Abstract
Syntheses of the 6′-N-(2-hydroxyethyl) and 1-N-(4-amino-2S-hydroxybutyryl) derivatives of the 4,6-aminoglycoside sisomicin and that of the doubly modified 1-N-(4-amino-2S-hydroxybutyryl)-6′-N-(2-hydroxyethyl) derivative known as plazomicin are reported together with their antibacterial and antiribosomal activities and selectivities. The 6′-N-(2-hydroxyethyl) modification results in a moderate increase in prokaryotic/eukaryotic ribosomal selectivity, the 1-N-(4-amino-2S-hydroxybutyryl) modification has the opposite effect. When combined in plazomicin the effects of the two groups on ribosomal selectivity cancel each other out leading to the prediction that plazomicin will exhibit comparable ototoxicity to the parent and to the current clinical aminoglycoside antibiotics gentamicin and tobramycin, as borne out by ex-vivo studies with mouse cochlear ex-plants. The 6′-N-(2-hydroxyethyl) modification restores antibacterial activity in the presence of the AAC(6′) aminoglycoside-modifying enzymes, while the 1-N-(4-amino-2S-hydroxybutyryl) modification overcomes resistance to the AAC(2′) class, but is still affected to some extent by the AAC(3) class. Neither modification is able to circumvent the ArmA ribosomal methyltransferase-induced aminoglycoside resistance. The use of phenyltriazenyl protection for the secondary amino group of sisomicin facilitates synthesis of each derivative and their characterization through the provision of sharp NMR spectra for all intermediates.
Keywords: Aminoglycosides, Structure-Activity Relationship, Aminoglycoside modifying enzymes, ribosomal methyltransferases, cell-free translation assays
Graphical Abstract
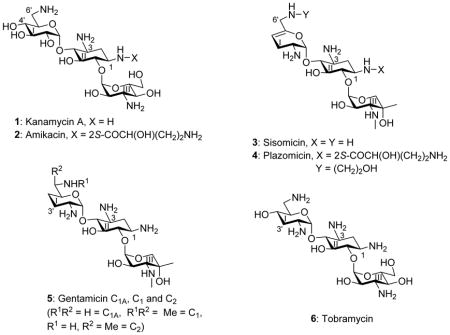
The growing threat to public health posed by the spread of multidrug-resistant infectious diseases necessitates the development of new antibiotic substances.1–7 This may be achieved either by the discovery of new classes of antibiotic molecules or by further development of existing antibiotic classes. The aminoglycoside antibiotics (AGAs) are an attractive starting point for the latter avenue as, with more than fifty years of development and clinical application, an extensive knowledge of their mechanism of action, pharmacology, and chemistry provides a strong foundation for rational development.8–23 The decades of clinical use have also given rise to extensive resistance to AGAs, which is mostly due to the aminoglycoside-modifying enzymes (AMEs) or the ribosomal methyltransferases (RMTases).24–29 AMEs can be thwarted by AGA redesign to prevent modification without compromising antibacterial activity. Indeed, this has long been a strategy in the pharmaceutical industry and led to a series of semisynthetic AGAs, of which the kanamycin A 1 derivative amikacin 2 was the last to enter the clinic in the early 1990s,19–22,30,31 and which continues with the sisomicin 3 derivative plazomicin 4 that is currently in phase III clinical trials.32–34 Plazomicin was designed to overcome the action of most AMEs, but it is still a target for the RMTases and most notably the G1405 methyl transferases.35 AGA modification can also impact selectivity by reducing affinity for the eukaryotic ribosomes, particularly the mitochondrial ribosome for which drug affinity underlies the major side effect of ototoxicity.36–38 Unfortunately, little is known about the manner in which AGA modifications affect affinity for and thus selectivity among the bacterial and eukaryotic ribosomes. In our laboratories, using cell-free translation assays with bacterial wild type ribosomes and hybrid bacterial ribosomes carrying humanized decoding A sites,39 we have begun to address these issues with particular emphasis on modification of ring I in the 2-deoxystreptamine class of AGAs.40–48
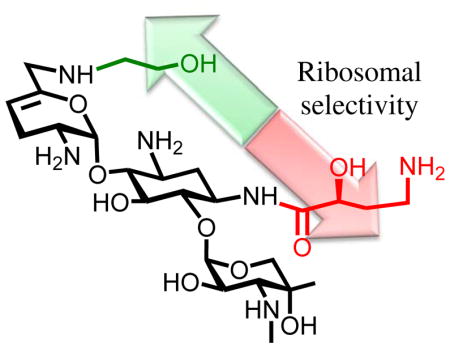
Continuing our studies of the relation between structure, activity, and selectivity of the AGAs, we report expedient methods for the synthesis of sisomicin derivatives modified at either or both of the N1 or the N6′-positions. These syntheses incorporate phenyltriazene protection of the secondary amino group in ring III of the parent system facilitating spectral characterization of intermediates, a notable advantage over the classical carbamate-based protecting schemes. The consequent effective availability of plazomicin 4, a semisynthetic AGA possessing two previously known individual structural modifications (the 6′-N (2-hydroxyethyl and the 1-N-(4-amino-2S-hydroxybutyryl) substituents) of the parent drug, and of the two individual modifications 10 and 11 enable us to report on the contribution of these modifications, alone and in combination, to antibacterial activity and ribosomal selectivity. We further report on ex-vivo studies with mouse cochlear explants leading to the conclusion that the cochleotoxicity of plazomicin is comparable to that of typical AGAs including sisomicin 3, gentamicin 5, and tobramycin 6.
Results and Discussion
Synthesis
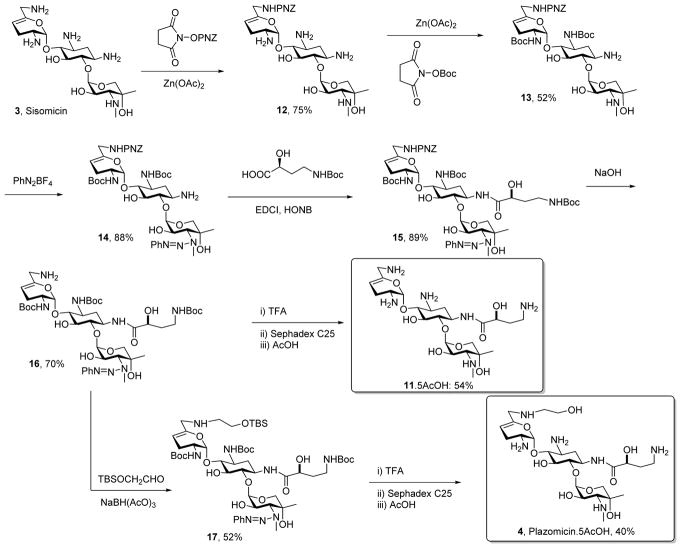
Adopting the use of the N-phenyl triazenes described earlier for the selective protection of secondary amines in the presence of their primary counterparts, we oxidized the known sisomicin derivative 749–51 with selenium dioxide in pyridine to afford the α,β-unsaturated aldehyde 8 in 77% yield.52 Reductive amination of 8 with ethanolamine then afforded derivative 9 in 63% yield, from which the known sisomicin derivative 1051 was obtained by Staudinger reduction of the azides,53 cleavage of the triazene with trifluoroacetic acid,49 and purification by ion exchange chromatography over Sephadex (Scheme 1).
Scheme 1.
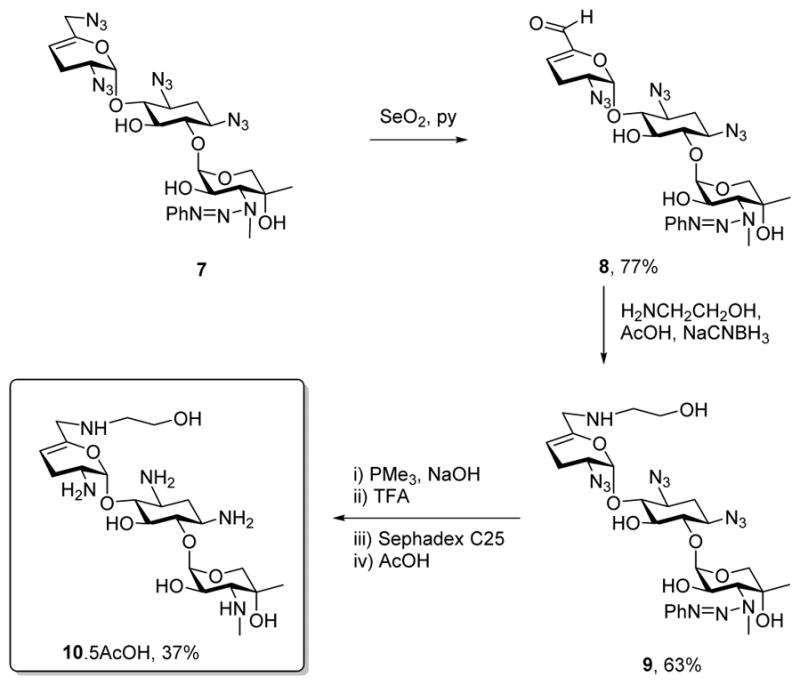
Synthesis of 6′-N-(2-hydroxyethyl)sisomicin 10
The preparation of the 1-N-(4-amino-2(S)-hydroxybutyryl sisomicin 11, a compound previously described in the patent literature54,55 and characterized as an impurity in plazomicin from the Achaogen synthesis,56 began with the selective protection of the 6′-NH2 group of sisomicin 3 using 4-nitrobenzyl N-hydroxysuccinimidyl carbonate33,57 and zinc acetate giving 6′-N-(4-nitrobenzyloxycarbonyl)sisomicin 12 in 75% yield. The amino substituents at positions 2′ and 3 were then selectively protected as the Boc derivatives by temporary protection of the other amines in the form of zinc chelates, resulting in the formation of 13 in 52% yield.58 Reaction of 13 with a 10 mol % excess of phenyldiazonium tetrafluoroborate in acetonitrile in the presence of potassium carbonate gave the desired triazene 14 in 88% yield. The remaining amino group was then coupled to N-Boc-4-amino-2(S)-hydroxy-butyric acid (LHABA)59 under standard carbodiimide conditions in the presence of N-hydroxy-5-norbornene-2,3-dicarboximide (HONB),60 and afforded the amide 15 in 89% yield. The 4-nitrobenzyl carbamate group was then cleaved with aqueous sodium hydroxide in dioxane to yield 70% of the amine 16, which on treatment with TFA followed by ion exchange chromatography over Sephadex afforded the target 1-LHABA sisomicin 11. Alternatively, reductive amination of tert-butyldimethylsilyloxy acetaldehyde with 16 and sodium triacetoxyborohydride61 gave 17 in 52% yield. This reaction was conducted in the presence of Hünig’s base62 so as to avoid premature cleavage of the triazene group. Finally, removal of the Boc, silyl, and triazene groups from 17 was achieved with 50% trifluoroacetic acid in dichloromethane and gave plazomicin 4 in 40% yield after purification by chromatography over Sephadex and lyophilisation (Scheme 2).
Scheme 2.
Synthesis of 1-N-LHABA sisomicin 11 and of plazomicin 4
The syntheses of 4, 10, and 11 all feature the use of the phenyltriazene group for the protection of secondary amines. In addition to the convenient protection of secondary amines in the presence of their primary counterparts, and because of the absence of the rotamer problems associated with the more common carbamates, the use of this protecting group affords sharp, well-resolved NMR spectra which facilitate structural elucidation. We also note that this synthesis affords the opportunity for full characterization of plazomicin, as the published synthesis provides neither experimental details, nor characterization data,32 and the patent literature33 reveals only high resolution mass spectrometric measurements.
Antibacterial Screening
Compounds 10, 11 sisomicin 3 and plazomicin 4 were screened for activity against two methicillin-resistant clinical isolates of the Gram-positive Staphylococcus aureus (MRSA) and against two clinical isolates of the Gram negative Escherichia coli (E. coli) from the Diagnostic Division of the Institute of Medical Microbiology (Table 1). Gentamicin C 5, tobramycin 6 and the structurally unusual apramycin 18, characterized by activity in the presence of most common resistance determinants and low levels of ototoxicity,40,63,64 were also screened against the same panel for comparison. For both the Gram-positive and the Gram-negative organisms the installation of the 6′-N-(2-hydroxyethyl) substituent on sisomicin as in compound 10 results in a modest 2–4 fold reduction in antibacterial activity, consistent with our earlier observations on the influence of the same substituent as applied to the 4,5-aminoglycoside neomycin B.47,65 Similarly, the 1-N-LHABA sisomicin derivative 11 exhibits a small 2–4-fold decrease in activity against the same clinical strains of MRSA and E. coli (Table 1). Not surprisingly, therefore, the combination of both substituents into the single compound plazomicin 4 also results in a modest decrease in antibacterial activity against wild type MRSA and E. coli (Table 1). The moderately reduced antibacterial activity of plazomicin, as compared to the parent sisomicin, is consistent with the literature data.32 Broad antibacterial screening was not conducted as the activity of plazomicin is widely reported in the literature,32,66–68 nevertheless all compounds were screened against a small panel of ESKAPE pathogens (Table 2).
Table 1.
Antibacterial Activities (MIC, μg/mL)a
|
|
||||
|---|---|---|---|---|
| MRSA | E. coli | |||
| AG038 | AG039 | AG001 | AG055 | |
|
|
|
|||
| Sisomicin 3 | 0.25 | 0.25 | 0.5 | 0.25 |
| 6′-(2-Hydroxyethyl)sisomicin 10 | 1 | 1 | 1–2 | 1 |
| 1-LHABA sisomicin 11 | 0.5 | 1 | 2 | 0.5 |
| Plazomicin 4 | 2 | 2 | 2 | 2 |
| Gentamicin 5 | 0.25 | 0.25 | 1–2 | 0.5 |
| Tobramycin 6 | 0.5–1 | >128 | 4–8 | 2 |
| Apramycin 18 | 4 | 4 | 4 | 4 |
All values were determined in duplicate using twofold dilution series.
Table 2.
Activity Against ESKAPE Pathogens (MIC, μg/mL)a
| E. coli (AG212) | K. pneu (AG215) | A. baum (AG225) | E. cloacae (AG290) | P. aerug (AG220) | |
|---|---|---|---|---|---|
| Sisomicin 3 | 0.25–0.5 | 0.125 | 0.5 | 0.25–0.5 | 0.25–0.5 |
| 6′-(2-Hydroxyethyl)sisomicin 10 | 1 | 0.5 | 8–16 | 0.5–1 | 2–4 |
| 1-LHABA sisomicin 11 | 0.25–0.5 | 0.125 | 1 | 0.5 | 1 |
| Plazomicin 4 | 1 | 0.25–0.5 | 2–4 | 0.5–1 | 0.5–1 |
| Gentamicin 5 | 0.5–1 | 0.25 | 0.5–1 | 0.25 | 1 |
| Tobramycin 6 | 1 | 0.25–0.5 | 1 | 0.5 | 0.5 |
| Apramycin 18 | 4 | 1–2 | 4 | 2–4 | 4 |
All values were determined in duplicate using twofold dilution series.
All compounds were screened for antibacterial activity against a panel of wild-type, recombinant, and clinical strains of E. coli carrying specific resistance determinants in order to distinguish the influence of the individual modifications (Table 3). Noteworthy is the fact that the 6′-N-(2-hydroxyethyl) modification restores activity to compound 10 in the presence of the aminoglycoside-modifying enzyme AAC(6′)-1 that causes a very significant drop in the activity of the parent, and of the clinical AGA tobramycin. This result is consistent with the early indications from Mallams and coworkers,51 and with application of the same modification to neomycin B and to related 4,6-AGAs.69,70 Modification of sisomicin with the 1-LHABA group 11 largely restores activity in the presence of AAC(3)-I, and is also effective in protecting against the action of AAC(2′) and AAC(6′)-I (Table 3). Consistent with these observations, and those of the Achaogen group,32 plazomicin retains most of its activity in the face of the AAC(2′), AAC(3)-I, and AAC(6′)-IAMEs. Most importantly, however, as members of the 4,6-class of AGAs, all of these compounds including plazomicin succumb to resistance arising from modification of the decoding A site of helix 44 of bacterial 16S ribosomal RNA by the RMTase ArmA acting on N5 of G1405.24,35,71–73 Apramycin 18 on the other hand, despite its moderately lower activity against wild-type bacteria (Tables 1 and 2), retains full activity in the presence of most resistance determinants including ArmA (Table 3).
Table 3.
Antimicrobial Data Against Wild Type, Engineered Strains, and Clinical Strains of E. coli Carrying Specific Resistance Determinants (MIC, μg/mL)a
| Strain | AG006b | AG007b | AG105b | AG036b | AG037b | AG103b | ATCC25922c | AG175c |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Resistance Mechanism | WT | AAC(3)-I, | AAC(2′) | ANT(4′,4″) | APH(3′)-IIIa | ArmA | WT | AAC(6′)-I |
| Sisomicin 3 | ≤0.25 | 16 | >64 | ≤0.125 | ≤0.125 | >64 | 0.5 | 16–32 |
| 6′-(2-Hydroxyethyl)sisomicin 10 | 0.5 | >64 | >64 | ≤0.125 | 0.25 | >64 | 1 | 1–2 |
| 1-LHABA sisomicin 11 | 0.25 | 1 | 0.25–0.5 | ≤0.125 | ≤0.125 | >64 | 0.5 | 1–2 |
| Plazomicin 4 | 0.5 | 2–4 | 1 | 0.25 | 0.25 | >64 | 1 | 1–2 |
| Gentamicin 5 | 0.25 | 32 | 64 | ≤0.125 | ≤0.125 | >64 | 0.5–1 | 1 |
| Tobramycin 6 | 0.5 | 2 | 2 | 8 | 0.5 | >64 | 1 | 64 |
| Apramycin 18 | 4 | 4–8 | 4 | 4 | 4 | 2 | 4–8 | nd |
All values were determined in duplicate using twofold dilution series.
Genetically engineered strain from Pasteur Institute.
Clinical isolates
Antiribosomal Activity and Selectivity
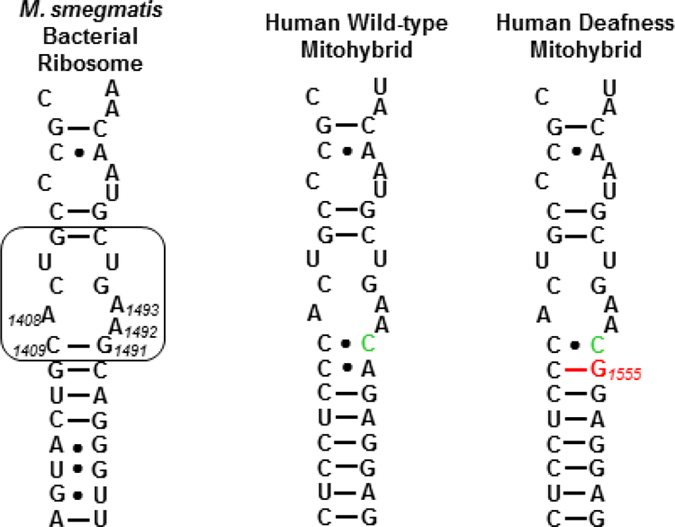
In a series of cell-free translation assays, 6′-N-(2-hydroxyethyl)sisomicin 10, 1-N-LHABA sisomicin 11, plazomicin 4, sisomicin 3, and the comparators gentamicin 5, tobramycin 6, and apramycin 18 were screened for inhibition of luciferase production by the bacterial ribosome. Inhibitory activities against hybrid bacterial ribosomes carrying either the complete mitochondrial decoding A site (Wild-type mitohybrid) or its A1555G mutant (Mutant deafness mitohybrid) (Figure 1), that confers hypersusceptibility to drug-induced hearing loss, were also determined (Table 4).36,37,39 Selectivities for the bacterial decoding A site over the wild-type and mutant mitochondrial were then calculated (Table 4).
Figure 1.
Decoding A sites of bacterial, human wild-type mitohybrid, and human deafness mitohybrid ribosomes. The AGA binding pocket is boxed. The bacterial numbering scheme is illustrated for the AGA binding pocket. Changes from the bacterial ribosome binding pocket are coloured green. The A1555G mutant conferring hypersusceptibility to AGA ototoxicity is coloured red.
Table 4.
Antiribosomal Activities (IC50 μg/mL)a and Selectivities
| Antiribosomal Activitya IC50 μg/mL |
Selectivityb | ||||
|---|---|---|---|---|---|
| Bacterial | Wild-type Mitohybrid | Mutant Deafness Mitohybrid | Wild-type Mitohybrid | Mutant Deafness Mitohybrid | |
|
| |||||
| Sisomicin 3 | 0.01 | 9.8 | 0.6 | 980 | 60 |
|
| |||||
| 6′-(2-Hydroxyethyl)sisomicin 10 | 0.06 | 59 | 6.6 | 983 | 110 |
|
| |||||
| L-HABA Sisomicin 11 | 0.01 | 4.7 | 0.25 | 470 | 25 |
|
| |||||
| Plazomicin 4 | 0.06 | 50 | 2.8 | 833 | 47 |
|
| |||||
| Gentamicin 5 | 0.015 | 9 | 0.5 | 600 | 33 |
|
| |||||
| Tobramycin 6 | 0.02 | 15 | 0.5 | 750 | 25 |
|
| |||||
| Apramycin 18 | 0.08 | 68 | 38 | 850 | 475 |
All values were determined in duplicate using twofold dilution series.
Selectivity = eukaryotic/prokaryotic antiribosomal activity
Compound 10, the 6′-(N-2-hydroxyethyl) derivative of sisomicin, displays largely comparable selectivity to 3 over the wild type mitochondrial ribosomes and a two fold increase in selectivity over the A1555G mutant mitochondrial ribosomes, suggestive of modest reduction in ototoxicity and largely consistent with the influence exerted by this modification in the 4,5-aminoglycoside neomycin B.47 The sisomicin derivative 11 carrying the 1-N-LHABA modification, on the other hand exhibits reduced selectivity over both the wild type and mutant mitochondrial ribosomes. Thus, at least for sisomicin the 1-N-LHABA modification used to overcome the action of AAC(2′) and AAC(3) AMEs attenuates ribosomal selectivity. The opposing influences of the two modifications on ribosomal selectivity are canceled by their combination in plazomicin 4, resulting overall in comparable selectivity to that exhibited by the parent, and suggesting that plazomicin and sisomicin will display comparable cochleotoxicity. As reported previously,40 apramycin 18 exhibits excellent selectivity for the bacterial ribosome over both the human mitochondrial and mutant mitochondrial ribosomes (Table 4).
Cochleotoxicity
The cochleotoxicity of plazomicin 4, and of comparators tobramycin 5, gentamicin 6, and apramycin 18, was examined using the mouse cochlear explant model, as described previously for apramycin,40 in 24 and 72 h exposures, enabling the measure of μM EC50 values (Table 5).
Table 5.
Ex-vivo Ototoxicity
| EC50 μM | ||
|---|---|---|
| 24 h | 72 h | |
|
| ||
| Plazomicin 4 | 75–150 | 16.4 |
|
| ||
| Tobramycin 5 | 125–250 | 9.3 |
|
| ||
| Gentamicin 6 | 150 | 4.5 |
|
| ||
| Apramycin 18 | 3500 | 85.5 |
The difference in EC50 values for the 24 and 72 h exposures clearly indicates the importance of exposure time in these studies. While 24 h exposure requires suprapharmacological drug concentrations, the longer 72 h period better models the clinical conditions, requiring at least an order of magnitude less drug to kill 50% of the sensory cells in the explant (EC50). Regardless of exposure time the data demonstrate that plazomicin has cochleotoxicity largely comparable to the clinical AGA tobramycin. The ototoxicity of tobramycin is similar to that of amikacin and sisomicin in an in-vivo guinea pig model,74 and moderately lower than that of gentamicin. This observation is consistent with a preliminary report by Achaogen suggesting that plazomicin may be less ototoxic than gentamicin in the guinea pig model,75 albeit only a single relatively low concentration of 80 mg/kg−1 was employed that does not allow for firm conclusions in this regard. It is clear from Table 5 that plazomicin is significantly more cochleotoxic than apramycin, a compound that is known to exhibit very low levels of ototoxicity in the guinea pig model.40
Conclusion
The 6′-N-(2-hydroxyethyl) and 1-N-(4-amino-2S-hydroxybutyryl) modifications of the 4,6-aminoglycoside sisomicin have been prepared and characterized, and exhibit contrasting influences on the ribosomal selectivity of the parent. Combination of the two modifications in a single derivative, the experimental drug plazomicin, results in effective cancellation of their effects on ribosomal selectivity and the prediction that plazomicin will exhibit comparable ototoxicity to the parent sisomicin and other AGAs in current clinical practice. This conclusion is clearly borne out ex-vivo studies with mouse cochlear ex-plants.
Supplementary Material
Acknowledgments
We are grateful to Sven N. Hobbie, University of Zurich for assistance with microbiology, and Patrice Courvalin, Institut Pasteur, for the gift of engineered strains of E. coli. We thank the NIH (AI123352) for support of this work, and acknowledge the NSF (MRI-084043) for funds in support of the purchase of the 600 MHz NMR spectrometer in the Lumigen Instrument Center at Wayne State University.
Footnotes
Notes
The authors declare the following competing financial interest(s): A.V., E.C.B., and D.C. are cofounders of and have an equity interest in Juvabis, a biotech startup working in the aminoglycoside area.
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website at DOI:
Full experimental details and copies of 1H and 13C NMR spectra for all intermediates; 1H and 13C NMR and 2D NMR spectra for 4, 10, and 11 (PDF)
References
- 1.Holdren JP, Lander ES. Report to the President on Combatting Antibiotic Resistance. President’s Council of Advisors on Science and Technology; Washington DC. 2014. pp. 1–65. [Google Scholar]
- 2.Antibiotic Resistance Threats in the United States, 2013. Center for Disease Control and Prevention; Atlanta: 2013. pp. 1–114. [Google Scholar]
- 3.Fischbach MA, Walsh CT. Antibiotics for Emerging Pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connell KMGO, Hodgkinson JT, Sore HF, Welch M, Salmond GPC, Spring DR. Combating Multidrug-Resistant Bacteria: Current Strategies for the Discovery of Novel Antibacterials. Angew Chem Int Ed. 2013;52:10706–10733. doi: 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- 5.Gualerzi CO, Brandi L, Fabbretti A, Pon CL, editors. Antibiotics: Targets, Mechanisms and Resistance. Wiley-VCH; Weinheim: 2014. [Google Scholar]
- 6.Wright PM, Seiple IB, Myers AG. The Evolving Role of Chemical Synthesis in Antibacterial Drug Discovery. Angew Chem Int Ed. 2014;53:8840–8863. doi: 10.1002/anie.201310843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JF, Mobashery S. Endless Resistance. Endless Antibiotics? Med Chem Commun. 2016;7:37–49. doi: 10.1039/C5MD00394F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter AP, Clemons WM, Brodersen DE, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. Functional Insights from the Structure of the 30S Ribosomal Subunit and Its Interactions with Antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 9.Greber BJ, Bieri P, Leibundgut M, Leitner A, Aebersold R, Boehringer D, Ban N. The Complete Structure of the 55S Mammalian Mitochondrial Ribosome. Science. 2015;348:303–308. doi: 10.1126/science.aaa3872. [DOI] [PubMed] [Google Scholar]
- 10.Garreau de Loubresse N, Prokhorova I, Holtkamp W, Rodnina MV, Yusupova G, Yusupov M. Structural Basis for the Inhibition of the Eukaryotic Ribosome. Nature. 2014;513:517–523. doi: 10.1038/nature13737. [DOI] [PubMed] [Google Scholar]
- 11.Amunts A, Brown AD, Toots J, Scheres SHW, Ramakrishnan V. The Structure of the Human Mitochondrial Ribosome. Science. 2015;348:95–98. doi: 10.1126/science.aaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arya DP, editor. Aminoglycoside Antibiotics: From Chemical Biology to Drug Discovery. Wiley; Hoboken: 2007. [Google Scholar]
- 13.Forge A, Schacht J. Aminoglycoside Antibiotics. Audiology Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 14.Huth ME, Ricci AJ, Cheng AG. Mechanisms of Aminoglycoside Ototoxicity and Targets of Hair Cell Protection. Int J Otolaryngol. 2011:937861. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Böttger EC, Schacht J. The Mitochondrion: A Perpetrator of Acquired Hearing Loss. Hearing Res. 2013;303:12–19. doi: 10.1016/j.heares.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houghton JL, Green KD, Chen W, Garneau-Tsodikova S. The Future of Aminoglycosides: The End or Renaissance? ChemBioChem. 2010;11:880–902. doi: 10.1002/cbic.200900779. [DOI] [PubMed] [Google Scholar]
- 17.Becker B, Cooper MA. Aminoglycoside Antibiotics in the 21st Century. ACS Chem Biol. 2013;8:105–115. doi: 10.1021/cb3005116. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong ES, Kostrub CF, Cass RT, Moser HE, Serio AW, Miller GH. Aminoglycosides. In: Dougherty TJ, Pucci MJ, editors. Antibiotic Discovery and Development. Springer Science+Business Media; New York: 2012. pp. 229–269. [Google Scholar]
- 19.Umezawa S. Structures and Syntheses of Aminoglycoside Antibiotics. Adv Carbohydr Chem Biochem. 1974;30:111–182. doi: 10.1016/s0065-2318(08)60264-4. [DOI] [PubMed] [Google Scholar]
- 20.Haddad J, Kotra LP, Mobashery S. Aminoglycoside Antibiotics: Structures and Mechanisms of Action. In: Wang PG, Bertozzi CR, editors. Glycochemsitry: Principles, Synthesis, and Applications. Dekker; New York: 2001. pp. 307–351. [Google Scholar]
- 21.Haddad J, Liu M-Z, Mobashery S. Methodologies in Syntheses of Aminoglycoside Antibiotics. In: Wang PG, Bertozzi CR, editors. Glycochemistry: Principles, Synthesis, and Applications. Dekker; New York: 2001. pp. 353–424. [Google Scholar]
- 22.Wang J, Chang C-WT. Design, Chemical Synthesis, and Antibacterial Activity of Kanamycin and Neomycin Class Aminoglycoside Antibiotics. In: Arya DP, editor. Aminoglycoside Antibiotics. Wiley; Hobeken: 2007. pp. 141–180. [Google Scholar]
- 23.Berkov-Zrihen Y, Fridman M. Synthesis of Aminoglycosides. In: Werz DB, Vidal S, editors. Modern Synthetic Methods in Carbohydrate Chemistry; from Monosaccharides to Complex Glycoconjugates. Wiley; Weinheim: 2014. pp. 161–190. [Google Scholar]
- 24.Magnet S, Blanchard JS. Molecular Insights into Aminoglycoside Action and Resistance. Chem Rev. 2005;105:477–497. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 25.Lambert T. Aminoglycosides and Gram-Negative Bacteria. In: Courvalin P, Leclercq R, Rice LB, editors. Antibiogram. ASM Press; 2010. pp. 225–242. [Google Scholar]
- 26.Bismuth R, Courvalin P. Aminoglycosides and Gram-Positive Bacteria. In: Courvalin P, Leclercq R, Rice LB, editors. Antibiogram. ASM Press; 2010. pp. 213–224. [Google Scholar]
- 27.Bacot-Davis VR, Bassenden AV, Berghuis IM. Drug-Target Networks in Aminoglycoside Resistance: Hierarchy of Priority in Structural Drug Design. Med Chem Commun. 2016;7:103–113. [Google Scholar]
- 28.Garneau-Tsodikova S, Labby KJ. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Med Chem Commun. 2016;7:11–27. doi: 10.1039/C5MD00344J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zárate SG, De la Cruz Claure ML, Benito-Arenas R, Revuelta R, Santana AG, Bastida A. Overcoming Aminoglycoside Enzymatic Resistance: Design of Novel Antibiotics and Inhibitors. Molecules. 2018;23:284. doi: 10.3390/molecules23020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price KE, Godfrey JC, Kawaguchi H. Effect of Structural Modifications on the Biological Properties of Aminoglycoside Antibiotics Containing 2-Deoxystreptamine. Adv Appl Microbiol. 1974;18:191–307. doi: 10.1016/s0065-2164(08)70572-0. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Ye XS. Development of Aminoglycoside Antibiotics Effective against Resistant Bacterial Strains. Curr Top Med Chem. 2010;10:1898–1926. doi: 10.2174/156802610793176684. [DOI] [PubMed] [Google Scholar]
- 32.Aggen JB, Armstrong ES, Goldblum AA, Dozzo P, Linsell MS, Gliedt MJ, Hildebrandt DJ, Feeney LA, Kubo A, Matias RD, Lopez S, Gomez M, Wlasichuk KB, Diokno R, Miller GH, Moser HE. Synthesis and Spectrum of the Neoglycoside ACHN-490. Antimicrob Agent Chemother. 2010;54:4636–4642. doi: 10.1128/AAC.00572-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggen JB, Goldblum AA, Sell M, Dozzo P, Mozer HE, Hildebrandt D, Gliedt M. Antibacterial Aminoglycoside Analogs. 067692 A1. WO Patent. 2009
- 34.A Study of Plazomicin Compared With Colistin in Patients With Infection Due to Carbapenem-Resistant Enterobacteriaceae (CRE) 2014 https://www.clinicaltrials.gov/ct2/show/NCT01970371.
- 35.Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. Activity of Aminoglycosides, Including ACHN-490: against Carbapenem-resistant Enterobacteriaceae Isolates. J Antimicrob Chemother. 2011;66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- 36.Prezant TR, Agapian JV, Bohlman MC, Bu X, Öztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, Shohat M, Fischel-Ghodsian N. Mitochondrial Ribosomal RNA Mutation Associated with Both Antibiotic-Induced and Non-Syndromic Deafness. Nat Genetics. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 37.Hobbie SN, Bruell CM, Akshay S, Kalapala SK, Shcherbakov D, Böttger EC. Mitochondrial Deafness Alleles Confer Misreading of the Genetic Code. Proc Natl Acad Sci, USA. 2008;105:3244–3249. doi: 10.1073/pnas.0707265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobbie SN, Akshay S, Kalapala SK, Bruell C, Shcherbakov D, Böttger EC. Genetic Analysis of Interactions with Eukaryotic rRNA Identify the Mitoribosome as Target in Aminoglycoside Ototoxicity. Proc Natl Acad Sci, USA. 2008;105:20888–20893. doi: 10.1073/pnas.0811258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hobbie SN, Kalapala SK, Akshay S, Bruell C, Schmidt S, Dabow S, Vasella A, Sander P, Böttger EC. Engineering the rRNA Decoding Site of Eukaryotic Cytosolic Ribosomes in Bacteria. Nucl Acids Res. 2007;35:6086–6093. doi: 10.1093/nar/gkm658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matt T, Ng CL, Lang K, Sha SH, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Böttger EC. Dissociation of Antibacterial Activity and Aminoglycoside Ototoxicity in the 4-Monosubstituted 2-Deoxystreptamine Apramycin. Proc Natl Acad Sci, USA. 2012;109:10984–10989. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Fernandez D, Shcherbakov D, Matt T, Leong NC, Kudyba I, Duscha S, Boukari H, Patak R, Dubbaka SR, Lang K, Meyer M, Akbergenov R, Freihofer P, Vaddi S, Thommes P, Ramakrishnan V, Vasella A, Böttger EC. 4′-O-Substitutions Determine Aminoglycoside Selectivity at the Drug Target Level. Nature Commun. 2014;5:4112/4111–4112/4111. doi: 10.1038/ncomms4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duscha S, Boukari H, Shcherbakov D, Salian S, Silva S, Kendall A, Kato T, Akbergenov R, Perez-Fernandez D, Bernet B, Vaddi S, Thommes P, Schacht J, Crich D, Vasella A, Böttger EC. Identification and Evaluation of Improved 4′-O-(Alkyl) 4:5-Disubstituted 2-Deoxystreptamines as Next Generation Aminoglycoside Antibiotics. mBio. 2014;5 doi: 10.1128/mBio.01827-01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandhapati AR, Shcherbakov D, Duscha S, Vasella A, Böttger EC, Crich D. Importance of the 6′-Hydroxy Group and Its Configuration for Apramycin Activity. ChemMedChem. 2014;9:2074–2083. doi: 10.1002/cmdc.201402146. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Matsushita T, Shcherbakov D, Boukari H, Vasella A, Böttger EC, Crich D. Synthesis, Antiribosomal and Antibacterial Activity of 4′-O-Glycopyranosyl Paromomycin Aminoglycoside Antibiotics. MedChemCommun. 2014;5:1179–1187. [Google Scholar]
- 45.Matsushita T, Chen W, Juskeviciene R, Teo Y, Shcherbakov D, Vasella A, Böttger EC, Crich D. Influence of 4′-O-Glycoside Constitution and Configuration on Ribosomal Selectivity of Paromomycin. J Am Chem Soc. 2015;137:7706–7717. doi: 10.1021/jacs.5b02248. [DOI] [PubMed] [Google Scholar]
- 46.Kato T, Yang G, Teo Y, Juskeviciene R, Perez-Fernandez D, Shinde HM, Salien S, Bernet B, Vasella A, Böttger EC, Crich D. Synthesis and Antiribosomal Activities of 4′-O-, 6′-O-, 4″-O-, 4′,6′-O- and 4″,6″-O-Derivatives in the Kanamycin Series Indicate Differing Target Selectivity Patterns between the 4,5- and 4,6-Series of Disubstituted 2-Deoxystreptamine Aminoglycoside Antibiotics. ACS Infect Dis. 2015;1:479–486. doi: 10.1021/acsinfecdis.5b00069. [DOI] [PubMed] [Google Scholar]
- 47.Sati GC, Shcherbakov D, Hobbie S, Vasella A, Böttger EC, Crich D. N6′, N6‴, and O4′-Modifications to Neomycin Affect Ribosomal Selectivity without Compromising Antibacterial Activity. ACS Infect Dis. 2017;3:368–376. doi: 10.1021/acsinfecdis.6b00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandhapati AR, Yang G, Kato T, Shcherbakov D, Hobbie SN, Vasella A, Böttger EC, Crich D. Structure-Based Design and Synthesis of Apramycin-Paromomycin Analogues. Importance of the Configuration at the 6′-Position and Differences between the 6′-Amino and Hydroxy Series. J Am Chem Soc. 2017;139:14611–14619. doi: 10.1021/jacs.7b07754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonousi A, Crich D. Selective Protection of Secondary Amines as the N-Phenyl Triazenes. Application to Aminoglycoside Antibiotics. Org Lett. 2015;17:4006–4009. doi: 10.1021/acs.orglett.5b01902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagabhushan TL. Process for the Manufacture of 6′-N-Alkyl Derivatives of Sisomycin and Verdamicin; Novel Intermediates Useful Therein, and Novel 6′-N-Alkylverdamcins Prepared Thereby. 3,997,524. US Patent. 1976
- 51.Davies DH, Mallams AK. Semisynthetic Aminoglycoside Antibacterials. 6. Synthesis of Sisomicin, Antibiotic G-52, and Novel 6′-Substituted Analogues of Sisomicin from Aminoglycoside 66–40c. J Med Chem. 1978;21:189–193. doi: 10.1021/jm00200a009. [DOI] [PubMed] [Google Scholar]
- 52.This oxidation mimics one previously reported by the Hanessian lab with a related N3″-Fmoc or acetamido derivatives Hanessian S, Szychowski J, Maianti JP. Synthesis and Comparative Antibacterial Activity of Verdamicin C2 and C2a. A New Oxidation of Primary Allylic Azides in Dihydro[2H]pyrans. Org Lett. 2009;11:429–432. doi: 10.1021/ol802421d.
- 53.Pathak R, Perez-Fernandez D, Nandurdikar R, Kalapala SK, Böttger EC, Vasella A. Synthesis and Evaluation of Paromomycin Derivatives Modified at C(4′) Helv Chim Acta. 2008;91:1533–1552. [Google Scholar]
- 54.Daniels PJL. Aminoacyl Derivatives of Aminoglycoside Antibiotics. 4,117,221. US Patent. 1978
- 55.Cron MJ, Keil JG, Lin JS, Ruggeri M, Walker DW. Preparation of 1-n-[ω-Amino-α-Hydroxyalkanoyl]-Aminoglycoside Polysilylated Antibiotics and Products Obtained Therefrom. 4,347,354. US Patent. 1982
- 56.Tan L, Wlasichuk KB, Schmidt DE, Campbell RL, Hirtzer P, Cheng L, Karr DE. A High pH Based Reversed-Phase High Performance Liquid Chromatographic Method for the Analysis of Aminoglycoside Plazomicin and Its Impurities. J Pharm Biomed Anal. 2012;66:75–84. doi: 10.1016/j.jpba.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 57.McKeever B, Pattenden G. Total Synthesis of the Cytotoxic Cyclopeptide Mollamide, Isolated from the Sea Squirt Didemnum Molle. Tetrahedron. 2003;59:2701–2712. [Google Scholar]
- 58.Lee SH, Cheong CS. Selective Reactions of Reactive Amino Groups in Polyamino Compounds by Metal-Chelated or -Mediated Methods. Tetrahedron. 2001;57:4801–4815. [Google Scholar]
- 59.Banfi L, Basso A, Giardini L, Riva R, Rocca V, Guanti G. Tandem Ugi MCR/Mitsunobu Cyclization as a Short, Protecting-Group-Free Route to Benzoxazinones with Four Diversity Points. Eur J Org Chem. 2011:100–109. [Google Scholar]
- 60.Fujino M, Kobayashi S, Obayashi M, Fukuda T, Shinagawa S, Nishimura O. The Use of N-Hydroxy-5-Norbornene-2:3-Dicarboximide Active Esters in Peptide Synthesis. Chem Pharm Bull. 1974;22:1857–1863. doi: 10.1248/cpb.22.1857. [DOI] [PubMed] [Google Scholar]
- 61.Abdel-Magid AF, Carson KG, Harris BD, Maryanoff CA, Shah RD. Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures. J Org Chem. 1996;61:3849–3862. doi: 10.1021/jo960057x. [DOI] [PubMed] [Google Scholar]
- 62.Hudson JA, Sweeney JB. An Efficient Method for Reductive Amination of Carbonyl Compounds under Nonacidic Conditions. Synlett. 2012;23:2176–2178. [Google Scholar]
- 63.Smith KP, Kirby JE. Evaluation of Apramycin Activity against Carbapenem-Resistant and -Susceptible Strains of Enterobacteriaceae. Diagn Microbiol Infect Dis. 2016;86:439–441. doi: 10.1016/j.diagmicrobio.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Kang AD, Smith KP, Eliopoulos GM, Berg AH, McCoy C, Kirby JE. In Vitro Apramycin Activity against Multidrug-Resistant Acinetobacter Baumannii and Pseudomonas Aeruginosa. Diagn Microbiol Infect Dis. 2017;88:188–191. doi: 10.1016/j.diagmicrobio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 65.In contrast Mallams and coworkers reported that sisomicin and the same derivative had identical activity against wild type S. aureus and E. coli, albeit different strains.51
- 66.Reyes N, Aggen JB, Kostrub CF. In Vivo Efficacy of the Novel Aminoglycoside ACHN-490 in Murine Infection Models. Antimicrob Agent Chemother. 2011;55:1728–1733. doi: 10.1128/AAC.00862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhanel GG, Lawson CD, Zelenitsky S, Findlay B, Schweizer F, Adam H, Walkty A, Rubinstein E, Gin AS, Hoban DJ, Lynch JP, Karlowsky JA. Comparison of the Next-Generation Aminoglycoside Plazomicin to Gentamicin, Tobramycin and Amikacin. Expert Rev Anti-infect Ther. 2012;10:459–473. doi: 10.1586/eri.12.25. [DOI] [PubMed] [Google Scholar]
- 68.Walkty A, Adam H, Baxter M, Denisuik A, Lagacé-Wiens P, Karlowsky JA, Hoban DJ, Zhanel GG. In Vitro Activity of Plazomicin against 5,105 Gram-Negative and Gram-Positive Clinical Isolates from Patients in Canadian Hospitals as Part of the Canward Study, 2011–2012. Antimicrob Agent Chemother. 2014;58:2554–2563. doi: 10.1128/AAC.02744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swayze EE, Hanessian S, Szychowski J, Adhikari SS, Pachamuthu K, Wang X, Migawa MT, Griffey RH. Antibacterial 4,5-Substituted Aminoglycoside Analogs Having Multiple Substituents. 064954 (A2) WO Patent. 2007
- 70.Goldblum AA, Dozzo P, Kane TR, Aggen JB, Linsell MS, Hildebrandt DJ, Gliedt MJ. Antibacterial Aminoglycoside Analogs. 0122809 A1. US Patent. 2012
- 71.Shakya T, Wright GD. Mechanisms of Aminoglycoside Antibiotic Resistance. In: Arya DP, editor. Aminoglycoside Antibiotics: From Chemical Biology to Drug Discovery. Wiley; Hoboken: 2007. pp. 119–140. [Google Scholar]
- 72.Doi Y, Arakawa Y. 16S Ribosomal RNA Methylation: Emerging Resistance Mechanism against Aminoglycosides. Clin Infect Dis. 2007;45:88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 73.Wachino J-i, Arakawa Y. Exogenously Acquired 16S rRNA Methyltransferases Found in Aminoglycoside-Resistant Pathogenic Gram-Negative Bacteria: An Update. Drug Res Updates. 2012;15:133–148. doi: 10.1016/j.drup.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Brummett RE, Fox KE, Bendrick TW, Himes DL. Ototoxicity of Tobramycin, Gentamicin, Amikacin and Sisomicin in the Guinea Pig. J Antimicrob Chemother. 1978;4(suppl A):73–83. doi: 10.1093/jac/4.suppl_a.73. [DOI] [PubMed] [Google Scholar]
- 75.Kostrub CF, Dolan DF, Altschuler RA, Tapp RL, Baird TJ, Boggs JA, Bruss JB. Ototoxic Potential of ACHN-490 Compared to Gentamcin and Amikacin. ECCMID. 2010 Poster-1249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.