit development and connectivity do not have obvious behavioral manifestations at birth. Despite the lack of behavioral manifestations, the neural circuits underlying more complex behaviors that will appear later in childhood are being constructed (3,4). For example, behaviors such as working memory, set-shifting, multi-tasking, and attention that are not present in the neonate nevertheless have their neural ontogenies prior to term birth.
Therefore, attention to neurodevelopment in preterm and term infants is important not only for immediate function, but also for scaffolding for later-developing structures and circuits. The time period starting in the last trimester of pregnancy and progressing through the first 12-24 months post-term is characterized by the development of primary circuits that serve important functions. The brain regions and processes that are rapidly developing during this time period include the hippocampus and striatum that support fundamental declarative and implicit learning, respectively, myelination that supports speed of processing, and monoamine neurotransmitter systems that support reward processing (4,6). Optimal construction of these primary systems is key to proper development of later emerging higher order neural systems that rely on the fidelity of the early developing systems (1). For example, the later developing prefrontal cortex relies on initial connections from striatum and hippocampus early in life. Perinatal events that affect hippocampus and striatal integrity, such as nutrient/substrate restriction, result not only in abnormal function of these primary areas, but also in abnormal frontal lobe function. Thus, it is not surprising that attention deficit/hyperactivity behavioral phenotypes in middle childhood are more prevalent following intrauterine growth restriction (7).
Principles of Nutrient-Brain Interactions
The principles that govern how nutrients regulate brain development during prenatal and postnatal life are outlined in the following sections. These principles are key to reading and understanding the frequently contradictory literature on nutrition and brain development (1).
The Metabolic Demand of the Rapidly Growing Fetal/Neonatal Brain
The growth rate of the brain in this time period is among the highest during life span. The neonatal brain in the human consumes 60% of the body’s total oxygen and therefore caloric consumption (8). This truly remarkable value far exceeds that of non-human species including monkeys, sheep and rodents as well as that of the adult human (8). Rapidly growing organs are more vulnerable to damage if critical nutritional substrates that support that growth are not provided in adequate amounts (9). In the case of the brain, however, this increased vulnerability is counterbalanced by a higher degree of plasticity and amenability to repair (10). Overall, there is consensus that vulnerability outweighs plasticity, a principle that translates into a practice that encourages staying on developmental trajectory rather than relying on “catch-up” after a period of deprivation. During normal development, the brain goes from a highly plastic but functionally non-specific organ to a highly specific but much less plastic one (10). The specification of the brain is manifested by the remarkable behaviors it sub-serves. However, it appears that the cost of specification is loss of plasticity and ability to repair itself after injury. This period of specification is characterized by rapid growth and development and is often referred to as a critical or sensitive period (1). These two terms are often used interchangeably, but important conceptual differences distinguish them. Critical periods refer to epochs of development with a sharply defined time limit after which repair of an abnormally developed system is no longer possible and thus the neurodevelopmental effects are irreversible (1,10). Sensitive periods refer to broader time epochs when a developing system is particularly responsive to being shaped by stimuli, eg nutrients, but where the effects are not necessarily permanent (1,11). Nutrient effects on brain development can exhibit either characteristic.
The Importance of Timing, Dose and Duration
Another major principle is that the positive or negative effects of nutrients on the brain are based on the timing, dose and duration of the exposure (9). This principle resides biologically in the fact that the brain is not a homogenous organ. Rather, it is made up of distinct regions (eg, hippocampus, cortex, striatum, cerebellum) and processes (eg, myelination, neurotransmitters), each of which has a different developmental trajectory and set of nutrient requirements. The vulnerability of the brain and thus the behavioral phenotype that results as a consequence of a nutrient deficit is a function of two factors: when the nutrient deficit is likely to occur in the pediatric lifespan and the region’s requirement for the nutrient at that time. Thus, nutrient deficits do not typically show a “signature” brain-behavior effect because timing plays such a large role in determining the behavioral phenotype. Based on this regionalization hypothesis, nutrients can have global (eg, protein, energy, iodine) or regional (eg, iron) effects on the brain.
Design and Interpretation of the Nutrition-Brain Development Literature
Clinical studies of the effect of nutritional interventions or nutritional status on neurobehavioral outcomes must be designed and interpreted with the following rules in mind, or true connections between nutrient and developmental outcome may be obscured. Specifically, for a nutrient-brain connection to be verified or, alternately, unsupported, the following must be considered: 1) the time (age) to start supplementation of a given nutrient must be concomitant with development of the brain structures or circuitry known to be dependent on that nutrient. Beginning an intervention too early or too late will likely have no measureable effect on the outcome of interest; 2) the dose and duration of supplementation must be considered, as must be the current nutritional status of the population of interest. It is important to note that provision of more of a dietary source of a nutrient or medicinal supplementation of an already replete population, may not necessarily lead to better outcomes; and 3) the appropriate neurobehavioral tests and outcome measures that test neural circuits potentially altered by the nutrient must be applied at the correct assessment age. Null findings on global tests (e.g., Bayley Scales of Infant Development; Wechsler Preschool and Primary Scale of Intelligence) following nutrient supplementation trials are common and may mask true, but more subtle nutrient-brain connections measureable by a more specialized test.
Because of their required nuance in design and outcome assessment, these clinical nutrient-brain relationships are typically not best assessed with meta-analyses. In an effort to increase statistical power with greater sample size, this analytic method frequently combines studies of different ages of the start of intervention, different dosages of nutrient and length of supplementation period, and different neurobehavioral assessment tools, inevitably leading to greater variability, increased type II (beta) error, and overall null findings (12).
For example, a recent Cochrane Review (13) concluded that iron treatment in young children under three years of age with iron deficiency anemia has no effect on psychomotor development or cognitive function underscores the problems inherent in using meta-analyses to examine nutrient and brain interactions. In that review, six studies providing iron for less than 30 days to iron-deficient anemic children under the age of three were combined statistically, with each study using a global test (Bayley Scales) to assess cognitive and psychomotor development.
Although the specific age ranges of the included studies were not specified, birth to three years includes a significant span of time–12 - 36 months–when brain iron deficiency would not be expected to affect global outcome. The brain structures dependent on iron that would dictate later global outcomes (the hippocampus and striatum) would have already developed during the last trimester of pregnancy and the first 12 post-natal months. Repletion of iron status in iron-deficient and anemic toddlers would instead be expected to improve behavioral measures such as wariness and hesitancy that are secondary to the insults to dopaminergic signaling secondary to iron deficiency that can occur at any age. Behavioral outcomes were not assessed in the meta-analysis. Further, the duration of iron therapy was short (< 30 days) in all studies, and the amount or formulation of iron given was not specified or known to be the same.
Studies assessing nutrient and brain interactions require careful design that aligns the timing of peak brain need for a nutrient with simultaneous provision of the adequate amounts of nutrient. These studies also require alignment of the timing, dose, and duration of the nutrient with circuit-specific outcome measures. Attention to these nuances is difficult to ensure with a meta-analysis, but is requisite for accurate assessment of brain and nutrient relationships.
Nutrients that Affect Brain Development in the Ex Utero Fetus and Term Neonate
All nutrients are important for structural and functional brain development, but those that support energy, carbohydrate, protein, and fat metabolism are of particular importance (14). Substrates that support mitochondrial health are relevant because mitochondrial function appears to be programmable in early life and thus may influence lifespan health. Table 1 presents macronutrients, micronutrients, and vitamins/cofactors that are particularly important for the developing brain. Each has been shown in humans or in pre-clinical models to exhibit a critical or sensitive period early in life. Many demonstrate the property that an early life deficiency results in life-long brain dysfunction. Epigenetic mechanisms have been evinced for some nutrients’ long-term effects on genes regulating adult brain function.
Table 1.
Nutrients with Particularly Large Effects on Early Brain Development and Subsequent Adult Function.
| Nutrient | Main Brain Process(es) Affected | Evidence for Critical Period(s) for Neuro-development | Early Deficiency Results in Adult Dysfunction | Epigenetic Mechanism of Long-term Effects |
|---|---|---|---|---|
| Protein | Structure Growth Factors Neuro-transmitters |
Yes | Yes | Suspected |
| LC-PUFA | Membrane integrity Signaling |
Yes | Yes | Yes |
| Glucose | Energetics | Yes | Yes | No |
| Iron | Energetics Myelination Monoamine Neuro-transmission |
Yes | Yes | Yes |
| Zinc | Growth Factors Synaptic Efficacy |
Yes | Yes | No |
| Copper | Energetics Myelination Neurotransmission |
Yes | Yes | No |
| Iodine | Thyroid-dependent Myelination, Synaptogenesis, & Energy Metabolism |
Yes | Yes | No |
| Vitamin B12 | Neuronal structure Myelination |
Yes | Yes | Yes |
| Folate | Neural Tube Closure Neuronal Structure |
Yes | Yes | Yes |
| Choline | Neurotransmitters Myelination |
Yes | Yes | Yes |
Whereas it is tempting to think of nutrient effects on the brain as predominantly “neuronal”, non-neuronal cells including oligodendrocytes, astrocytes, and microglia can also be affected (15, 16). Nutrients certainly affect anatomical structure through signaling pathways such as mammalian Target of Rapamycin (mTOR) (17). The mTOR pathway senses the status of important metabolic substrates such as amino acids, iron, glucose, and oxygen and integrates their inputs through a multiple kinase-driven system to determine rates of actin polymerization, protein translation, DNA transcription, and autophagy (17). Actin polymerization is key to axonal and dendritic structural development and it is well recognized that dendritic structural complexity correlates closely with function. Other nutrients that have profound effects on brain anatomy include iodine, zinc, copper, choline, vitamin A, and long-chain polyunsaturated fatty acids (LC-PUFAs) (14). Nutrients also affect the function of the brain through their effects on neurotransmitter concentrations, receptors, and re-uptake mechanisms (18). Nutrients that particularly affect neurotransmitter function include protein, iron, zinc, copper, and choline. Nutrients also affect the electrophysiologic potential of neurons through their effects on metabolic rate. The electrical potential generated by neurons is a highly energy taxing process and relies on healthy mitochondria generating adequate amounts of ATP (19). Thus, nutrients that support oxidative and glycolytic metabolism are in high demand in the developing brain and include glucose, protein, iron, and zinc (14).
Developmental Origins and the Brain: the true cost to society of early life malnutrition
Altered nutrient status in fetal and early postnatal life can cause acute brain dysfunction only during the period of deficiency through the mechanisms described above. If repletion of a deficiency resolved all of the neurologic issues, catch-up nutritional management could be relied upon to return the child to the normal developmental trajectory in order to optimize outcomes. However, substantial evidence in pre-clinical models exists for several nutrients including protein, energy, iron, choline, and LC-PUFAs (20,21,22,23,24) that early life deficiencies confer neurologic risk well beyond the time of deficiency and in spite of complete nutrient repletion (Table 1). This risk includes altered developmental trajectory resulting in adult dysfunction.
The adult dysfunction, quantified in some studies as loss of educational and job potential, is the true cost to society of early life malnutrition. It has been estimated that eradication of the three most common micronutrient deficiencies (i.e., iron, zinc, and iodine) would shift the world’s IQ by 10 points to the positive (25). Generalized fetal malnutrition as manifested by intrauterine growth restriction reduces IQ by 7 points at age 7 and increases the risk of schizophrenia in adulthood (26). Fetal or early postnatal iron deficiency increases the risk of autism, schizophrenia, depression, anxiety, and poorer executive function in adulthood (27, 28, 29). The role of early life nutrient status in the development of psychopathologies in adulthood are supported by preclinical models as well as by these epidemiologic studies. This conceptualization constitutes the mental health version of the Developmental Origins of Health and Disease with respect to adult cardiovascular risk. The mechanisms by which these long-term effects occur are an active area of research because prevention of them would have high health and economic reward for societies. Two theories, which are not mutually exclusive, can account for the observed long-term loss of synaptic plasticity by early-life nutrient deficits.
Loss of Adult Neural Plasticity Through Residual Structural Deficits
One theory is that, in the brain, residual brain regional structural deficits from the neonatal period permanently alter the integrity and function of the neural circuits containing the affected brain region(s). These residual deficits appear to be the result of nutrient deficiencies that occur during and beyond the critical period of development of a particular brain region, implying that the capacity for plasticity was no longer present when the nutrient was repleted (10). Preclinical models of hippocampal neuron specific iron deficiency demonstrate complete structural and functional recovery if iron is repleted during the critical period of rapid growth, but not afterward (30).
Loss of Adult Neural Plasticity Through Epigenetic Modification of Chromatin
The second theory is that nutrients can alter regulation of synaptic plasticity genes through epigenetic modification of chromatin (Figure 1). Nutrients can affect both histone biology (eg, methylation, acetylation) and DNA CpG island methylation. Several fetal/neonatal nutritional conditions have been associated with brain epigenetic modifications that last into adulthood in rodent models. Intrauterine growth restriction (IUGR) represents a state of generalized fetal macro- and micronutrient malnutrition and has been associated with disruption of hippocampal H4K20 histone methylation (20). LC-PUFA stats also modified DNA methylation of BDNF (21). Iron deficiency alters the activity of JARID-containing histone demethylases which also regulate BDNF expression and DNA methylation in the hippocampus (23, 24). Choline, which can act as a methyl donor (22), reverses BDNF suppression induced by iron deficiency when it is given in one of two critical periods (31). Understanding the mechanisms behind the long-term effects provides an opportunity for better timed interventions with nutrient supplements (eg, methyl diets) that can work as potential epigenetic work-arounds to protect the developing brain when nutrient supplementation is not possible.
Figure 1.
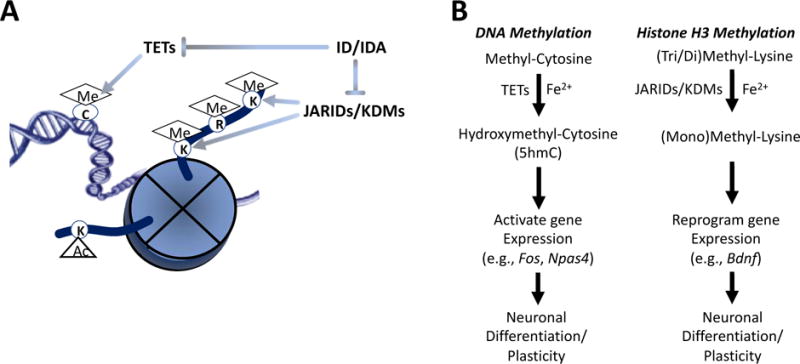
Iron-dependent epigenetic modifications (A) that potentially modify genes that affect neuronal differentiation during development and adult neural plasticity (B) (23,24,31)
Clinical Studies of Early Nutritional Status and Neurodevelopment
In general, clinical studies in humans have reinforced the findings from pre-clinical animal models of connections between many key nutrients and early brain development (Table 1). The clinical literature on early provision of sufficient protein and iron and resultant brain outcomes is perhaps most expansive, but work supporting a role for other macronutrients, including polyunsaturated fatty acids (PUFAs), and micronutrients, including zinc, iodine, and vitamin B12 and brain development is substantial and growing. The following sections discuss the effects of macro and micronutrients provided during early life on short- and long-term neurodevelopmental outcome, with an emphasis on the effects of protein and iron.
Macronutrient Effects on Brain Development
Protein
Macronutrient status and its anthropometric correlate, growth velocity (see below), follow the timing, dose, and duration rules as they relate to brain development. Improved linear growth is an important finding because linear growth prenatally and in early infancy is a consistent predictor of neurobehavioral outcomes. The importance of protein intake in early life was established by the landmark studies in Guatemala by Pollitt and colleagues (32) who demonstrated that children whose mothers consumed a high-energy, high-protein beverage in during pregnancy and who continued to consume the beverage until they had reached two years of age had higher scores of general intellectual abilities, including better information processing, numeracy, and vocabulary, compared to children exposed to a beverage containing no protein.
Improving protein intake and linear growth in early life is critical to later cognition. Pongcharoen et al. related the Intelligence Quotient (IQ), as measured by the Wechsler Intelligence Scale, of 560 9-year-old Thai children to indices of birth size and rate of postnatal growth (33). Researchers found that infant length at birth and throughout the first 12 months of life were strongly positively correlated with child IQ at 9 years of age. Early infancy (birth to 4 months) weight was also associated with IQ at 9 years. No index of growth after 12 months was related to IQ in later childhood.
A supply of adequate protein and other nutrients that support growth and brain development is critical even before birth. In a large cohort of Nepalese children who underwent testing for cognitive testing at age 7-9 months (34), low birthweight was significantly associated with poorer scores on the Universal Nonverbal Intelligence Test, tests of executive function, and the Movement Assessment Battery for Children. Further analyses revealed that intra-uterine growth restriction [IUGR, defined as being born small-for-gestational age (SGA), or < 10th percentile of the reference], rather than being born preterm, drove the association between low birthweight and poorer test scores. Given that the prevalence of SGA was more than 50% among the 7-9 year-old children tested, the impact on the collective developmental trajectory of these children cannot be overstated. Optimizing maternal nutrition during pregnancy would thus help prevent intrauterine growth restriction and associated cognitive deficits present in the early school years of childhood.
Long-chain polyunsaturated fatty acids (LCPUFAs)
The effects of early-life supplementation of LCPUFA’s on child development has been extensively studied. Although recent meta-analyses of LCPUFAs during gestation, infancy, and early childhood report no significant benefit with regard to cognition and attention (35, 36), several smaller studies suggest that benefit is apparent on more specialized tasks assessed in older children rather than in infants and toddlers. One series of studies (37-39) examined the effect of maternal supplementation with arachidonic acid (ARA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3) from week 18 of pregnancy to 3 months postpartum on infant cognitive outcomes. Supplementation with ARA as compared to DHA provided no additional benefit on infant cognitive outcomes or growth at 3 months of age (37), but infants whose mothers received DHA during pregnancy exhibited better mental processing scores and 4 years of age (38) and better sequential processing scores at 7 years of age (39). Similarly, a recent study found no benefit of LCPUFA-fortified formula in infants at 18 months on standardized developmental tests, but reported benefit in children at 4-6 years of age on more specific tasks (40), such as rule-learning, inhibition, and vocabulary tests. These findings collectively emphasize the need for continued follow-up of cohorts through later childhood when children can be tested on more complex behaviors.
Micronutrient Effects on Brain Development
The clinical literature with regard to early life provision of other macronutrients, including, and other micronutrients, including iodine, zinc, and vitamin B12, and brain development is also growing.
Iron
Iron deficiency is a good paradigm for demonstrating the importance of timing, dose and duration effects of nutrient deficiencies in humans. It is the most common nutritional deficiency in the world and one of the top four causes of lost developmental potential among children in low- and middle-income countries (41). The risk of iron deficiency is greatest in pregnancy and early infancy and childhood, coinciding with periods of peak need for iron for the developing fetal and infant brain. The importance of the timing of iron supplementation and maintenance of early life iron sufficiency on distinct neurobehavioral outcomes is clearly demonstrated in two sets of studies: 1) a series of studies in Nepal that assessed cognitive and motor function in a group of 7-9 year-old Nepalese children whose mothers had participated in a large, randomized controlled of prenatal micronutrient supplementation and who were themselves part of a micronutrient supplementation study between the ages of 12 and 35 months (42, 43, 44); and 2) a large study in China that assessed pregnant women and their offspring in order, in part, to assess the independent effects of prenatal vs. postnatal iron status on motor and cognitive development (45,46).
In the Nepal studies, school-aged children whose mothers received daily iron/folic acid from early pregnancy to 12 weeks postpartum scored significantly better on tests of working memory, inhibitory control, and fine-motor functioning compared to children whose mothers received no iron/folic acid (42). However, daily iron/folic acid to children 12-36 months of age whose mothers had received iron/folic acid in pregnancy conferred no additional benefit 43), and daily iron/folic acid from 12-36 months in toddlers whose mothers received no supplementation in pregnancy did not significantly affect intellectual, executive, or motor functioning at 7-to-9 years of age (44). These results collectively support the known benefit of prenatal iron, specifically in the last trimester, to support the hippocampal and striatal development requisite for memory and executive functioning later in postnatal life.
The findings of the China study mirrored these findings and gave additional insight into prenatal vs. postnatal effects of iron on cognitive and motor functioning. Geng et al. paired a post-natal iron supplementation study with an infancy iron supplementation study and found that infants born iron-deficient exhibited slower recognition of their mother’s voice at two months of age, as measured by event-related potentials, than children born iron-sufficient (46). Such poorer auditory recognition memory likely reflects the effect of iron deficiency effects on the hippocampus predicted by developmentally timed pre-clinical models and is consistent with the hippocampus developing rapidly between 28 weeks gestation and the first postpartum year in humans.
A further analysis of the China study data exemplifies the differential effects that the timing of introduction of iron can have on brain development and behavioral phenotypes (45). Regardless of whether their mothers received iron in pregnancy, children who received iron supplementation in infancy (between 6 weeks and 9 months) exhibited better gross motor scores at 9 months than children who did not receive iron in infancy. This finding is in apparent contrast to the demonstrated benefit of iron supplementation in pregnancy on cognitive outcomes, but is in line with the fact that motor development starts after the hippocampal and striatal development that underlies cognition. Motor control in infants shifts from the primitive reflexes driven by the brain stem and midbrain to more coordinated movements driven by the motor cortex around 3-4 months of age. This movement is supported by the ongoing process of myelination that begins around 36 weeks gestation and continues throughout the first two postnatal years.
The findings of the Nepalese and Chinese studies are thus consistent with established principles of nutrient-brain interaction including developmental timing and circuit specific effects. Supplementation after 12 months of age to improve cognitive outcomes or supplementing only during pregnancy to improve motor outcomes would be outside of the sensitive or critical windows of development for the structures and processes underlying these functions. These relationships also underscore the necessity of selecting the correct brain assessment tool. For example, it is possible that iron supplementation between 12 and 36 months in the Nepal studies would have improved socioemotional behavior, which is known to be altered in iron deficiency, secondary to deficits in dopaminergic signaling. Disruptions of this set of behaviors is apparent in infancy following prenatal iron deficiency (47) and the behaviors continue to be dependent on iron throughout life (48,49).
The studies demonstrating long-lasting consequences of un-treated iron deficiency in infancy, i.e., before 12 months of age, reinforce the supplementation studies showing benefit of early introduction of iron. Such consequences include poorer inhibitory control (diminished attentional control and greater risk taking) at 10 years of age and persistent alternations in brain functional connectivity in adults who were iron-deficient as infants (50, 51). Pre-conceptional iron supplementation to optimize maternal and fetal iron status in high-risk pregnant women is being recognized as an important strategy because it may contribute to improved neurobehavioral outcomes by ensuring that sufficient iron is available for building brain architecture before the opening of critical windows of development (52).
Iodine
Iodine deficiency is the leading cause of preventable impaired mental function worldwide, affecting an estimated two billion people. The developing brain is most susceptible to iodine deficiency during the first trimester, when fetal T3 production depends entirely upon supply of maternal T4. The harmful impact of severe iodine deficiency (populations in which 30% of school-aged children have goiter and median population urinary iodine concentration is below 20) on brain development is established. Women who give birth in these areas often have children with cretinism, a form of severe mental impairment, marked by deficits in hearing, speech, and gait, and IQ of approximately 30 (53). The effectiveness of iodine supplementation in preventing cretinism was established in 1972, when in a study of 165,000 people in Papua New Guinea who lived in an area of severe iodine deficiency and endemic cretinism, injection of iodized before conception or in early pregnancy significantly reduced the incidence of cretinism and improved cognitive and motor outcomes compared to women who received placebo (54, 55). Similar results were also found in China, with children of pregnant women given iodized oil early in pregnancy having significantly better cognitive outcomes at two and five years of age as compared to children of women who received iodized oil later in pregnancy (56).
The effects of mild and moderate iodine deficiency during pregnancy on child neurobehavioral outcomes have been less studied, although two recent studies both support the importance of optimal iodine status from the beginning of pregnancy to best ensure child developmental outcomes. In a study of pregnant Spanish women, Berbel at al. (57) divided the women into three groups, and started iodine supplementation at either 4-6, 12-14, or 37-40 weeks of gestation. The authors observed a significantly improvement in developmental quotient score at child age 18 months with earlier supplementation, with the highest scores observed among children whose mothers’ supplementation began at 4-6 weeks gestation and the lowest score among women whose mothers began supplementation at 37-40 weeks. Similarly, Velasco et al. (58) report higher psychomotor development scores among two-year-old children born to mothers who received iodine supplementation that started before 10 weeks gestations as compared to those who began supplementation in the last month of pregnancy.
Zinc
Meta-analyses have failed to find a significant effect of early zinc supplementation on child cognitive and motor outcomes, although disparate study designs and effect sizes in the combined datasets were noted (59). Nevertheless, fetuses of zinc-deficient mothers demonstrate decreased movement, lower heart rate variability, and altered autonomic nervous system stability. They additionally demonstrate decreased preferential looking behavior, but do not display differences in global cognitive tests.
Vitamin B12
Sufficient vitamin B12 is required for neuronal development and myelination. In a review of 48 case reports of infant vitamin B12 deficiency, Dror and Allen (60), report that all cases resulted from maternal deficiency, and two-thirds of the 48 reports reported developmental regression in infants with B12 deficiency, marked by nerve demyelination and cerebral atrophy. A recent study of maternal B12 supplementation reported no difference in global cognitive scores at 9 months in infants of supplemented mothers (61). However, unsupplemented mothers had higher plasma homocysteine concentrations that were associated with poorer infant scores in some domains.
Multiple nutrients including copper, folate, and choline that demonstrate an important role in brain development and long-term consequences of deficiency are understudied with respect to neurodevelopmental outcomes in young humans (Table 1). Prospective studies designed to account for the nutrient-brain interaction principles of timing, dose, and duration are thus needed for each nutrient with the appropriate assessment tool applied at the correct age to test the structure or circuitry of interest.
Assessment of Nutritional Effects on Brain Development
The positive or negative effects of nutrients on early brain development can be obvious or subtle (Table 1). The fact that early life nutrient effects on the brain can last into adulthood means that the tools that assess nutrient status and its relationship to developmental outcome should be as sensitive and specific as possible to detect acute effects and predict long-term sequelae (Table 2). The vast majority of the nutrient assessment-brain development literature in children has focused on the relationship of neurodevelopmental outcome to overall growth rates. Whereas micronutrient deficiencies can certainly suppress growth, failure to thrive has been generally associated with macronutrient deficiency.
Table 2.
Strengths and Limitations of Growth Assessments as Predictors of Neurodevelopmental Outcomes
| Assessment | Strength | Limitation |
|---|---|---|
| Weight | Easily obtained | Only a general connection to brain growth |
| Length | Close connection to brain growth | Difficult to obtain (accurately) |
| Head Circumference | Early sign of moderate malnutrition | Recovers first (spared) and thus not sensitive to mild malnutrition |
| Body Proportionality | Indication of stunting or underweight | Values are relative |
| Body Composition | Indication of metabolic risk | Interpolated or expensive |
Traditionally, early growth has been used as a surrogate for nutritional status and nutrient sufficiency. For healthy term born breastfed infants, expected growth patterns throughout early life have been investigated and published as the World Health Organization (WHO) curves (62). The curves were a great advancement in nutritional assessment because they represent a standard, as opposed to a population, reference curve for humans. They are currently being used in research studies to assess the relationship between early growth patterns and relevant long-term health outcomes. However, determining optimal growth goals for other populations, such as preterm infants, still requires collection of long-term outcome data on neurodevelopment and metabolic status as a function of growth (63). For example, the American Academy of Pediatrics has recommended for decades that growth goals for the preterm infant replicate that of fetal growth in all anthropometric parameters; however, whether this goal is achievable or optimal with respect to long-term neurodevelopmental or metabolic health for this population remains unclear 30 years after the recommendation was made (64).
The Relationship of Weight Gain to Neurodevelopment
Weight gain is the traditional metric of growth and represents the balance between energy intake and expenditure. It is also the easiest and most accurate measure to obtain, and for this reason adequate and/or optimal growth has been defined as weight gain for some time. However, weight does not give a complete picture of the overall nutritional state of the infant, and can be frequently confounded by non-nutritional weight gain. For this reason, it is important to consider the impact of growth in other parameters, including length, head circumference and body composition as well, when assessing the impact of growth on long-term neurodevelopment.
Preterm infants around the world continue to accrue macronutrient deficits and undergo initial growth restriction during the first weeks of their hospitalization (65). Unfortunately, they do not recover from these early deficits quickly and largely remain small compared to healthy term counterparts at term corrected age. In 2013, approximately 50% of very low birth weight infants were discharged with a weight below the 10th percentile and approximately 30% below the 3rd percentile (66). This growth failure occurs at a time when many important neurological processes are occurring, and the association of poor neonatal growth and suboptimal long-term neurodevelopment has been well documented.
Poor weight gain prior to term (while in the neonatal intensive care unit) has been associated with worsened neurodevelopmental outcomes. Preterm infants in the highest quartile of weight gain (21 grams/kg/day) had an 8-fold reduction in cerebral palsy and a 2.5-fold reduction in any neurodevelopmental impairment at 18-22 months corrected age for prematurity when compared to those in the lowest quartile of weight gain (12 grams/kg/day) (67). A group of adults, who were born very low birth weight in Finland, and had faster weight gain prior to term also had better neurocognitive abilities, executive functioning, visual memory, and verbal flexibility (68). In contrast, faster weight gain from term to 12 months corrected age did not show the same neurodevelopmental benefit. Similarly, faster weight gain velocities both prior to term and from term to 4 months corrected age, but not between 4 and 12 months was associated with higher scores on developmental testing with the Bayley Scales of Infant Development in a group of preterm infants born prior to 33 weeks gestation (69). Weight gain out of proportion to length, as indexed by an increased BMI, prior to term was associated with improved outcomes. However, after term, this association was no longer present.
Early catch-up growth in weight gain prior to term and likely until at least 4 months of age appears to be beneficial for long-term neurodevelopment in the preterm population. Given the ease of weight measurement, and therefore wide availability of data on weight gain, along with its association with neurodevelopmental outcomes, this remains an important measure to follow. However, because this metric does not give a complete picture of the overall nutritional state of the infant and can be frequently confounded by non-nutritional weight gain (eg, edema), other metrics should be utilized when using growth as a metric to predict later neurodevelopmental outcomes.
The Relationship of Head Circumference to Neurodevelopment
Head circumference is also a relatively easy measure to obtain, is directly related to brain volume, and is widely available clinically. Neonatal head growth, similar to weight gain, has been associated with later IQ. In a study involving more than 250 preterm infants, head circumference gains during multiple time periods in early life were associated with higher IQ scores at 5 years of age (70). The time period between hospital discharge and 3 months corrected age was an especially critical time period, reiterating the importance of close growth monitoring that continues beyond hospital discharge and is not focused solely on weight gain. In this study, suboptimal head growth, defined as > 1 SD but < 2 SD below the norm was associated with lower IQ (90 vs. 98; p <0.001) compared to infants whose head circumference was <1 SD from the norm in either direction. Similarly, in the Finnish study, increased head circumference gains prior to term were associated with increased IQ score and improved verbal flexibility, visual memory, and executive function in adulthood (68). Faster head growth from term to 12 months of age was also associated with increased performance IQ in adulthood in this group who was born at very low birth weight. Head growth up to 5 years, but particularly prior to term, is critical for optimizing neurodevelopment.
The Relationship of Linear Growth and Fat Free Mass (FFM) to Neurodevelopment
Linear growth, which represents lean body mass and protein accretion, and is more closely associated with organ growth than overall weight gain, is underutilized in growth assessment. Length measurements are more difficult to obtain and require appropriate equipment (e.g., length boards) to obtain accurate repeated measures (71). Linear growth, and fat free mass (FFM) gains are being more closely investigated as predictive biomarkers of later neurodevelopment because of their closer relationship to neurodevelopmental outcome than weight gain and fat mass.
Slower linear growth, irrespective of weight gain, is common among growth restricted children and is associated with poorer neurodevelopmental outcomes in children who experienced stunting prior to or after 40 weeks post-conceptional age (33, 72, 73). Linear growth restriction in preterm infants is often more severe and persistent than poor weight gain or restriction in head growth (72). Amongst very low birth weight preterm infants, faster linear growth in the first year after hospital discharge, even after controlling for weight and head growth, is associated with improved cognitive and language scores on the Bayley Scales of Infant Development administered at 24 months corrected age for prematurity (72). When tested in later childhood and adulthood, faster linear growth from term to 4 months corrected age is associated with a lower risk of IQ being less than 85 at age 8 and 18years in a large cohort of low birth weight preterm infants (74). Faster postnatal linear growth from birth to 2 years in preterm infants is associated with lower rates of cerebral palsy, as well as improved cognitive and motor scores, among very low birth weight preterm infants (75). Institutionalized children in Romania and children in Thailand exhibit a similar growth pattern to preterm born infants, with significant linear growth stunting out of proportion to weight suppression and also show comparable relationships between linear growth suppression and poorer neurodevelopmental outcomes (33,73).
Recent technology has led to more research focused on describing infant body composition changes in preterm infants and their relationship to later neurodevelopmental outcomes. The main compositional components that have been studied are FFM and fat mass. FFM, similar to linear growth, indexes protein status and organ growth. FFM gains prior to term, but also throughout early childhood have been associated with better neurodevelopmental outcomes and may be a more specific marker of brain growth than weight gain or linear growth alone (76,77,78). Of note, gains in fat mass during these same time periods do not show the same associations with neurodevelopment.
Faster gains in FFM prior to term and while hospitalized in the neonatal intensive care unit are associated with higher standardized development scores in motor and cognitive domains measured at 12 months of age by the Bayley Scales of Infant Development (76). Increased FFM gains prior to discharge from the NICU and throughout the first 4 months after discharge are associated with faster speed of brain processing in infancy (measured via event related potentials) (77). When tested at 4 years of age, early gains in FFM continued to be associated with improved neurodevelopment on standardized testing, specifically, improved working memory, faster speed of processing and higher IQ (78). These findings support the hypothesis that early life FFM gains are an important biomarker for brain growth and can serve as a predictor of later neurodevelopment. Overall, associations between linear growth/FFM accretion and neurodevelopmental outcomes could be explained by the positive effects of protein intake on protein accretion and thus on neuronal differentiation. Optimal protein status may have a direct effect of providing necessary amino acids for dendrite structure, but also may act through stimulating the synthesis of important neural growth factors such as insulin like growth factor-1 (IGF-1) and brain derived neurotrophic factor. Further study is underway to better understand the role of the growth hormone axis in linking growth to neurodevelopmental outcomes, as both IGF-1 levels and growth hormone administration have been shown to be neuroprotective and neurostimulatory in animal models, healthy children and growth hormone deficient children (79,80).
Improved growth in early life, a time of rapid growth and development of the brain, is associated with improved neurodevelopment. Specifically, increases in weight, length, head circumference and FFM gains improve speed of processing, language, cognitive and motor scores and decrease rates of cerebral palsy. These findings emphasize the importance of optimizing early growth in order to optimize neurodevelopmental outcomes among vulnerable populations.
Summary
Whereas all nutrients are needed for the development and function of the brain, certain nutrients have high impact on early brain development, including protein fats, iron, zinc, iodine, and vitamin B12. The impact is greater in the fetal and early postnatal period because of the high metabolic demands of the brain at that age. The positive or negative neurobehavioral effects of these nutrients depends on the timing, dose, and duration of provision or deprivation. Timing appears to play an important role because of the non-homogenous nature of regional brain development and because of the unequal distribution of prevalence of nutrient deficits in a population. Ensuring adequate fetal loading of nutrients through better maternal care during pregnancy via provision of adequate nutrition and reduction of obesity, hypertension, and glucose intolerance appears to be key to fostering postnatal nutrient sufficiency. For the term neonate, human milk provides the optimal support for neurodevelopment, whereas for at risk populations such as preterm infants, early identification and correction of nutrient deficits is essential to maintain the brain on trajectory through critical periods of development.
Key Notes.
Nutrients and growth factors regulate late fetal and early neonatal brain development.
Early life nutritional deficits can affect brain function across the lifespan and this loss of brain function is the true cost to society of early malnutrition.
Early provision of nutrition and accurate assessment of nutritional status are key to optimizing long-term development.
Acknowledgments
The authors are grateful to Phu Tran, PhD for generating Figure 1.
Funding
Funded in part by grants to MKG (NIH: HD-29421), SER (March of Dimes: 12-FY13-295) and SEC (NIH: HD-074262)
Abbreviations
- PCA
post-conceptional age
- IQ
Intelligence Quotient
- LCPUFA
Long-chain polyunsaturated fatty acids
- IGF-1
Insulin like growth factor-1
- FFM
Fat Free Mass
Footnotes
Conflict of Interest
The authors have no disclosures and no conflicts of interest to resolve.
References
- 1.Wachs TD, Georgieff M, Cusick S, McEwan B. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience and psychological research. Annals of the New York Academy of Sciences. 2014;1308:89–106. doi: 10.1111/nyas.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cusick SE, Georgieff MK. The role of nutrition in brain development: The golden opportunity of the first 1000 days. J Pediatrics. 2016;175:16–21. doi: 10.1016/j.jpeds.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagercrantz H. Origins of the Mind and Basic Construction of the Brain. In: Lagercrantz H, editor. Infant Brain Development. Springer; 2016. pp. 1–14. [Google Scholar]
- 4.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 5.deRegnier RA, Nelson CA, Thomas K, Wewerka S, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr. 2000;137:777–784. doi: 10.1067/mpd.2000.109149. [DOI] [PubMed] [Google Scholar]
- 6.Bhide PG. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol. 2009;20:395–402. doi: 10.1016/j.semcdb.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Lahti J, Räikkönen K, Kajantie E, et al. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47:1167–74. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuzawa CW. Adipose tissue in human infancy and childhood: An evolutionary perspective. Yearbook of Physical Anthropology. 1998;41:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. Am J Clin Nut. 1996;63:997S–1001S. doi: 10.1093/ajcn/63.6.997. [DOI] [PubMed] [Google Scholar]
- 10.Hensch T. Critical period regulation. Annual Reviews Neuroscience. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 11.Bornstein MH. Sensitive periods in development: Structural characteristics and causal interpretations. Psychol Bull. 1989;105:179–197. doi: 10.1037/0033-2909.105.2.179. [DOI] [PubMed] [Google Scholar]
- 12.Barnard ND, Willet WC, Ding EL. The misuse of meta-analyses in nutrition research. JAMA. 2017 doi: 10.1001/jama.2017.12083. Epub September 18, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Zhan S, Gong T, Lee L. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database of Systematic Reviews. (6) 2013 doi: 10.1002/14651858.CD001444.pub2. Art. No.: CD001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao R, Georgieff MK. The nutritionally deprived fetus and newborn infant. In: Shevell, Miller, editors. International Review of Child Neurology Series: Acquired Brain Injury in the Fetus & Newborn. Mac Keith Press; 2012. pp. 277–287. [Google Scholar]
- 15.Todorich B, Zhang X, Connor JR. H-ferritin is the major source of iron for oligodendrocytes. Glia. 2011;59:927–35. doi: 10.1002/glia.21164. [DOI] [PubMed] [Google Scholar]
- 16.Greminger AR, Lee DL, Shrager P, Mayer-Pröschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J Nutr. 2014;144:1058–66. doi: 10.3945/jn.113.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 19.Bastian TW, von Hohenberg WC, Mickelson DJ, Lanier LM, Georgieff MK. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism and dendrite complexity. Dev Neurosci. 2016;38:264–276. doi: 10.1159/000448514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grissom NM, Reyes TM. Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci. 2013;31:406–14. doi: 10.1016/j.ijdevneu.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyagi E, Zhuang Y, Agrawal R, Ying Z, Gomez-Pinilla F. Interactive actions of Bdnf methylation and cell metabolism for building neural resilience under the influence of diet. Neurobiol Dis. 2015;73:307–18. doi: 10.1016/j.nbd.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisel SH. A brief history of choline. Ann Nutr Metab. 2012;61:254–8. doi: 10.1159/000343120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran PV, Kennedy BC, Lien YC, Simmons RA, Georgieff MK. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am J Physiol Integr Comp Phys. 2015;308:R276–R282. doi: 10.1152/ajpregu.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Condon DE, Tran PV, Lien Y-C, Schlug J, Georgieff MK, Simmons RA, Won KJ. Defiant: (DMRs: Easy, Fast, Identification and ANnoTation) identifies differentially methylated regions from iron-deficient rat hippocampus. BMC Bioinformatics. 2018 doi: 10.1186/s12859-018-2037-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris SS, Cogill B, Uauy R. Effective international action against undernutrition: why has it proven so difficult and what can be done to accelerate progress? Lancet. 2008;371:608–21. doi: 10.1016/S0140-6736(07)61695-X. [DOI] [PubMed] [Google Scholar]
- 26.Eide MG, Moster D, Irgens LM, Reichborn-Kjennerud T, Stoltenberg C, et al. Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol Med. 2013;43:2057–66. doi: 10.1017/S003329171200267X. [DOI] [PubMed] [Google Scholar]
- 27.Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch Gen Psychiatry. 2008;65:1136–44. doi: 10.1001/archpsyc.65.10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt RJ, Tancredi DJ, Krakowiak P, Hansen RL, Ozonoff S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am J Epidemiol. 2014;180:890–900. doi: 10.1093/aje/kwu208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 30.Fretham SJB, Carlson ES, Wobken J, Tran PV, Petryk A, Georgieff MK. Temporal manipulation of transferrin receptor-1 dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus. 2012;22:1691–1702. doi: 10.1002/hipo.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran PV, Kennedy BC, Pisansky MT, Won K-J, Gewirtz JC, Simmons RA, Georgieff MK. Prenatal choline supplementation diminishes early–life iron deficiency induced preprogramming of networks associated with behavioral abnormalities in the adult rat hippocampus. J Nutrition. 2016;146:484–93. doi: 10.3945/jn.115.227561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollitt E, Gorman KS, Engle PL, Riveras JA, Martorell R. Nutrition in early life and the fulfillment of intellectual potential. J Nutr. 1995;125:1111S–1118S. doi: 10.1093/jn/125.suppl_4.1111S. [DOI] [PubMed] [Google Scholar]
- 33.Pongcharoen T, Ramakrishnan U, DiGirolamo AM, Winichagoon P, Flores R, Singkhornard J, et al. Influence of prenatal and postnatal growth on intellectual functioning in school-aged children. Arch Pediatr Adolesc Med. 2012;166:411–6. doi: 10.1001/archpediatrics.2011.1413. [DOI] [PubMed] [Google Scholar]
- 34.Christian P, Murray-Kolb LE, Tielsch JM, Katz J, Le Clerq SC, Khatry SK. Associations between preterm birth, small-for-gestational age, and neonatal morbidity and cognitive function among school-age children in Nepal. BMC Pediatrics. 2014;14:58. doi: 10.1186/1471-2431-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jasani B, Simmer K, Patole SK, Rao SC. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Reviews. 2017;(3) doi: 10.1002/14651858.CD000376.pub4. Art. No.: CD000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delgado-Noguera MF, Calvache JA, Bonfill Cosp X. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2010;12:CD007901. doi: 10.1002/14651858.CD007901.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Helland IB, Saugstad OD, Smith L, Saarem K, Solvoll K, Ganes T, Drevon CA. Similar effects on infants of n-3 and n-6 fatty acid supplementation to pregnant and lactating women. Pediatrics. 2001;108:E82. doi: 10.1542/peds.108.5.e82. [DOI] [PubMed] [Google Scholar]
- 38.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39–44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 39.Helland IB, Smith L, Blomén B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children’s IQ and body mass index at 7 years of age. Pediatrics. 2008;122:e472–9. doi: 10.1542/peds.2007-2762. [DOI] [PubMed] [Google Scholar]
- 40.Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, et al. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr. 2013;98:403–12. doi: 10.3945/ajcn.112.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, International Child Development Steering Group Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christian P, Murray-Kob LE, Khatry SK, Katz J, Schaefer BA, Cole PM, et al. Prenatal Micronutrient Supplementation and Intellectual and Motor Function in Early School-aged Children in Nepal. JAMA. 2010;304:2716–23. doi: 10.1001/jama.2010.1861. [DOI] [PubMed] [Google Scholar]
- 43.Christian P, Morgan ME, Murray-Kolb L, LeClerq SE, Khatry SK, Schaefer B, et al. Preschool iron-folic acid and zinc supplementation in children exposed to iron-folic acid in utero confers no added cognitive benefit in early school-age. J Nutr. 2011;141:2042–8. doi: 10.3945/jn.111.146480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC. Preschool micronutrient supplementation effects on intellectual and motor function in school-aged Nepalese children. Arch Pediatr Adolesc Med. 2012;166:404–410. doi: 10.1001/archpediatrics.2012.37. [DOI] [PubMed] [Google Scholar]
- 45.Angula-Barroso RM, Li M, Santos DCC, Bian Y, Sturza J, Jiang Y, et al. Iron supplementation in pregnancy or infancy and motor development: A randomized controlled trial. Pediatrics. 2016;137:e20153547. doi: 10.1542/peds.2015-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff MK, et al. Impact of fetal-neonatal iron deficiency on recognition memory at two months of age. J Pediatr. 2015;167:1226–32. doi: 10.1016/j.jpeds.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- 48.Berglund SK, Chmielewska A, Starnberg J, Westrup B, Hägglöf B, Norman M, Dommellöf M. Effects of iron supplementation of low-birth-weight infants on cognition and behavior at 7 years: a randomized controlled trial. Pediatr Res. 2017 doi: 10.1038/pr.2017.235. [DOI] [PubMed] [Google Scholar]
- 49.Perez EM, Hendricks MK, Beard JL, Murray-Kolb LE, Berg A, Tomlinson M, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850–5. doi: 10.1093/jn/135.4.850. [DOI] [PubMed] [Google Scholar]
- 50.Algarin C, Nelson CA, Peirano P, Westerlund A, Reyes S, Lozoff B. Iron-deficiency anemia in infancy and poorer cognitive inhibitory control at age 10 years. Dev Med Child Neurol. 2013;55:453–8. doi: 10.1111/dmcn.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Algarin C, Karunakaran KD, Reyes S, Morales C, Lozoff B, Peirano P, et al. Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Front Aging Neurosci. 2017;9:54. doi: 10.3389/fnagi.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen PH, Gonzalez-Casanova I, Young MF, Truong TV, Hoang H, Nguyen H, et al. Preconception micronutrient supplementation with iron and folic acid compared with folic acid alone affects linear growth and fine motor development at 2 years of age: a randomized controlled trial in Vietnam. J Nutr. 2017;147:1593–601. doi: 10.3945/jn.117.250597. [DOI] [PubMed] [Google Scholar]
- 53.Skeaff S. Iodine deficiency in pregnancy: the effect on neurodevelopment in the child. Nutrients. 2011;3:265–273. doi: 10.3390/nu3020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pharoah POD, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet. 1971;297:308–10. doi: 10.1016/s0140-6736(71)91040-3. [DOI] [PubMed] [Google Scholar]
- 55.Pharoah PO, Connolly KJ. A controlled trial of iodinated oil for the prevention of endemic ctreinism: a long-term follow-up. Int J Epidemiology. 1987;16:68–73. doi: 10.1093/ije/16.1.68. [DOI] [PubMed] [Google Scholar]
- 56.O’Donnell KJ, Rakeman MA, Zhi-Hong D, Xue-Yi C, Mei ZY, DeLong N, et al. Effects of iodine supplementation during pregnancy on child growth and development at school age. Dev Med Child Neurol. 2002;44:76–81. doi: 10.1017/s0012162201001712. [DOI] [PubMed] [Google Scholar]
- 57.Berbel P, Mestre JL, Santamaria A, Palazon I, Franco A, Graells M, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19:511–19. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 58.Velasco I, Carreira M, Santiago P, Muela JA, Garcia-Fuentes E, Sanchez-Munoz B, et al. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab. 2009;94:3234–41. doi: 10.1210/jc.2008-2652. [DOI] [PubMed] [Google Scholar]
- 59.Gogia S, Sachdev HS. Zinc supplementation for mental and motor development in children. Cochrane Database Syst Rev. 2012 Dec 12;12:CD007991. doi: 10.1002/14651858.CD007991.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev. 2008;66:250–255. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- 61.Srinivasan K, Thomas T, Kapenee ARM, Ramthal A, Bellinger DC, Bosch RJ, et al. Effects of maternal vitamin B12 supplementation on early infant neurocognitive outcomes: a randomized controlled clinical trial. Matern Child Nutr. 2017;13:e12325. doi: 10.1111/mcn.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Onis M, Garza C, Onyango AW, Borghi E. Comparison of the WHO child growth standards and the CDC 2000 growth charts. J Nutr. 2007;137:144–8. doi: 10.1093/jn/137.1.144. [DOI] [PubMed] [Google Scholar]
- 63.Raiten DJ, Steiber AL, Carlson SE, Griffin I, Anderson D, Hay WW, Jr, Robins S, Neu J, Georgieff MK, Groh-Wargo S, Fenton TR, Pre-B Consultative Working Groups Working group reports: evaluation on the evidence to support practice guidelines for nutritional care of preterm infants - the Pre-B Project. Am J Clin Nutr. 2016;103:648S–78S. doi: 10.3945/ajcn.115.117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976–86. [PubMed] [Google Scholar]
- 65.Cole TJ, Statnikov Y, Santhakumaran S, Pan H, Modi N, Neonatal Data Analysis Unit and the Preterm Growth Investigator Group Birth weight and longitudinal growth in infants born below 32 weeks’ gestation: a UK population study. Arch Dis Child Fetal Neonatal Ed. 2014;99:F34–40. doi: 10.1136/archdischild-2012-303536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000-2013. Pediatrics. 2015;136:e84–92. doi: 10.1542/peds.2015-0129. [DOI] [PubMed] [Google Scholar]
- 67.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birthweight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 68.Sammallahti S, Pyhälä R, Lahti M, Lahti J, Pesonen AK, Heinonen K, et al. Infant growth after preterm birth and neurocognitive abilities in young adulthood. J Pediatr. 2014;165:1109–1115.e3. doi: 10.1016/j.jpeds.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 69.Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899–906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neubauer V, Fuchs T, Griesmaier E, Kager K, Pupp-Peglow U, Kiechl-Kohlendorfer U. Poor postdischarge head growth is related to a 10% lower intelligence quotient in very preterm infants at the chronological age of five years. Acta Paediatr. 2016;105:501–7. doi: 10.1111/apa.13336. [DOI] [PubMed] [Google Scholar]
- 71.Wood AJ, Raynes-Greenow CH, Carberry AE, Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J Paediatr Child Health. 2013;49:199–203. doi: 10.1111/jpc.12119. [DOI] [PubMed] [Google Scholar]
- 72.Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff M. The relationship of poor linear growth velocity with neonatal illness and two year neurodevelopment in preterm infants. Neonatology. 2012;102:19–24. doi: 10.1159/000336127. [DOI] [PubMed] [Google Scholar]
- 73.Johnson DE, Guthrie D, Smyke AT, Koga SF, Fox NA, Zeanah CH, et al. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs. ongoing institutional care. Arch Pediatr Adolesc Med. 2010;164:507–16. doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr. 2013;163:1564–9.e2. doi: 10.1016/j.jpeds.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr. 2003;143:163–7. doi: 10.1067/S0022-3476(03)00243-9. [DOI] [PubMed] [Google Scholar]
- 76.Ramel SE, Gray H, Christiansen E, Boys C, Georgieff MK, Demerath EW. Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr. 2016;173:108–15. doi: 10.1016/j.jpeds.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE. An exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res. 2013;74:576–83. doi: 10.1038/pr.2013.138. [DOI] [PubMed] [Google Scholar]
- 78.Scheurer J, Zhang L, Plummer E, Hultgren S, Demerath EW, Ramel SE. Body composition changes in preterm children from infancy to 4 years are associated with early childhood cognition. Under review. doi: 10.1159/000487915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansen-Pupp I, Hövel H, Löfqvist C, Hellström-Westas L, Fellman V, Hüppi PS, et al. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr Res. 2013;74:564–9. doi: 10.1038/pr.2013.135. [DOI] [PubMed] [Google Scholar]
- 80.Gunnell D, Miller LL, Rogers I, Holly JM, ALSPAC Study Team Association of insulin-like growth factor and insulin-like growth factor-binding protein-3 with intelligence quotient among 8 to 9-year old children in the Avon longitudinal study of parents and children. Pediatrics. 2005;116:e681–6. doi: 10.1542/peds.2004-2390. [DOI] [PubMed] [Google Scholar]


