Abstract
Western blot is routinely used to quantify differences in the levels of target proteins in tissues. Standard methods typically use measurements of housekeeping proteins to control for variations in loading and protein transfer. This is problematic, however, when housekeeping proteins also are affected by experimental conditions such as injury, disease, and/or gonadal hormone manipulations. Our goal was to evaluate an alternative and perhaps superior method for conducting Western blot analysis of brain tissue homogenates from rats with distinct physiologically relevant gonadal hormone states. Tissues were collected from the hippocampus, frontal cortex, and striatum of young adult female rats that either were ovariectomized to model surgical menopause, or were treated with the ovatotoxin 4-vinylcyclohexene diepoxide (VCD) to model transitional menopause. Tissues also were collected from rats with a normal estrous cycle killed at proestrus when estradiol levels are high, and at diestrus when estradiol levels are low. Western blot detection of α-tubulin, β-actin, and GAPDH was performed and were compared for sensitivity and reliability with a fluorescent total protein stain (REVERT®). Results show that the total protein stain was much less variable across samples and had a greater linear range than α-tubulin, β-actin, or GAPDH. The stain was stable and easy to use, and did not interfere with the immunodetection or multiplexed detection of the housekeeping proteins. In addition, we show that normalization of our data to total protein, but not to GAPDH, revealed significant differences in α-tubulin expression in the hippocampus as a function of treatment, and that gel-to-gel consistency in measuring differences between paired samples run on multiple gels was significantly better when data were normalized to total protein than when normalized to GAPDH. These results demonstrate that the REVERT® total protein stain can be used in Western blot analysis of brain tissue homogenates to control for variations in loading and protein transfer, and provides significant advantages over the use of housekeeping proteins for quantifying changes in the levels of multiple target proteins.
Keywords: Menopause, Accelerated Ovarian Failure, VCD, Estradiol, Hippocampus, Striatum, Frontal Cortex
1. Introduction
Gonadal hormones affect the brain through a multitude of mechanisms involving all regions of the cell. Estradiol in particular has been shown to produce well-documented effects on cellular metabolism, synaptic plasticity, brain growth and development, neuronal maintenance and repair, sex and social behaviors, and learning and memory [1-8]. These effects are mediated by estrogen receptors (ERs) and correspond with changes in both gene and protein expression in specific regions of the brain.
Several methods are available to study changes in protein expression in brain. These include spectrometry methods such as HPLC and LC/MS; and also antibody-dependent methods such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA) and Western blot. Western blot evolved from northern blotting with the addition of electrophoretic transfer and the use of secondary antibodies to allow protein quantification by optical densitometry [9, 10]. Further advances such as the acquisition of images using high dynamic range detectors (such as CCDs) and the use of fluorescently-labeled secondary antibodies have led to increased detection sensitivity, a broader linear range of signal quantification, and have enabled multiplex detection of multiple proteins simultaneously [11-13]. Quantitative Western blot became a common and well-established methodology as a result of being easy to perform, cost-efficient, and its ability to be used with a variety of visualization methods for quantifying relative amounts of protein.
A common practice in quantitative Western blot analysis is to normalize the intensity of bands representing target proteins to a house-keeping protein (HKP) that is present in high abundance and assumed to be unaffected by treatment. This controls for variations in loading and transfer that can affect band intensity and quantification. In studies involving estrogen regulation of proteins in the central nervous system, it is common practice to normalize target proteins to one of three HKPs, β-actin, GAPDH, or α-tubulin.
To verify how common this is, we conducted a search of publications using the Pubmed advanced search engine to identify publications in which (a) circulating estrogens were manipulated in rats or mice by either ovariectomy and/or estradiol treatment, and (b) Western blot methods were used to quantify changes in one or more target proteins in the brain. Our analysis was limited to 50 papers published in the last 4 years (2013-2017). The specific HKPs that were used to normalize Western blot data were noted and frequency of use was quantified. The results of this survey are summarized in supplementary table 1. Of the 50 publications surveyed, 32 (64%) normalized target proteins to actin, 9 (18%) normalized to GAPDH, and 9 (18%) normalized to tubulin.
Table 1. Summary of serum hormone levels in cvcling. ovariectomized and VCD-treated rats.
| Hormone | Proestrus [pg/ml] | Diestrus [pg/ml] | OVX-1 [pg/ml] | OVX-6 1 [pg/ml] | VCD-1 [pg/ml] | VCD-6 [pg/ml] |
|---|---|---|---|---|---|---|
| n=8 | n=7 | n=7 | n=7 | n=8 | n=8 | |
| 17β-Estradiol | 52.6±4.5 | 16.3±1.7* | - | - | 16.4±7.6** | 8.0±4.9 ** |
| Testosterone | 210.5±41.8 | 78.8±6.4* | - | - | 88.2±46.5** | 66.0±19.7** |
| Androstenedione | 220.9±40.8 | 71.5±6.6* | - | - | 87.3±31.8** | 55.7±16.3** |
Values indicate mean ± SEM. Hyphen mark values below detection limit and asterisks shows significant difference from proestrus.
p<0.02, two-tailed t-test.
p<0.001, one-way ANOVA.
Studies have reported, however, that, in many tissues, these same HKPs are themselves targets of estrogen regulation [5]. This raises concerns about the use of HKPS in the estrogen field as normalization controls for Western blot analyses, and about the interpretation of data based on these methods.
An alternative approach is to use total protein (TP) staining in place of HKPs as an internal control. TP stain enables one to combine signal densitometry from all proteins in a defined MW range in order to represent the TP content. Some TP stains can be applied on a gel to represent amount of loaded protein; while some can be applied directly on polyvinylidene difluoride (PVDF) or nitrocellulose membrane. In each case, signal generated by the TP stain represents the whole-sample proteome. Therefore, any set of changes caused by hormonal manipulations will be proportional to the TP content and less variable between samples. Indeed, a number of studies have reported on the use of TP as a reliable loading control for Western blot quantification [14-23]. Despite these advantages and the lack of a reliable HKP, normalization by TP stain has not yet been evaluated as a suitable loading control for protein quantification in brain samples obtained at different stages of the estrus cycle or following other gonadal or hormonal manipulations.
Here we evaluated the REVERT® (LI-COR, Inc) TP stain as an internal control in Western blot analyses. This was compared with commonly used HKPs to quantify changes in the levels of target proteins in the brains of rats being used to model surgical vs. transitional menopause, as well as in tissues collected at proestrus and diestrus.
Our results demonstrate significant benefits of using TP to normalize loading when quantifying protein levels by Western blot. In addition, we detected significant effects of menopausal model on HKPs, but not TP, and show that these differences are of sufficient magnitude to alter conclusions about effects of ovarian manipulations on protein expression. We conclude that the described TP stain is a more reliable and precise method than the alternative of using a single common HKP as an internal control when evaluating effects of hormone manipulations on target proteins in the central nervous system.
2. Materials and Methods
2.1 Ethics statement
All procedures were carried out in accordance with PHHS policies on the use of animals in research, and with the approval of the University of Pittsburgh's Institutional Animal Care and Use Committee.
2.2 Animals
Young adult (3mo old) female Sprague-Dawley rats were purchased from Harlan Sprague-Dawley Laboratories, Inc. Rats were housed individually with free access to food and water, and maintained on a 12-hr light/dark cycle.
2.3 Experimental design and treatments
Rats were randomly assigned to one of four groups, proestrus (P), diestrus (D), 4-vinylcyclohexene diepoxide (VCD)-treatments and ovariectomy (OVX). Regularly cycling animals underwent daily vaginal smears to assess cycle stage [24]. Rats were euthanized on proestrus (n=8) or diestrus (n=7) and brain tissues were collected. Additional rats were used to model surgical and transitional menopause. Transitional menopause was modeled by administering daily injections of the ovatotoxin VCD at 80 mg/kg diluted in sesame oil at a volume of 2.5 μl/g body weight for 30 days (n=16). This regimen has been shown to destroy >95% of primary ovarian follicles with little toxicity to stromal cells or to other organs, and no other lasting toxic effects [25-28]. An additional group of intact rats underwent daily vehicle injections of sesame oil. After 30 days of injection, the vehicle-treated rats underwent bilateral ovariectomy (OVX). Ovariextomy was conducted in-house using a lateral approach. Rats were anesthetized with ketamine (70 mg/Kg) and xylazine (14 mg/Kg). An incision was made and the apical tip of the uterus and ovaries were exposed. The apical tip of the uterus was tied off with 6-0 suture silk and the ovaries removed. The peritoneum was then sutured shut with 6-0 suture silk and the skin closed with two 9 mm suture clips. Rats that had received 30-days VCD treatment underwent a sham surgery which included anesthesia and the abdominal incisions, but no removal of the ovaries. Animals were placed onto a warm heating pad during recovery. Ketofen (3mg/Kg, i.p.) was administrated twice per day for three days to reduce discomfort. Topical antibiotic ointment was applied for 3-days to prevent infection. At 1 or 6 weeks following surgery, rats were euthanized with an overdose of ketamine (280 mg/kg) and xylazine (56 mg/kg) and tissues were collected for analysis. This resulted in a total of 6 treatment groups: P, D, Ovx-1 W, Ovx-6W, VCD-1W, and VCD-6W.
2.4 Collection of Tissues
Rats were anesthetized and decapitated. Brains were rapidly removed and dissected on an ice-cold petri dish. Tissues from the hippocampus (HPC), striatum (STR), and frontal cortex (FCX) were collected and snap frozen on dry ice. Serum was also collected and stored at -20° C for quantification of hormone levels.
2.5 Analysis of Hormone Levels by UPLC-MS-MS
Serum levels of 17β-estradiol (E2), testosterone (T) and and rostenedione (AD) were quantified by UPLC-MS/MS as described [29]. Briefly, for E2 analysis, samples were spiked with internal standard 25 μl 2,4,16,16,17-d5-17 beta-estradiol (1 ng/ml in methanol). After extraction samples were derivatized with dansyl chloride. E2 was eluted using a Waters Acquity UPLC BEH C1 8, 1.7 μm, 2.1 × 150 mm reversed-phase column, with an acetonitrile: water (0.1% formic acid) gradient. MS detection and quantification were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via an electrospray ionization (ESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 506 → 171 for E2, and 511 →171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards.
Testosterone (T) and Androstenedione (AD) levels were quantified by a modification of the method described by Cawood [30] and using methods similar to the quantification of E2 described above. Briefly, samples were spiked with 0.25 ng/ml D3-testosterone or D7-androstenedione as the internal standard. T and AD was eluted from the same column as E2, with a methanol: water (0.1% formic acid and 2 mM ammonium acetate) gradient from 50 to 85% methanol. Transitions used for T analysis were 289 →97 for T and 292 →97 for the deuterated T; transitions used for AD analysis were 287→100 for AD and 294→ 100 for the deuterated AD. The lower limits of detection were 2.5 pg/mL for E2 and 10.0 pg/mL for T and AD.
2.6 Processing and Analysis of Brain Tissues Samples
Brain tissues were thawed in freshly-prepared cold lysis buffer (10 μl buffer per 1 μg tissue) containing: 1% protease inhibitor cocktail, 0.5% Sodium deoxycholate, 0.001M ethylenediaminetetraacetic acid (EDTA), 0.15M NaCl, 0.01M Sodium fluoride, 0.1% Sodium dodecyl sulfate (SDS), Tris-HCl (50mM, pH=7.4), 0.001M Sodium orthovanadate (Na3VO4) and 0.001M phenylmethylsulfonyl fluoride (PMSF). All reagents were purchased from Sigma-Aldrich. Samples were then sonicated at 4°C 6 times at 30-seconds ea. for total of 3-minutes. An aliquot of each sample was used to determine protein concentration by Bradford assay [31].
2.7 SDS-PAGE
Samples were diluted to 2.4 μg/μl in sonication buffer mixed with an equal volume of 2× Laemmli Sample Buffer (BIO-RAD Cat#1610737) containing 5% (v/v) 2-mercaptoethanol. Samples were then heated to 98 C for 7-minutes. Samples were loaded onto commercially-prepared 12% Mini-PROTEAN® TGX™ gels. Samples were loaded at a volume of 10 μl per lane to achieve a total of 12 μg protein per lane. This loading concentration was confirmed to ensure that signals from each of our targets fell within their linear detection range. In addition, for each brain region, a dilution series was generated using a mixture of homogenates containing twenty samples to equally represent each of our animal models. The combined mixture was used to test and compare the range for linear detection and quantification of TP and individual targets. The Chameleon 700 pre-stained protein ladder (LI-COR) was included on all gels. Samples were run for 2-hours at 100 V using a running buffer containing: 25 mM Tris-HCL, 192 mM glycine and 0.1% SDS. Upon completion of the run, samples were transferred (2-hr, 100 V, 4°C) to Immobilon FL PVDF membrane (Millipore) using a transfer buffer (25 mM Tris-HCl, 192 mM glycine, 10% v/v methanol, pH 8.3).
2.8 Total Protein Membrane Stain
After transfer, PVDF membranes were directly stained for TP detection using LI-COR REVERT™ Kit according to the manufacturer's instructions. Briefly, membranes were submerged in REVERT for 5 minutes, washed 2× for 30 seconds each with wash solution (LI-COR, 6.7% (v/v) glacial acetic acid, 30% (v/v) methanol, in water) and imaged immediately. Membranes were scanned using the 700 nm channel of the Odyssey imaging system (LI-COR) at a scanning resolution of 169 μm and a ‘normal’ image quality setting. TP stain was then removed by 5 minutes incubation in reversal solution (LI-COR, 0.1 M sodium hydroxide, 30% (v/v) methanol, in water) with gentle shaking and then imaged again to verify that no residual signal remained.
Membranes were than blocked for 1 hour at room temperature in Odyssey® TBS blocking buffer (LI-COR, 927-50000) and incubated overnight at 4°C with mixture of primary antibodies: against α-tubulin (Abcam, ab52866, rabbit monoclonal anti-α-tubulin, 1:5000), β-Actin (Abcam, ab6276, mouse polyclonal anti-β-actin, 1:5000), and GAPDH (Abcam, ab181602, rabbit polyclonal anti-GAPDH, 1:5000).
The next morning, membranes were washed 3× for 10 min each with TBS-T (50 mM Tris-HCL at pH=7.4, 150 mM NaCl and 0.1% Tween-20) and incubated for 1 hr at room temperature in TBS-T containing secondary antibodies of goat anti-mouse IgG (LI-COR IRDye 800CW, cat#32210) and goat anti-rabbit IgG (LI-COR IRDye800CW, cat#32211) each at 1:5,000 dilution.
2.9 Image Acquisition and Data Analysis
All PVDF-membranes were analyzed using the Odyssey application software (Ver 3.0.30) by acquiring integrated intensity values of defined bands. Densitometry of single-band targets (e.g. GAPDH) were measured in a rectangular area automatically defined by the software. Manual adjustments were made to contain all detectable signal for a band and background was subtracted using the software's averaging protocol. Signal from the TP stain was measured in accordance with manufactures protocol by manually applying a rectangular area at a select range of molecular weights (e.g.50-90KDa; see below). For comparison of samples on the same gel, relative quantification was achieved by normalizing each target to the value of either TP or to an individual HKP. In some cases these ratios were then normalized to an average of the same target from proestrus animals on the same gel, to facilitate between-gel comparisons.
All linear ranges of quantification were obtained by graphing integrated intensities of serial dilutions and choosing a range of dilutions with the highest linearity represented by least-squares analysis.
2.10 Analysis of the consistency of TP densitometry across different molecular ranges
The TP stain produced bands of varied intensities depending on loading and molecular weight. Signals for specific molecular weight ranges were analyzed for consistency. The following ranges were evaluated: 30-160KDa, 15-160KDa, 30-90KDa, 30-50KDa, 50-160KDa and 50-90KDa. Signal consistency for each range was determined by calculating the percent coefficient of variation (%CV). Samples representing each of the treatment groups were loaded onto the same gel (total n=12; OVX (n=4), VCD-treated (n=4), diestrus (n=2), proestrus (n=2); each at 12 μg/lane). The %CV was calculated for the mean of all 12 samples per gel for each of 2 gels.
2.11 Analysis of the consistency of the signals for each of the HKPs vs TP
Analysis of the consistency of the HKP signals across treatment groups was evaluated similarly to above. Samples representing each of the treatment groups were loaded onto the same gel (12/gel). %CV for each of the HKPs and for TP was calculated from the same membrane by multiplexing images. This experiment was repeated twice per brain region.
2.12 Statistical comparison of normalizing to GAPDH vs TP when evaluating effects on α-tubulin
Evidence for effects of ovarian status on α-tubulin was detected. We therefore set out to test whether the effects were significantly different when data were normalized to TP vs. the commonly used HKP GAPDH. Densitometry data for α-tubulin was normalized to GAPDH and separately to TP using values obtained simultaneously from the same PVDF membrane. This was repeated twice. Differences between proestrus and diestus were evaluated by two-tailed T-test. In addition, differences among all 6 groups were evaluated by one-way ANOVA. Statistical comparisons were conducted using JMP Pro software (SAS, version 13.0.0) and statistical significance was defined as p <0.05.
3. Results
3.1 Hormone levels
Serum levels of E2, T and AD are summarized in Table 1. As expected, levels of all three hormones were significantly higher at proestrus than at diestrus (E2: t(13)=7.14, p<0.0001; T: t(13)=2.91, p<0.02; AD: t(13)=3.38, p<0.005). Following ovariectomy, the levels of all three hormones were below the levels of detection. In rats treated with VCD, levels of E2, T, and AD were significantly lower than levels detected in proestrus rats (p<0.05 in all cases), and did not differ significantly from levels detected in diestrus rats. However, levels of both androgens were much higher than detected in Ovx rats. This was the case at both 1-w and at 6-w following the completion of VCD treatments. This is consistent with the selective depletion of ovarian follicles and the preservation of surrounding interstitial cells following VCD treatment [27, 32-35].
3.2 Consistency of the REVERT® TP stain across samples and for different ranges of molecularweight
Consistency of the TP signal across samples from different treatment groups is illustrated in Figure 1 and demonstrates a distinctive detection pattern. Further analysis of the consistency of the TP staining is summarized in Figure 2. TP staining produced a consistent densitometry pattern with higher optical density in areas where more proteins are present (Fig 1B and Fig 2A). The lowest fluorescence intensity was in the range between 50 to 90 KDa while the widest range (15 to 160 KDa) produce approximately 3.5× more signal (Fig 2B). Regardless of these differences the %CV for each MW range fell between 5.22% and 6.17% (Fig 2C). Highest variability was observed in the 30 to 160 KDa range while lowest variability was observed in the 50 to 90 KDa range. These values remained consistent regardless of treatment (not shown). Thus, all subsequent analyses used the MW range of 50-90 kDA for normalizing targets to TP.
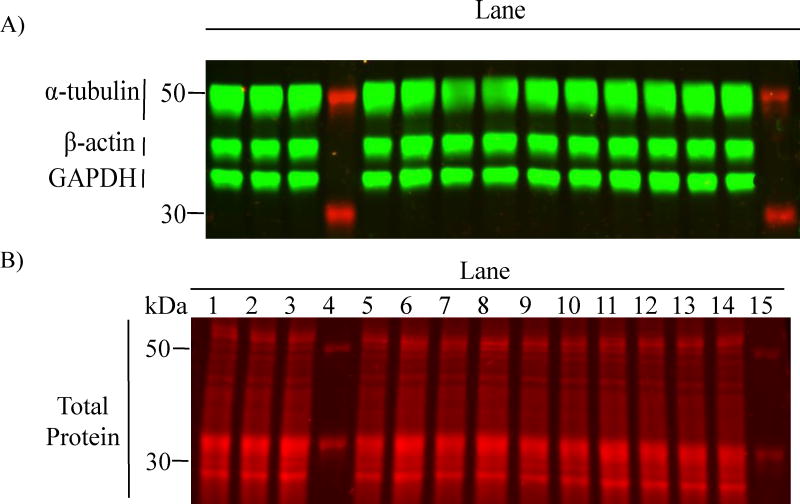
Fig 1.
Fluorescence signal densitometry of TP stain is less variable than individual HKPs. (A) representative image of PVDF membrane with different bands of individual HKPs (green): GAPDH (36KDa), β-actin (42KDa) and α-tubulin (50KDa); and (B) a TP stain produced on the same membrane (red). Note the apparent consistency in TP signal across lanes loaded with different samples (12μg-protein/lane).
Figure 2.
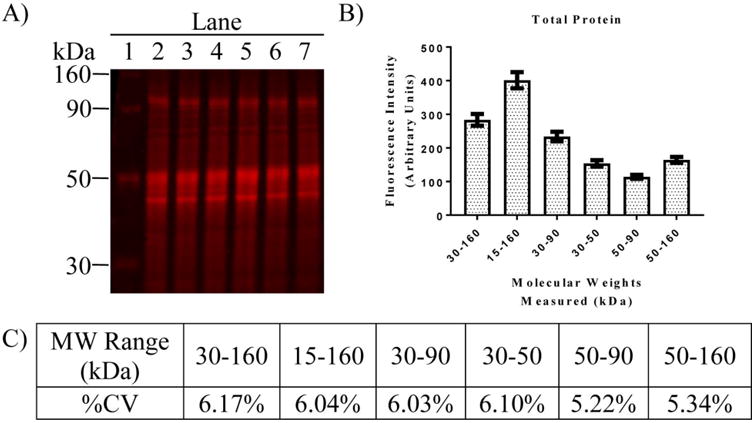
Comparative analysis of the variability in TP signal across different ranges of MW for samples of regional brain homogenates. (A) Image of PVDF membrane showing TP stain of six different hippocampal homogenates for MWs ranging from 30-160 KD. (B) Analysis of fluorescence intensity for different ranges of molecular weight from 12 samples run on a single gel (not shown). Bars represent the mean fluorescence intensity ± SEM for each MW range. (C) %CV calculated from the same 12 samples summarized in B. Note the consistent and low variability in TP measurements across multiple MW ranges.
3.3 Comparison of the linear dynamic range for measuring TP vs. HKPs
Figure 3 shows an image of TP and HKPs detected in lanes loaded with different amounts of protein. Linear dynamic range was evaluated by plotting fluorescence intensity vs. protein loading. To avoid possible bias associated with treatment, twenty samples were selected across all treatment groups and were combined. This was done for each brain region. These combined samples were then loaded at concentrations ranging from 0.5 to 80 μg protein per lane. Results obtained from all three brain regions were similar. Representative graphs are shown in Figure 3. For TP, signal remained linear across a wide range from 0.5 to 40 μg/lane (Fig 3D, R2>0.98). In comparison, the effective linear range for the three HPKs was much more limited. GAPDH: 5 to 15 μg/lane (Fig 3E, R2=0.93); β-actin: 5 to 25 μg/lane (Fig 3F, R2=0.91); α-tubulin: 2 to 25 μg/lane (Fig 3G, R2>0.97). Based on these data, lanes were loaded with 15 μg protein for all subsequent analyses.
Fig 3.
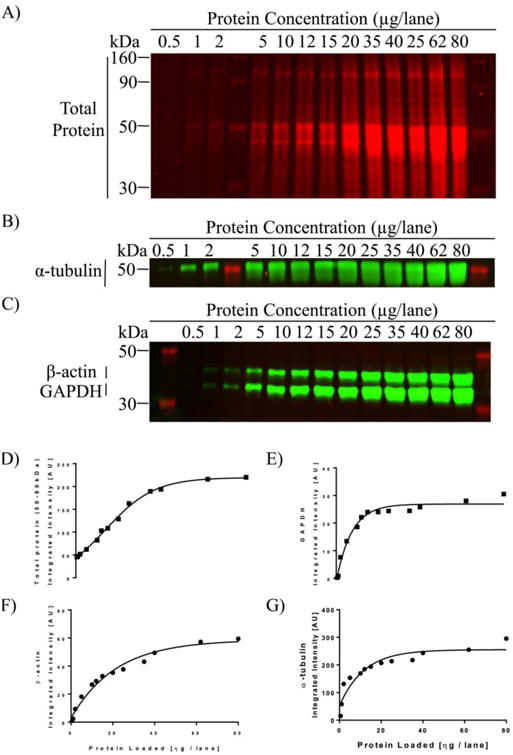
Analysis of linear range of detectability for TP and each HKP. Representative images of PVDF membranes stained with total-protein (A) and three HKPs (B,C) in a serial dilution of regional rat brain homogenates ranging from 0.5 μg to 80 μg per lane. (D-G) Graphs showing densitometric analysis. Lines show least-squares best fit across all points. For each curve, a dynamic range for quantification was defined by choosing the linear portion of the curve that yields the highest correlation. For example, note the rapid saturation of α-tubulin signal at 25μg/lane and the limited linear range of GAPDH (5 to 15 μg/lane) and P-actin (5 to 25 μg/lane). In contrast, REVERT® TP signal had a much wider linear range (0.5 to 40 μg/lane).
3.4 Analysis of the consistency of TP vs. HKP staining
The consistency of TP staining vs. staining for each of the HKPs was evaluated and compared for each of the three brain regions (Table 2, Fig 1). Note that the values represent %CV for data collected across all treatment groups. Data show that %CV for TP staining was substantially less than the %CV for each of the HKPs, regardless of brain region. In most cases, %CV for the HKP was 2-5× the %CV for TP staining. This is consistent with the supposition that levels of HKPs are affected by changes in ovarian status, thus resulting in greater variability when data are combined across treatment groups. The fact that variability for TP staining was very consistent across treatment groups supports the suitability of using TP rather than HKPs for normalization.
Table 2. Summary of calculated %CV for quantification of TP and individual HKPs.
| Brain Region | Gel 1 | Gel 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| TP | GAPDH | β-actin | α-tubulin | TP | GAPDH | β-actin | α-tubulin | |
| FCX | 5.43% | 16.71% | 20.21% | 9.54% | 5.22% | 27.51% | 14.12% | 18.87% |
| STR | 7.37% | 34.82% | 31.08% | 28.22% | 11.10% | 40.20% | 36.09% | 49.48% |
| HPC | 7.84% | 32.03% | 20.74% | 17.01% | 13.03% | 39.60% | 30.20% | 17.69% |
Data obtained from multiplexing densitometry of TP, GAPDH, β-actin and α-tubulin signals from 2 gels. For each target, the %CV was calculated from 12 samples/gel representing OVX (n=4), VCD (n=4), diestrus (n=2) and proestrus (n=2). Note that %CV is consistently lower for measurements of TP vs. each of the HKPs.
3.5 Effects of treatment on relative levels of α-tubulin
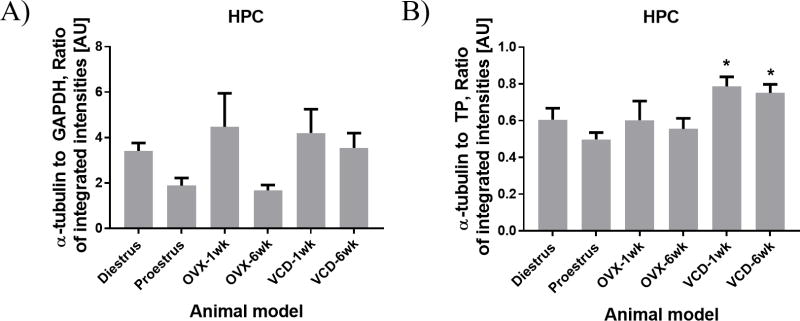
Based on the supposition that HKPs can be affected by changes in ovarian status, we evaluated differences in relative levels of α-tubulin as a function of treatment. In addition, we compared results obtained by normalizing the α-tubulin data to TP vs. GAPDH. The results are summarized in Figure 4. After normalizing to TP, a significant increase in α-tubulin was detected in the HPC of VCD-treated rats relative to levels at proestrus (Fig 4A; F(5,20)=4.62, p<0.006). This effect was observed at both 1-week and 6-weeks following VCD treatment. In contrast, analysis of these same data normalized to GAPDH produced no significant effect (Fig 4B; F(5,20)=2.63, p=0.055). This is likely due to the added variability associated with the GAPDH signal, and the added variability of data normalized to GAPDH. No significant effects of VCD treatment on α-tubulin were detected in the STR or FCX regardless of normalization (not shown). Hence the effect of VCD treatment on α-tubulin was region specific, and was detected only when data were normalized to TP and not to GAPDH.
Fig 4.
Effect of VCD treatment on α-tubulin expression in the HPC. Bars represent the mean ratio of integrated intensities ± SEM from n=5 per group. (A) Data normalized to GAPDH. (B) Same data normalized to TP. Note significant increase in α-Tubulin detected in VCD-treated rats relative to proestrus in (B). In contrast, no significant effect was detected in (A). *p<0.05 relative to proestrus by one-way ANOVA.
3.6 Reproducibility of results across gels
A concern in Western blot analysis is the reproducibility of results and the ability to combine results obtained from different gels. While signal intensity can vary greatly from gel-to-gel, it should be the case that the measurement of relative levels of a target protein in two different samples should be relatively consistent from one gel to another. We predicted that the gel-to-gel variability in such measurements would be lower when targets are normalized to TP than when normalized to an HKP. To test this, we used our existing data set and identified pairs of samples that were run on the same gel on at least two separate occasions. We identified a total of 32 samples, serving as 16 pairs from different treatments. For each pair, within the same gel, the percent difference between samples was calculated twice: once normalized to TP (100*[(Sample 1/TP) – (Sample 2/TP)]/(Sample 1/TP)), and once normalized to GAPDH (100* [(Sample 1/GAPDH) – (Sample 2/GAPDH)]/(Sample 1/GAPDH)). Identical calculations were made for the same pair of samples on a second gel (supplemental table 2).
To compare the reliability of the two normalization procedures we calculated the intraclass correlation coefficients (ICCs) for the same 16 pairs when normalized by TP or by GAPDH. These ICC values reflect the variation of our data by each normalization method. The intraclass correlation coefficients were calculated using the reliability analysis platform on IBM SPSS Statistics Software Version 25. ICCs models were defined as two-way mixed effects, absolute agreement with multiple raters and 95% confidence interval. The calculated ICC value for samples normalized to TP was 0.892 (i.e., excellent reliability) while the calculated ICC value for samples normalized to GAPDH was 0.215 (i.e., poor reliability).
To determine whether the two calculated ICC values differ significantly, we used the Konishi-Gupta modified Z-test (TZ) where the null hypothesis H0 is that two ICCs are equal [36-38]. Our TZ test was calculated using raters values of k1=k2=2, intraclass estimates of r1A and r2A were substitutes with p1=1.26, p2=0.29 correspondingly and an inter class p12=0.44. The final approximation to the Fisher's Z-transformation was Tz=Tzm=2.83 with proportions of TZ >1.65 showing significance at p<0.05 level where H0 is rejected. We also compared our ICC Cronbach alpha values by a second method of Diedenhofen and Musch [39]. The correlation between our underlying scores of the Cronbach alphas (0.95 and 0.50) was 0.2 and applied to the on-line web interface based on R-code resulting in χ2; = 4.6, df=1 and p<0.05 level where H0 is rejected.
Thus, the ICC value obtained when data were normalized to TP was in the excellent reliability range (0.892) and differed significantly from the ICC valuate obtained when data were normalized to GAPDH (0.215).
4. Discussion
Our goal was to evaluate an alternative method for conducting Western blot analysis of brain homogenates from rats with distinct physiologically relevant gonadal hormone states. To accomplish this, experimental groups included models of surgical (OVX) and transitional (VCD-treated) menopause, and tissues from normal cycling rats collected at proestrus and at diestrus. Hormone measurements confirmed significantly higher serum levels of E2, T, and AD on proestrus than on diestrus and undetectable levels of all three hormones in OVX rats. In VCD-treated rats, E2 levels were undetectable which is consistent with the loss of ovarian follicles. In contrast, levels of T and AD were elevated compared with diestrus, consistent with the continued presence of ovarian interstitial cells which produce androgens [32]. These data are consistent with prior validations of this model [32, 33, 35, 40] and confirm that we successfully produced an array of physiologically relevant gonadal hormone conditions with which to evaluate the methodology.
Results show that direct application of the REVERT™ TP stain to PVDF membranes containing samples from the HPC, STR and FCX produced a stable fluorescence signal, with low variability and a wide linear range (0.5-40 μg/lane). In comparison, the linear range for quantification of GAPDH, β-actin, or α-tubulin was much more limited. In addition, the TP signal across several ranges of molecular weights produced lower coefficients of variation (%CV) than individual HKPs. The higher variability in the HKP signals across treatments is consistent with literature showing that these HKPs can be affected by gonadal hormone status (discussed below). The impact of these findings is made evident by the observation that normalization of our data to TP, but not to GAPDH, detected significant differences in α-tubulin expression in the HPC as a function of treatment. Finally, we showed that gel-to-gel consistency in measuring differences between paired samples run on multiple gels was significantly better when data were normalized to TP than when normalized to GAPDH. These findings are consistent with other recent reports describing the benefits of TP stains for normalizing Western blot analysis of rodent samples of other tissues including cerebrospinal fluid [16], sciatic nerve [14], and liver [17]. Our data extend these findings by demonstrating the utility of the REVERT® TP stain in Western blot analysis of brain tissues from rats of varying gonadal hormone status.
4.1 Evidence that HKPs are affected by gonadal hormone status
The fact that relatively high variability in HKP measurement was observed across treatment groups is not surprising given the many studies that have demonstrated significant regulation of housekeeping genes by gonadal hormones in a variety of tissues. For example, differences in the expression of GAPDH, actin and tubulin mRNAs were reported in samples from brain and other tissues of males vs. females, in obesity models, and in ovariectomized rats with and without estrogen treatment [41-45]. Estradiol has been shown to regulate actin polymerization in a variety of tissues [46-48], to alter microtubule stability in cancer cells and in hippocampal neurons [46, 49], and to alter the expression of GAPDH in endometrium and liver [17, 50]. Estradiol also has been shown to bind to and induce rapid phosphorylation of GAPDH in hippocampal neurons and to increase GAPDH activity [51]. Proteomic studies in brain samples revealed estradiol regulation of proteins involved in glucose utilization (including GAPDH), the branching of actin filaments, and post-translational modification of tubulin [5, 52]. These changes in expression were brain region-specific, varied with treatment, and were affected by pregnancy, estrus- and menstrual- cycles.
Recent proteomic studies have attempted to identify suitable HKPs for Western blot quantification [53, 54]. Analysis of the human proteomic database ProteomicsDB identified 20 protein candidates with %CV<20% that have constitutive and ubiquitous expression. Notably, in this analysis GAPDH, β-actin and β-tubulin had %CV>20% qualifying them as unreliable for protein normalization [53]. Another analysis of two different proteomic studies screened a total of 1297 proteins from 12 tissues and found that 107 of them had %CV < 30% variation within the same tissue [54]. After comparing expression levels between the two studies, only 34 proteins consistently had %CV < 30% across the 12 tissues. Of these 34 proteins, an analysis of gene expression showed that only 14 had gene expression levels with %CV < 20% across 6 microarray studies. Note that this is still much greater than the variations observed in TP signal.
Collectively, these data demonstrate significant effects of gonadal status on GAPDH, actin and tubulin, as well as the difficulty in identifying HKPs suitable for normalizing Western blot data. Nevertheless, our small survey of recent reports suggests that the vast majority of Western blot analyses of hormonal effects on protein expression continue to use actin, tubulin, and GAPDH as normalization controls, often without first demonstrating that the HPKs are not also affected by treatment. Hence the need for a more consistent and reliable method.
4.2 Considerations in Selecting a TP stain to normalize Western blot data
When selecting a TP stain for use in Western blot analysis a number of factors need to be considered. Chief among these are an appropriate linear range consistent with the targets of interest and low sample-to-sample variability. Many of the currently available stains meet these criteria. Other factors include (1) reversibility, which can be an important consideration for samples of limited volume or if the stain interacts with subsequent immunodetection or spectrometric analysis of targets, (2) the ability to be used on membranes vs. gels, as membrane staining can account for inconsistencies in loading as well as transfer, and (3) compatibility with multiplexing for simultaneous detection of multiple targets. Many stains are available and each has its limitations. For example, Ponceau S is easily reversed, but reportedly loses sensitivity below 200 ng [55]. In addition, Janes et al. [55] reported that the zero intercept of the Ponceau S densitometry could not be accurately estimated from a blank region of the PVDF membrane. This could compromise the ability to use Ponceau S for relative protein quantification. Coomasie Blue and Amido Black are sensitive down to 50 ng [56], but have higher %CV than other TP stains and may block epitopes required for subsequent immunodetection [18, 19, 23, 57]. SYPRO Ruby is highly sensitive and is compatible with immunodetection techniques, but is irreversible [58]. These are just a few of the TP stains that have been commonly used in Western blot analysis.
The REVERT® TP stain displayed many desired characteristics of a non-specific TP stain including a broad linear range and excellent sample-to-sample consistency. In addition, the stain was stable for hours, yet easily reversed, could be applied directly to PVDF membranes thus controlling for inconsistencies in both protein loading and transfer, and was highly suited for use in the quantification of multiple targets on a single gel. Importantly, we detected no bleed-through from the 700 nm channel to the 800 nm channel (Fig 1), and no apparent interference with the immunodetection of our targets. Normalization to the REVERT® TP stain also resulted in excellent gel-to-gel consistency in the detection of relative differences in the expression of a target protein between pairs of samples. These findings suggest that this methodology overcomes the limitations of HKPs and is an easy and reliable method of normalization for Western blot analysis of brain tissue homogenates.
4.3 Limitations
This study demonstrates some advantages of the REVERT® TP stain over HKPs for use in quantitative Western blot analysis of brain tissue homogenates; however, there are some limitations. (1) We make no claims as to the superiority of REVERT® TP stain over any other TP stains that are commonly available for use in Western blot analysis. Direct comparison with other TP stains was not conducted and was not a goal of this study. It should be noted that several other TP reagents have also been shown to produce high accuracy, low %CV and excellent detection sensitivity [14-17, 23, 59]. (2) Our studies were conducted using PVDF membrane and not on other media types such as nitrocellulose or nylon. It is possible that performance will vary on other media types. (3) While we did not detect any binding of the TP stain to our targets or interference with immunodetection of the targets, it is impossible to rule out such interactions with other targets. Such interactions need to be tested empirically and, if observed, may require modification of the protocol to the specific application.
4.4 Summary
Our goal was to evaluate an alternative method for conducting Western blot analysis of brain homogenates from rats with distinct physiologically relevant gonadal hormone states. Our results demonstrate that the REVERT™ TP stain can be applied directly to PVDF membranes for multiplex fluorescence detection with target proteins. The method is easy to apply and shows excellent sensitivity, linearity, and reproducibility. Detection of TP showed much less variability across samples than the detection of HKPs. In addition, we provide direct evidence that normalizing to TP can enable detection of an effect that is otherwise masked by the added variability associated with normalizing to an HKP. Finally, we show that gel-to-gel consistency in measuring differences between paired samples run on multiple gels was significantly better when data were normalized to TP than when normalized to GAPDH. Given the evidence that gonadal hormones can influence the expression and activity of HKPs in many tissues including brain, we strongly recommend the use of this methodology for quantifying protein expression by Western blot in studies involving gonadal hormone manipulations or different gonadal hormone states.
Supplementary Material
Highlights.
REVERT® total protein (TP) stain had many advantages over housekeeping proteins (HKPs) for WB quantification.
Staining had greater linear range and less variability than HKPs.
Staining was stable, reversible, and easy to use in WB.
Staining worked well with immunodetection and fluorescence multiplexing.
Normalizing target data to TP improved reliability of quantification across gels.
Acknowledgments
This study was supported by NIH grant # 1R21AG043817. In addition, we wish to thank Tao Long, Junyi Li, and Doug Nelson for their contributions to the animal treatments, surgeries and tissue collection, and to Tao Long for collecting the vaginal smears and staging the estrous cycles of the gonadally intact rats. We also acknowledge Junyi Li for developing and validating the hormone assays and for analyzing hormone levels in the serum samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heberden C. Sex steroids and neurogenesis. Biochem Pharmacol. 2017;141:56–62. doi: 10.1016/j.bcp.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113(Pt B):652–660. doi: 10.1016/j.neuropharm.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TV, Ducharme S, Karama S. Effects of Sex Steroids in the Human Brain. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0198-3. [DOI] [PubMed] [Google Scholar]
- 4.Coyoy A, Guerra-Araiza C, Camacho-Arroyo I. Metabolism Regulation by Estrogens and Their Receptors in the Central Nervous System Before and After Menopause. Horm Metab Res. 2016;48(8):489–96. doi: 10.1055/s-0042-110320. [DOI] [PubMed] [Google Scholar]
- 5.Hansberg-Pastor V, et al. Sex Hormones Regulate Cytoskeletal Proteins Involved in Brain Plasticity. Front Psychiatry. 2015;6:165. doi: 10.3389/fpsyt.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinton RD, et al. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasconsuelo A, Milanesi L, Boland R. Actions of 17beta-estradiol and testosterone in the mitochondria and their implications in aging. Ageing Res Rev. 2013;12(4):907–17. doi: 10.1016/j.arr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31(2):224–53. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alwine JC, Kemp DJ, Stark GR. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977;74(12):5350–4. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renart J, Reiser J, Stark GR. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979;76(7):3116–20. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morseman JP, et al. PBXL-1: a new fluorochrome applied to detection of proteins on membranes. Biotechniques. 1999;26(3):559–63. doi: 10.2144/99263pf02. Epub 1999/03/26. [DOI] [PubMed] [Google Scholar]
- 12.Poor ML, Santa PF, Sittampalam GS. Visualization of multiple protein bands on the same nitrocellulose membrane by double immunoblotting. Anal Biochem. 1988;175(1):191–5. doi: 10.1016/0003-2697(88)90377-6. Epub 1988/11/15. [DOI] [PubMed] [Google Scholar]
- 13.Sternberg Biomedical Image Processing. Computer. 1983;16(1):22–34. doi: 10.1109/mc.1983.1654163. [DOI] [Google Scholar]
- 14.Eaton SL, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS One. 2013;8(8):e72457. doi: 10.1371/journal.pone.0072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SC, et al. A defined methodology for reliable quantification of Western blot data. Mol Biotechnol. 2013;55(3):217–26. doi: 10.1007/s12033-013-9672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins MA, et al. Total protein is an effective loading control for cerebrospinal fluid western blots. J Neurosci Methods. 2015;251:72–82. doi: 10.1016/j.jneumeth.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivero-Gutierrez B, et al. Stain-free detection as loading control alternative to Ponceau and housekeeping protein immunodetection in Western blotting. Anal Biochem. 2014;467:1–3. doi: 10.1016/j.ab.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Moritz CP, et al. Epicocconone staining: a powerful loading control for Western blots. Proteomics. 2014;14(2-3):162–8. doi: 10.1002/pmic.201300089. [DOI] [PubMed] [Google Scholar]
- 19.Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res. 2011;10(3):1416–9. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 20.Zeng L, et al. Direct Blue 71 staining as a destaining-free alternative loading control method for Western blotting. Electrophoresis. 2013;34(15):2234–9. doi: 10.1002/elps.201300140. [DOI] [PubMed] [Google Scholar]
- 21.Gilda JE, Gomes AV. Stain-Free total protein staining is a superior loading control to beta-actin for Western blots. Anal Biochem. 2013;440(2):186–8. doi: 10.1016/j.ab.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Calvo I, et al. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401(2):318–20. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 23.Moritz CP. Tubulin or Not Tubulin: Heading Toward Total Protein Staining as Loading Control in Western Blots. Proteomics. 2017;17(20) doi: 10.1002/pmic.201600189. [DOI] [PubMed] [Google Scholar]
- 24.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer PB, et al. Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model. Toxicol Pathol. 2001;29(1):91–9. doi: 10.1080/019262301301418892. [DOI] [PubMed] [Google Scholar]
- 26.Muhammad FS, et al. Effects of 4-vinylcyclohexene diepoxide on peripubertal and adult Sprague-Dawley rats: ovarian, clinical, and pathologic outcomes. Comp Med. 2009;59(1):46–59. [PMC free article] [PubMed] [Google Scholar]
- 27.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: a novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–87. doi: 10.1016/j.brainres.2010.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Kempen TA, et al. Characterization of neural estrogen signaling and neurotrophic changes in the accelerated ovarian failure mouse model of menopause. Endocrinology. 2014;155(9):3610–23. doi: 10.1210/en.2014-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, et al. A microsomal based method to detect aromatase activity in different brain regions of the rat using ultra performance liquid chromatography-mass spectrometry. J Steroid Biochem Mol Biol. 2016;163:113–20. doi: 10.1016/j.jsbmb.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Cawood ML, et al. Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem. 2005;51(8):1472–9. doi: 10.1373/clinchem.2004.044503. [DOI] [PubMed] [Google Scholar]
- 31.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 32.Mayer LP, et al. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71(1):130–8. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 33.Mayer LP, et al. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol. 2005;25(9):1910–6. doi: 10.1161/01.ATV.0000175767.46520.6a. [DOI] [PubMed] [Google Scholar]
- 34.Acosta JI, et al. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150(9):4248–59. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koebele SV, et al. Cognitive changes across the menopause transition: A longitudinal evaluation of the impact of age and ovarian status on spatial memory. Horm Behav. 2017;87:96–114. doi: 10.1016/j.yhbeh.2016.10.010. Epub 2016/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konishi S. Normalizing and variance stabilizing transformations for intraclass correlations. Annals of the Institute of Statistical Mathematics. 1985;37(1):87–94. [Google Scholar]
- 37.Konishi S, Gupta A. Inferences about interclass and intraclass correlations from familial data. Advances in the statistical sciences. 1987;4:225–233. [Google Scholar]
- 38.Donner A, Zou G. Testing the Equality of Dependent Intraclass Correlation Coefficients. Journal of the Royal Statistical Society Series D (The Statistician) 2002;51(3):367–379. [Google Scholar]
- 39.Diedenhofen B, Musch J. cocron: A Web Interface and R Package for the Statistical Comparison of Cronbach's Alpha Coefficients. International Journal of Internet Science. 2016;11:51–60. [Google Scholar]
- 40.Wright LE, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res. 2008;23(8):1296–303. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B, et al. Identification of optimal reference genes for RT-qPCR in the rat hypothalamus and intestine for the study of obesity. Int J Obes (Lond) 2014;38(2):192–7. doi: 10.1038/ijo.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Beamonte R, et al. Selection of reference genes for gene expression studies in rats. J Biotechnol. 2011;151(4):325–34. doi: 10.1016/j.jbiotec.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Taki FA, Abdel-Rahman AA, Zhang B. A comprehensive approach to identify reliable reference gene candidates to investigate the link between alcoholism and endocrinology in Sprague-Dawley rats. PLoS One. 2014;9(5):e94311. doi: 10.1371/journal.pone.0094311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroder AL, Pelch KE, Nagel SC. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine. 2009;35(2):211–9. doi: 10.1007/s12020-009-9154-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu NK, Xu XM. beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. J Neurotrauma. 2006;23(12):1794–801. doi: 10.1089/neu.2006.23.1794. [DOI] [PubMed] [Google Scholar]
- 46.Briz V, Baudry M. Estrogen Regulates Protein Synthesis and Actin Polymerization in Hippocampal Neurons through Different Molecular Mechanisms. Front Endocrinol (Lausanne) 2014;5:22. doi: 10.3389/fendo.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Y, et al. Estrogen receptor alpha and beta regulate actin polymerization and spatial memory through an SRC-1/mTORC2-dependent pathway in the hippocampus of female mice. J Steroid Biochem Mol Biol. 2017 doi: 10.1016/j.jsbmb.2017.08.003. doi:. doi: 10.1016/j.jsbmb.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A, et al. Estrogen and androgen regulate actin-remodeling and endocytosis-related genes during rat spermiation. Mol Cell Endocrinol. 2015;404:91–101. doi: 10.1016/j.mce.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda H, et al. The estrogen receptor influences microtubule-associated protein tau (MAPT) expression and the selective estrogen receptor inhibitor fulvestrant downregulates MAPT and increases the sensitivity to taxane in breast cancer cells. Breast Cancer Res. 2010;12(3):R43. doi: 10.1186/bcr2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flamini MI, et al. Differential actions of estrogen and SERMs in regulation of the actin cytoskeleton of endometrial cells. Mol Hum Reprod. 2009;15(10):675–85. doi: 10.1093/molehr/gap045. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez VD, Kipp JL, Joe I. Estradiol, in the CNS, targets several physiologically relevant membrane-associated proteins. Brain Research Reviews. 2001;37(1-3):141–152. doi: 10.1016/s0165-0173(01)00114-x. [DOI] [PubMed] [Google Scholar]
- 52.Szego EM, et al. Estrogen regulates cytoskeletal flexibility, cellular metabolism and synaptic proteins: A proteomic study. Psychoneuroendocrinology. 2010;35(6):807–19. doi: 10.1016/j.psyneuen.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Lee HG, et al. State-of-the-art housekeeping proteins for quantitative western blotting: Revisiting the first draft of the human proteome. Proteomics. 2016;16(13):1863–7. doi: 10.1002/pmic.201500344. [DOI] [PubMed] [Google Scholar]
- 54.Higdon R, Kolker E. Can “normal” protein expression ranges be estimated with high-throughput proteomics? J Proteome Res. 2015;14(6):2398–407. doi: 10.1021/acs.jproteome.5b00176. [DOI] [PubMed] [Google Scholar]
- 55.Janes KA. An analysis of critical factors for quantitative immunoblotting. Sci Signal. 2015;8(371):rs2. doi: 10.1126/scisignal.2005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harper S, Speicher DW. Detection of proteins on blot membranes. Curr Protoc Protein Sci. 2001;Chapter 10 doi: 10.1002/0471140864.ps1008s00. Unit 10 8. [DOI] [PubMed] [Google Scholar]
- 57.Tovey ER, Ford SA, Baldo BA. Protein blotting on nitrocellulose: some important aspects of the resolution and detection of antigens in complex extracts. Journal of Biochemical and Biophysical Methods. 1987;14(1):1–17. doi: 10.1016/0165-022x(87)90002-9. [DOI] [PubMed] [Google Scholar]
- 58.Berggren K, et al. A luminescent ruthenium complex for ultrasensitive detection of proteins immobilized on membrane supports. Anal Biochem. 1999;276(2):129–43. doi: 10.1006/abio.1999.4364. [DOI] [PubMed] [Google Scholar]
- 59.Thacker JS, et al. Total protein or high-abundance protein: Which offers the best loading control for Western blotting? Anal Biochem. 2016;496:76–8. doi: 10.1016/j.ab.2015.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.