Abstract
Objectives
To examine associations of race/ethnicity and purported risk factors with hospitalized allopurinol-associated severe cutaneous adverse reactions (AASCARs).
Methods
We used US Medicaid data to identify incident allopurinol users between 1999 and 2012. We examined the risk of hospitalized AASCARs according to race/ethnicity and purported key risk factors and calculated relative risks (RR).
Results
Among 400,401 allopurinol initiators, we documented 203 hospitalized AASCAR cases (1 in 1,972 initiators). The average AASCAR hospitalization was 9.6 days and 43 individuals (21%) died. The multivariable-adjusted RRs for AASCARs among Blacks, Asians, and Native Hawaiians/Pacific Islanders compared with Whites or Hispanics were 3.00 (95% confidence interval [CI], 2.18 to 4.14), 3.03 (95% CI, 1.72 to 5.34), and 6.68 (95% CI, 4.37 to 10.22), respectively. Female sex, older age (≥60 years), chronic kidney disease (CKD), and initial allopurinol dose (>100 mg/day) were independently associated with a 2.5-, 1.7-, 2.3-, and 1.9-fold higher risk of AASCAR, respectively. In our combined demographic analysis, older women (≥60 years) of a high-risk race/ethnicity (Blacks, Asians, or Native Hawaiians/Pacific Islanders) had over a 12-fold higher risk of hospitalized AASCARs than younger men of a low-risk race/ethnicity (Whites or Hispanics) (multivariable-adjusted RR, 12.25; 95% CI, 6.46 to 23.25).
Conclusions
This racially-diverse (yet mostly White) cohort study indicates that the risk of hospitalized AASCAR is rare overall, although Blacks, Asians, and Native-Hawaiians/Pacific-Islanders have a substantially higher risk of hospitalized AASCARs, particularly among older women. These data also support the practice of initiating allopurinol at a low dose (e.g., ≤100 mg/day).
Keywords: Race, Allopurinol, Hypersensitivity Syndrome, Gout, Severe Cutaneous Adverse Reactions
INTRODUCTION
Allopurinol is the predominant, first-line urate-lowering drug (ULD) worldwide for the treatment of gout.[1–4] Although allopurinol is generally well-tolerated, an uncommon yet feared adverse reaction is severe allopurinol hypersensitivity syndrome (AHS), manifesting as severe cutaneous adverse reactions such as Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). Severe cutaneous adverse reactions due to AHS frequently involve major organs, with long-term sequelae such as corneal damage and renal insufficiency, and can be fatal in up to 32% of cases.[5,6] A previous study found allopurinol to be the most common cause of both SJS and TEN in Europe and Israel.[7]
Understanding the risk factors for AASCARs is essential to mitigate the risk of this severe adverse drug reaction; however, relevant data are scarce. Beyond the established risk of AASCARs associated with chronic kidney disease (CKD),[8,9] the HLA-B*5801 allele has also been found to portend a substantially higher risk of severe cutaneous adverse reactions.[10–16] A meta-analysis reported that the risk of developing severe cutaneous adverse reactions was 97 times higher among allopurinol users with the HLA-B*5801 allele compared to those without the allele.[17] Furthermore, the allele frequency of HLA-B*5801 varies substantially among different races/ethnicities. For example, the allele frequency in the US has been estimated to be 7.4%, 4%, 1%, and 1% among Asians, Blacks, Whites, and Hispanics, respectively. [18] These varying frequencies of HLA-B*5801 could lead to substantial variations in the risk of AASCARs across different races and ethnicities; however, relevant data are scarce. Furthermore, female sex, older age, asymptomatic hyperuricemia, and diuretic use have been purported to increase the risk of AASCARs [5,8,9,19]. Such information, together with knowledge of other independent risk factors, can help identify high-risk patients to target with potential preventive measures. For example, a recent prospective study showed that screening for the HLA-B*5801 allele in Taiwanese patients (who have an HLA-B*5801 carriage rate of 20%), coupled with the use of an alternative ULD for those deemed to be carriers, substantially reduced the incidence of AASCARs.[20]
We examined the risk of AASCARs according to race/ethnicity and other candidate risk factors [5,8,9,19] in a large, racially diverse population and evaluated their potential independent associations.
METHODS
Source Population
Our source population was the Medicaid Analytic eXtract database, an administrative data system containing all billing claims for Medicaid enrollees in 47 US states and the District of Columbia from January 1, 1999 to December 31, 2012. The database contains clinical, demographic, and death status information for beneficiaries as well as Medicaid claims for covered health care services including pharmacy benefits and hospitalizations from the time of a person’s Medicaid eligibility until death or the end of Medicaid eligibility. As about 17% of Medicaid beneficiaries are also enrolled in Medicare,[21] Medicare data were obtained to ensure complete data capture in dually-eligible beneficiaries. This study was exempted by the Partners Institutional Review Board.
Study Population and Design
We conducted a cohort study among adults (i.e., 18 to 90 years of age) who had at least 180 days of Medicaid eligibility and at least one outpatient or inpatient claim present before the first prescription of allopurinol. We identified new allopurinol users starting from January 1, 1999, excluding individuals who had a history of severe cutaneous adverse reactions prior to allopurinol initiation. We followed patients from allopurinol initiation until: 1) hospitalization for a severe cutaneous adverse reaction, 2) the end of Medicaid eligibility, 3) the end of the study period, or 4) death, whichever came first.
Assessment of Endpoints
The primary endpoint of interest was incident cases of hospitalized AASCARs with a principal hospital discharge diagnosis of a relevant ICD-9-CM code, occurring within the first three months after filling the first prescription for allopurinol, and followed by discontinuation of the drug after the episode.[5] The employed ICD-9-CM codes for the definition consisted of those used by a recent claims database study (i.e., dermatitis due to drugs and medicines [693.0]; erythema multiforme, SJS, and TEN [695.1]; unspecified erythematous conditions [695.9]; and other specified erythematous conditions [695.89]).[5] The three-month time window was employed because AHS predominantly occurs within the first three months of drug exposure (Figure 1).[5,12,22] The aforementioned study evaluated the accuracy of this definition and found that all 33 cases of AASCARs meeting the definition were confirmed by a medical record review conducted by experienced dermatologists.[5] Our secondary definition of hospitalized severe cutaneous adverse reactions restricted the principal hospital discharge diagnosis to the ICD-9-CM code 695.1x but did not require any other conditions, as was adopted by an earlier claims database study.[6] This definition was found to have a positive predictive value of >90% for hospitalized severe cutaneous adverse reactions.[6,23–25] Finally, we examined the risk of AASCAR mortality, defined as death within two months of the AASCAR hospitalization date.[5]
Figure 1.
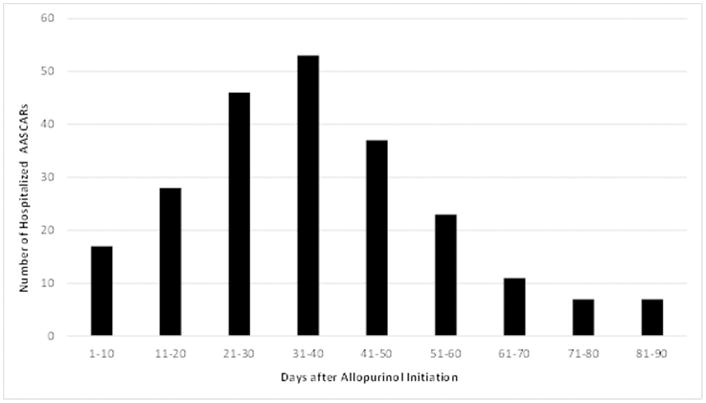
Frequency of Hospitalized Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs)
Assessment of Race/Ethnicity and Covariates
Our primary risk factor of interest was race/ethnicity, consisting of White, Black, Asian, Native Hawaiian or other Pacific Islander, and Hispanic or Latino as reported by patients in the Medicaid database. Patients for whom information on race or ethnicity was unclassifiable or missing (e.g., “more than one race” or “other/unknown”) were excluded from these analyses. Covariates of interest consisted of purported risk factors, including demographic factors (i.e., age and sex), presence of CKD (ICD-9-CM codes 580–586), presence of gout (ICD-9-CM code 274), use of diuretics, and initial allopurinol dose [>100 mg/day vs. ≤100 mg/day]).[5,8,9,19]
Statistical Analyses
We assessed the timing of AASCAR incidence after initiating allopurinol by graphically displaying the number of events every 10 days. The overall risk of hospitalized AASCARs per 1,000 allopurinol initiators and corresponding 95% confidence interval (CI) were calculated. We then estimated the risk of hospitalized AASCARs per 1,000 allopurinol initiators according to race/ethnicity and other purported risk factors. We used Poisson regression models to determine the relative risk (RR) of AASCARs in relation to race/ethnicity while adjusting for covariates. Our final multivariable models included variables that were significantly associated with the risk of hospitalized AASCARs in age- and sex-adjusted analyses or affected the RR estimate for any race by at least 10%. Our reference group consisted of Whites and Hispanics, given their similarly low frequencies of HLA-B*5801 (both 1% in the US population) [18] and similar risks of AASCARs observed in the current study. We further examined the risk and relative risk of hospitalized AASCARs according to combined demographic profiles of age, sex, and race/ethnicity. In compliance with the Healthcare Cost and Utilization Project Data Use Agreement, [26] we did not report data when the tabulated cell size was less than 11. All p-values were 2-sided with a significance threshold of p<0.05. Statistical analyses were performed using SAS Version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Baseline Characteristics
Our cohort consisted of 400,401 allopurinol initiators. The baseline characteristics of these initiators are summarized in Table 1. Sixty-two percent of allopurinol initiators were White, 53% were male, and 52% were younger than 60 years of age. Five percent of initiators had CKD at initiation and 61% were prescribed allopurinol at an initial dose greater than 100 mg/day.
Table 1.
Risk of Hospitalized Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs) according to Race/Ethnicity and Other Risk Factors
| Variable | Allopurinol Initiators N (%) |
Hospitalized AASCARs N |
Risk of Hospitalized AASCARs (/1000 persons) | Age-, Sex-Adjusted Relative Risk | Multivariable-Adjusted Relative Risk* |
|---|---|---|---|---|---|
| All | 400,401 (100) | 203 | 0.51 (0.45 to 0.59) | – | – |
| Race/Ethnicity | |||||
| White/Hispanic | 248,501 (62) | 64 | 0.26 (0.20 to 0.33) | 1.0 | 1.0 |
| Black | 111,619 (28) | 91 | 0.82 (0.66 to 1.00) | 3.02 (2.20 to 4.17) | 3.00 (2.18 to 4.14) |
| Asian | 21,442 (5) | 15 | 0.70 (0.39 to 1.15) | 2.94 (1.67 to 5.17) | 3.03 (1.72 to 5.34) |
| Native Hawaiian/Pacific Islander | 18,839 (5) | 33 | 1.75 (1.21 to 2.46) | 6.54 (4.28 to 10.00) | 6.68 (4.37 to 10.22) |
| Sex | |||||
| Male | 213,041 (53) | 61 | 0.29 (0.22 to 0.37) | 1.0 | 1.0 |
| Female | 187,360 (47) | 142 | 0.76 (0.64 to 0.89) | 2.37 (1.74 to 3.21) | 2.49 (1.83 to 3.38) |
| Age | |||||
| <60 years | 208,151 (52) | 70 | 0.34 (0.26 to 0.42) | 1.0 | 1.0 |
| ≥60 years | 192,250 (48) | 133 | 0.71 (0.60 to 0.84) | 1.73 (1.29 to 2.33) | 1.66 (1.23 to 2.24) |
| Chronic Kidney Disease | |||||
| No | 381,561 (95) | 184 | 0.48 (0.42 to 0.56) | 1.0 | 1.0 |
| Yes | 18,840 (5) | 19 | 1.01 (0.63 to 1.54) | 2.11 (1.32 to 3.39) | 2.33 (1.44 to 3.77) |
| Initial Allopurinol Dose (>100 mg/d) | |||||
| No | 157,138 (39) | 58 | 0.37 (0.28 to 0.47) | 1.0 | 1.0 |
| Yes | 243,263 (61) | 145 | 0.60 (0.51 to 0.70) | 1.74 (1.28 to 2.36) | 1.85 (1.36 to 2.51) |
Mutually adjusted for the variables in this table.
Risk of Hospitalized AASCARs after Allopurinol Initiation according to Race/Ethnicity
Among allopurinol initiators, we documented 203 hospitalized AASCAR cases (Table 1). The risk of hospitalized AASCARs was apparent within 10 days of allopurinol initiation, peaked around one month after initiation, and declined progressively thereafter, reaching its nadir at the end of the third month (Figure 1). The average length of an AASCAR hospitalization was 9.6 days and 43 patients (21%) died.
The risk of hospitalized AASCARs was 1 in 3,883 initiators among Whites and Hispanics, whereas the risk was 1 in 1,227, 1,429, and 571 initiators among Blacks, Asians, and Native Hawaiians/Pacific Islanders, respectively (Table 1). The baseline characteristics according to race/ethnicity are summarized in Supplemental Table 1. The multivariable-adjusted RRs for AASCARs among Blacks and Asians as compared with Whites and Hispanics were 3.00 (95% CI, 2.18 to 4.14) and 3.03 (95% CI, 1.72 to 5.34), respectively. The corresponding multivariable-adjusted RR of hospitalized AASCARs was 6.68 (95% CI, 4.37 to 10.22) among Native Hawaiian/Pacific Islanders (Table 1). After adjusting for age as a continuous variable, these RRs remained similarly large (Supplemental Table 2). Furthermore, after applying our secondary definition of hospitalized severe cutaneous adverse reactions, [6] these RRs remained similar (Supplemental Table 3). Finally, there was no significant subgroup effect according to the presence of gout (p for interaction = 0.36).
Risk of Hospitalized AASCARs according to Other Risk Factors
All covariates that were statistically significant in the age- and sex-adjusted analyses remained independently associated with the risk of hospitalized AASCARs in our multivariable analyses, including female sex (multivariable-adjusted RR, 2.49; 95% CI, 1.83 to 3.38), age ≥60 years (multivariable-adjusted RR, 1.66; 95% CI, 1.23 to 2.24), CKD (multivariable-adjusted RR, 2.33; 95% CI, 1.44 to 3.77), and initial allopurinol dose >100 mg/day (multivariable-adjusted RR, 1.85; 95% CI, 1.36 to 2.51) (Table 1). In our analysis using age as a continuous variable, a 10-year increase was associated with a 29% higher risk of hospitalized AASCAR (multivariable-adjusted RR, 1.29; 95% CI, 1.16 to 1.42) (Supplemental Table 2). Neither a history of gout nor diuretic use was significantly associated with the risk of AASCAR in age- and sex-adjusted analyses (RRs, 1.21 [95% CI, 0.91 to 1.60] and 1.38 [95% CI, 0.99 to 1.92], respectively) or significantly affected the RR estimate for any race/ethnicity category.
Risk of Hospitalized AASCARs according to Combined Demographic Profiles
We further examined the risk of hospitalized AASCARs according to combined demographic profiles by classifying Whites and Hispanics as low-risk race/ethnicities, and Blacks, Asians, and Hawaiians/Pacific Islanders as high-risk races/ethnicities (Figure 2 and Table 2). Older women (≥60 years) of a high-risk race/ethnicity had over a 12-fold higher risk of hospitalized AASCARs than younger men (<60 years) of a low-risk race/ethnicity (multivariable-adjusted RR, 12.25; 95% CI, 6.46 to 23.25). Older men of a high-risk race/ethnicity had over a 6-fold higher risk of hospitalized AASCARs than younger men of a low-risk race/ethnicity (multivariable-adjusted RR, 6.59; 95% CI, 3.28 to 13.26) (Figure 2 and Table 2).
Figure 2.
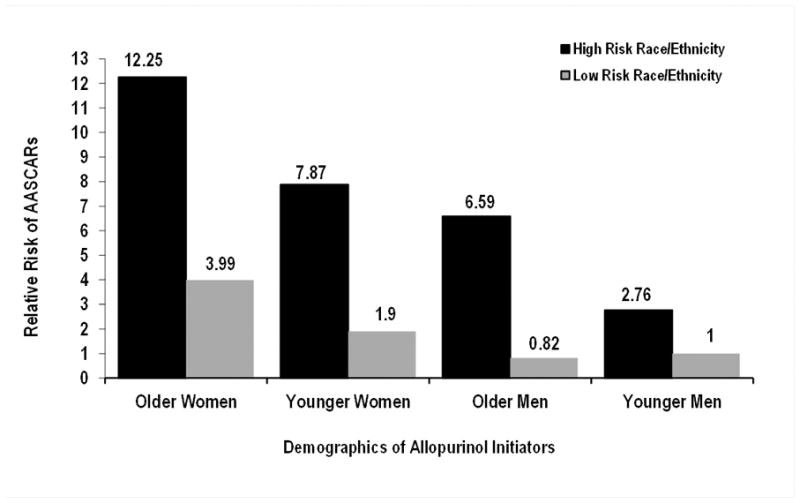
Relative Risk of Hospitalized Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs) according to Demographic Factors. Older age defined as ≥60 years; high-risk race/ethnicity = Blacks, Asians, or Native Hawaiians/Pacific Islanders; low-risk race/ethnicity = Whites or Hispanics.
Table 2.
Risk of Hospitalized Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs) according to Combined Demographic Profiles
| Variable | High-Risk Race/Ethnicity | Low-Risk Race/Ethnicity | |||
|---|---|---|---|---|---|
|
| |||||
| Risk of Hospitalized AASCARs (/1000 persons) | Multivariable-Adjusted Relative Risk* | Risk of Hospitalized AASCARs (/1000 persons) | Multivariable-Adjusted Relative Risk* | ||
| Female | ≥60 years | 1.50 (1.16 to 1.90) | 12.25 (6.46 to 23.25) | 0.53 (0.38 to 0.72) | 3.99 (2.01 to 7.89) |
| <60 years | 1.01 (0.70 to 1.41) | 7.87 (3.95 to 15.66) | 0.24 (0.13 to 0.42) | 1.90 (0.82 to 4.38) | |
| Male | ≥60 years | 0.83 (0.56 to 1.18) | 6.59 (3.28 to 13.26) | 0.10 (0.03 to 0.23) | 0.82 (0.29 to 2.37) |
| <60 years | 0.47 (0.21 to 0.57) | 2.76 (1.28 to 5.95) | 0.13 (0.07 to 0.23) | 1.0 (reference) | |
Adjusted for chronic kidney disease and initial allopurinol dose.
Risk of Hospitalized AASCARs according to Race/Ethnicity Combined with CKD Status and Initial Allopurinol Dose
We also examined the risk of hospitalized AASCARs according to race/ethnicity combined with CKD status and initial allopurinol dose (Table 3). CKD patients of a high-risk race/ethnicity who received an initial allopurinol dose >100mg/day had over a 9-fold higher risk of hospitalized AASCARs compared with non-CKD patients of a low-risk race/ethnicity who received an initial allopurinol dose ≤100mg/day (multivariable-adjusted RR, 4.37; 95% CI, 2.27 to 8.42).
Table 3.
Risk Race/Ethnicity and Risk of Hospitalized Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs) according to Chronic Kidney Disease (CKD) and Initial Allopurinol Dose
| Variable | High-Risk Race/Ethnicity | Low-Risk Race/Ethnicity | |||
|---|---|---|---|---|---|
|
| |||||
| Initial Allopurinol Dose | Risk of Hospitalized AASCARs (/1000 persons) | Multivariable-Adjusted Relative Risk* | Risk of Hospitalized AASCARs (/1000 persons) | Multivariable-Adjusted Relative Risk* | |
| CKD | >100 mg/d | 1.63 (0.60 to 3.55) | 9.08 (3.58 to 22.77) | 1.12 (0.36 to 2.60) | 5.65 (2.10 to 15.22) |
| ≤100 mg/d | 0.78 (0.21 to 1.99) | 4.10 (1.37 to 12.13) | 0.72 (0.20 to 1.85) | 3.62 (1.23 to 10.71) | |
| No CKD | >100 mg/d | 1.14 (0.92 to 1.39) | 5.86 (3.54 to 9.68) | 0.25 (0.17 to 0.34) | 1.32 (0.76 to 2.34) |
| ≤100 mg/d | 0.55 (0.38 to 0.78) | 2.69 (1.51 to 4.79) | 0.20 (0.12 to 0.32) | 1.0 (reference) | |
Adjusted for age, and sex.
Mortality of AASCARs according to Race/Ethnicity and Other Risk Factors
Compared to patients of a low-risk race/ethnicity (Whites/Hispanics), allopurinol initiators of a high-risk race/ethnicity had a 3.65-fold higher risk of mortality from AASCARs (multivariable-adjusted RR, 3.65; 95% CI, 1.90 to 6.99) (Table 4). Other significant independent risk factors for AASCAR mortality included female sex (multivariable-adjusted RR, 1.96; 95% CI, 1.02 to 3.74) and age ≥60 years (multivariable-adjusted RR, 2.79; 95% CI, 1.39 to 5.59) (Table 4).
Table 4.
Mortality of Allopurinol-Associated Severe Cutaneous Adverse Reactions (AASCARs) according to Race/Ethnicity and Purported Risk Factors
| Variable | Allopurinol Initiators N (%) |
Mortality Risk of Hospitalized AASCARs (/1000 persons) | Age-, Sex-Adjusted Relative Risk | Multivariable-Adjusted Relative Risk* |
|---|---|---|---|---|
| All | 400,401 (100) | 0.11 (0.08 to 0.14) | – | – |
| Race/Ethnicity | ||||
| White/Hispanic | 248,501 (62) | 0.05 (0.03 to 0.09) | 1.0 | 1.0 |
| Black/Asian/Native Hawaiian/Pacific Islander | 151,900 (38) | 0.20 (0.14 to 0.28) | 3.65 (1.90 to 6.99) | 3.65 (1.90 to 6.99) |
| Sex | ||||
| Male | 213,041 (53) | 0.07 (0.04 to 0.11) | 1.0 | 1.0 |
| Female | 187,360 (47) | 0.16 (0.11 to 0.22) | 1.93 (1.01 to 3.70) | 1.96 (1.02 to 3.74) |
| Age | ||||
| <60 years | 208,151 (52) | 0.05 (0.03 to 0.09) | 1.0 | 1.0 |
| ≥60 years | 192,250 (48) | 0.17 (0.12 to 0.23) | 2.75 (1.37 to 5.53) | 2.79 (1.39 to 5.59) |
| Chronic Kidney Disease | ||||
| No | 381,561 (95) | 0.10 (0.07 to 0.14) | 1.0 | 1.0 |
| Yes | 18,840 (5) | 0.22 (0.07 to 0.52) | 2.16 (0.77 to 6.04) | 2.16 (0.77 to 6.09) |
| Initial Allopurinol Dose (>100 mg/d) | ||||
| No | 157,138 (39) | 0.09 (0.05 to 0.15) | 1.0 | 1.0 |
| Yes | 243,263 (61) | 0.12 (0.08 to 0.17) | 1.47 (0.77 to 2.78) | 1.57 (0.83 to 2.98) |
Mutually adjusted for the variables in this table.
DISCUSSION
In the present study of 400,401 allopurinol initiators, we observed substantial variations in the incidence of hospitalized AASCARs according to race/ethnicity. Blacks, Asians, and Native Hawaiians/Pacific Islanders had a three- to six-times higher risk of AASCARs compared with Whites and Hispanics. These associations persisted similarly after adjusting for age, sex, presence of CKD, and initial allopurinol dose >100 mg daily, all of which we also found to be independently associated with the risk of hospitalized AASCARs. However, the presence of gout or the use of diuretics were not significantly associated with this risk. In our combined demographic analysis, elderly high-risk race/ethnicity women had over a 12-fold higher risk of hospitalized AASCARs compared with young White or Hispanic men. These findings support the use of extra caution among Native Hawaiians/Pacific Islanders, Asians, and Blacks when considering allopurinol (including screening for HLA-B*5801[2]), particularly among elderly women with CKD. Importantly, a low initial allopurinol dose (e.g., ≤100 mg/day) was the only modifiable risk factor, which is readily implementable and is also recommended by the latest rheumatology guidelines.[1,2]
The risk of AASCARs was over six times higher among Native Hawaiians/Pacific Islanders compared to Whites in this study. To our knowledge, this study provides the first evidence that this racial/ethnic group has a high risk of AASCARs. This finding corroborates the allele frequency of HLA-B*5801 (e.g., 5.8%[27,28] vs. <1–1.9%[18] in US Pacific Islanders and Whites, respectively). In other Pacific Island countries, the allele frequencies and prevalence of positive carriage are even higher (e.g., 6–12% and 11–22%, respectively, in Malaysia), [18] and thus, one would expect their risk of AASCARs to be at least as high as that observed in the current study. The recommendation to screen for HLA-B*5801[20] or to consider the use of an alternative ULD would be applicable to Native Hawaiians/Pacific Islanders prior to initiating allopurinol therapy, particularly when additional AASCAR risk factors are present (e.g., in the case of being an elderly woman with CKD).
The increased risk of AASCARs in Blacks in the current study expands on the findings of the recent US Nationwide Inpatient Sample study that showed a higher risk of SJS/TEN hospitalizations in Blacks compared to Whites, [19] although that study was not able to definitively determine the drug responsible for the increased risk. To that end, the current study identifies allopurinol as the culprit drug while also confirming the previously observed association.[19] These findings are also reflective of the allele frequencies of HLA-B*5801 (e.g., 2.6–6.4% vs. <1–1.9% in US Blacks and Whites, respectively[18]). Furthermore, the allele frequencies among Blacks in Africa are known to be even higher (e.g., 7 to 10% in Kenya and 8% in South Africa), suggesting a higher risk of AASCARs among those populations as well.[18]
The higher risk of AASCARs in Asians in the current study is consistent with the high incidence rate of AASCARs in Taiwan, [5] the increased risk of this adverse reaction among Chinese descendants compared with European descendants, [8] and the high carriage prevalence of HLA-B*5801 in Asian countries (e.g., 20% in Taiwan[20]). Accordingly, the Taiwanese Food and Drug Administration has recently adopted an alternative first-line ULD for patients with CKD.[5] Furthermore, a recent prospective study screened the HLA-B*5801 allele among 2,926 Taiwanese allopurinol initiators and was able to reduce the incidence of AASCARs from seven expected cases to zero (P = 0.003), demonstrating the effectiveness of such a practice.[20] In contrast, despite the sufficient sample size of Hispanic allopurinol initiators at our study baseline, there was an obviously low risk of AASCARs, similar to that seen in Whites (Supplemental Table 4). These data were similar to the recent US Nationwide Inpatient Sample study[19] and are concordant with the low frequency of HLA-B*5801 reported among Hispanics in the US and among Mexicans (i.e., ~1%).[18]
We also found that female sex was associated with a 2.5 times higher risk of AASCARs than male sex, even after adjusting for other risk factors. The aforementioned Taiwanese study also found a 45% higher risk of AHS among Chinese women compared with Chinese men.[5] The mechanism behind the difference in sex remains speculative, including the potential role of female hormones, which calls for further study. Older age was also independently associated with an increased risk of AASCARs, which was also consistent with the Taiwanese study findings among Chinese patients.[5] While this could reflect an aging and vulnerable immune system or a slower metabolic rate (which would predispose older individuals to develop this type of severe hypersensitivity reaction), further mechanistic clarification is needed. Finally, our analysis confirmed a strong independent association with CKD and the initial allopurinol dose of >100mg/day. This provides support for the latest rheumatology guideline recommendations to initiate allopurinol at a dose ≤100 mg daily.[1,2]
The main strength of this study is the use of 400,401 allopurinol initiators in a nationwide database, which provides information relevant to AASCAR outcomes. The racial and ethnic diversity of the US Medicaid study population made it possible to directly compare different racial/ethnic groups, which is not feasible in homogenous populations. Similar to the aforementioned study, [5] our study examined all allopurinol initiators regardless of gout status. As such, proportions of males and those with CKD tended to be lower than those of a typical gout cohort. While our analysis did not suggest a significant subgroup effect by the presence of gout, larger studies conducted specifically among gout patients would be valuable. We used pharmacy claims data, which are considered to be one of the best data sources for drug exposure. Also, because our administrative censoring for AASCARs was at the end of three months, potential issues associated with discontinuation of the medication are expected to be minimal. Because the Medicaid database is an administrative database, a certain degree of diagnostic code misclassification is inevitable. However, as mentioned, a recent study found our endpoint definition to have a high level of accuracy according to dermatologists’ medical record review.[5] As we further narrowed our primary endpoint definition to a primary discharge diagnosis of hospitalized cases, we expect the specificity to be even higher. Moreover, the high AASCAR-associated mortality rate as well as the long hospital stay corroborate its validity and our secondary definition of AASCARs [6] led to similar results. As our case definition did not include potential outpatient cases of AASCARs, the risk estimates for AASCARs in our study should be interpreted as conservative. Regardless, this would not have affected the RR measures and instead further guarded against the misclassification of cases.
In conclusion, these findings from a large, racially-diverse cohort indicate that Native Hawaiians/Pacific Islanders, Asians, and Blacks all have a substantially higher risk of hospitalized AASCARs compared with Whites and Hispanics, calling for heightened vigilance when initiating allopurinol in these racial/ethnic groups. Furthermore, female sex, older age, CKD, and an initial allopurinol dose >100 mg/day are all independent risk factors for hospitalized AASCARs and should also be considered when initiating allopurinol to help prevent this severe and potentially fatal adverse reaction.
Supplementary Material
Acknowledgments
Funding information: This work was supported in part by a grant from the National Institutes of Health (R01AR065944).
Footnotes
Competing Interests: HKC has received research grants from Pfizer and Astra-Zeneca to Massachusetts General Hospital for unrelated studies and served as a consultant for Takeda Pharmaceuticals, Selecta, Horizon, and Ironwood. SCK has received research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Bristol-Myers Squibb and Roche for unrelated studies.
Statement for Reports of Original Data: SFK, NL, and HKC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributorship: All authors participated in the conception, design and analyses of the study. SFK, NL, and HKC drafted the manuscript and are guarantors. All authors contributed to interpretation of the results.
Ethical approval information: Exempted from the Partners Institutional Review Board.
Data Sharing Statement: At this moment there are no additional unpublished data.
References
- 1.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 2.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jutkowitz E, Choi HK, Pizzi LT, et al. Cost-effectiveness of allopurinol and febuxostat for the management of gout. Ann Intern Med. 2014;161:617–26. doi: 10.7326/M14-0227. [DOI] [PubMed] [Google Scholar]
- 4.Stern RJ. Reducing Life-Threatening Allopurinol Hypersensitivity. JAMA Intern Med. 2015;175:1558. doi: 10.1001/jamainternmed.2015.3546. [DOI] [PubMed] [Google Scholar]
- 5.Yang CY, Chen CH, Deng ST, et al. Allopurinol Use and Risk of Fatal Hypersensitivity Reactions: A Nationwide Population-Based Study in Taiwan. JAMA Intern Med. 2015;175:1550–7. doi: 10.1001/jamainternmed.2015.3536. [DOI] [PubMed] [Google Scholar]
- 6.Kim SC, Newcomb C, Margolis D, et al. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: a population-based cohort study. Arthritis Care Res (Hoboken) 2013;65:578–84. doi: 10.1002/acr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halevy S, Ghislain PD, Mockenhaupt M, et al. Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58:25–32. doi: 10.1016/j.jaad.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Stamp LK, Taylor WJ, Jones PB, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: A proposed safe starting dose of allopurinol. Arthritis Rheum. 2012 doi: 10.1002/art.34488. [DOI] [PubMed] [Google Scholar]
- 9.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 10.Kang HR, Jee YK, Kim YS, et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet Genomics. 2011;21:303–7. doi: 10.1097/FPC.0b013e32834282b8. [DOI] [PubMed] [Google Scholar]
- 11.Jung JW, Song WJ, Kim YS, et al. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26:3567–72. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 12.Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9:1617–22. doi: 10.2217/14622416.9.11.1617. [DOI] [PubMed] [Google Scholar]
- 14.Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 15.Lonjou C, Borot N, Sekula P, et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18:99–107. doi: 10.1097/FPC.0b013e3282f3ef9c. [DOI] [PubMed] [Google Scholar]
- 16.Zineh I, Mummaneni P, Lyndly J, et al. Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics. 2011;12:1741–9. doi: 10.2217/pgs.11.131. [DOI] [PubMed] [Google Scholar]
- 17.Somkrua R, Eickman EE, Saokaew S, et al. Association of HLA-B*5801 allele and allopurinol-induced Stevens Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Med Genet. 2011;12:118. doi: 10.1186/1471-2350-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.[http://www.allelefrequencies.net/].
- 19.Lu N, Rai SK, Terkeltaub R, et al. Racial disparities in the risk of Stevens-Johnson Syndrome and toxic epidermal necrolysis as urate-lowering drug adverse events in the United States. Semin Arthritis Rheum. 2016 doi: 10.1016/j.semarthrit.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko TM, Tsai CY, Chen SY, et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ. 2015;351:h4848. doi: 10.1136/bmj.h4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark WD, Hulbert MM. Research Issues: Dually Eligible Medicare and Medicaid Beneficiaries, Challenges and Opportunities. Health Care Financ Rev. 1998;20:1–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Chung WH, Chang WC, Stocker SL, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis. 2015;74:2157–64. doi: 10.1136/annrheumdis-2014-205577. [DOI] [PubMed] [Google Scholar]
- 23.Strom BL, Carson JL, Halpern AC, et al. A population-based study of Stevens-Johnson syndrome. Incidence and antecedent drug exposures. Arch Dermatol. 1991;127:831–8. [PubMed] [Google Scholar]
- 24.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126:43–7. [PubMed] [Google Scholar]
- 25.Strom BL, Carson JL, Halpern AC, et al. Using a claims database to investigate drug-induced Stevens-Johnson syndrome. Stat Med. 1991;10:565–76. doi: 10.1002/sim.4780100408. [DOI] [PubMed] [Google Scholar]
- 26.https://www.hcup-us.ahrq.gov/team/NationwideDUA.jsp.
- 27.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–88. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 28.https://bioinformatics.bethematchclinical.org/hla-resources/haplotype-frequencies/high-resolution-hla-alleles-and-haplotypes-in-the-us-population/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


