Abstract
Environmental and socioeconomic changes over the past thirty years have contributed to a dramatic rise in the worldwide prevalence of obesity. Heart disease is among the most serious health risks of obesity, with increases in both atherosclerotic coronary heart disease and heart failure among obese individuals. In this review, we focus on primary myocardial alterations in obesity that include hypertrophic remodeling and diastolic dysfunction. Obesity-associated perturbations in myocardial and systemic lipid metabolism are important contributors to cardiovascular complications of obesity. Accumulation of excess lipid in non-adipose cells of the cardiovascular system can cause cell dysfunction and cell death, a process known as lipotoxicity. Lipotoxicity has been modeled in mice using high fat diet feeding, inbred lines with mutations in leptin receptor signaling, and in genetically engineered mice with enhanced myocardial fatty acid uptake, altered lipid droplet homeostasis, or decreased cardiac fatty acid oxidation. These studies, along with findings in cell culture model systems, indicate that the molecular pathophysiology of lipid overload involves endoplasmic reticulum stress, alterations in autophagy, de novo ceramide synthesis, oxidative stress, inflammation, and changes in gene expression. We highlight recent advances that extend our understanding of the impact of obesity and altered lipid metabolism on cardiac function.
Introduction
In large epidemiological studies, obesity is associated with increased incidence of heart failure. Analysis of the Framingham Heart Study (FHS) revealed that obese individuals, as defined by a body mass index of 30 kg/m2 or more, had a doubling of the risk of heart failure over a mean follow-up of 14 years [1]. Similarly, over a mean follow-up of 16 years in the Atherosclerosis Risk in Communities Study (ARIC), obesity (defined by body mass index, waist hip ratio, or waist circumference) correlated with increased incidence of heart failure [2]. Increased heart failure risk was observed in both men and in women in these two studies, and the ARIC study indicated that this association was present in racially diverse populations. Although the FHS and ARIC studies examined community-based samples from the United States, similar associations have been reported in other parts of the developed world [3].
Obese individuals also have increased incidence of coronary heart disease risk factors, including hypertension, hyperlipidemia and diabetes [4, 5]. Thus, it is not surprising that obesity is associated with subclinical atherosclerotic vascular disease and with incident coronary heart disease events [6–10]. Because myocardial infarction is a common cause of heart failure, increased obstructive coronary heart disease is a likely contributor to heart failure in obese subjects. Nonetheless, the FHS and ARIC studies demonstrated a significant association between obesity and incident heart failure, even when analyses were adjusted for baseline covariates known to increase the risk of coronary heart disease, including diabetes, smoking, and hypertension, and when analyses were adjusted for myocardial infarction. This indicates an additional risk for heart failure in obesity that is independent of coronary heart disease and suggests that the myocardium undergoes primary maladaptive changes in obesity [1, 2, 10].
Pathophysiology of myocardial disease in obesity
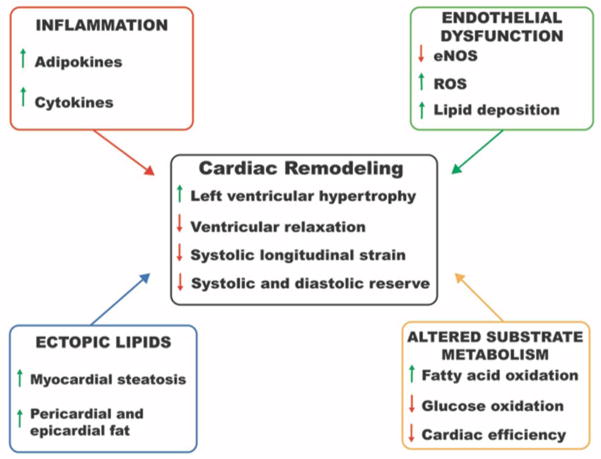
Obesity is accompanied by abnormalities of both structure and function of the heart. These changes reflect pathophysiological alterations in obesity that are both extrinsic and intrinsic to the myocardium. In this section, we summarize some of the major myocardial changes in obesity and contributing pathophysiological processes (Figure 1).
Figure 1. Alterations in myocardial structure and function associated with obesity.
Remodeling of myocardial structure and impaired diastolic and systolic function are observed in obese individuals. Contributions of altered lipid metabolism, inflammation and endothelial dysfunction are highlighted. eNOS, endothelial nitric oxide synthase; ROS, reactive oxygen species.
Structural and functional myocardial alterations
Obesity is associated with cardiac remodeling that affects all chambers of the heart. Young obese patients typically exhibit increased relative wall thickness, concentric remodeling, and hypertrophy of the left ventricle, even in the absence of hypertension [11]. However, long-standing and/or severe obesity can also be associated with left ventricular chamber enlargement and eccentric hypertrophy [12, 13]. Hemodynamic changes associated with obesity, including increased blood volume and cardiac output, contribute to these alterations in cardiac morphology [14]. In addition, the hyperinsulinemia, hyperleptinemia, and elevated renin-angiotensin-aldosterone system activity that often accompany obesity may drive left ventricular hypertrophy through the addition of new sarcomeres, and in some instances, through cardiac fibrosis [15]. Left atrial enlargement is also common and may result from changes in left ventricular structure and function as well as altered loading conditions [16]. Right ventricular enlargement correlates with obesity and relates to the pathophysiology of sleep disordered breathing and/or left ventricular dysfunction [13].
Myocardial dysfunction in obesity manifests primarily with diastolic abnormalities. Noninvasive cardiac imaging with conventional and tissue Doppler modalities demonstrate abnormal left ventricular filling patterns and decreased longitudinal relaxation velocity (e′) in obese subjects relative to lean subjects [11, 17, 18]. The degree of diastolic function correlates with body mass index. Although systolic function, as quantified by ejection fraction or fractional shortening, is typically normal, subclinical systolic abnormalities can be detected by both tissue Doppler (s′) and strain imaging, and the degree of impairment of s′ is predicted by increasing body mass index [11, 19, 20]. Abnormalities of strain measures in the right ventricle and left atrium have also been reported [21]. Furthermore, obese individuals may demonstrate reduced myocardial systolic and diastolic reserve as measured by stress echocardiography [15].
Altered Myocardial Substrate Metabolism
Under normal physiological conditions, β-oxidation of fatty acids provides the majority of energy for the myocardium [22]. Nonetheless, the heart is a metabolic omnivore that also readily metabolizes glucose, amino acids, and ketone bodies. This metabolic flexibility helps to preserve contractile function under a variety of physiologic and pathophysiologic conditions. In obesity and diabetes, elevated circulating free- and very low-density lipoprotein (VLDL)-derived fatty acids cause myocardial lipid uptake to increase dramatically [11, 18]. Although this augments flux through the β-oxidation pathway, fatty acids accumulate in a metabolic low-turnover pool and triglyceride content increases, indications of mismatch between substrate uptake and metabolism. The limited ability of cardiomyocytes to safely store excess fatty acids in triglyceride pools may be a key factor in the development of lipotoxicity. Increases in fatty acid metabolism also profoundly suppress glucose oxidation through substrate competition (Randle cycle) and induction of cardiac insulin resistance [23, 24]. Since metabolism of fatty acids produces less ATP per molecule of oxygen consumed than metabolism of glucose, the hearts of obese subjects produce energy less efficiently and consume more oxygen than the hearts of lean controls [18].
Inflammation
Obesity is characterized by elevated levels of circulating pro-inflammatory cytokines, many of which are produced by adipocytes and macrophages within visceral adipose depots [25]. Not only do cytokines serve as biomarkers of the altered metabolic state, but they also they can directly impact insulin and AMPK signaling, glucose and fatty acid metabolism, and fibrosis in the myocardium [26, 27]. Inflammatory markers may also provide prognostic information about heart failure risk in obesity. In the Multiethnic Study of Atherosclerosis, IL-6 expression was upregulated in obese patients and correlated strongly with incidence of heart failure [28].
Endothelial Dysfunction
Obesity is associated with impaired endothelium-dependent vasodilation and increased carotid intimal thickness, key indicators of vascular dysfunction and subclinical atherosclerosis [29–31]. Chronic low-grade inflammation and elevated circulating free fatty acids contribute synergistically to endothelial dysfunction through multiple mechanisms, including generation of ROS and suppression of insulin signaling in endothelial cells and activation of immune cells such as monocytes [32–34]. This milieu, coupled with elevated VLDL and low density lipoprotein (LDL) production, promotes cholesterol deposition, macrophage activation, and atherosclerosis [32]. In the absence of obstructive coronary heart disease, microvascular dysfunction has the potential to impair myocardial performance. Although myocardial blood flow, as quantified in positron emission tomography studies, is increased young obese women [18], lower coronary microvascular density observed in left ventricular biopsies from older obese patients could also contribute to impairment of myocardial function [35].
Ectopic Lipid Accumulation
Overload of adipose depots, dysregulation of lipolysis, and/or failure to suppress hepatic lipogenesis cause hyperlipidemia in obesity that results in accumulation of triglyceride in lipid droplets in many non-adipose tissues. Ectopic lipid deposition has been detected in the hearts of obese individuals with impaired glucose tolerance by 1H magnetic resonance spectroscopy [36, 37]. In these studies, steatosis was observed in the absence of diabetes or symptomatic heart failure, but it was associated with increased left ventricular mass, evidence of remodeling, and depressed septal wall thickening, suggesting subclinical functional impairment. At the time of autopsy, hearts of obese individuals with heart failure show a trend for greater lipid deposition compared to non-obese, non-failing subjects [38]. In addition to accumulation of lipid within cardiomyocytes, visceral adiposity is associated with expansion of epicardial and pericardial fat. The association of pericardial fat with atrial fibrillation and coronary disease and epicardial fat with increased left ventricular mass suggests these depots may not only provide fatty acid substrates to the myocardium, but also elaborate pro-inflammatory cytokines and adipokines that impact coronary and myocardial function [15, 39, 40].
Models that provide insights into underlying mechanisms
In order to dissect molecular mechanisms and delineate their causality in obesity-associated pathophysiological changes in the heart, investigators have leveraged in vivo and in vitro models. Here, we discuss some of the major models that have provided important insights into the myocardial effects of obesity and lipotoxicity (Table 1).
Table 1.
Murine Models of Myocardial Lipotoxicity
| Model | Mechanism | Phenotype | Reference(s) |
|---|---|---|---|
| High fat diet | Weight gain, fat mass expansion, insulin resistance, hyperlipidemia, cardiac steatosis | Cardiomyocyte hypertrophy, systolic and diastolic dysfunction | 41, 43, 44 |
| db/db, and ob/ob | Leptin receptor signaling deficiency, weight gain, fat mass expansion, insulin resistance, hyperlipidemia, cardiac steatosis | Diastolic dysfunction, reduced systolic reserve (db/db) or impaired systolic function (ob/ob) | 45–52 |
| Long chain acyl-CoA synthetase 1 (Acsl1) transgenic | Excessive cardiomyocyte fatty acid uptake; cardiac steatosis, inflammation, altered mitochondrial dynamics | Cardiac hypertrophy and dilation, systolic dysfunction, premature death | 54, 58, 105 |
| Fatty acid transport protein 1 (FATP) transgenic | Excessive myocardial fatty acid uptake, fatty acid accumulation, and metabolism | Cardiac hypertrophy with diastolic dysfunction | 55 |
| GPI-anchored lipoprotein lipase (LPLGPI) transgenic | Excessive myocardial uptake of lipoprotein-derived fatty acid, ceramide accumulation on high fat diet | Left ventricular dilatation, systolic dysfunction | 56 |
| PPARα transgenic | Excessive myocardial fatty acid uptake and oxidation, steatosis, oxidative stress, ceramide accumulation | Cardiomyocyte hypertrophy, systolic dysfunction | 61,62 |
| PPARδ knockout | Reduced myocardial mitochondrial biogenesis and fatty acid oxidation, steatosis, oxidative stress | Cardiac dilation, systolic and diastolic dysfunction, premature death | 63, 64 |
| PPARγ transgenic | Myocardial steatosis, ceramide accumulation | Cardiac hypertrophy, systolic dysfunction | 71 |
| Perilipin 5 knockout | Enhanced myocardial lipolysis and fatty acid oxidation, oxidative stress | Systolic dysfunction | 69 |
| Adipose triglyceride lipase (Atgl) knockout | Blunted myocardial lipolysis, impaired mitochondrial respiration, steatosis | Cardiac hypertrophy, systolic function, premature death | 73, 112 |
| CGI-58 knockout | Impaired myocardial triglyceride hydrolysis, steatosis | Cardiac hypertrophy, systolic dysfunction, premature death | 74 |
| HDAC3 knockout | Decreased myocardial expression of mitochondrial lipid metabolism genes, decreased fatty acid oxidation, steatosis | Cardiac hypertrophy, systolic dysfunction, and premature death on high fat diet | 60 |
Insights from murine models of obesity
Diets containing 40 to 60% fat and variable amounts of sucrose have long been used for the study of obesity in mouse models. These models produce rapid, substantial increases in body weight and fat mass, recapitulating the energy surplus that underlies obesity [41]. As early as 24 hours after initiation of a high fat diet, C57BL/6 mice show signs of glucose intolerance that progresses over subsequent weeks [42]. Insulin-stimulated glucose uptake, insulin signaling, and expression of the GLUT4 glucose transporter are diminished in the myocardium following 1.5 weeks of high fat diet, prior to the onset of whole body insulin resistance and hyperinsulinemia at three weeks [41]. By 15 to 25 weeks of high fat diet, systolic function as quantified by transthoracic echocardiography is reduced by 10 to 40% with evidence of cardiac and cardiomyocyte hypertrophy and diastolic dysfunction [41, 43, 44]. Cowart and colleagues found that a milk fat-based diet that is enriched in myristic acid has a particularly robust negative inotropic effect, indicating that the source of fat is an important determinant of phenotype in these models [43].
The db/db and ob/ob mouse inbred strains, which harbor mutations in the leptin signaling pathway, are genetic models of extreme obesity. As early as 4 weeks of age, these mice are obese and insulin resistant, and both strains develop hyperglycemia, although this occurs earlier in the db/db model [45]. In both models at 4 to 8 weeks of age, myocardial fatty acid oxidation rates and oxygen consumption are increased while glucose oxidation is suppressed. Myocardial efficiency is impaired, with evidence for mitochondrial uncoupling that is independent of changes in expression of uncoupling proteins [46, 47]. In ob/ob mice, there is evidence of cardiac insulin resistance and metabolic inflexibility, with failure to modulate myocardial metabolism in response to changes in insulin or substrates [48]. In both strains, these early metabolic changes in the heart are accompanied by increased contractility and relaxation [45]. The ob/ob heart maintains normal systolic function beyond 10 weeks of age despite cardiac steatosis, but demonstrates impaired functional reserve during dobutamine challenge [49]. By contrast, in the db/db heart, decreased systolic function accompanies the development of hyperglycemia by 8 weeks of age [45, 50–52].
Transgenic and knockout murine models of obesity-related myocardial disease
In obesity, high circulating levels of free fatty acids and triglycerides cause enhanced lipid delivery to the myocardium that exceeds its ability to metabolize these substrates [53]. Excess lipid nutrient delivery has been modeled in several transgenic mouse models in which cardiomyocyte expression of lipid transport proteins drives lipid uptake, even in the absence of systemic metabolic disturbances. Cardiomyocyte overexpression of long chain acyl-CoA synthetase 1 (Acsl1), fatty acid transport protein 1 (Fatp1) or glycosylphosphatidylinositol anchored lipoprotein lipase (LplGPI) cause fatty acid uptake in excess of utilization that results in triglyceride, fatty acid, diacylglycerol and ceramide accumulation [54–56]. Fatp1-mediated increases in myocardial lipid increases fatty acid oxidation and results in lipid-induced diastolic dysfunction [55]. On the other hand, models with either overexpression of Acsl1 or LplGPI have profound systolic dysfunction. These models are robust, specific for disturbances of cardiac lipid metabolism, and have served as platforms for evaluation of underlying mechanisms and testing interventions to mitigate cardiac lipotoxicity. In the Acsl1 and LplGPI models, treatments to decrease oxidative stress, inflammation, and de novo ceramide synthesis improve heart function, suggesting these pathways are critical for the pathogenesis of cardiac lipotoxicity [57–59].
Whether excess lipid is engineered in models that promote lipid uptake or supplied in the diet, it is clear that the heart has evolved strategies to protect against the deleterious consequences of metabolic overload. The heart is metabolically flexible, with mechanisms for upregulating lipid metabolism in the face of lipid excess [22]. For example, genes involved in fatty acid uptake and β-oxidation are coordinately regulated in cardiac muscle by histone deacetylase 3 (HDAC3) and peroxisome-proliferator activated receptors (PPAR) α and δ. Loss of function of HDAC3 causes inefficient fatty acid utilization and high fat diet-induced cardiomyopathy [60]. Gain of function of PPARα, a nuclear receptor that drives expression of genes for fatty acid uptake and utilization, results in cardiac steatosis, oxidative stress, and heart failure that is particularly sensitive to long-chain triglycerides [61, 62]. This suggests that forced expression of PPARα induces lipid uptake that outpaces upregulation of enzymes of fatty β-oxidation. Conversely, loss-of-function of PPARα protects against diabetes-associated adverse myocardial remodeling. On the other hand, the down regulation of fatty acid oxidation that results from cardiomyocyte-specific loss of function of PPARδ promotes lipid accumulation and heart failure [63, 64]. These studies indicate that complex transcriptional mechanisms balance utilization of substrate uptake and metabolism in the myocardium. Intriguingly, beyond its ability to regulate expression of lipid metabolic genes, the heart expresses several genes required for lipoprotein secretion, suggesting that lipid export from the heart could serve an adaptive role in the face of lipid excess. Overexpression of apolipoprotein B in cardiomyocytes enhances triglyceride secretion from the heart, effectively ameliorating pathological lipid accumulation in disease models of inborn errors of metabolism and diabetes [65–67].
Cardiac steatosis is a well-recognized pathological feature of obesity and lipid overload states, and it may be a proverbial double-edged sword. In some models, the capacity for triglyceride accumulation is associated with less myocardial damage and improved cardiac function. For example, overexpression of diacylglycerol acyl transferase 1 (Dgat1) promotes triglyceride accumulation, decreases ceramide accumulation, and preserves cardiac function in the Acsl1 lipotoxic cardiomyopathy model [68]. Conversely, loss of function of the lipid droplet protein, perilipin 5, in the myocardium decreases myocardial triglyceride, increases fatty acid oxidation and evidence of oxidative stress, and is associated decline in systolic function with age and following ischemia-reperfusion [69, 70]. These studies suggest a model in which sequestration of excess fuel in lipid droplet stores is cytoprotective. On the other hand, overexpression of PPARγ in the myocardium increases cardiac accumulation of triglyceride, FFA, and ceramide that is associated with dilated cardiomyopathy [71]. Although cardiac specific-overexpression of adipose tissue triglyceride lipase (Atgl) protects from high fat diet induced cardiomyopathy [72], the cardiac triglyceride accumulation that results from loss of function of Atgl or its activator, Comparative gene identification-58 (CGI-58), is associated with marked myocardial steatosis and cardiomyopathy [73, 74]. The distinct outcomes of these different genetic manipulations indicate that a model of cytoprotective storage of lipid within in inert triglyceride droplets in the myocardium is overly simplified.
Cellular Models
Many insights into mechanisms of lipid-induced tissue damage have come from in vitro studies in which the culture medium of primary cells or cell lines is supplemented with concentrations of free fatty acids to model pathophysiological conditions. Because long-chain free fatty acids are relatively water-insoluble and albumin, an abundant plasma protein with multiple fatty acid binding sites, serves as an acceptor for lipoprotein lipase-liberated fatty acids [75], cell culture studies have commonly relied on supply of exogenous fatty acids complexed to albumin. Typical experimental conditions model pathophysiological concentrations of unbound free fatty acids in the sub-micromolar range [76]. By adding fatty acid-albumin complexes to normal growth medium, it is possible to avoid the potential confounder of concomitant serum starvation. Using such experimental approaches, many findings regarding the effects of exogenous fatty acids on cardiomyocytes, cardiomyoblasts, fibroblasts and endothelial cells are mirrored by observations in other non-adipose cell types that share a relatively limited capacity for storage of this excess nutrient [77]. From studies in which one or a few fatty acid species are provided, a general paradigm has emerged that saturated long chain fatty acids are particularly toxic, leading to cell death within 24 to 48 hours.
Cellular mechanisms that contribute to cardiac lipotoxicity
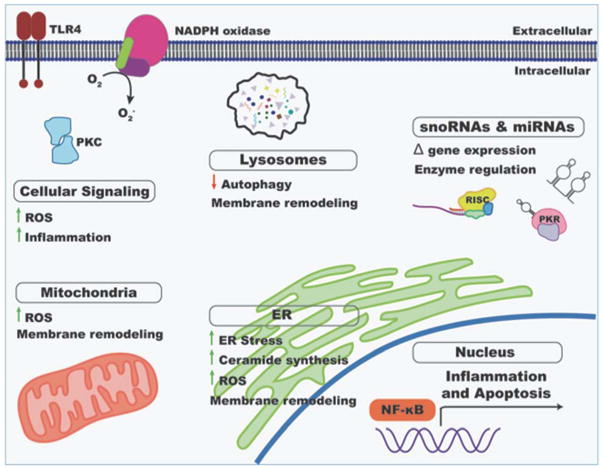
Many insights into mechanisms of lipotoxicity have emerged from the study of animal models and cultured cells. We focus here on mechanisms that have particular relevance to the lipid overloaded heart (Figure 2).
Figure 2. Mechanisms of myocardial lipotoxicity.
Excess delivery of fatty acids to the myocardial impacts many different aspects of normal cardiomyocyte function. Effects on cellular signaling, expression of protein-coding genes and non-coding RNAs, and organelle integrity and function are depicted. ROS, reactive oxygen species; ER, endoplasmic reticulum.
Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is central to cellular lipid homeostasis. It is the primary site of sterol and phospholipid synthesis and many proteins that regulate lipid metabolism localize to ER membranes. The critical role of the ER in orchestrating cellular responses to lipid overload is the focus of an excellent recent review [78]. In the ob/ob mouse model, induction of ER stress as evidenced by expression of chaperone GRP78, splicing of X-box binding protein-1 (XBP-1), and phosphorylation of eIF2a and PERK, leads to suppression of hepatic insulin signaling. Reducing ER stress by treatment with the chemical chaperone tauroursodeoxycholic acid (TUDCA) or by XBP-1 deletion ameliorates insulin resistance and restores systemic glucose control [79, 80]. TUDCA also decreases cardiac hypertrophy and improves cardiac function in this model [81]. ER stress has also been implicated in several transgenic models in which myocardial lipid overload causes heart failure [82, 83]. The observation that ER stress is diminished by PPARβ/δ signaling or sequestration of excess lipid in triglyceride stores suggests that diverting excess lipid away from the ER abrogates a key step in lipotoxicity. These in vivo findings are mirrored by evidence for fatty acid-induced ER stress in cultured cardiomyocytes and in myotubes prior to cell death [82, 84, 85] that is mitigated by treatment with TUDCA [81].
Pleiotropic mechanisms likely underlie saturated fatty acid-induced ER stress. Reactive oxygen species (ROS) generated during lipotoxicity oxidize ER proteins and lipids, aggravate ER stress signaling, and promote calcium efflux and caspase activation [78]. Moreover, saturated fatty acid-induced sphingolipid remodeling impairs protein trafficking from the ER, thus contributing to protein overload in the secretory pathway [86]. Moreover, exposure to high concentrations of saturated fatty acids promotes incorporation of excess lipid into membrane phospholipid pools, leading to membrane remodeling. In the ER, such remodeling can promote ER stress, impact the activity of transmembrane proteins, and impair membrane integrity [87, 88]. Membrane remodeling in phospholipid pools of other organelles is also likely to contribute to mitochondrial and lysosomal dysfunction [89, 90].
Autophagy
The autophagy pathway is central for adaptation to the nutritional environment and regulates many metabolic pathways including the response to lipid overload [91]. In response to some high fat diets, initiation of autophagy and autophagic flux are decreased, and this downregulation contributes to increased susceptibility to ischemia-reperfusion injury [92, 93] Consistent with this finding, studies in cardiomyocytes indicate that the saturated fatty acid palmitate decreases expression of transcription factor EB, a master regulator of lysosomal biogenesis, and impairs lysosomal function [93, 94]. On the other hand, a milk-fat based diet increases myocardial autophagy in a sphingolipid-dependent manner [43]. Understanding the complex relationships between myocardial lipid overload and regulation of autophagy will be an important area for future investigation.
Ceramide Synthesis
Sphingolipids are important membrane components and signaling molecules, particularly during cell stress and apoptosis. The saturated fatty acids palmitate and stearate are precursors for de novo ceramide biosynthesis, and this pathway is upregulated during lipid overload [95]. Given their well-appreciated role in promoting apoptosis [96, 97], ceramides have been extensively investigated as drivers of lipotoxicity. Although inhibitors of ceramide biosynthesis blunt lipotoxicity in skeletal myocytes, endothelial cells, and pancreatic β-cells [98–100], in fibroblasts and hepatoma cells, blockade of the de novo synthesis pathway does not prevent palmitate-induced cell death [101, 102]. In diet and transgenic mouse models of cardiac lipid overload, myocardial ceramide content is markedly increased [43, 54, 56]. Inhibitors of de novo ceramide synthesis decrease myocardial ceramide content and improved function and survival in the lipid-overloaded hearts of LplGPI mice [59]. Although knockdown of ceramide synthase 5 prevents lipid-induced cardiomyocyte hypertrophy in vitro [43], it has been difficult to determine whether the benefits of systemic administration of ceramide synthesis inhibitors relates to inhibition of ceramide synthesis specifically in cardiomyocytes and/or other relevant cell types in the heart. Conditional knockout in cardiomyocytes of the rate limiting enzyme serine in de novo ceramide synthesis, palmitoyltransferase (subunit 2), causes cardiomyopathy by seven months of age, complicating assessment of effects on lipid-induced cardiomyopathy [103].
Oxidative Stress
Lipid overload is associated with ROS generation in multiple cellular compartments. In many cell types exposure to excess fatty acids stimulates β-oxidation and ROS production through the mitochondrial respiratory chain [104]. In mice with cardiac overexpression of Acsl1, mitochondrial ROS induces alterations in mitochondrial membrane dynamics [105]. Lipotoxic conditions also activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to generate cytosolic superoxide, through activation of protein kinase C signaling pathways in endothelial cells and through direct activation of the enzyme by lipid metabolites including phosphatidic acid and diacylglycerol [99, 106, 107]. Lipid-induced ER protein misfolding also promotes ER and nuclear oxidative stress [108].
In both cell culture models and in vivo, lipotoxic conditions generate lipid peroxidation products including 4-hydroxy nonenal (4-HNE), 4-hydroxy hexenal, and oxidized cardiolipin that damage cellular membranes and impair organelle function (recently reviewed in [109]). While highly reactive lipid aldehydes can impact proteins and membranes of all cellular organelles, oxidative modification of cardiolipin has profound consequences on function of mitochondria where this lipid is concentrated. Lipotoxicity induces damage to mitochondrial membranes with release of cytochrome C, and mitochondrial DNA damage appears to be a critical mechanism of lipotoxicity in cultured muscle cells, as reduction of mitochondrial DNA damage or inhibition of c-Jun N-terminal kinase prevent lipid-induced cell death [110]. In vivo, high fat/high sucrose diet induced cardiac hypertrophy and fibrosis is accompanied by evidence for increased 4-HNE adducts and protein carbonyls, changes that are further exacerbated by haploinsufficiency of glutathione peroxidase 4 [111]. The hallmarks of oxidative stress also accompany lipid overload in db/db hearts, with evidence of increased mitochondrial ROS production [47]. Furthermore, in the Atgl knockout and perilipin 5 knockout animals, profound lipid overload is accompanied by evidence for oxidative stress [69, 112]. While there is a growing appreciation of the pathways through which ROS may be generated in lipotoxic states, few studies have provided evidence that targeting ROS in vivo ameliorates heart function [113].
Inflammation and signaling pathways
Similar to observations in other tissues, lipid overload stimulates low grade inflammation, also known as metainflammation, in the heart [25]. In the Acsl1 model of lipotoxic cardiomyopathy, a myocardial mononuclear infiltrate precedes cardiac dysfunction, and systemic depletion of macrophages improves fractional shortening [58]. In vitro exposure of endothelial cells, myocytes and macrophages to pathophysiological concentrations of saturated fatty acids induces Toll-like receptor 4 signaling, secretion of pro-inflammatory cytokines, expression of cellular adhesion molecules, and down-stream activation of NFkB signaling pathways [89, 114, 115]. The contributions of lipid-induced inflammatory signaling that initiates from within the myocardium and lipid-induced inflammatory infiltrates suggest that approaches targeting metainflammation may be beneficial.
Many other signaling pathways are activated by lipid overload in non-adipose tissues and cells. For example, protein kinase B is upregulated in isolated hearts perfused with palmitate [116]. In vitro, palmitate induces apoptosis through death receptor signaling in hepatocytes [117]. Understanding the contributions of these and other signaling pathways to lipotoxicity in the heart could inform new approaches for improving cardiac function in obesity.
Gene expression and non-coding RNAs
Lipid overload induces broad changes in expression of protein-coding genes. Not surprisingly, exposure of adult cardiomyocytes to lipotoxic levels of saturated fatty acids induces a time-dependent increase in abundance of mRNAs involved in ER and oxidative stress and apoptosis [118]. Simultaneous induction of genes involved in mitochondrial and peroxisomal fatty acid oxidation and phospholipid and triglyceride synthesis may reflect induction of pathways for lipid disposal, although increased mitochondrial metabolism is likely to also increase ROS generation. Transcription factors may serve as important regulatory nodes to direct these changes in gene expression. For example, activation of the transcription factor FoxO1 plays a critical role in metabolic stress-induced cardiomyopathy in high fat-fed mice [44]. The observation that haploinsufficiency of RNASET2 protects against lipotoxicity indicates that gene expression during metabolic stress may also be regulated at the level of RNA degradation [119]. Emerging evidence for palmitate regulation of translation in other cell types suggests that changes in gene expression in the lipid overloaded heart are likely to occur at many levels in the heart [120].
Noncoding RNAs also regulate metabolic homeostasis during lipotoxicity. MicroRNAs that are repressed or induced by lipid overload in cell culture have been shown to regulate the progress of lipid-induced cell death through interaction with specific RNA targets [121, 122]. Interestingly, miR-451 is induced in cardiomyocytes treated with palmitate and in the hearts of mice with diabetes. Cardiac-specific deletion of miR-451 increases AMPK signaling and prevents high fat diet-induced cardiac hypertrophy. Several long non-coding RNAs (lncRNAs), including Malat1 and Gadd7, are also upregulated during lipotoxicity, and Gadd7 is essential for lipotoxicity-induced oxidative stress and cell death in cultured fibroblasts [123, 124]. Small nucleolar RNAs (snoRNAs) are another class of non-coding RNAs that have been implicated in regulation of lipotoxicity. These short (~80 nucleotide) RNAs form ribonucleoproteins that canonically function within the nucleolus to direct covalent modifications of nascent ribosomal RNAs. A genetic screen for palmitate resistance in fibroblasts revealed that snoRNAs U32a, U33, U34, and U35a encoded within the introns of the Rpl13a locus are critical for palmitate-induced cell death and progression of lipid-induced ROS in the liver [125]. Because these snoRNAs accumulate outside the nucleus during metabolic stress, it is possible that they target additional classes of cellular RNAs for covalent modifications during metabolic stress. Interestingly, snoRNAs bind to and activate the RNA-dependent protein kinase (PKR) [126]. In obesity and under lipotoxic conditions, activated PKR regulates translational responses and insulin receptor signaling, whereas knockout of PKR prevents insulin resistance in obesity [127, 128]. Whether specific snoRNAs, including those from the Rpl13a locus, function in these physiological responses will be an interesting area for future studies.
Conclusions and implications for diagnosis and treatment
As the prevalence of obesity increases worldwide, so too do complications that affect longevity, quality of life, and medical costs. The untoward consequences of obesity on myocardial structure and function likely contribute to the observed increased prevalence and incidence of heart failure in obese individuals. Multiple mechanisms have been implicated in the pathogenesis of heart failure in individuals who are obese. These include mechanisms external to the heart (e.g., increased hypertension and vascular dysfunction) as well as mechanisms intrinsic to the myocardium, including lipotoxicity. Insights gained into the biology of lipid overload in the heart from the studies of mouse models and cultured cells have the potential to provide new approaches to manage cardiometabolic disease and improve cardiac function in obesity.
Acknowledgments
Work in the authors’ laboratories is supported by grants from the NIH (P30 HL113444 to JES, R34 HL138253 to LRP), Fondation Leducq (12CVD04 to JES), and the Barnes Jewish Hospital Foundation (to LRP).
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.Loehr LR, Rosamond WD, Poole C, et al. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 5.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 6.McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 1995;15:431–40. doi: 10.1161/01.atv.15.4.431. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DS, Dietz WH, Tang R, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28:159–66. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- 8.Fernberg U, Fernstrom M, Hurtig-Wennlof A. Arterial stiffness is associated to cardiorespiratory fitness and body mass index in young Swedish adults: The Lifestyle, Biomarkers, and Atherosclerosis study. Eur J Prev Cardiol. 2017 doi: 10.1177/2047487317720796. 2047487317720796. [DOI] [PubMed] [Google Scholar]
- 9.Rabkin SW, Mathewson FA, Hsu PH. Relation of body weight to development of ischemic heart disease in a cohort of young North American men after a 26 year observation period: the Manitoba Study. Am J Cardiol. 1977;39:452–8. doi: 10.1016/s0002-9149(77)80104-5. [DOI] [PubMed] [Google Scholar]
- 10.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–77. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 11.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. Journal of the American College of Cardiology. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Alpert MA, Lambert CR, Panayiotou H, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. The American Journal of Cardiology. 1995;76:1194–7. doi: 10.1016/s0002-9149(99)80338-5. [DOI] [PubMed] [Google Scholar]
- 13.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Translational research : the journal of laboratory and clinical medicine. 2014;164:345–56. doi: 10.1016/j.trsl.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 14.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64:477. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 15.Kosmala W, Sanders P, Marwick TH. Subclinical Myocardial Impairment in Metabolic Diseases. JACC Cardiovascular imaging. 2017;10:692–703. doi: 10.1016/j.jcmg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Lavie CJ, Amodeo C, Ventura HO, Messerli FH. Left atrial abnormalities indicating diastolic ventricular dysfunction in cardiopathy of obesity. Chest. 1987;92:1042–6. doi: 10.1378/chest.92.6.1042. [DOI] [PubMed] [Google Scholar]
- 17.Chakko S, Mayor M, Allison MD, Kessler KM, Materson BJ, Myerburg RJ. Abnormal left ventricular diastolic filling in eccentric left ventricular hypertrophy of obesity. The American Journal of Cardiology. 1991;68:95–8. doi: 10.1016/0002-9149(91)90718-z. [DOI] [PubMed] [Google Scholar]
- 18.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 19.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 20.Wang YC, Liang CS, Gopal DM, et al. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CY, O’Moore-Sullivan T, Leano R, Hukins C, Jenkins C, Marwick TH. Association of subclinical right ventricular dysfunction with obesity. Journal of the American College of Cardiology. 2006;47:611–6. doi: 10.1016/j.jacc.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–13. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 23.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet (London, England) 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 24.Alrob OA, Sankaralingam S, Ma C, et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovascular Research. 2014;103:485–97. doi: 10.1093/cvr/cvu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ertunc ME, Hotamisligil GS. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res. 2016;57:2099–114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schram K, Sweeney G. Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends in cardiovascular medicine. 2008;18:199–205. doi: 10.1016/j.tcm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovascular research. 2008;79:279–86. doi: 10.1093/cvr/cvn115. [DOI] [PubMed] [Google Scholar]
- 28.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. Journal of the American College of Cardiology. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 29.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 30.Arcaro G, Zamboni M, Rossi L, et al. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–42. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. Journal of Clinical Investigation. 1996;97:2601–10. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A, Gao L, Thakur A, Siu PM, Lai CWK. Role of free fatty acids in endothelial dysfunction. Journal of Biomedical Science. 2017;24:50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang WY, Schwartz E, Wang Y, Attrep J, Li Z, Reaven P. Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:514–9. doi: 10.1161/01.ATV.0000200226.53994.09. [DOI] [PubMed] [Google Scholar]
- 34.Williams MJ, Sutherland WH, McCormick MP, de Jong SA, Walker RJ, Wilkins GT. Impaired endothelial function following a meal rich in used cooking fat. Journal of the American College of Cardiology. 1999;33:1050–5. doi: 10.1016/s0735-1097(98)00681-0. [DOI] [PubMed] [Google Scholar]
- 35.Campbell DJ, Somaratne JB, Prior DL, et al. Obesity is associated with lower coronary microvascular density. PLoS One. 2013;8:e81798. doi: 10.1371/journal.pone.0081798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGavock JM, Lingvay I, Zib I, et al. Cardiac Steatosis in Diabetes Mellitus. Circulation. 2007;116:1170. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 37.Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magnetic Resonance in Medicine. 2003;49:417–23. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S, Adrogue JV, Golfman L, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 39.Mahabadi AA, Massaro JM, Rosito GA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JJ, Yin X, Hoffmann U, Fox CS, Benjamin EJ. Relation of Pericardial Fat, Intrathoracic Fat, and Abdominal Visceral Fat With Incident Atrial Fibrillation (from the Framingham Heart Study) Am J Cardiol. 2016;118:1486–92. doi: 10.1016/j.amjcard.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–40. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 42.Fisher-Wellman KH, Ryan TE, Smith CD, et al. A Direct Comparison of Metabolic Responses to High-Fat Diet in C57BL/6J and C57BL/6NJ Mice. Diabetes. 2016;65:3249–61. doi: 10.2337/db16-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–30. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battiprolu PK, Hojayev B, Jiang N, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–18. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 46.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 47.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 48.Mazumder PK, O’Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 49.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–90. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 50.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–13. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 51.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–82. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 52.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–41. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 53.Peterson LR, Saeed IM, McGill JB, et al. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 2012;20:802–10. doi: 10.1038/oby.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–33. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 56.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–26. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem Biophys Res Commun. 2006;344:446–52. doi: 10.1016/j.bbrc.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 58.Schilling JD, Machkovech HM, Kim AH, Schwendener R, Schaffer JE. Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2012;303:H1366–73. doi: 10.1152/ajpheart.00111.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park TS, Hu Y, Noh HL, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–12. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Z, Singh N, Mullican SE, et al. Diet-induced lethality due to deletion of the Hdac3 gene in heart and skeletal muscle. J Biol Chem. 2011;286:33301–9. doi: 10.1074/jbc.M111.277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng L, Ding G, Qin Q, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 64.Wang P, Liu J, Li Y, et al. Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circ Res. 2010;106:911–9. doi: 10.1161/CIRCRESAHA.109.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bjorkegren J, Veniant M, Kim SK, et al. Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem. 2001;276:38511–7. doi: 10.1074/jbc.M106839200. [DOI] [PubMed] [Google Scholar]
- 66.Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem. 2002;277:27014–20. doi: 10.1074/jbc.M203458200. [DOI] [PubMed] [Google Scholar]
- 67.Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–11. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–23. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuramoto K, Okamura T, Yamaguchi T, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–63. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng P, Xie Z, Yuan Y, et al. Plin5 alleviates myocardial ischaemia/reperfusion injury by reducing oxidative stress through inhibiting the lipolysis of lipid droplets. Sci Rep. 2017;7:42574. doi: 10.1038/srep42574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Son NH, Park TS, Yamashita H, et al. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulinilkunnil T, Kienesberger PC, Nagendran J, Sharma N, Young ME, Dyck JR. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int J Obes (Lond) 2014;38:205–15. doi: 10.1038/ijo.2013.103. [DOI] [PubMed] [Google Scholar]
- 73.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 74.Zierler KA, Jaeger D, Pollak NM, et al. Functional cardiac lipolysis in mice critically depends on comparative gene identification-58. J Biol Chem. 2013;288:9892–904. doi: 10.1074/jbc.M112.420620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16:165–79. [PubMed] [Google Scholar]
- 76.Kleinfeld AM, Prothro D, Brown DL, Davis RC, Richieri GV, DeMaria A. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78:1350–4. doi: 10.1016/s0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 77.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han J, Kaufman RJ. The role of ER stress in lipid metabolism and lipotoxicity. J Lipid Res. 2016;57:1329–38. doi: 10.1194/jlr.R067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 80.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceylan-Isik AF, Sreejayan N, Ren J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol. 2011;50:107–16. doi: 10.1016/j.yjmcc.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Borradaile NM, Buhman KK, Listenberger LL, Magee CJ, Morimoto ET, Ory DS, Schaffer JE. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol Biol Cell. 2006;17:770–8. doi: 10.1091/mbc.E05-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Son NH, Yu S, Tuinei J, et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–54. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peter A, Weigert C, Staiger H, et al. Individual stearoyl-coa desaturase 1 expression modulates endoplasmic reticulum stress and inflammation in human myotubes and is associated with skeletal muscle lipid storage and insulin sensitivity in vivo. Diabetes. 2009;58:1757–65. doi: 10.2337/db09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bosma M, Dapito DH, Drosatos-Tampakaki Z, et al. Sequestration of fatty acids in triglycerides prevents endoplasmic reticulum stress in an in vitro model of cardiomyocyte lipotoxicity. Biochim Biophys Acta. 2014;1841:1648–55. doi: 10.1016/j.bbalip.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boslem E, Weir JM, MacIntosh G, Sue N, Cantley J, Meikle PJ, Biden TJ. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic beta-cells. J Biol Chem. 2013;288:26569–82. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proceedings of the National Academy of Sciences. 2013;110:4628–33. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 89.Schilling JD, Machkovech HM, He L, Diwan A, Schaffer JE. TLR4 activation under lipotoxic conditions leads to synergistic macrophage cell death through a TRIF-dependent pathway. J Immunol. 2013;190:1285–96. doi: 10.4049/jimmunol.1202208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elezaby A, Sverdlov AL, Tu VH, et al. Mitochondrial remodeling in mice with cardiomyocyte-specific lipid overload. J Mol Cell Cardiol. 2015;79:275–83. doi: 10.1016/j.yjmcc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaishy B, Abel ED. Lipids, lysosomes, and autophagy. J Lipid Res. 2016;57:1619–35. doi: 10.1194/jlr.R067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sciarretta S, Zhai P, Shao D, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 2012;125:1134–46. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaishy B, Zhang Q, Chung HS, et al. Lipid-induced NOX2 activation inhibits autophagic flux by impairing lysosomal enzyme activity. J Lipid Res. 2015;56:546–61. doi: 10.1194/jlr.M055152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trivedi PC, Bartlett JJ, Perez LJ, et al. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim Biophys Acta. 2016;1861:1893–910. doi: 10.1016/j.bbalip.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 95.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dbaibo GS, Pushkareva MY, Rachid RA, Alter N, Smyth MJ, Obeid LM, Hannun YA. p53-dependent ceramide response to genotoxic stress. J Clin Invest. 1998;102:329–39. doi: 10.1172/JCI1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dbaibo GS, El-Assaad W, Krikorian A, et al. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS letters. 2001;503:7–12. doi: 10.1016/s0014-5793(01)02625-4. [DOI] [PubMed] [Google Scholar]
- 98.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 99.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–45. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 100.Shimabukuro M, Higa M, Zhou Y-T, Wang M-Y, Newgard CB, Unger RH. Lipoapoptosis in Beta-cells of Obese Prediabeticfa/fa Rats: ROLE OF SERINE PALMITOYLTRANSFERASE OVEREXPRESSION. Journal of Biological Chemistry. 1998;273:32487–90. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 101.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 102.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–5. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 103.Lee SY, Kim JR, Hu Y, et al. Cardiomyocyte specific deficiency of serine palmitoyltransferase subunit 2 reduces ceramide but leads to cardiac dysfunction. J Biol Chem. 2012;287:18429–39. doi: 10.1074/jbc.M111.296947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura S, Takamura T, Matsuzawa-Nagata N, et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem. 2009;284:14809–18. doi: 10.1074/jbc.M901488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsushima K, Bugger H, Wende AR, et al. Mitochondrial Reactive Oxygen Species in Lipotoxic Hearts Induces Post-Translational Modifications of AKAP121, DRP1 and OPA1 That Promote Mitochondrial Fission. Circ Res. 2017 doi: 10.1161/CIRCRESAHA.117.311307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 107.Palicz A, Foubert TR, Jesaitis AJ, Marodi L, McPhail LC. Phosphatidic acid and diacylglycerol directly activate NADPH oxidase by interacting with enzyme components. J Biol Chem. 2001;276:3090–7. doi: 10.1074/jbc.M007759200. [DOI] [PubMed] [Google Scholar]
- 108.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants & redox signaling. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 109.Hauck AK, Bernlohr DA. Oxidative stress and lipotoxicity. J Lipid Res. 2016;57:1976–86. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yuzefovych LV, Solodushko VA, Wilson GL, Rachek LI. Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology. 2012;153:92–100. doi: 10.1210/en.2011-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katunga LA, Gudimella P, Efird JT, et al. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Molecular metabolism. 2015;4:493–506. doi: 10.1016/j.molmet.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–85. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 114.Pillon NJ, Arane K, Bilan PJ, Chiu TT, Klip A. Muscle cells challenged with saturated fatty acids mount an autonomous inflammatory response that activates macrophages. Cell Commun Signal. 2012;10:30. doi: 10.1186/1478-811X-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pillon NJ, Azizi PM, Li YE, et al. Palmitate-induced inflammatory pathways in human adipose microvascular endothelial cells promote monocyte adhesion and impair insulin transcytosis. Am J Physiol Endocrinol Metab. 2015;309:E35–44. doi: 10.1152/ajpendo.00611.2014. [DOI] [PubMed] [Google Scholar]
- 116.Soltys CL, Buchholz L, Gandhi M, Clanachan AS, Walsh K, Dyck JR. Phosphorylation of cardiac protein kinase B is regulated by palmitate. Am J Physiol Heart Circ Physiol. 2002;283:H1056–64. doi: 10.1152/ajpheart.00275.2002. [DOI] [PubMed] [Google Scholar]
- 117.Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–70. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lockridge JB, Sailors ML, Durgan DJ, et al. Bioinformatic profiling of the transcriptional response of adult rat cardiomyocytes to distinct fatty acids. J Lipid Res. 2008;49:1395–408. doi: 10.1194/jlr.M700517-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caputa G, Zhao S, Criado AE, Ory DS, Duncan JG, Schaffer JE. RNASET2 is required for ROS propagation during oxidative stress-mediated cell death. Cell Death Differ. 2016;23:347–57. doi: 10.1038/cdd.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hatanaka M, Maier B, Sims EK, Templin AT, Kulkarni RN, Evans-Molina C, Mirmira RG. Palmitate induces mRNA translation and increases ER protein load in islet beta-cells via activation of the mammalian target of rapamycin pathway. Diabetes. 2014;63:3404–15. doi: 10.2337/db14-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, Gores GJ. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. Journal of Lipid Research. 2011;52:1517–25. doi: 10.1194/jlr.M014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuwabara Y, Horie T, Baba O, et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res. 2015;116:279–88. doi: 10.1161/CIRCRESAHA.116.304707. [DOI] [PubMed] [Google Scholar]
- 123.Yan C, Chen J, Chen N. Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. 2016;6:22640. doi: 10.1038/srep22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem. 2009;284:7446–54. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Michel CI, Holley CL, Scruggs BS, et al. Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 2011;14:33–44. doi: 10.1016/j.cmet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Youssef OA, Safran SA, Nakamura T, Nix DA, Hotamisligil GS, Bass BL. Potential role for snoRNAs in PKR activation during metabolic stress. Proc Natl Acad Sci U S A. 2015;112:5023–8. doi: 10.1073/pnas.1424044112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakamura T, Arduini A, Baccaro B, Furuhashi M, Hotamisligil GS. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 2014;63:526–34. doi: 10.2337/db13-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamura T, Furuhashi M, Li P, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–48. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]