Abstract
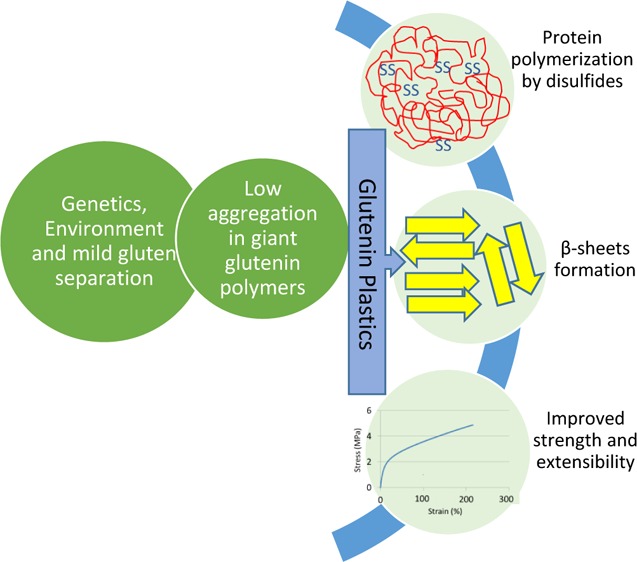
A combination of genotype, cultivation environment, and protein separation procedure was used to modify the nanoscale morphology, polymerization, and chemical structure of glutenin proteins from wheat. A low-polymerized glutenin starting material was the key to protein–protein interactions mainly via SS cross-links during film formation, resulting in extended β-sheet structures and propensity toward the formation of nanoscale morphologies at molecular level. The properties of glutenin bioplastic films were enhanced by the selection of a genotype with a high number of cysteine residues in its chemical structure and cultivation environment with a short grain maturation period, both contributing positively to gluten strength. Thus, a combination of factors affected the structure of glutenins in bioplastic films by forming crystalline β-sheets and propensity toward the ordered nanostructures, thereby resulting in functional properties with high strength, stiffness, and extensibility.
Introduction
Fossil fuel-based polymers are known for their high carbon footprint affecting the environment negatively. Thus, renewable alternatives to replace fossil fuel-based polymers have received increased attention in recent years.1,2 Proteins are biological macromolecules with intriguing chemical properties of relevance for bioplastics production.3 For example, glutenin proteins from wheat possess unique polymeric properties and have therefore been considered as a potential alternative to replace fossil fuel-based polymers in some applications.4
Glutenin proteins consist of high-molecular-weight glutenin subunits (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS).5 These GS are known to polymerize, e.g., in bread-making processes through disulfide cross-links, and thereby form one of the largest polymers in nature.6 These giant macromolecular polymer networks contribute to the strength and elasticity of the wheat dough during the bread-baking process.7 Polymerization and macromolecular rearrangements into nanostructures have been suggested as important processes for the successful production of high-quality protein-based plastic materials and foams.8,9 However, as for the glutenin-derived bioplastics, such structural rearrangements have so far been a challenge due to the high aggregation of the polymeric protein network. The glutenins have appeared as an unbreakable aggregated mass of polymers, and a unique combination of strength and elasticity associated with the structures as for bread making has so far not been achieved.10 Materials from glutenins have been reported as fragile with limited elasticity compared to other wheat protein materials.10
Bioplastic applications for films, foams, etc., using other protein types, such as gliadins, whey, and soy protein, have clearly shown a correlation between protein molecular reorganization, including chemical cross-linking, and functional properties.11−13 Previous studies have pointed out key parameters, such as selection of genetic materials (G) and cultivation environments (E), for a successful molecular organization and polymerization of plant proteins.14,15 Furthermore, protein separation methods have been found to significantly influence the aggregation level of proteins as well as their ability to depolymerize before processing.16,17
Previous successful work on the use of plants as a “green factory” for developing high-strength gluten films led us to further focus on the selection of protein source and treatments to resolve the understanding of protein material properties and their relationship to protein aggregation. Therefore, the aim of this study was to combine different factors, such as genetics, environment, and protein separation procedures, and to evaluate whether and how these factors could be used to reshape and steer the secondary structure and nanoscale morphologies in glutenin proteins. We hypothesized that suitable modifications of the structural morphologies of glutenin proteins would unravel new functional potentials of the glutenins. Therefore, two genotypes differing in protein composition (HMW-GS 5 + 10 and 2 + 12) were cultivated under two different growing conditions (E treatments; Table 1). The selection of the two growing conditions was based on previous studies, where high nitrogen and low temperature resulted in weak gluten and low (early) nitrogen and high temperature (during grain filling) resulted in strong gluten.15 The weaker gluten in the former was due to high gliadin-to-glutenin ratio.4,15 HMW-GS 5 + 10 has 12 cysteine residues, hence resulting in more possible disulfide bonds in its chemical structure, leading to higher strength compared to HMW-GS 2 + 12, which possesses 11 cysteines.18,19 Two gluten separation methods, i.e., harsh (H-gluten), used in industrial scale for commercially available gluten, and mild (M-gluten),16 were used to obtain glutenins with different aggregation levels.
Table 1. Nitrogen Application and Corresponding Cultivation Temperatures for Wheat Genotypes (2 + 12 and 5 + 10)a.
| nitrogen
applied (mg/plant) |
|||||
|---|---|---|---|---|---|
| E treatment | spike 44–48 | anthesis 65–69 | temperature (°C) | development time | gluten strength |
| A | 20 | 20 | 18 | long | weak |
| B | 20 | 0 | 25 | short | strong |
E; environment.
By the use of the selected parameters, we expected to process and tune glutenin proteins to form suitable secondary and nanoscale morphologies with attractive mechanical properties for bioplastic applications.
Materials and Methods
Wheat cultivars, i.e., Diskette (5 + 10) and Puntari (2 + 12), used in this study were obtained from Lantmännen, Sweden. Besides the differences between the two HMW GS, the cultivars differ in other HMW GS as well (Table S1). These subunits are expected to play a minor role in the structural and functional properties of glutenins in bioplastic films as the differences in gluten strength due to these subunits are small and they are scored of similar strength in previous studies.18
Wheat plants were grown in trays, with nine plants in each tray in biotron facility at the Swedish University of Agricultural Sciences, Alnarp, Sweden. The soil used was Krukväxtjord, teca (Weibull Trädgård AB, Landskrona, Sweden), with the amount of N being 980 gm–3. The day/night temperature used for plants was 18/14 °C until the initiation of spike and also a dose of nitrogen (20 mg/plant) were provided to all plants. After the spike initiation, the plants were divided into two groups (A and B). Group A plants continued growing at 18/14 °C and were also supplied with nitrogen to prolong the grain maturation period. Group B plants were shifted to a chamber set at 25/19 °C and were not supplied with nitrogen to shorten the grain maturation period. The plants were kept growing until fully matured spikes were ready for harvesting. Details of the nitrogen application pattern and amount as well as cultivation temperatures are given in Table 1 and grouped as two E treatments (A, B). The aim of these treatments was to develop plants with variation in grain maturation period20 (also described in Table 1). The nitrogen source used was Yara Mila (NPK: 24-4-5) manufactured by Yara AB, Landskrona, Sweden. Harvesting was done at full maturation of spikes and threshed using a laboratory thresher. The seeds were milled to white flour in the analysis laboratory, Lantmännen, Sweden, by a laboratory mill (Brabender Quadrumat Senior).
Glutenin Modification and Separation in a Chemical/Shear-Free Green Process
The structure and strength of glutenin protein were modified in wheat plants by the use of two genotypes, each under two sets of environmental interactions (Table 1). The separation of glutenins from the obtained wheat flour was done in two sequential steps. First, gluten was separated from the flour in a milder way, by washing the flour in a muslin cloth under a stream of water until a rubbery mass of gluten was obtained, which was freeze-dried (Cool Safe, Scanvae, Denmark) and milled to fine powder (IKA A10, IKA-Werke) afterward. The gluten powder (32 g) was mixed with 70% ethanol (400 mL), stirred for 30 min, and then kept under shaking at 300 rpm for 2 h at room temperature (22 °C). The mixture was centrifuged and glutenin was collected as rubbery pellet, chopped, lyophilized, and milled to fine powder.
Glutenin Separation from Harshly Treated Industrial Gluten
Industrial gluten separation (harsh) starts with the wet milling of grains into flour. Thereafter, an intense mixing, with high energy inputs (∼8 kJ/(kg min)) and shear forces, of water with the flour is carried out to create a lump-free dough. For this purpose, high-pressure homogenizers are used and the dough is processed at 80–100 bar pressure. Furthermore, the centrifugal separation of components (starch and gluten) and drying of gluten by a ring dryer at very high inlet (∼140 °C) and outlet (∼65 °C) temperatures are carried out (personal communication with the industry). The gluten powder that was prepared industrially by harsh separation procedure was obtained from Lantmännen Reppe AB, Lidköping Sweden. A sample of 32 g of this gluten powder was mixed with 70% ethanol (400 mL) and then agitated at 300 rpm for 2 h at 22 °C. The mixture was centrifuged and glutenins were collected as a rubbery pellet. The pellet was chopped into small pieces, lyophilized, and milled to a fine powder.
Processing of Glutenin into Bioplastic Films
Compression Molding
Glutenin films were produced by compression molding glutenin–glycerol mixtures. Each of the glutenin powder (7 g) was mixed with 3 g of glycerol (99.5%) in a laboratory grinder (IKA A10, IKA-Werke) using four pulses of 10 s each for thorough mixing. The glutenin–glycerol blend was placed in the center of an aluminum frame (100 mm × 100 mm × 0.5 mm) between two preheated aluminum plates and pressed for 10 min at 130 °C in a hydraulic press (Schwabenthan Polystat 400 s). Glycerol was used as a plasticizer as the films without glycerol are too brittle.
Fourier Transform Infrared (FT-IR) Spectroscopy
Infrared spectra were recorded for predried (72 h drying in desiccator) glutenin powder and glutenin films using a PerkinElmer Spectrum 2000 FT-IR spectrometer (PerkinElmer) equipped with a single reflection ATR accessory, Golden Gate from Specac (Kent, England). The spectra were recorded from 4000 to 600 cm–1 and averaged over 32 scans. The data were normalized to the total amide 1 band intensity from 1700 to 1600 cm–1. The FT-IR absorbance spectra were first Fourier self-deconvoluted using the spectrum software with γ = 2 and a smoothing factor of 70%. Peak fitting was carried out by the peak position assignments to different structures described by Cho et al.,21 and seven to nine Gaussian peaks were fitted between 1600 and 1700 cm–1 using the software Fityk v0.9.8.
Small- and Wide-Angle X-ray Scattering (WAXS)
Small-angle X-ray scattering (SAXS) measurements of the glutenin films were performed at the beamline I911-4 of the MAX IV Laboratory, Lund, Sweden, with a monochromatic beam of λ = 0.91 Å and a sample-to-detector distance of 1325 mm. The scattering vector q range was 0.01–0.7 Å–1 (where q = (4π/λ) sin(θ) and 2θ is the scattering angle). The sample exposure time was 2 min. A two-dimensional hybrid pixel array detector (Pilatus 1M, Dectris) was used to register the data. The collected data were reduced with the software bli911-4 and normalized by the integral incident intensity and sample transmission. Background was subtracted using an empty cell signal. Wide-angle X-ray scattering analysis was carried out at the MAX IV Laboratory, Lund, Sweden, using the beamline I911-2 with a wavelength of 1.0384 Å and a sample-to-detector distance of 150 mm with a MarResearch 165 detector. The sample exposure time was 1 min. The data obtained were analyzed by the software FIT2D. Silicon powder was used as a standard for calibration.
Size Exclusion High-Performance Liquid Chromatography (SE-HPLC)
To measure the soluble amount of proteins and their size distribution, a three-step extraction procedure with sodium dodecyl sulfate (SDS)–phosphate buffer (pH 6.9) and sonication was used. Glutenin films were chopped into small pieces by a scalpel, and three replicates containing 16.5 mg of each sample were prepared. Extraction buffer (1.4 mL) was added to each sample with shaking at 2000 rpm for 5 min, followed by centrifugation (30 min, 12 000g, room temperature). The supernatant was collected into HPLC vials, while the pellet was subjected to two sequential extractions with sonication: first with 30 s sonication and centrifugation and second with multiple sonication steps (30 + 60 + 60 s). Then, the supernatants were collected by centrifugation for each extraction.
To measure the soluble amounts and size distribution of proteins, each extraction was run on an HPLC system (Waters, Milford) by an isocratic flow of 50% acetonitrile and 50% water (both containing 0.1% trifluoroacetic acid (TFA)) and the proteins were eluted at a flow rate of 0.2 mL/min. UV absorption at 210 nm was used to detect the proteins. The chromatograms were divided into low molecular weight (0–13.5 elution time) and high molecular weight (13.5–25.0 elution time) for both the unprocessed WG (raw powder) and bio-based films, according to Blomfeldt et al.11
Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
Protein solubility of unprocessed glutenin and the corresponding films was measured by RP-HPLC in various nonreducing and reducing solvents. The films were chopped into small pieces using a scalpel, and three replicates, each containing 100 mg of every sample, were prepared to carry out the analysis. Six sequential extraction steps were adopted from Kuktaite et al.22 and modified as described by Rasheed et al.8 Each extraction step was followed by centrifugation at 12 000g to collect the supernatant for analysis by the RP-HPLC system (Waters, Milford) equipped with a precolumn (5 μm, 2 cm × 4.0 mm, Discovery BIO Wide) and a main column (5 μm, 25 cm × 4.6 mm, Discovery BIO Wide, Supelco). A 50 μL sample injection volume was used for 40 min protein separation, followed by 15 min cleaning by mobile phase (50% acetonitrile–0.1% TFA and 50% H2O–0.1% TFA). Gradient flow (28–72%) was used to elute the proteins at 0.8 mL/min, and chromatograms were collected at 210 nm and integrated for 6.5–35 min for each extraction.
Tensile Testing
The films were conditioned for 48 h at 23 °C and 50% relative humidity (RH). After conditioning, nine dumbbell-shaped samples were cut from each film (sample cutter, ISO 37, type 3). The thickness of the test area was calculated as an average of five random measurements (Mitutoyo IDC 112B). The samples were tensile-tested at 23 °C and 50% RH on an Instron 5581 universal test machine (Instron Corp. Ltd., MN) at a crosshead speed of 100 mm/min using a load cell of 50 N. Stress was calculated from the applied force divided by the cross-sectional area of the narrow section, and strain was calculated by crosshead displacement divided by the narrow section length (16 mm). Young’s modulus was calculated by the initial slope of the stress–strain curve after toe compensation (ASTM D638-08).
Statistics
Statistical analysis software (version 9.2) was used to calculate the mean and standard deviation values.
Results and Discussion
Impact of Modification Parameters on Glutenin Secondary Structures
A remarkable difference was observed for the intermolecular β-sheet content of the glutenin films depending on the G × E treatment and the separation method. A sharp β-sheet peak (1618–1625 cm–1)23 was observed for all glutenin films based on M-gluten, and clearly, the most intense peak was recorded from the 5 + 10-B films, whereas the glutenin films from H-gluten showed a slightly developed shoulder for β-sheets (Figure 1). The peak size data (Table 2) indicated a variation in the relative amount of strong intermolecular β-sheet contents of different films. The lowest amount of intermolecular β-sheets was found in glutenin films from H-gluten (26%), and the highest amount was found in 5 + 10-B films (53%). In general, our results showed a high relative amount of β-sheets for all glutenin films from mildly separated gluten (M-gluten), whereas glutenins from H-gluten showed a relatively high proportion of α-helices and random coil structures (Table 2). Thus, the protein separation procedure turned out to be the most important parameter to affect the ability of glutenin proteins to form dominant β-sheet structures.
Figure 1.
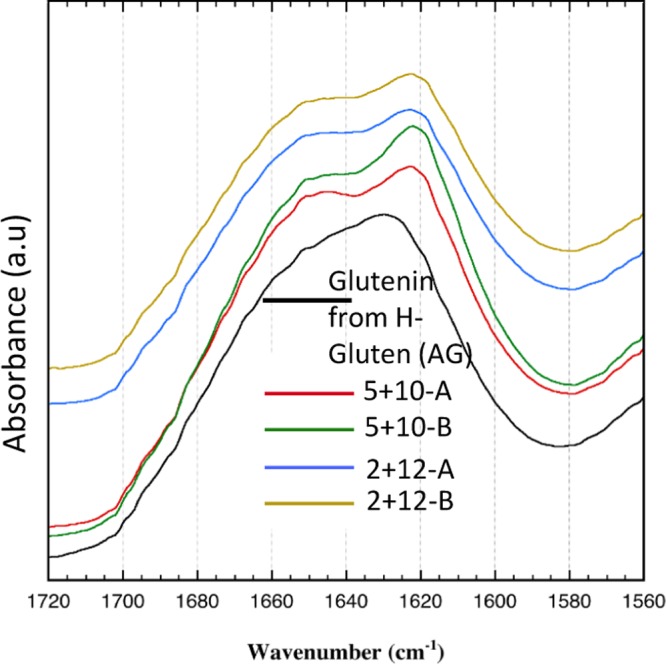
FT-IR curves for glutenin films from H-gluten and 2 + 12 and 5 + 10 glutenins (H-gluten-derived films are later designated as aggregated glutenins).
Table 2. Quantification of Various Secondary Structures in Films Obtained by FT-IR Deconvoluted Absorbance Spectraa,b.
| position (cm–1) | structure | 5 + 10-A | 5 + 10-B | 2 + 12-A | 2 + 12-B | AG |
|---|---|---|---|---|---|---|
| 1691 (± 2.76) | β-turns | 2.5 | 2.4 | 2.3 | 0.9 | 2.7 |
| 1680 (± 2.65) | β-sheets (weakly hydrogen-bonded peptide groups) | 3.1 | 4.0 | 3.3 | 9.3 | 2.9 |
| 1667 (± 1.82) | β-turns | 20.1 | 12.1 | 18.0 | ||
| 1658–1660 (± 0.18) | α-helices | 0.3 | 0.3 | 0.5 | ||
| 1651 (±2.08) | α-helices and random coils | 23.4 | 30.7 | 45.4 | 58.7 | 49.2 |
| 1644 (± 2.36) | unordered | 0.1 | 0.1 | 0.3 | ||
| 1625 (± 1.07) | β-sheets (strongly hydrogen-bonded peptide groups) | 49.2 | 49.5 | 1.0 | 3.3 | 26.4 |
| 1618 (± 2.36) | β-sheets (strongly hydrogen-bonded peptide groups) | 1.1 | 3.1 | 37.1 | 27.7 |
Relative area of Gaussian components.
Values in parenthesis represent the variation in peak positions between different samples.
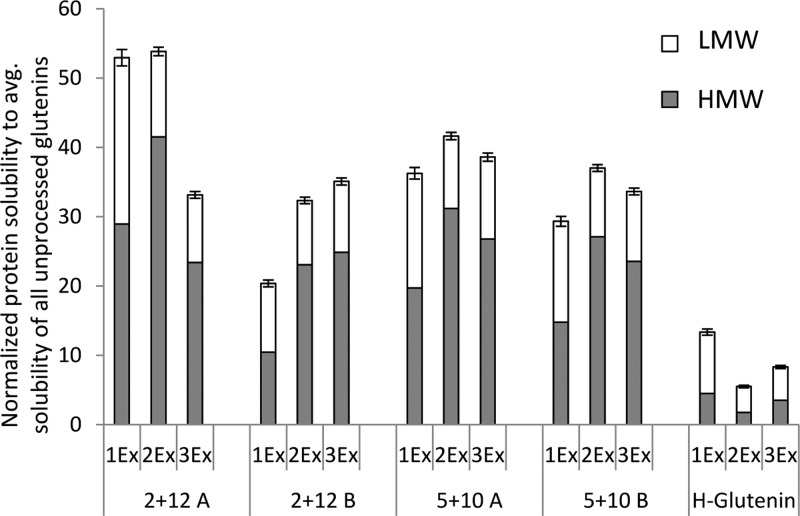
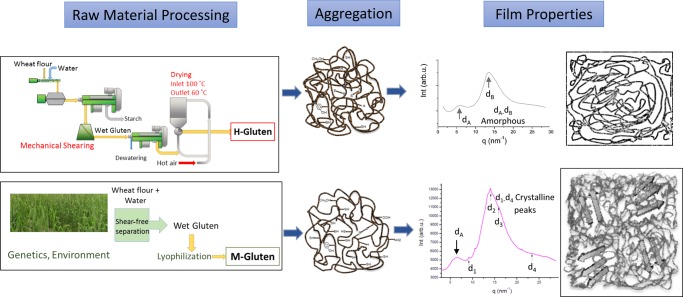
Previous reports suggest that the development of β-sheets in wheat-based protein films is positively correlated to protein solubility (as a result of the protein separation method) of the corresponding unprocessed raw material.15,16 Therefore, to understand the background of the dominant β-sheet formation in glutenin films from M-gluten, the protein solubility of glutenin powders was evaluated. The highest protein solubility was observed for the 2 + 12-A glutenins, followed by the 2 + 12-B and 5 + 10 (A, B) glutenins, and the lowest solubility was observed for glutenins separated from H-gluten (Figure 2).9,11,24 Therefore, glutenins from H-gluten showed a highly aggregated structure and is designated as “aggregated glutenin” (AG) throughout this article. A low aggregation in glutenin powder increased the opportunities of protein–protein interactions and molecular cross-linking rearrangements during protein unfolding and refolding events while thermo-molded into films. Preaggregated glutenin powder instead hindered such cross-linking rearrangements as observed for the AG films.
Figure 2.
Protein solubility measured in three steps via SE-HPLC in unprocessed glutenin (powders) to observe the extent of depolymerization. LMW: low molecular weight; HMW: high molecular weight; AG: aggregated glutenin; 1Ex: SDS–phosphate buffer; 2Ex: SDS–phosphate buffer + 30 s sonication; 3Ex: SDS–phosphate buffer + 30 + 60 + 60 s sonication. The error bars represent standard deviations.
Wheat glutenins, in their pristine form, belong to a category of intrinsically disordered proteins8,25 and lack ordered secondary structures such as β-sheets. The ability of proteins to form secondary structural conformations is influenced by the types and order of amino acids present in the proteins.26 Aromatic amino acids (tryptophan, tyrosine, and phenylalanine) together with valine and threonine preferably adopt β-sheet conformations.27 Glutenins in general possess low amount of β-sheets forming amino acids as observed in their amino acid sequence8 and also the order in which they occur. The sequence of glutenins does not support helical or β-sheet conformations, but are instead related to specific functions of the proteins.28 Mild gluten separation combined with glutenins having a high amount of cysteines in their chemical composition tends to develop the most β-sheet-rich protein network; 5 + 10 films showed a higher β-sheet content compared to 2 + 12 films (Figure 1, Table 2). Thus, a higher number of cysteines contribute to the formation of β-sheets via disulfide cross-links together with H-bonding within the protein backbone. Also previous reports have shown a positive impact of disulfide cross-linking on the formation of β-sheets.29,30 Growing condition B implying early grain maturation4,14 and high gluten strength resulted in a higher degree of β-sheets compared to growing condition A, implying late grain maturation and weak gluten.4 Thus, glutenins can be tuned to form secondary structures, such as β-sheets, when a combination of parameters, such as a mild separation procedure, is used to enable a low degree of polymerization in the starting material combined with cultivation conditions and a genotype with the ability to form additional disulfide bonds during film processing.
Atomic- and Molecular-Level Structural Rearrangements in Glutenin
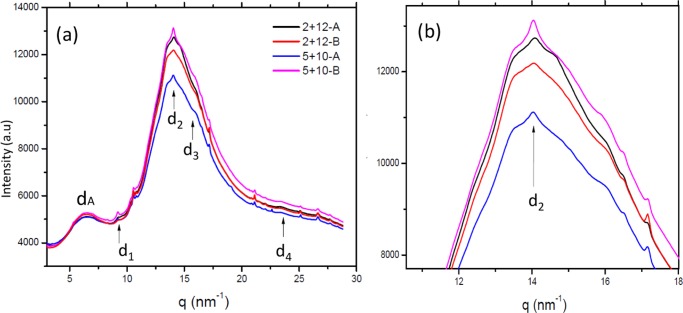
WAXS diffraction patterns for glutenin films from M-gluten displayed reflections of β-sheet structures with a distinctive d2 peak corresponding to the characteristic interstrand distance of 4.47 Å (0.45 nm)31,32 (Figure 3a,b, Supporting Information Figure S1). The intensity of the d2 peaks (Figure 3b) was compared for different samples, evaluating the difference between the maximum intensity of the peak and its baseline, DI = Imax – Ib. The DI values obtained were: 5 + 10-A: 32.7 nm; 5 + 10-B: 58.9 nm; 2 + 12-A: 36.9 nm; and 2 + 12-B: 19.6 nm (the estimated error is 5%). The results show maximum values for the sample 5 + 10-B and minimum values for the sample 2 + 12-B. The difference in intensities of d2 peaks indicates a relationship with the changes in the β-sheet content (strongly hydrogen-bonded peptide groups) observed by FT-IR data (Table 2). Nanoconfined β-sheet structures as observed by WAXS in our study are known to enhance the mechanical properties of other protein systems such as silk.33 Therefore, the relationship between the amounts of β-sheets observed by FT-IR spectroscopy and the intensity of the d2 peak by WAXS provides an indication of the improved mechanical strength of M-gluten-based films over H-gluten-derived films.
Figure 3.
(a) WAXS diffractograms of 2 + 12 and 5 + 10 films. (b) Zoomed-in region around the d2 peak (interstrand distance peak).
In addition, three crystalline peaks, d1, d3, and d4, were also observed. The d-spacing does not change significantly for the different samples (Table 3), but intensity variations were noted especially for peak d1. Similar d-spacing values were observed for silk I and silk II β-sheet crystals from fibrous proteins.34 The existence of d1–d4 reflections in our glutenin samples may indicate extended β-sheet crystals embedded in a dominant amorphous matrix. Importantly, the dominant scattering features on the WAXS data were two broad peaks, dA centered between 0.97 and 0.96 nm and dB (0.45 nm, superimposed by d2) corresponding to the amorphous phase (Figure 3a). For AG films, WAXS diffraction recorded only amorphous phases with two broad peaks (Supporting Information Figure S2) corresponding to a low β-sheet content and random coil structures as also observed in FT-IR spectra. The other peaks (besides d1–d4 and dA and dB peaks) on the WAXS patterns (Figure 3) not considered as part of the β-sheet structure are due to small amounts of crystalline material, most probably starch residues, with a preferential orientation.
Table 3. Characteristic Distances d1–d4 for all Glutenin Films Observed by WAXSa.
| films | d1 (nm) | d2 (nm) | d3 (nm) | d4 (nm) | dA (nm) |
|---|---|---|---|---|---|
| 2 + 12-A | 0.68 (±0.001) | 0.45 (±0.001) | 0.39 (±0.003) | 0.27 (±0.003) | 0.96 (±0.01) |
| 2 + 12-B | 0.68 (±0.001) | 0.45 (±0.001) | 0.39 (±0.003) | 0.27 (±0.003) | 0.97 (±0.01) |
| 5 + 10-A | 0.68 (±0.001) | 0.45 (±0.001) | 0.39 (±0.003) | 0.27 (±0.003) | 0.96 (±0.01) |
| 5 + 10-B | 0.68 (±0.001) | 0.45 (±0.001) | 0.39 (±0.003) | 0.27 (±0.003) | 0.97 (±0.01) |
dA is the distance associated with the peak position of the first ring. Values in parenthesis represent standard deviations.
The X-ray scattering data at low angles (SAXS) can provide complementary structural information on the WAXS data in the nanometric scale. SAXS intensity curves presented in the Iq2 vs q plot show two peaks (blue arrows, dbroad and ds1; Figure 4a), the first one intense and broad and the second one with lower intensity and reduced width, for all 2 + 12 and 5 + 10 films under both E treatments (A–B, Table 1). One more peak, ds2, additional to the previously mentioned one, was observed for 5 + 10 films (red arrow, Figure 4b). In AG films, only one broad peak was observed (Figure 4a), which refers to an aggregated and disordered protein morphology on a nanoscale.8 The d-spacing of the peak ds1 remained the same for all films with HMW GS 2 + 12 and HMW GS 5 + 10 (Supporting Information Table S2). However, the ds2 peak was only observed for 5 + 10 films (Figure 4). The existence of additional scattering peak in 5 + 10 films could indicate the formation of nanomorphology correlated with the existence of extended β sheet crystals (WAXS data) not previously described for glutenin-based system. No obvious relationship can be found between the d-values on the SAXS regime, so no attempt of unit cell definition is presented in this article.
Figure 4.
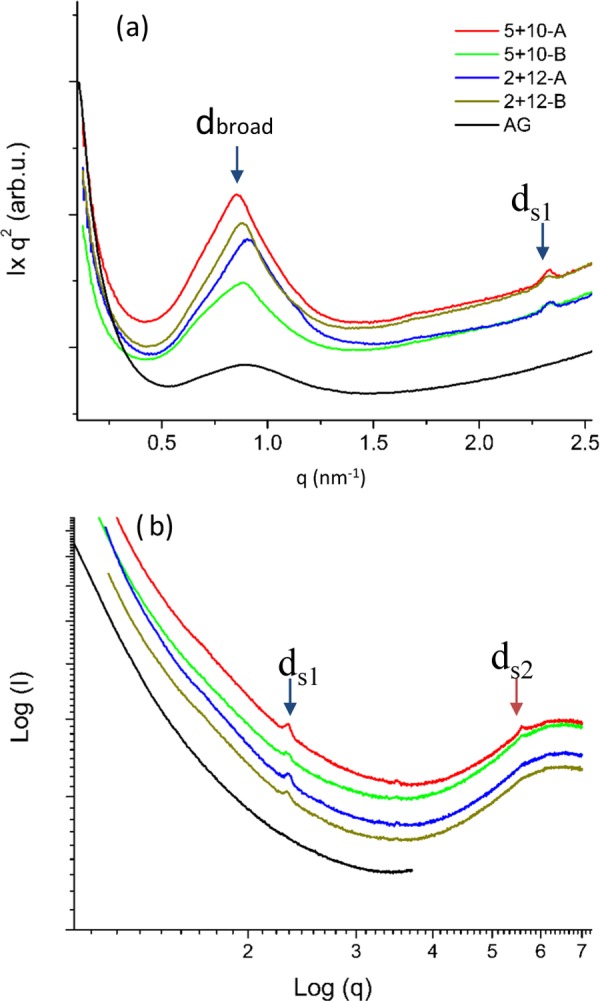
One-dimensional SAXS curves for glutenin films. (a) I × q2 vs q plots showing the dbroad and ds1 peaks (blue arrows). (b) log I vs log q plots indicating the presence of low-intensity peaks in the system.
The d-spacing for the broad peak, calculated from the position of the maxima and through the relationship d = 2π/qmax, showed a slight variation among different films (Supporting Information Table S2) by SAXS. The lowest d-spacing was observed for the 2 + 12-A film, where dbroad = 6.89 nm and the highest d-spacing was recorded for the 5 + 10 films, where dbroad = 7.32 nm. Our results correspond well with a previous study, where dbroad for glutenin was reported to be 7.01 nm (70.10 Å).8
The protein separation procedure along with the specific genotype turned out to be the most important factor to influence the ability of glutenin proteins to form nanomorphologies with extended β-sheet structures. A mild protein separation method enabled low polymerization of glutenins, which during film processing allowed structural rearrangements via molecular cross-linking by complete unfolding and refolding of proteins into compact β-sheet structures, as observed by FT-IR and WAXS data. Genotypes such as 5 + 10 further enhanced the nanostructural organization of the system by providing additional cysteine residues to form disulfide cross-links assisting the formation of stable structures.
Rearrangements in Glutenin Chemical Structure
A difference in the protein cross-linking pattern was observed by measuring the solubility of glutenin films in various solvents. All protein films exhibited extremely low solubility compared to the corresponding glutenin powders (Figures 2 and 5a), indicating glutenin polymerization upon thermomolding.8,35 The AG films showed the lowest solubility among all of the films in both the reducing and nonreducing solvents (Figure 5a,b). It can be suggested that a preexisting protein network via SS cross-links in AG powder (indicated by lowest solubility in AG powder, Figure 2) complemented with irreversible cross-links, such as S- and peptide bonds12 (as indicated by low solubility in extraction steps 5 and 6, Figure 5b), during film formation resulted in highly aggregated protein structure. Irreversible cross-linking has been related to poor film properties in previous studies.12 The very low glutenin solubility observed in the films corresponds well with the results observed for the solubility of industrial glutenins reported in previous studies.8,24
Figure 5.
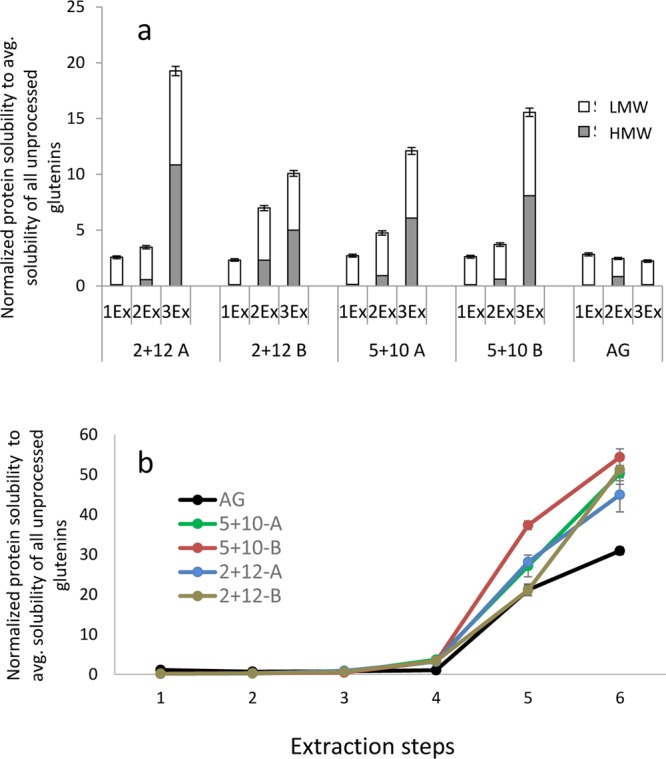
Protein solubility in glutenin films. (a) Protein size distribution and solubility measured by SE-HPLC. LMW: low molecular weight; HMW: high molecular weight; AG: aggregated glutenin; 1Ex: SDS–phosphate buffer; 2Ex: SDS–phosphate buffer + 30 s sonication; 3Ex: SDS–phosphate buffer + 30 + 60 + 60 s sonication. (b) Protein extractability measured by RP-HPLC in different solvents: (1) 70% ethanol, (2) 50% propanol, (3) 50% propanol, 60 °C, (4) 50% propanol 0.5% SDS, 60 °C, (5) 50% propanol 1% dithiothreitol (DTT), 60 °C, and (6) 1% DTT, 1% SDS, 6 M urea solution, 100 °C. Protein solubility was normalized to average total solubility of all unprocessed glutenin samples. The error bars represent standard deviations.
The relatively higher solubility with reducing solvents in 5 + 10 films compared to 2 + 12 films (Figure 5b; ext. 5 and 6) is explained by a relatively higher amount of cysteine residues in the 5 + 10 genotype compared to the 2 + 12 one, known for the formation of disulfide cross-links. Among the two cultivation environments, treatment B (low N, high T) contributed the most to higher polymerization of glutenins at film formation (Figure 5a,b) as also indicated by intermolecular β-sheet development, resulting in a strong glutenin network governed by a positive correlation between the short grain maturation period of wheat plants and polymer complexity.4,14
A synchronized pattern among protein structural parameters, such as chemical cross-linking, β-sheet content at molecular level, and nanomorphology, is obvious from the above results. AG films with the lowest amount of SS cross-links (low values at ext. 5 and 6; Figure 5b) and poor β-sheet content also indicated an unorganized nanoscale morphology.10 The 5 + 10 films with the highest amount of SS cross-links (Figure 5b; ext. 5 and 6) and dominant β-sheet content showed high propensity toward the formation of ordered nanomorphologies.
In previous reports, the giant molecular mass of glutenins was described as the restricting factor to obtain high-order structures, such as β-sheets in glutenins, due to limited possibilities of molecular rearrangements during thermomolding.8,10 However, in the present study, we were able to determine that the selection of particular factors, such as separation method, particular genotype, and cultivation environment, can significantly improve the quality of the raw material and influence the properties of the final product down to atomic and molecular levels.
Relationship between Protein Structures and Protein Functionality in Materials
Protein separation procedures were clearly able to influence the tensile properties of glutenin-based films. A relationship was seen between the nanoscale morphologies, secondary structures, and tensile properties of the films. All glutenin films from M-gluten showed a 100% higher Young’s modulus (E-modulus) compared to the AG films (Figure 6a). The former also showed high tensile strength (maximum stress) as well as high elongation at break compared to the AG films (Figure 6b,c). Previous studies on protein-based films have shown a general pattern of an increase in tensile strength and stiffness being connected with a decrease in extensibility.36,37 In our study, however, the improvement in Young’s modulus and maximum stress did not lower the elongation at break, which is an important aspect if one is looking to design a material with improved strength and stiffness without affecting elongation. It has been demonstrated via the macromolecular elastomer theory that chemical cross-links and secondary structures, such as extended β-sheets, contribute to the protein network behavior.38 It may be speculated for the present materials that chemical cross-links among glutenin polypeptides via SS and hydrogen bonds and extended β-sheets created a cohesive/dense protein network.39 This cohesive protein network not only added strength and stiffness to the films,40 but also added improved resistance to crack formation and early failure of the materials when exposed to stress (leading to higher extensibility). Our study thereby proposes a possible relationship among the mechanical properties of the films and the amount of ordered secondary structures.10,41
Figure 6.
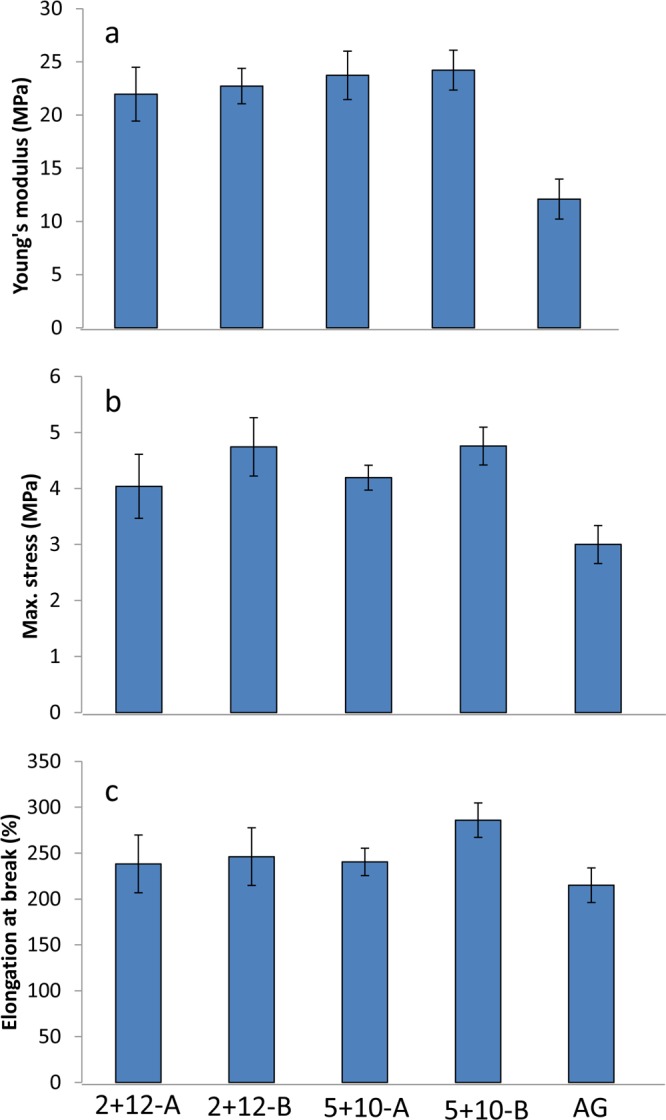
Mechanical properties of glutenin films. (a) E-modulus, (b) maximum stress, and (c) elongation at break. The error bars represent standard deviations.
Besides the significant differences observed among the M-gluten and H-gluten films, the mechanical properties did not differ significantly among genotypes and E treatments in this study. The lack of such differences may be the result of relatively fewer structural interchanges in the glutenin molecule being triggered by the genotype and cultivation environment than by the separation procedure. The separation procedure influenced the glutenin structure the most at both the secondary structural and nanostructural levels compared to the cultivation treatments. The glutenin films from M-gluten in the present study, in general, revealed improved mechanical properties, particularly in terms of E-modulus and strength compared to those of previously reported glutenin materials modified with chemical additives.10 This study showed opportunities to tune the protein structure at molecular levels by using a mild separation of proteins in combination with the genotype and cultivation environment to obtain novel nanostructural and secondary structural organizations.
Our results indicate a key relationship among the properties of the starting glutenin powder and resulting structural morphologies and mechanical profile of the films (Scheme 1). A starting material able to depolymerize and repolymerize during processing resulted in reorganization of molecular cross-links, leading to nanoscale morphologies and a high β-sheet content. Among the G and E interactions, a 5 + 10 genotype with cultivation conditions increasing the short grain maturation period resulted in strong glutenins. When processed into materials, these glutenins showed the highest SS cross-links and hence the highest amount of β-sheets, as well as being relatively strong and elastic material. The present study indicates that selection of genotype with the ability to form a large number of SS cross-links and later using a mild protein separation to obtain a low-polymerized material (Scheme 1) can result in a valuable low-cost raw material suitable to produce high-quality bioplastics for various applications. Protein-based materials, in particular those which are strong and extensible, are very attractive as next-generation bio-based materials. Also, the production of specific qualities of protein raw materials without the addition of chemical modifiers will assist in developing new green pathways for the bioplastics industries.
Scheme 1. Schematic Summary of the Impact of Starting Raw Material Properties on the Final Product.
The harshly processed gluten (H-gluten) leads to the formation of aggregated glutenins, resulting in amorphous protein structure when processed into bio-based films with low mechanical properties. Specifically produced M-gluten via mild protein separation leads to low aggregation in glutenins with high amount of β-sheets in amorphous matrix.
Acknowledgments
Cereal Quality Laboratory, Lantmännen Lantbruk, is acknowledged for milling of wheat grains. Tina Henriksson and Pernilla Vallenback are thanked for providing information about HMW-GS in the wheat cultivars. The MAX IV Laboratory Synchrotron, Lund, is acknowledged for the provision of beam time at the beamlines I911-2 and I911-4 under proposal no. 20130028. William R. Newson is thanked for his kind help with the drawing of H-gluten separation process scheme. The Swedish Research Council (VR), Trees and Crops for Future (TC4F), and VINNOVA are acknowledged for financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b02081.
Two-dimensional WAXS images clearly depicting d2 peaks for β-sheet structures (Figure S1); WAXS curve for the AG film (Figure S2); deconvoluted FT-IR data fitted to the Gaussian peak (Figure S3); different HMW GS in the wheat cultivars (Table S1); and scattering distance calculated for maxima of each peak studied by SAXS (Table S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gonzalez-Gutierrez J.; Partal P.; Garcia-Morales M.; Gallegos C. Development of highly-transparent protein/starch-based bioplastics. Bioresour. Technol. 2010, 101, 2007–2013. 10.1016/j.biortech.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Dang K. M.; Yoksan R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. 10.1016/j.carbpol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Cuq B.; Gontard N.; Guilbert S. Proteins as agricultural polymers for packaging production. Cereal Chem. J. 1998, 75, 1–9. 10.1094/CCHEM.1998.75.1.1. [DOI] [Google Scholar]
- Johansson E.; Malik A. H.; Hussain A.; Rasheed F.; Newson W. R.; Plivelic T.; Hedenqvist M. S.; Gällstedt M.; Kuktaite R. Wheat Gluten Polymer Structures: The Impact of Genotype, Environment, and Processing on Their Functionality in Various Applications. Cereal Chem. J. 2013, 90, 367–376. 10.1094/CCHEM-08-12-0105-FI. [DOI] [Google Scholar]
- Wieser H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Wrigley C. W. Biopolymers-Giant proteins with flour power. Nature 1996, 381, 738–739. 10.1038/381738a0. [DOI] [PubMed] [Google Scholar]
- Shewry P. R.; Tatham A. S. Disulphide bonds in wheat gluten proteins. J. Cereal Sci. 1997, 25, 207–227. 10.1006/jcrs.1996.0100. [DOI] [Google Scholar]
- Rasheed F.; Newson W. R.; Plivelic T. S.; Kuktaite R.; Hedenqvist M. S.; Gällstedt M.; Johansson E. Structural architecture and solubility of native and modified gliadin and glutenin proteins: non-crystalline molecular and atomic organization. RSC Adv. 2014, 4, 2051–2060. 10.1039/C3RA45522J. [DOI] [Google Scholar]
- Kuktaite R.; Plivelic T. S.; Cerenius Y.; Hedenqvist M. S.; Gällstedt M.; Marttila S.; Ignell R.; Popineau Y.; Tranquet O.; Shewry P. R.; Johansson E. Structure and Morphology of Wheat Gluten Films: From Polymeric Protein Aggregates toward Superstructure Arrangements. Biomacromolecules 2011, 12, 1438–1448. 10.1021/bm200009h. [DOI] [PubMed] [Google Scholar]
- Rasheed F.; Newson W. R.; Plivelic T. S.; Kuktaite R.; Hedenqvist M. S.; Gällstedt M.; Johansson E. Macromolecular changes and nano-structural arrangements in gliadin and glutenin films upon chemical modification: Relation to functionality. Int. J. Biol. Macromol. 2015, 79, 151–159. 10.1016/j.ijbiomac.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Blomfeldt T. O.; Kuktaite R.; Plivelic T. S.; Rasheed F.; Johansson E.; Hedenqvist M. S. Novel freeze-dried foams from glutenin-and gliadin-rich fractions. RSC Adv. 2012, 2, 6617–6627. 10.1039/c2ra20946b. [DOI] [Google Scholar]
- Tilley K. A.; Benjamin R. E.; Bagorogoza K. E.; Okot-Kotber B. M.; Prakash O.; Kwen H. Tyrosine cross-links: molecular basis of gluten structure and function. J. Agric. Food Chem. 2001, 49, 2627–2632. 10.1021/jf010113h. [DOI] [PubMed] [Google Scholar]
- Lacroix M.; Le T.; Ouattara B.; Yu H.; Letendre M.; Sabato S.; Mateescu M.; Patterson G. Use of γ-irradiation to produce films from whey, casein and soya proteins: structure and functionals characteristics. Radiat. Phys. Chem. 2002, 63, 827–832. 10.1016/S0969-806X(01)00574-6. [DOI] [Google Scholar]
- Johansson E.; Nilsson H.; Mazhar H.; Skerritt J.; MacRitchie F.; Svensson G. Seasonal effects on storage proteins and gluten strength in four Swedish wheat cultivars. J. Sci. Food Agric. 2002, 82, 1305–1311. 10.1002/jsfa.1185. [DOI] [Google Scholar]
- Rasheed F.; Kuktaite R.; Hedenqvist M. S.; Gällstedt M.; Plivelic T. S.; Johansson E. The use of plants as a green factory to produce high strength gluten-based materials. Green Chem. 2016, 18, 2782–2792. 10.1039/C5GC03111G. [DOI] [Google Scholar]
- Rasheed F.; Hedenqvist M. S.; Kuktaite R.; Plivelic T. S.; Gällstedt M.; Johansson E. Mild gluten separation-A non-destructive approach to fine tune structure and mechanical behavior of wheat gluten films. Ind. Crops Prod. 2015, 73, 90–98. 10.1016/j.indcrop.2015.04.007. [DOI] [Google Scholar]
- Newson W. R.; Prieto-Linde M. L.; Kuktaite R.; Hedenqvist M. S.; Gällstedt M.; Johansson E. Effect of extraction routes on protein content, solubility and molecular weight distribution of Crambe abyssinica protein concentrates and thermally processed films thereof. Ind. Crops Prod. 2017, 97, 591–598. 10.1016/j.indcrop.2016.12.037. [DOI] [Google Scholar]
- Shewry P.; Halford N.; Tatham A. High molecular weight subunits of wheat glutenin. J. Cereal Sci. 1992, 15, 105–120. 10.1016/S0733-5210(09)80062-3. [DOI] [Google Scholar]
- Lawrence G.; Moss H.; Shepherd K.; Wrigley C. Dough quality of biotypes of eleven Australian wheat cultivars that differ in high-molecular-weight glutenin subunit composition. J. Cereal Sci. 1987, 6, 99–101. 10.1016/S0733-5210(87)80045-0. [DOI] [Google Scholar]
- Malik A. H.; Prieto-Linde M. L.; Kuktaite R.; Andersson A.; Johansson E. Individual and interactive effects of cultivar maturation time, nitrogen regime and temperature level on accumulation of wheat grain proteins. J. Sci. Food Agric. 2011, 91, 2192–2200. 10.1002/jsfa.4439. [DOI] [PubMed] [Google Scholar]
- Cho S. W.; Gällstedt M.; Johansson E.; Hedenqvist M. S. Injection-molded nanocomposites and materials based on wheat gluten. Int. J. Biol. Macromol. 2011, 48, 146–152. 10.1016/j.ijbiomac.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kuktaite R.; Larsson H.; Johansson E. Variation in protein composition of wheat flour and its relationship to dough mixing behaviour. J. Cereal Sci. 2004, 40, 31–39. 10.1016/j.jcs.2004.04.007. [DOI] [Google Scholar]
- Wellner N.; Mills E. C.; Brownsey G.; Wilson R. H.; Brown N.; Freeman J.; Halford N. G.; Shewry P. R.; Belton P. S. Changes in protein secondary structure during gluten deformation studied by dynamic Fourier transform infrared spectroscopy. Biomacromolecules 2005, 6, 255–261. 10.1021/bm049584d. [DOI] [PubMed] [Google Scholar]
- Kuktaite R.; Newson W. R.; Rasheed F.; Plivelic T. S.; Hedenqvist M. S.; Gällstedt M.; Johansson E. Monitoring Nanostructure Dynamics and Polymerization in Glycerol Plasticized Wheat Gliadin and Glutenin Films: Relation to Mechanical Properties. ACS Sustainable Chem. Eng. 2016, 4, 2998–3007. 10.1021/acssuschemeng.5b01667. [DOI] [Google Scholar]
- Tompa P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005, 579, 3346–3354. 10.1016/j.febslet.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Voet D.; Voet J. G.. Biochemistry, 4th ed.; John Wiley and Sons: New York, 2001. [Google Scholar]
- Dedmon M. M.; Patel C. N.; Young G. B.; Pielak G. J. FlgM gains structure in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 12681–12684. 10.1073/pnas.202331299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejri M.; Roge B.; Bensouissi A.; Michels F.; Mathlouthi M. Effects of some additives on wheat gluten solubility: A structural approach. Food Chem. 2005, 92, 7–15. 10.1016/j.foodchem.2004.07.021. [DOI] [Google Scholar]
- De Simone A.; Berisio R.; Zagari A.; Vitagliano L. Limited tendency of α-helical residues to form disulfide bridges: a structural explanation. J. Pept. Sci. 2006, 12, 740–747. 10.1002/psc.809. [DOI] [PubMed] [Google Scholar]
- Weisman S.; Okada S.; Mudie S. T.; Huson M. G.; Trueman H. E.; Sriskantha A.; Haritos V. S.; Sutherland T. D. Fifty years later: The sequence, structure and function of lacewing cross-beta silk. J. Struct. Biol. 2009, 168, 467–475. 10.1016/j.jsb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Makin O. S.; Serpell L. C. Structural characterisation of islet amyloid polypeptide fibrils. J. Mol. Biol. 2004, 335, 1279–1288. 10.1016/j.jmb.2003.11.048. [DOI] [PubMed] [Google Scholar]
- Keten S.; Xu Z.; Ihle B.; Buehler M. J. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk. Nat. Mater. 2010, 9, 359–367. 10.1038/nmat2704. [DOI] [PubMed] [Google Scholar]
- Lu Q.; Hua X.; Wang X.; Kluge J. A.; Lu S.; Cebe P.; Kaplan D. L. Water-insoluble silk films with silk I structure. Acta Biomater. 2010, 6, 1380–1387. 10.1016/j.actbio.2009.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gällstedt M.; Mattozzi A.; Johansson E.; Hedenqvist M. S. Transport and tensile properties of compression-molded wheat gluten films. Biomacromolecules 2004, 5, 2020–2028. 10.1021/bm040044q. [DOI] [PubMed] [Google Scholar]
- Cao N.; Fu Y.; He J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocolloids 2007, 21, 1153–1162. 10.1016/j.foodhyd.2006.09.001. [DOI] [Google Scholar]
- McHugh T. H.; Krochta J. M. Sorbitol-vs glycerol-plasticized whey protein edible films: integrated oxygen permeability and tensile property evaluation. J. Agric. Food Chem. 1994, 42, 841–845. 10.1021/jf00040a001. [DOI] [Google Scholar]
- Van Kleef F.; Boskamp J.; Van den Tempel M. Determination of the number of cross-links in a protein gel from its mechanical and swelling properties. Biopolymers 1978, 17, 225–235. 10.1002/bip.1978.360170118. [DOI] [PubMed] [Google Scholar]
- Newson W. R.; Rasheed F.; Kuktaite R.; Hedenqvist M. S.; Gällstedt M.; Plivelic T. S.; Johansson E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerisation and tensile properties. RSC Adv. 2015, 5, 32217–32226. 10.1039/C5RA00662G. [DOI] [Google Scholar]
- Woerdeman D. L.; Veraverbeke W. S.; Parnas R. S.; Johnson D.; Delcour J. A.; Verpoest I.; Plummer C. J. Designing new materials from wheat protein. Biomacromolecules 2004, 5, 1262–1269. 10.1021/bm034530+. [DOI] [PubMed] [Google Scholar]
- Türe H.; Gällstedt M.; Kuktaite R.; Johansson E.; Hedenqvist M. S. Protein network structure and properties of wheat gluten extrudates using a novel solvent-free approach with urea as a combined denaturant and plasticiser. Soft Matter 2011, 7, 9416–9423. 10.1039/c1sm05830d. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.