Abstract
Objective
Inflammation in adipose tissues in obesity promotes insulin resistance and metabolic disease. The Duffy antigen receptor for chemokines (DARC) is a promiscuous non-signaling receptor expressed on erythrocytes and other cell types that modulates tissue inflammation by binding chemokines such as monocyte chemoattractant protein-1 (MCP-1) and by acting as a chemokine reservoir. DARC allelic variants are common in humans, but the role of DARC in modulating obesity-related metabolic disease is unknown.
Methods
We examined body weight gain, tissue adiposity, metabolic parameters and inflammatory marker expression in wild-type and DARC knockout mice fed a chow diet (CD) and high fat diet (HFD).
Results
Compared to wild-type mice, HFD-fed DARC knockout mice developed glucose intolerance and insulin resistance independent of increases in body weight or adiposity. Interestingly, insulin sensitivity was also diminished in lean male DARC knockout mice fed a chow diet. Insulin production was not reduced by DARC gene deletion, and plasma leptin levels were similar in HFD fed wild-type and DARC knockout mice. MCP-1 levels in plasma rose significantly in the HFD fed wild-type mice, but not in the DARC knockout mice. Conversely, adipose tissue MCP-1 levels were higher, and more macrophage crown-like structures were detected, in the HFD fed DARC knockout mice as compared with the wild-type mice, consistent with augmented adipose tissue inflammation that is not accurately reflected by plasma levels of DARC-bound MCP-1 in these mice.
Keywords: DARC, High fat diet, Obesity, Insulin resistance, Inflammation
1. Introduction
Obesity, which afflicts approximately one-third of the US population, is associated with increased risk for the development of the metabolic syndrome characterized by insulin resistance, abnormal blood glucose, hypertension, and dyslipidemia (Flegal et al., 2012). Diseases associated with obesity and the metabolic syndrome include diabetes, cardiovascular disease, stroke, and certain cancers. The health care costs related to obesity impose an economic burden of up to $190 billion annually (Cawley and Meyerhoefer, 2012) that is projected to increase by $48–66 billion each year by 2030 (Wang et al., 2011).
Obesity is accompanied by chronic low grade inflammation that originates from increased production of pro-inflammatory cytokines and chemokines in adipose tissues. The accumulating chemokines recruit additional immune cells, most notably macrophages, which in turn produce more chemokines in a viscous cycle of chronic inflammation. Certain adipocytokines and chemokines, such as tissue necrosis factor alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1), have been causally linked to the development of insulin resistance, a key feature of the metabolic syndrome, and type 2 diabetes (Xu et al., 2003; Kwon and Pessin, 2013). Indeed, genetic deletion or pharmacological inhibition of TNF-α (Hotamisligil et al., 1993; Uysal et al., 1997) or MCP-1 (Weisberg et al., 2006; Kanda et al., 2006) was shown to improve insulin sensitivity in mice in the setting of obesity. MCP-1 levels were also reported to be elevated in plasma of obese patients (Huber et al., 2008), correlating with the degree of insulin resistance (Kim et al., 2006). Conversely, in other studies, MCP-1 deficiency in mice failed to restrain macrophage recruitment to adipose tissues or ameliorate insulin resistance during diet-induced obesity (Kirk et al., 2008). These disparate findings suggest that unidentified biological variables modulate the function of individual chemokines in obesity-related metabolic disease.
Chemokines bind not only to their respective signaling receptors, but also to several non-signaling receptors, the most noteworthy of which is the atypical chemokine receptor 1 (ACKR1), also known as the Duffy antigen receptor for chemokines (DARC). DARC is a promiscuous chemokine receptor with the unique ability to bind both C-C and C-X-C class chemokines. In humans, DARC is expressed on erythrocytes, capillary and post-capillary endothelial cells, lymphatic endothelial cells, littoral cells of splenic sinusoids, lung epithelium, kidney collecting ducts, and cerebellar Purkinje cells (Hansell et al., 2011). Lacking the triplet sequence Asp-Arg-Tyr (DRY motif) in its second intracellular loop, DARC cannot activate G-protein coupled signaling pathways (de Brevern et al., 2005) and is thought to act primarily as a chemokine modulator by sequestering chemokines (i.e., ‘buffer-sink’ function) or by regulating their local concentration at sites of inflammation. Among various chemokines, DARC has strong binding affinity for C-C motif chemokine ligand 2 (CCL2)/MCP-1 (Hansell et al., 2011), and humans that lack functional DARC expression are more sensitive to MCP-1-induced monocyte mobilization (Mayr et al., 2009). Indeed, chemokines such as MCP-1 depend upon erythrocyte DARC to maintain plasma concentrations, and loss of functional DARC correlated with decreased levels of DARC-bound chemokines in the serum in both humans and mice (Schnabel et al., 2010; Lentsch, 2002). Erythrocyte DARC binds and clears chemokines from sites of inflammation to buffer inflammation while at the same time reducing receptor desensitization to counterbalance the buffering effect (Hansell et al., 2011). These findings illustrate the complex mechanisms whereby DARC regulates chemokine function.
The human population is characterized by three common alleles of the DARC gene: the ancestral FYB and the derived FYA and FYO alleles. The FYB and FYA alleles differ by a single amino acid (Asp42Gly), while the FYO allele is characterized by the lack of expression of DARC on erythrocytes (McManus et al., 2017). Blood donors carrying the FYO and FYA alleles exhibit reduced serum MCP-1 levels compared to FYB donors linked to reduced erythrocyte DARC binding affinity, suggesting that chemokine regulation is altered in these cohorts (Schnabel et al., 2010). Given the role of DARC in regulating chemokines that are thought to mediate obesity-related metabolic disease, and given the existence of common polymorphisms in the human population associated with differences in circulating chemokine levels, the role of the DARC in the development of obesity-related metabolic disease merits investigation.
Here, we investigated the impact of global DARC gene deletion on diet-induced obesity and insulin resistance in mice fed a high fat diet (HFD). DARC knockout mice fed a HFD exhibited marked impairments in glucose tolerance and insulin sensitivity compared to corresponding wild-type mice. Circulating levels of DARC-bound MCP-1 did not increase with HFD in DARC knockout mice as seen in wild-type controls; however, adipose tissue MCP-1 levels were higher in DARC knockout mice than in the wild-type mice, suggesting incongruence of plasma and adipose tissue levels of DARC-bound chemokines in DARC knockout mice. Surprisingly, moderate insulin resistance was also seen in male DARC knockout mice maintained on chow diet (CD) despite the lack of adipose tissue inflammation. Taken together, these data suggests that DARC regulates metabolic function and diet-induced obesity in mice.
2. Materials and methods
2.1. Mice
Male and female DARC knockout mice in the C57BL/6J background were obtained from Jackson Laboratories and bred in house to obtain littermates. Mice were housed in cages of 4–5 maintained on CD after weaning. At 8 weeks of age, mice were either maintained on CD (Harlan Teklad, LM-485) or switched to HFD (Research Diet, D12492, with 60% calories from fat) for up to 42 weeks. In a separate study, a weight-matched experiment in male non-littermate wild-type and DARC knockout mice was performed to control for body weight. Weight matched non-littermate wild-type mice (C57BL/6J) were either maintained on CD or switched to HFD for up to 26 weeks. Thereafter, mice were euthanized, blood was collected via cardiocentesis, and tissues were harvested following perfusion with ice-cold saline as previously described (Chatterjee et al., 2014). All animal studies were conducted using a protocol approved by the Institutional Animal Care and Use Committee of Augusta University, following appropriate guidelines.
2.2. Body fat and food intake measurements
Body weights were obtained weekly for mice fed CD or HFD. Body fat mass was measured in conscious mice fed CD or HFD one week prior to sacrifice using nuclear magnetic resonance (NMR) spectroscopy (Bruker Minispec LF90II) as described previously (Zhou et al., 2016). Food intake and metabolic energy expenditure were determined after 25 weeks on CD or HFD using a comprehensive laboratory animal monitoring system (CLAMS, Columbus Instruments, Columbus, OH) for 4 days (2 days of acclimation, followed by 2 days of measurement) as previously described (Zhou et al., 2016).
2.3. Examination of insulin and glucose tolerance
Glucose tolerance testing (GTT) was performed between 16 and 17 weeks (weight-matched cohort and female cohort) or 36 weeks (littermate cohort) on CD or HFD. Glucose levels were measured from tail veins immediately prior to and 30, 60, 90 and 120 min after intraperitoneal (IP) injection of glucose at 2 g/kg body weight in mice fasted for 12 h using glucose strips. Insulin sensitivity evaluated by insulin tolerance testing (ITT) was assessed at 20 weeks (weight-matched cohort and female cohort) or 38 weeks (littermate cohort) on CD or HFD by measurement of plasma glucose from tail veins at 0, 30, 60 and 90 min after IP injection of 0.75 U/kg body weight of porcine insulin in 6 hr-fasted mice.
2.4. Enzyme-linked immunosorbent assay (ELISA)
Plasma levels of adiponectin, leptin, and insulin were measured in mice after overnight fasting, utilizing commercially available ELISA kits (R&D Systems) as described previously (Unruh et al., 2015). Plasma levels of MCP-1 were quantified using mouse CCL2/JE/MCP-1 antibody (R&D Systems) according to the manufacturer’s protocol.
2.5. Histology
Pancreas, liver and adipose tissues were fixed by immersion in neutral buffered formalin (10%), dehydrated in ethanol and then transferred to xylene solution for embedding in paraffin. Five μm sections were stained with hematoxylin-eosin (H&E) or incubated with anti-insulin (Abcam, 1:100 dilution, to detect beta cells) or anti-F4/80 antibodies (Sigma-Aldrich, 1:100 dilution, to detect macrophages) for 4 h at 37 °C, and then processed with HistoMouse-SP kit (Invitrogen) or DAB Substrate kits (Vector Labs) according to the manufacturers’ protocols. The number of F4/80-positive crown-like structures were counted in 5 randomly selected high-power fields and normalized to mm2 for quantification.
2.6. RNA isolation, quantitative polymerase chain reaction (qPCR), and Western blotting
RNA was isolated utilizing RNeasy lipid mini kit (Qiagen), and qPCR quantification of mRNA levels was performed as described previously (Chatterjee et al., 2011) using Syber-green qPCR kit (Agilent). Arbp (acidic ribosomal phosphoprotein P0) mRNA was selected as a reference for normalization of transcripts under investigation. The primer sequences used in the qPCR assay are provided in Supplementary Table 1. Western blot analysis was performed as described previously (Chatterjee et al., 2011).
2.7. Statistical analysis
Data are expressed as mean ± SEM except for weight data (expressed as mean ± SD). Analysis was accomplished by one way ANOVA followed by Tukey or Bonferroni post-hoc analysis. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Increased adiposity and weight gain in male DARC knockout mice
Baseline weights did not differ amongst male wild-type and DARC knockout littermate mice. Over the course of the experiment, the CD-fed DARC knockout mice tended to gain more weight than their littermate counterparts, although the difference did not achieve statistical significance. HFD feeding resulted in progressive weight gain in both groups of mice (Fig. 1A), and under these conditions, the DARC knockout mice gained significantly more weight than did their wild-type littermates (Fig. 1A). Body composition by NMR spectroscopy showed that the CD-fed DARC knockout mice exhibited greater fat mass, and less lean mass, compared with their CD wild-type littermates (Fig. 1B). These differences were abolished by HFD feeding, during which both DARC knockout and wild-type mice exhibited dramatic increases in fat mass, and commensurate reductions in lean mass (Fig. 1B). Quantification of adipose depot weights (normalized to body weight) demonstrated that CD-fed DARC knockout mice possessed significantly more visceral adipose tissue, and a trend towards more subcutaneous adipose tissue, compared to their CD-fed wild-type littermates; however, following HFD feeding, percentage body fat and subcutaneous adipose mass increased proportionately in both groups of mice (Fig. 1B–C). The DARC knockout mice developed more liver enlargement during HFD feeding as compared with wild-type mice (Fig. 1D); percentage liver fat (quantified by NMR spectroscopy) tended to be higher in HFD-fed DARC knockout mice versus their littermate controls, although the difference was not statistically significant (Supplemental Fig. S1). Skeletal muscle mass was similar in DARC knockout and wild-type mice fed a CD or HFD, respectively (data not shown). Also, quantification of food consumption demonstrated no differences between wild-type and DARC knockout mice fed a CD or HFD, respectively (Fig. 1E).
Fig. 1. Increased adiposity, weight gain with impaired glucose tolerance, insulin sensitivity in male DARC knockout mice.
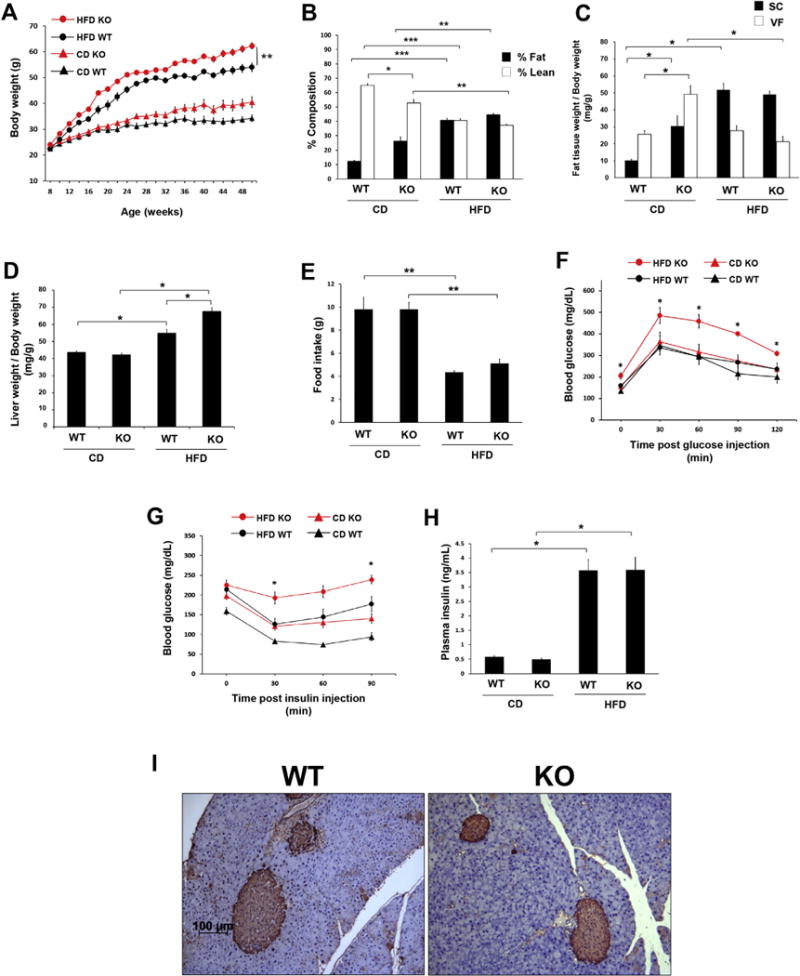
(A) Growth curves of male littermate DARC knockout (KO, red symbols) and wild-type (WT, black symbols) mice fed either HFD (open circles) or CD (closed circles) (**p < .01 vs HFD WT). (B) Lean mass (black bars) and fat mass (white bars) measured by whole body composition by nuclear magnetic resonance (*p < .05, **p < .01, ***p < .001, n = 5). (C) Inguinal (SC) and epidydimal (VF) fat pad weight normalized to body weight (*p < .05, n = 5). (D) Liver weight normalized to body weight (*p < .05, n = 5). (E) Food intake presented as g/mouse in WT and DARC knockout mice fed a CD or HFD (**p < .01, n = 4). (F) Glucose tolerance test after 32 weeks of CD or HFD (*p < .05 vs HFD WT, n = 5). (G) Insulin tolerance test after 36 weeks of CD or HFD (*p < .05 vs HFD WT, #p < .05 vs CD WT, n = 5). (H) Fasting plasma insulin levels measured by ELISA in the CD and HFD groups (*p < .05, n = 5). (I) Immunostaining for pancreatic beta cells in the HFD WT and KO groups. Representative images are shown (n = 3). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Impaired glucose tolerance and insulin sensitivity in HFD-fed DARC knockout mice
Next, we examined metabolic status in these mice by performing glucose and insulin tolerance testing. Glucose tolerance was similar in CD-fed wild-type and DARC knockout mice (Fig. 1F). Basal fasting glucose levels were elevated in the HFD-fed DARC knockout mice, and glucose levels rose significantly higher at all time points after glucose administration in these mice as compared with the wild-type mice (Fig. 1F). Interestingly, as compared to wild-type mice, CD-fed DARC knockout mice also exhibited a strong trend towards diminished insulin sensitivity (Fig. 1G). As expected, the HFD obese wild-type mice exhibited insulin resistance, the degree of which was amplified in the DARC knockout mice. Plasma insulin levels were similar in CD-fed wild-type and DARC knockout mice and were significantly but similarly elevated in both HFD groups (Fig. 1H). Likewise, we detected a similar degree of positive immunostaining for beta cells in pancreatic tissues from both groups of HFD-fed mice (Fig. 1I). These findings suggest that the impaired glucose tolerance and insulin resistance observed in the HFD-fed DARC knockout mice did not result from diminished capacity to produce insulin.
3.3. Controlling for body weight did not normalize the metabolic phenotype of HFD-fed male DARC knockout mice
The metabolic phenotype observed in the DARC knockout mice could have been explained, at least in part, by differences in body weight as compared to their wild-type littermates. Thus, we performed a weight-matched study in non-littermate wild-type and DARC knockout mice to control for this variable. As expected, during the course of the study, body weights did not differ amongst wild-type and DARC knockout mice fed a CD or HFD, respectively (Fig. 2A), nor were there differences in food intake (Supplemental Fig. S2A), locomotor activity (Supplemental Fig. S3A) or metabolic energy expenditure (Supplemental Fig. S3B). Likewise, fat mass and adipose tissue weights were similar in the weight-matched wild-type and DARC knockout mice (Fig. 2B and C). However, as was observed in the littermate study, liver enlargement was noted in the HFD-fed DARC knockout mice (Fig. 2D), and hepatic steatosis was detected in both wild-type and DARC knockout mice fed a HFD (Fig. 2E). Despite the similar weights and adiposity, the HFD fed DARC knockout mice exhibited impaired glucose tolerance as compared to the wild-type mice (Fig. 2F). Moreover, insulin sensitivity was significantly impaired in both CD-fed and HFD-fed DARC knockout mice as compared to their respective wild-type counterparts (Fig. 2G).
Fig. 2. Controlling for body weight did not normalize the metabolic phenotype of HFD fed male DARC knockout mice.
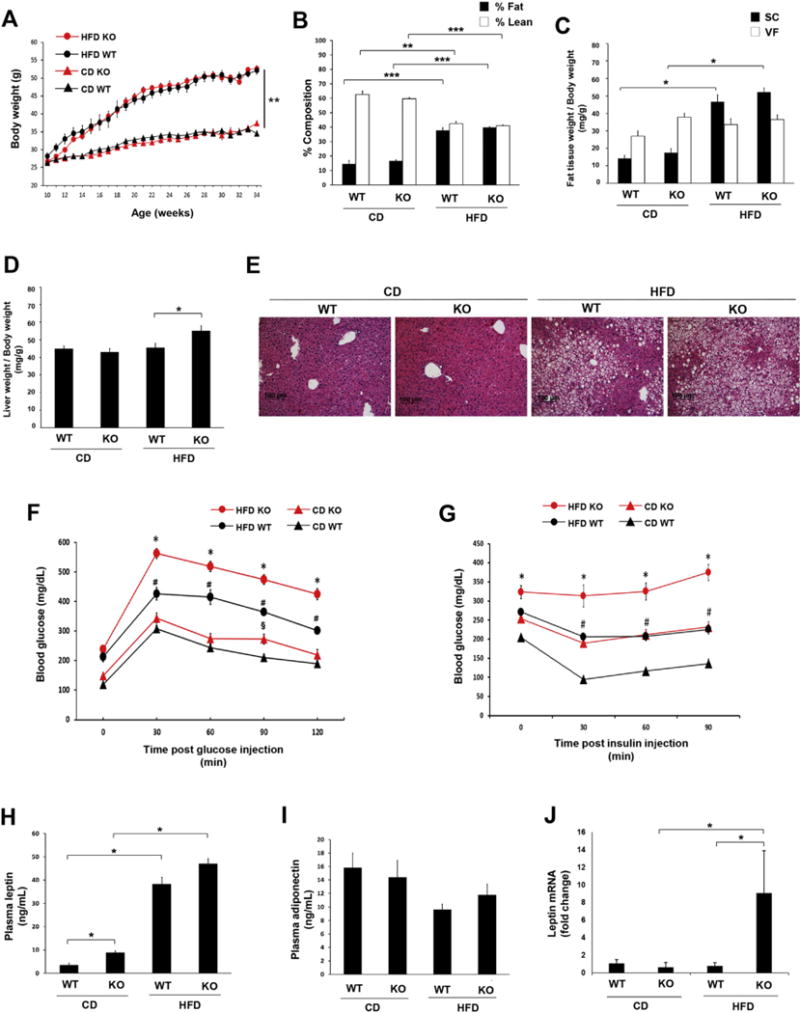
(A) Growth curves of male weight-matched DARC knockout mice (red symbols) and WT mice (black symbols) fed either HFD (open circles) or CD (closed circles) (**p < .01 vs CD WT and CD KO). (B) Lean mass (white bars) and fat mass (black bars) measured by whole body composition by NMR spectroscopy (***p < .001, **p < .01, n = 10). (C) Inguinal (SC) and epidydimal (VF) fat pad weight normalized to body weight (*p < .05, n = 10). (D) Liver weight normalized to body weight (*p < .05, n = 10). (E) Representative H&E staining images of liver tissues (n = 3). Glucose tolerance test (F) after 17 weeks on diet and insulin tolerance test (G) after 20 weeks on diet (§p < .05 vs CD WT, *p < .05 vs HFD WT, #p < .05 vs CD WT, n = 10). Plasma leptin (H) and adiponectin (I) from weight-matched male mice fed on diet for 24 weeks assayed by ELISA (*p < .05, n = 5). (J) Leptin mRNA expression in adipose tissues quantified by real-time PCR (*p < .05, n = 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we examined adipokine expression in the weight-matched cohort. Plasma leptin levels were slightly but significantly higher in the CD-fed DARC knockout mice than in wild-type mice (Fig. 2H) and tended to be higher in DARC knockout mice in response to HFD, although the differences were not statistically significant (Fig. 2H). Plasma adiponectin levels were similar in wild-type and DARC knockout mice in the CD and HFD groups (Fig. 2I). In adipose tissues, leptin mRNA levels were significantly increased in HFD-fed DARC knockout mice as compared to wild-type mice (Fig. 2J).
3.4. Increased adipose tissue inflammation in HFD-fed DARC knockout mice
DARC can bind and regulate the activity of MCP-1, a chemokine reported to promote adipose inflammation in obesity. Thus, we assayed MCP-1 in plasma of our weight-matched wild-type and DARC knockout mice. As expected, MCP-1 in plasma rose significantly during HFD feeding in the wild-type mice. However, the DARC knockout mice tended to exhibit lower plasma MCP-1 levels at baseline, and no significant increase was noted following HFD feeding (Fig. 3A). In contrast to the plasma MCP-1 findings, adipose MCP-1 (assayed by Western blotting) was significantly higher in HFD-fed DARC knockout mice as compared with wild-type mice (Fig. 3B). mRNA expression of TNFα and MCP-1 was likewise higher in visceral adipose tissues from HFD-fed DARC knockout mice as compared to wild-type (Fig. 2C&D), while interleukin (IL)-6 and CCL5 expression was similar in both groups of mice (Figure E&F). Adipose tissue immunostaining demonstrated few infiltrating F4/80 positive macrophages in visceral adipose tissues of CD-fed wild-type or DARC knockout mice (Fig. 3G&H). Following HFD feeding, increased macrophage staining was detected in adipose tissues of both wild-type and DARC knockout mice. However, significantly more crown-like structures were detected in the HFD-fed DARC knockout mice, consistent with increased adipose tissue inflammation (Fig. 3G&H).
Fig. 3. Increased adipose tissue inflammation in HFD fed DARC knockout mice.
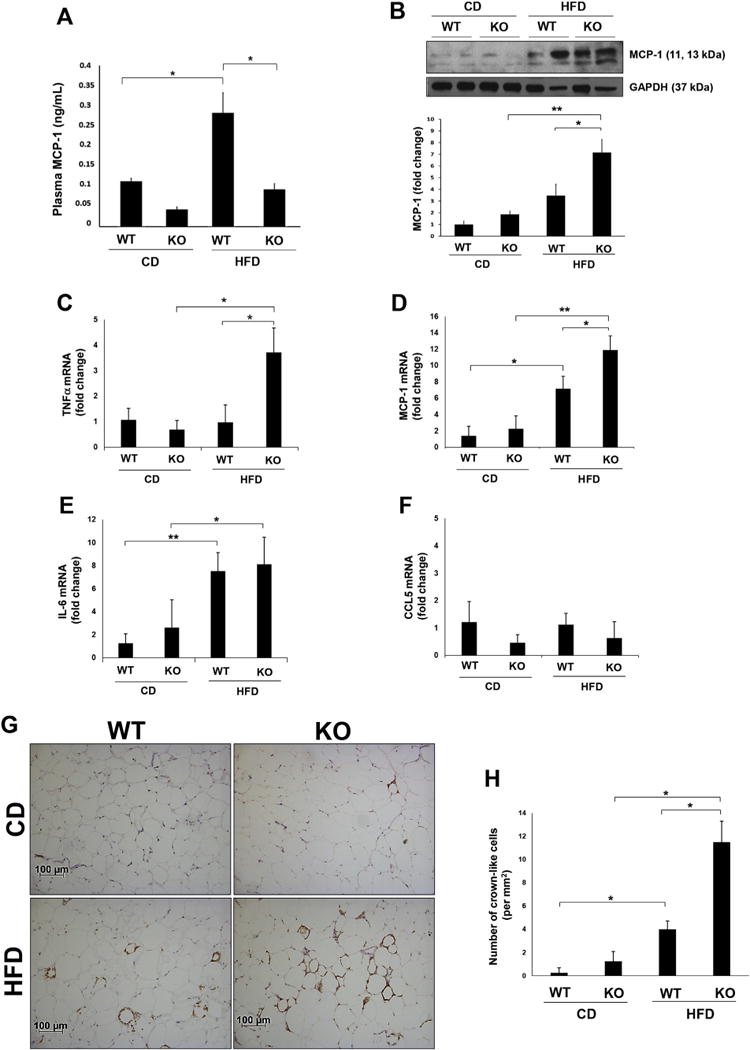
(A) Plasma MCP-1 concentration measured by ELISA following treatment of whole blood with heparin from weight-matched male mice on diet for 24 weeks (*p < .05, n = 4). (B) Representative Western blot and quantitated data showing MCP-1 protein in epididymal adipose tissue homogenates of WT and DARC knockout mice (*p < .05, **p < .01, n = 5). Adipose mRNA expression of TNFα (C), MCP-1 (D), IL-6 (E) and CCL5 (F) as examined by real time PCR (*p < .05, **p < .01, n = 4). (G) Representative images of F4/80 positive macrophage immunostaining in epididymal adipose tissue. Quantification of crown-like structures is shown in (H) (*p < .05, n = 4).
3.5. Impaired glucose tolerance and insulin sensitivity in HFD-fed female DARC knockout mice
Finally, we studied female wild-type and DARC knockout mice fed a CD or HFD. As was observed in the male mice, female DARC knockout mice gained more weight during the course of HFD feeding as compared to wild-type mice (Fig. 4A). However, we did not detect differences in body composition (Fig. 4B) or subcutaneous fat mass (Fig. 4C) between female DARC knockout and wild-type mice fed a CD or HFD, respectively. Also, unlike the male mice, female DARC knockout mice did not develop liver enlargement during HFD feeding (Fig. 4D). However, glucose tolerance (Fig. 4E) and insulin sensitivity (Fig. 4F) were significantly worse in the HFD-fed female DARC knockout mice as compared to the wild-type controls. Plasma insulin levels were similar in CD-fed wild-type and DARC knockout mice but were significantly higher in HFD-fed DARC knockout mice (Fig. 4G).
Fig. 4. Impaired glucose tolerance and insulin sensitivity in HFD-fed female DARC knockout mice.
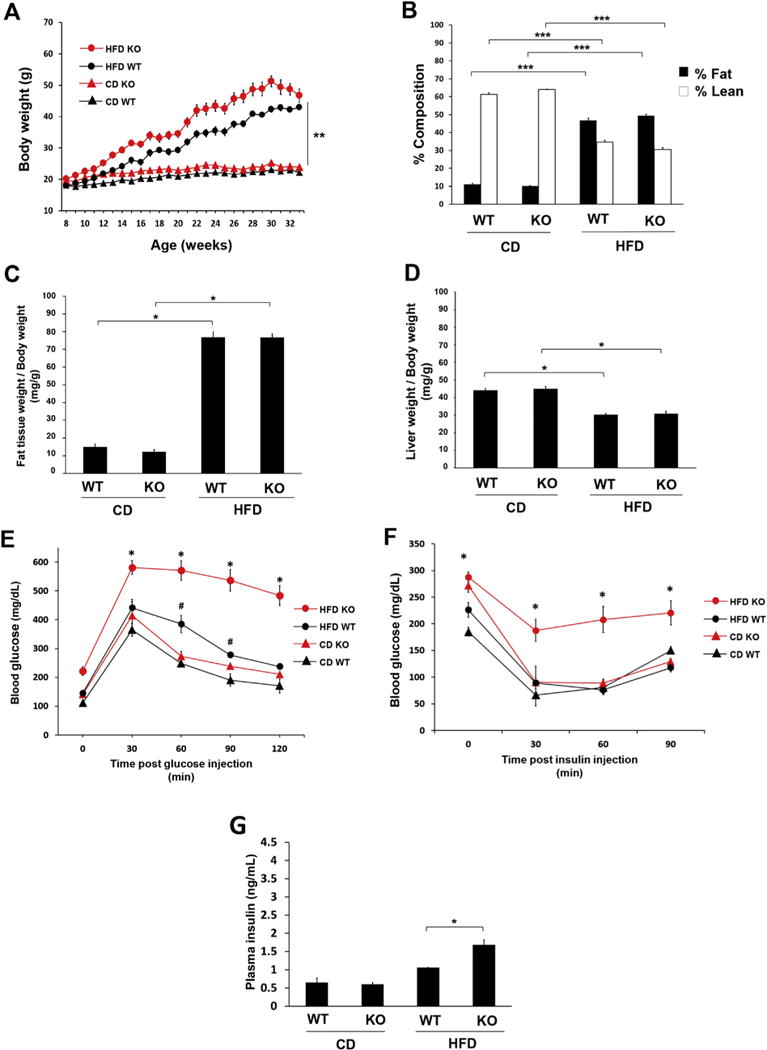
(A) Growth curves of female DARC knockout mice (red symbols) and WT mice (black symbols) fed either HFD (open circles) or CD (closed circles) (**p < .01 vs CD WT and CD KO). (B) Lean mass (white bars) and fat mass (black bars) measured by whole body composition by NMR spectroscopy (***p < .001, n = 5). (C) Inguinal (SC) fat pad weight normalized to body weight (*p < .05, n = 4). (D) Liver weight normalized to body weight (*p < .05, n = 5). Glucose tolerance test (E) at 17 weeks on diet and insulin tolerance test (F) at 20 weeks on diet (*p < .05 vs HFD WT, #p < .05 vs CD WT, n = 4). (G) Fasting plasma insulin levels measured by ELISA in the CD and HFD groups (*p < .05, n = 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Here, we investigated the impact of global DARC gene deletion on metabolic function and inflammation in diet-induced obesity. After HFD feeding, both male and female DARC knockout mice gained more weight and exhibited diminished glucose tolerance and insulin sensitivity compared to wild-type mice. In a weight-matched cohort, similar impairments in glucose tolerance and insulin sensitivity were observed, indicating body mass-independent metabolic dysfunction in the DARC knockout mice. While male DARC knockout mice displayed lower plasma levels of MCP-1, their visceral adipose tissues contained more MCP-1 protein and macrophage crown-like structures compared to the wild-type mice, consistent with heightened inflammation. Interestingly, male DARC knockout mice on CD also exhibited decreased insulin sensitivity despite the absence of tissue inflammation. Taken together, these data suggest that DARC plays a complex role in regulating systemic metabolism as well as adipose tissue inflammation during HFD feeding.
Because DARC acts as a chemokine regulator, its role has been investigated in a variety of pathological states. After lipopolysaccharide (LPS) challenge, DARC knockout mice exhibited increases in tissue inflammation compared to wild-type controls, suggesting a primary role for DARC as a buffer-sink in this acute inflammatory model (Hansell et al., 2011). Interestingly, blood monocytes in DARC knockout mice expressed significantly less tissue factor in response to intraperitoneally administered LPS (Østerud et al., 2015). Studies in DARC knockout mice and human cohorts lacking erythrocyte DARC expression have demonstrated increased incidence/severity of prostate cancer, attributed to the loss of DARC’s buffer-sink functionality to regulate tissue levels of angiogenic chemokines such as CXCl1 (Lentsch, 2002). The role of DARC in atherosclerosis has also been investigated by crossing DARC knockout mice with apolipoprotein E knockout mice. In this model, the loss of DARC conferred protection against atherosclerosis, presumably due to the lack of chemokines bound to erythrocytes that extravasated into atheromatous plaques (Wan et al., 2015). Thus, DARC appears able to play both pro- and anti-inflammatory roles depending on the specific disease. The role of DARC in regulating chronic low grade inflammation associated with diet-induced obesity, however, has not been previously investigated.
Weight gain leading to obesity induces a state of chronic inflammation that is associated with the development of metabolic syndrome and type 2 diabetes (Xu et al., 2003). As expected in the pro-inflammatory setting of obesity, male wild-type mice fed a HFD displayed a marked increase in circulating MCP-1 levels, concomitant with increased adipose tissue MCP-1 protein and macrophage infiltration. However, circulating MCP-1 levels remained low in the male DARC knockout mice following consumption of the HFD, despite the fact that their adipose tissues contained even more MCP-1 and macrophage crown-like structures than did the WT mice, indicative of enhanced adipose tissue inflammation. This phenotype of the DARC knockout mice could not be explained by differences in body weight or adiposity. Interestingly, male DARC knockout mice fed CD displayed insulin resistance comparable to that of HFD-fed WT mice despite a lack of adipose tissue inflammation. DARC gene deletion did not diminish fasting plasma insulin, and no gross changes in pancreatic beta cell expression were observed in the male DARC knockout mice, indicating that the ability to produce insulin was not impaired. Thus, the role of DARC in regulating insulin sensitivity is likely to be complex, extending beyond adipose tissue inflammation perhaps to insulin signaling. Further studies are required to determine whether DARC is expressed on insulin-sensitive cells, and if so what its function may be.
During metabolic testing in the CLAMS unit, both wild type and DARC knockout mice on HFD consumed less food (total grams or Kcal) when compared to their counterparts on CD (Supplemental Fig. S2A). This may be partially explained by the fact that the diets must be ground up to load into the CLAMS unit feeders, which turns the HFD into a dense, sticky paste that is harder for mice to consume compared to the powder-like CD. Second, it is possible that obese mice have a more difficult time accessing the CLAMS feeder due to their body habitus. These explanations are supported by a trend toward a higher percentage of body weight loss by both groups of mice on HFD during the CLAMS testing (Supplemental Fig. S2B).
One of the interesting findings in this study is the liver enlargement of male DARC knockout mice fed a HFD compared to wild-type control. We observed a strong trend toward increased percentage of liver fat in the DARC knockout mice compared to wild-type, suggesting the enlargement could in part be attributed to excess hepatic lipid accumulation in these mice. In contrast, liver enlargement was not seen in female DARC knockout mice during HFD feeding, despite their increased weight gain and insulin resistance, suggesting that female sex hormones and/or the estrous cycle could potentially modulate hepatic lipid flux in these mice. Future in-depth studies would be required to address this possibility.
Three main alleles of the DARC gene are present in the human population: FYB, FYA and FYO, the prevalence of which varies by region and ancestry (Howes et al., 2011). FYA is the most common allele globally and is most prevalent in those of Asian descent, FYB predominates in those of European descent, while FYO is most prevalent in those of African descent. The distribution of the DARC alleles is thought to result from selection pressure imposed by Plasmodium vivax malaria. P. vivax engages DARC to penetrate erythrocytes, and the absence of DARC on FYO-expressing erythrocytes blocks the entrance of P. vivax and thereby confers resistance to the parasite (Miller et al., 1976). Notably, P. vivax is largely absent from equatorial Africa, where the FYO allele is nearly fixed. FYA may have also emerged as an adaptation directed against P. vivax (King et al., 2011), and natural selection also appears to have acted on the FYA allele in India (Chittoria et al., 2012).
Evolutionary adaptations often carry fitness trade-offs. Indeed, the FYO allele has been associated with apparently maladaptive phenotypes, including increased risk of prostate cancer (Shen et al., 2006). Interestingly, neutrophil levels are also lower in carriers of the FYO allele (Reich et al., 2009). The present study found that DARC gene deletion exacerbated metabolic dysfunction in both male and female mice fed a HFD. Coincidentally, populations of African and Asian descent, respectively dominated by the FYO and FYA alleles, are at increased risk of metabolic disease compared to those of European ancestry (McNeely and Boyko, 2004; Brancati et al., 2000). Moreover, FYO and FYA human cohorts exhibit lower circulating MCP-1 levels (Schnabel et al., 2010), as was detected in DARC knockout mice in the present study, although it is unknown whether adipose tissue levels of MCP-1 in obese human FYO and FYA cohorts are disproportionately elevated. Given the results of this study, it is worth considering whether circulating MCP-1 levels provide an accurate representation of adipose inflammation in FYA and FYO populations. The extent to which our findings apply to human populations, however, remains unclear. The DARC knockout genotype does not equate to FYA or FYO carriers, and numerous environmental and genetic factors can impact circulating chemokine levels and metabolic disease risk in humans.
5. Conclusions
This study is the first to investigate the impact of DARC gene deletion on metabolic function during diet-induced obesity. Both male and female DARC knockout mice fed a HFD exhibited impaired glucose tolerance and insulin sensitivity, and increased adipose tissue inflammation, compared to HFD-fed wild-type mice. Increased adipose tissue inflammation alone does not appear to explain the observed phenotype, however, as male DARC knockout mice on CD also displayed moderate insulin resistance despite a lack of adipose tissue inflammation, suggesting a more complex role for DARC in metabolism beyond its role in regulating chemokines. Our study also reveals a disconnect between circulating and adipose tissue levels of DARC-bound chemokines in the DARC knockout mice which potentially may be relevant to human populations expressing common DARC allelic variants. Focused studies are needed to elucidate the potential impact of DARC polymorphisms on adipose tissue inflammation and metabolic disease in human populations.
Supplementary Material
Acknowledgments
This study was supported by NIH grants HL126949 and HL112640 (N.L.W.), HL134354 and AR070029 (Y.T. and N.L.W.), and Department of Defense grant NF140031 (B.K.S.).
Abbreviations
- DARC
Duffy antigen receptor for chemokines
- ACKR1
atypical chemokine receptor 1
- CCL
C-C motif chemokine ligand
- MCP-1
monocyte chemoattractant protein-1
- TNF-α
tissue necrosis factor alpha
- HFD
high fat diet
- CD
chow diet
- IP
intraperitoneal
- NMR
nuclear magnetic resonance
- CLAMS
comprehensive laboratory animal monitoring system
- IL
interleukin
- qPCR
quantitative polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- GTT
glucose tolerance test
- ITT
insulin tolerance test
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.mce.2018.01.006.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the atherosclerosis risk in communities study. J Am Med Assoc. 2000;283(17):2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Chatterjee TK, Idelman G, Blanco V, Blomkalns AL, Piegore MG, Jr, Weintraub DS, Kumar S, Rajsheker S, Manka D, Rudich SM, Tang Y, Hui DY, Bassel-Duby R, Olson EN, Lingrel JB, Ho SM, Weintraub NL. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem. 2011;286(31):27836–27847. doi: 10.1074/jbc.M111.262964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee TK, Basford JE, Knoll E, Tong WS, Blanco V, Blomkalns AL, Rudich S, Lentsch AB, Hui DY, Weintraub NL. HDAC9 knockout mice are protected from adipose tissue dysfunction and systemic metabolic disease during high-fat feeding. Diabetes. 2014;63(1):176–187. doi: 10.2337/db13-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittoria A, Mohanty S, Jaiswal YK, Das A. Natural selection mediated association of the Duffy (FY) gene polymorphisms with Plasmodium vivax malaria in India. PLoS One. 2012;7(9):e45219. doi: 10.1371/journal.pone.0045219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brevern AG, Wong H, Tournamille C, Colin Y, Le Van Kim C, Etchebest C. A structural model of a seven-transmembrane helix receptor: the Duffy antigen/receptor for chemokine (DARC) Biochim Biophys Acta. 2005;1724(3):288–306. doi: 10.1016/j.bbagen.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J Am Med Assoc. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Hansell CA, Hurson CE, Nibbs RJ. DARC and D6: silent partners in chemokine regulation? Immunol Cell Biol. 2011;89(2):197–206. doi: 10.1038/icb.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Narinder SS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, Ménard D, Williams TN, Weatherall DJ, Hay SI. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266. doi: 10.1038/ncomms1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93(8):3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006;30:1347–1355. doi: 10.1038/sj.ijo.0803259. [DOI] [PubMed] [Google Scholar]
- King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, Siddiqui A, Howes RE, da Silva-Nunes M, Ferreira MU, Zimmerman PA. Fya/Fyb antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci U S A. 2011;108(50):20113–20118. doi: 10.1073/pnas.1109621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EA, Sagawa ZK, McDonald TO, O’Brien KD, Heinecke JW. Monocyte chemoattractant protein-1 deficiency fails to restrain macrophage infiltration into adipose tissue. Diabetes. 2008;57(5):1254–1261. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) and prostate cancer. A role as clear as black and white? Faseb J. 2002;16(9):1093–1095. doi: 10.1096/fj.02-0066hyp. [DOI] [PubMed] [Google Scholar]
- Mayr FB, Spiel AO, Leitner JM, Firbas C, Schnee J, Hilbert J, Derendorf H, Jilma B. Influence of the Duffy antigen on pharmacokinetics and pharmacodynamics of recombinant monocyte chemoattractant protein (MCP-1, CCL-2) in vivo. Int J Immunopathol Pharmacol. 2009;22:615–625. doi: 10.1177/039463200902200307. [DOI] [PubMed] [Google Scholar]
- McManus KF, Taravella AM, Henn BM, Bustamante CD, Sikora M, Cornejo OE. Population genetic analysis of the DARC locus (Duffy) reveals adaptation from standing variation associated with malaria resistance in humans. PLoS Genet. 2017;13(3):e1006560. doi: 10.1371/journal.pgen.1006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks: the Duffy-blood-group genotype. FyFy N Engl J Med. 1976;295(6):302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- Østerud B, Unruh D, Olsen JO, Kirchhofer D, Owens AP, 3rd, Bogdanov VY. Procoagulant and proinflammatory effects of red blood cells on lipopolysaccharide-stimulated monocytes. J Thromb Haemostasis. 2015;13(9):1676–1682. doi: 10.1111/jth.13041. [DOI] [PubMed] [Google Scholar]
- Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, Mullikin J, Hsueh WC, Cheng CY, Coresh J, Boerwinkle E, Li M, Waliszewska A, Neubauer J, Li R, Leak TS, Ekunwe L, Files JC, Hardy CL, Zmuda JM, Taylor HA, Ziv E, Harris TB, Wilson JG. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel RB, Baumert J, Barbalic M, Dupuis J, Ellinor PT, Durda P, Dehghan A, Bis JC, Illig T, Morrison AC, Jenny NS, Keaney JF, Jr, Gieger C, Tilley C, Yamamoto JF, Khuseyinova N, Heiss G, Doyle M, Blankenberg S, Herder C, Walston JD, Zhu Y, Vasan RS, Klopp N, Boerwinkle E, Larson MG, Psaty BM, Peters A, Ballantyne CM, Witteman JC, Hoogeveen RC, Benjamin EJ, Koenig W, Tracy RP. Duffy antigen receptor for chemokines (Darc) polymorphism regulates circulating concentrations of monocyte chemoattractant protein-1 and other inflammatory mediators. Blood. 2010;115(26):5289–5299. doi: 10.1182/blood-2009-05-221382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Schuster R, Stringer KF, Waltz SE, Lentsch AB. The Duffy antigen/receptor for chemokines (DARC) regulates prostate tumor growth. Faseb J. 2006;20(1):59–64. doi: 10.1096/fj.05-4764com. [DOI] [PubMed] [Google Scholar]
- Unruh D, Srinivasan R, Benson T, Haigh S, Coyle D, Batra N, Keil R, Sturm R, Blanco V, Palascak M, Franco RS, Tong W, Chatterjee T, Hui DY, Davidson WS, Aronow BJ, Kalfa T, Manka D, Peairs A, Blomkalns A, Fulton DJ, Brittain JE, Weintraub NL, Bogdanov VY. Red blood cell dysfunction induced by high-fat diet: potential implications for obesity-related atherosclerosis. Circulation. 2015;132(20):1898–1908. doi: 10.1161/CIRCULATIONAHA.115.017313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Wan W, Liu Q, Lionakis MS, Marino AP, Anderson SA, Swamydas M, Murphy PM. Atypical chemokine receptor 1 deficiency reduces atherogenesis in ApoE-knockout mice. Cardiovasc Res. 2015;106(3):478–487. doi: 10.1093/cvr/cvv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Black SM, Benson TW, Weintraub NL, Chen W. Berardinelli-Seip congenital lipodystrophy 2/seipin is not required for brown adipogenesis but regulates brown adipose tissue development and function. Mol Cell Biol. 2016;36(15):2027–2038. doi: 10.1128/MCB.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


