Abstract
Context
Our pediatric diabetes center initiated insulin pump therapy for more than 250 patients with type 1 diabetes in 2014, but onboarding was inefficient.
Objective
To decrease time from the decision to initiate pump therapy to the ambulatory encounter after pump start (lead time) for new pump users from 132.5 days to less than 110 days within 5 months.
Design
Define, Measure, Analyze, Improve, Control method. We identified key problems: Long wait for training classes, unclear metrics, complicated scheduling, and nonstandardized processes. We then implemented 17 changes, including shortened classes, increased class offerings and space, clarified metrics, built a reporting dashboard, designated and cross-trained staff, created appeals letter templates, and educated clinicians. At project conclusion, we established a reaction plan if the processes were not performing as designed.
Main Outcome Measures
Outcomes of pump orders placed before and after improvements were implemented.
Results
During this project, 229 patients initiated the pump start process. Median lead time decreased from 132.5 to 98.5 days (p = 0.007). Patients with lead time under 110 days increased from 37% to 60% (p = 0.001). There were 31 pump nonstarters, with no significant association between group and whether the patient was a starter or nonstarter (p = 0.58). Nonstarters had a longer diabetes duration (median = 3.43 vs 2.05 years, p = 0.001).
Conclusion
Project goals were met. A high proportion of patients not starting pump therapy was discovered, but this was not affected by the project. We implemented further changes and a process-monitoring system.
INTRODUCTION
Continuous subcutaneous insulin infusion via insulin pump is a safe and effective treatment currently used by more than 55% of US pediatric patients with type 1 diabetes.1 Our pediatric diabetes clinic cares for 3500 patients with type 1 diabetes, with insulin pump use near the national rate. In the mid-1990s, we began developing a diabetes technology program that evolved over the years. Our program integrates staff across disciplines to provide nutritional support and a series of 3 training sessions to transition patients to insulin pumps: When the clinician, patient, and family jointly decide to transition to a pump and choose a specific pump, the provider or dietitian works with administrative staff to generate an order to send to the device company and initiate the insurance approval process. The patient and family then attend the Pump Preparation class, followed by the Saline Start class, where we fill the pump reservoir with saline to use in a practice mode for about a week. They then attend the Insulin Start class, at which the pump is loaded with insulin for the first time, and the patient suspends the regimen of insulin injections. For patients who live far from our center, some Saline Start and Insulin Start classes are completed by trainers employed by the device companies. Until a few months before this project, we had previously required patients starting an insulin pump to provide a 3-day dietary log with carbohydrate counts and insulin doses. Anticipating changes and acknowledging this step as unnecessary, our staff decided to remove this requirement before assembling the team.
In 2014, more than 250 patients from our clinic initiated insulin pumps. Despite having what we thought was a robust and appropriate process, miscommunication, rework, and unnecessary delays increasingly plagued the program to the point that a 6-month interval from the decision to use a pump to completion of training had become routine. It was unclear how often patients initiated but did not complete the transition. Therefore, in April 2015 we consulted a process improvement specialist to help us deconstruct, analyze, and rebuild our program into a more reliable and efficient process. We learned about Lean methods, a systematic approach to minimize waste without sacrificing productivity. Therefore, this project was characterized as a Lean project because of the focus on reducing overall time by reducing waste.2
Because best practices for starting a pediatric insulin pump are largely based on experience rather than rigorous scientific inquiry, the team maintained a commitment to our established training sequence. For this improvement project, we did not modify overall training content, choosing to focus on waste reduction.
METHODS
We assembled a project team of 17 stakeholders comprising physicians, advanced practice providers, dietitians, nurses, schedulers, administrative staff, and project facilitators with experience in collaborative problem solving using the Define, Measure, Analyze, Improve, Control method, a 5-step problem-solving tool for operational environments.3 The team met at 3 work sessions over 5 months. Using the framework of the Colorado Multiple Institutional Review Board, this quality improvement initiative did not meet the definition of human subjects research according to US Department of Health and Human Services regulations.
Results of Affinity Diagram Identifying Key Problems (Xs) and Root Causes of Inefficiencies in the Insulin Pump Initiation Process.
X1 = Wait for pump preparation class
Too few spaces per class
Lack of space for bigger class
Lack of staff to teach class
Staff scheduling
Poor utilization of available staff
Class offered only on
Fridays
Redundancies in training
Materials given to families unclear about scheduling
Higher volume of pump orders recently
X2 = Unclear metrics
No standard process to communicate pump orders to administrative staff (paper vs electronic)
Never tried to measure the process before
No schedule to assess and publish metrics
Many steps in the pump start process
X3 = Complicated scheduling of classes
Lack of staff
Complex practitioner/staff schedules
Fluctuating demand: End of calendar year, out of school in summer, vacations, illness
No clear system for scheduling
Difficult to organize and control the schedule
High volume
Many people involved in scheduling steps
Non-English-speaking families
X4 = Complex administrative process
Administrative process is unclear to staff
No standard procedures or protocols (eg, insurance denials)
Incomplete training
Inadequate cross-training
Exceptions: Long travel distance, other family member using pump
No clear contact person for families
Team Meeting 1: Define and Measure Phases
We assessed the current process in deliberate, open, and reflective discussions of “What is working well?” followed by “What is not working well?” Participants were encouraged to speak about the current process from their own perspectives, with the facilitator ensuring that each member was given an opportunity to speak. The results were a documented summary of the strengths and weaknesses of the current state and a shared sense of the impact it had on patients, clinicians, staff, and the clinic. One of the program’s strengths was having separate Saline Start and Insulin Start classes. Although some diabetes centers do not use a saline start, our trainers thought that the three hours of content is best received during two sessions, and families have better context after using the pump in a practice mode for a week. We also believed that these steps made the transition to an insulin pump safer. The most obvious shortcoming of our process was a long wait time to attend Pump Preparation class, so the team immediately decided to expand class size and frequency despite not having started the Improve phase.
The team then went on a Gemba Walk through the clinic.4 Gemba is a Japanese word meaning “place of work.” A Gemba Walk, a term from Lean methods, is the act of going together as a project team to see each step of the process firsthand. Team members were instructed to observe effective functioning; any flow disruptions, rework, barriers causing delays; and steps that involve waiting. This activity provided the team with current information and generated shared understanding about the process.
Next, we created a value stream map to visualize the steps of the process from start to finish5 and documented observed waste6 onto the value stream map. The key metric chosen was lead time,2 which we defined as the number of days from when the patient and clinician decided to pursue insulin pump therapy (measured by placing an order for a specific insulin pump) to the follow-up appointment 30 days after the Insulin Start class. We elected to start the measurement process with the order because that step demonstrates a firm commitment to prepare for a safe and effective transition. Our lead time measure provided a simple, patient-centered metric to capture a summary of the entire process. We also chose to measure time from the insulin pump order to Insulin Start class, but because some Insulin Start classes were completed off-site by device manufacturer representatives, these dates were more difficult to track. Some steps preceding the decision to transition to a pump include discussing options and recommendations from the health care provider and seeing the devices in person. Because this is usually an evolving series of poorly documented conversations during several ambulatory encounters and outside the clinic, we acknowledged it may be appropriate to address these steps, but we would have difficulty measuring changes. We completed the Define phase by generating a project charter summarizing the area of focus and scope.
For the Measure phase of the project, we established baseline performance through a manual review of 170 consecutive patients who had initiated an insulin pump in the 9 months before starting the project. We also began prospectively collecting data to measure days between various segments of the process and to identify any patients who ultimately did not start an insulin pump.
Team Meeting 2: Analyze and Improve Phases
The second meeting covered the Analyze phase and initiated the Improve phase of the project. On review of baseline data, a 110-day specification limit2 was set as the goal for patients to complete the entire process from placement of the insulin pump order to the ambulatory visit in clinic 1 month after initiating the pump. We arrived at this number by aiming to reduce the time from the order to Pump Preparation class by 50% from 3 months to 6 weeks, to allow 3 weeks until the Saline Start class, another week to the Insulin Start training, and 40 days for the follow-up encounter. The team believed these would be achievable targets, allowing reasonable expectations for insurance approval, device shipping, and trainer scheduling.
Using the value stream map, waste observations, and time segment data, the project team defined the factors that were unnecessarily prolonging lead time. These were organized into an affinity diagram.7 Four key problems were identified: 1) long wait for the Pump Preparation class, 2) unclear metrics, 3) complicated scheduling process for patient training, and 4) complex administrative processes. Root causes were identified (see Sidebar: Results of Affinity Diagram Identifying Key Problems (Xs) and Root Causes of Inefficiencies in the Insulin Pump Initiation Process) following the process outlined by the “Five Whys” analysis approach.6
For the Improve phase, team members brainstormed improvement ideas, aligning each idea to one of the key problems (see Sidebar: Affinity Diagram Identifying Improvement Ideas Aligned to Each of the Key Problems (Xs)). We estimated the effort required (< 1 week, about 2 weeks, or < 1 month) and potential impact each improvement would have on lead time (high, low), ultimately agreeing on 17 necessary improvements and assigning action steps and expected completion dates during the next 9 weeks. Improvements were not tested individually, but rather implemented as soon as feasible within the time allotted.
After Team Meeting 1, all patients for whom an insulin pump was ordered were prospectively tracked to allow measurement of time intervals between each step of the process and to identify patients who dropped out of the pump initiation process. Pump orders between the first team meeting and implementation of the 17 improvements were identified as the preintervention group. Pump orders after implementation were assigned to the postintervention group, to continue accruing patients for at least 4 months and a total of at least 100 patients.
Team Meeting 3: Control Phase
The final team meeting encompassed the Control phase. The team reviewed available lead time data and each of the implemented process changes. We documented how the forms of waste had been reduced or removed. This clarified the “future state” of the pump start process.
The final steps were to establish practices that would sustain the changes. Overall lead time and the days and number of patients in each interval in the process were identified as metrics that would continue to be collected and followed. Now that the process changes had also facilitated reliable, automated reporting of these metrics through the electronic medical record, regular reports were scheduled and a data visualization tool was designed. The team then established a reaction plan if the data began to suggest that the process was no longer performing as designed (Table 1).
Table 1.
Control plan
| Group | Critical element to sustain success | Goal | Frequency | Documentation | Monitoring | Staff responsible | Reaction plan |
|---|---|---|---|---|---|---|---|
| 1 | Track lead time and inventory of patients in new insulin pump process | Total lead time ≤ 110 calendar days | Data collected every 2 wks, starting Friday, September 11, 2015 | Spreadsheet, dashboard | Dashboard review meeting every 2 wks | Spreadsheet: Athena Dashboard: Raquel and Katie Skipper: Gail |
Address bottlenecks and wait times |
| 2 | Track time intervals between segments:
|
Segment 1 = 45 days Segment 2 = 21 days Segment 3 = 7 days Segment 4 = 40 days |
Data Analysis
All patients were tracked until at least 365 days after their pump order date. A Welch-Satterthwaite 2-sample t-test was used to test whether there was a significant difference in lead time, time to Insulin Start class, and diabetes duration between the 2 groups. Logistic regression was also implemented to determine whether there was a significant association between group and meeting the specification limit of 110 days. Linear regression was used to evaluate whether there was an association between lead time and diabetes duration.
Affinity Diagram Identifying Improvement Ideas Aligned to Each of the Key Problems (Xs).
X1 = Wait for pump preparation class
Reduce class length from 2.5 h to < 90 min
Increase number of class offerings
Increase slots per class (goal of 70–80 slots/mo)
Increase number of staff trained to teach class
X2 = Unclear metrics
Change record of patients in the pump process from paper to electronic spreadsheet
Determine data entry responsibilities
Create dashboard to display operational metrics: Weekly inventory of patients, monthly measure of time per segment
X3 = Complicated scheduling of classes
Develop list of acceptable outside trainers < 100 mi away from our campus
Require industry representatives to comply with our training schedule
Organize device demonstration kits to make them always available
X4 = Complex administrative process
Create “Pathway for insurance approval” document
Create template letters for insurance approvals and appeals
Prepare contact list for staff and patients to handle questions about insurance, training, etc
Develop new administrative protocols
Train on and communicate the new pump process to all staff and faculty at the center
Consolidate all orders to 1 staff member
Cross-train administrative staff to back up primary staff member (during vacation, illness)
We categorized those who had not started an insulin pump in more than 365 days as “nonstarters.” Logistic regression was used to determine whether there was a significant association between group and starting vs not starting a pump and whether there was a significant association between diabetes duration and starting vs not starting a pump.
RESULTS
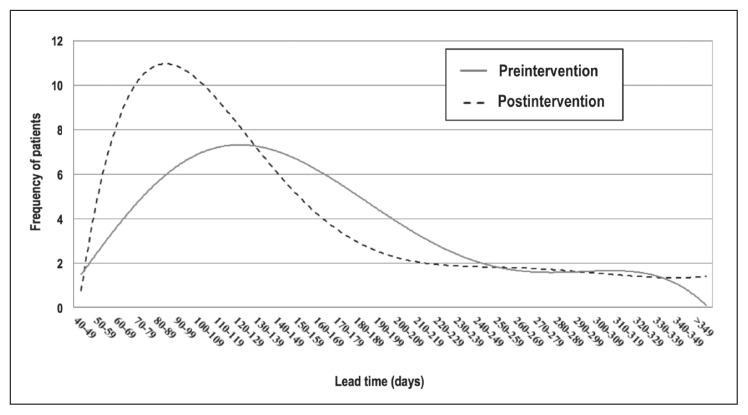
Only 22 of 170 patients who had initiated an insulin pump in the 9 months before this project met the specification limit goal of 110 days; the median lead time was 162 days. After starting this Lean project, we tracked the next 229 patients with new insulin pump orders from the time of pump order. There were 115 pump orders before implementation of the improvements. They were designated as Group 1, with the next 114 pump orders after implementation as Group 2 (Figure 1).
Figure 1.
Histogram of lead time for preintervention and postintervention groups. Median lead time was 132.5 and 98.5 days in the pre- and postintervention groups, respectively. Lead time was the number of days between when the patient and clinician decided to pursue insulin pump therapy (specific pump order) to the follow-up appointment 30 days after the Insulin Start class.
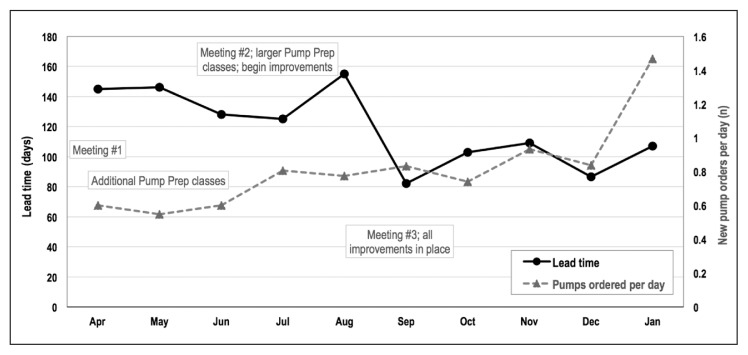
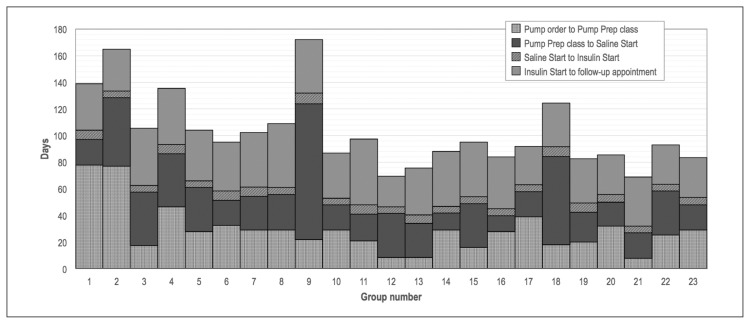
Among the 198 patients who started using an insulin pump within a year of the initial order, the median lead time decreased from 132.5 days (range = 40–311 days) to 98.5 days (range = 48–342 days, p = 0.007; Table 2). Marked decreases coincided with expansion of the Pump Preparation class in June and implementation of changes in September 2015 as the volume of insulin pump orders increased (Figure 2). Time from pump order to Pump Preparation class and time from Pump Preparation class to Saline Start class experienced the greatest decreases, whereas time from Saline Start class to Insulin Start class remained stable at 5 to 8 days (Figure 3).
Table 2.
Lead time, time to Insulin Start class, and diabetes duration by groupa
| Parameter | Entire cohort | Preintervention | Postintervention | p valueb |
|---|---|---|---|---|
| Lead time | ||||
| No. of patients | 198 | 98 | 100 | 0.007 |
| Mean (SD), d | 129.8 (62.77) | 141.89 (63.04) | 117.95 (60.49) | |
| Median (Q1, Q3), d | 112 (83, 156) | 132.5 (94, 175) | 98.5 (80.5, 140) | |
| Time to Insulin Start class | ||||
| No. of patients | 196 | 98 | 98c | 0.011 |
| Mean (SD), d | 80.38 (49.05) | 89.22 (52.85) | 71.54 (43.43) | |
| Median (Q1, Q3), d | 69 (47, 102) | 77 (52, 109) | 58 (42, 79) | |
| Diabetes duration | ||||
| No. of patients | 229 | 115 | 114 | 0.11 |
| Mean (SD), y | 3.53 (3.14) | 3.86 (3.3) | 3.19 (2.94) | |
| Median (Q1, Q3), y | 2.13 (1.66, 3.99) | 2.39 (1.85, 4.85) | 1.84 (1.46, 3.66) | |
Thirty-one patients did not start an insulin pump in less than 365 days.
Welch-Satterthwaite 2-sample t-test.
We were unable to ascertain an exact date of Saline Start class for 2 patients in the postintervention group.
Q1 = first quartile; Q3 = third quartile; SD = standard deviation.
Figure 2.
Median lead time and insulin pumps ordered, April 2015 to January 2016.
Prep = Preparation.
Figure 3.
Median days for each segment of the pump start process, by consecutive groups of 10 patients. All improvements were in place after the first 115 patients, which falls in the 12th group.
Prep = Preparation.
For 2 patients in the postintervention group, we were unable to determine the exact date of the Insulin Start class because they were scheduled and completed by a remote trainer, and the dates were not noted in the medical record. In the 196 patients with a documented Insulin Start date, median time from insulin pump order to Insulin Start class decreased from 77 days (range = 52–109 days) to 58 days (range = 42–79 days, p = 0.011; Table 2). In the logistic regression analysis, patients with lead time less than 110 days increased from 37% to 60% (p = 0.001). Each additional year of diabetes duration was significantly associated with an increase in lead time by a mean of 4.38 days (standard deviation = 1.486 days, p = 0.004).
Thirty-one patients did not start an insulin pump within 365 days of the insulin pump order, with no significant association between group (p = 0.58). These nonstarters had a longer diabetes duration (median = 3.43 vs 2.05 years, p = 0.001; Table 3). At subsequent ambulatory encounters, 13 patients reported continued interest in starting the pump but not yet being ready; 11 were no longer interested in pump therapy. At last contact, 7 patients had transferred care before starting the insulin pump. No patients were ultimately denied an insulin pump by their insurer, although clinicians may have prescribed according to knowledge of insurers’ policies. Five patients had pump orders initially denied by their insurer, but on appeal or prescription of a different device, they transitioned to pump therapy with a lead time less than 1 year. Four of these 5 patients had lead time greater than 110 days. There were also 2 patients whose pump orders were initially denied but decided to withdraw from the process without appealing the denial. These 2 patients were classified as nonstarters.
Table 3.
Descriptive statistics by cohort and starter vs nonstarter
| Diabetes duration | Entire cohort | Group 1 | Group 2 | |||
|---|---|---|---|---|---|---|
| Starter | Nonstarter | Starter | Nonstarter | Starter | Nonstarter | |
| No. of patients | 198 | 31 | 98 | 17 | 100 | 14 |
| Mean years (SD) | 3.29 (2.95) | 5.03 (3.86) | 3.5189 (2.92) | 5.82 (4.59) | 3.066 (2.99) | 4.071 (2.56) |
| Median years (Q1, Q3) | 2.05 (1.63, 3.64) | 3.43 (2.15, 6.29) | 2.2 (1.85, 3.84) | 4.85 (2.39, 6.29) | 1.785 (1.44, 3.20) | 3.135 (1.64, 5.86) |
| p valuea | 0.001 | 0.023 | 0.019 | |||
Welch-Satterthwaite 2-sample t-test.
Q1 = first quartile; Q3 = third quartile; SD = standard deviation.
DISCUSSION
Identifying and addressing sources of waste in the insulin pump initiation process reduced lead time by approximately one month and nearly doubled the percentage of patients meeting the 110-day specification limit goal. This occurred during a time when our clinic experienced an increased volume of patients transitioning to insulin pump therapy. There was also a sharp decrease in time to pump start in the two months preceding the project, most likely owing to removing the requirement for food logs, a step made as our staff began acknowledging that the process was inefficient.
Increasing the frequency of the Pump Preparation class offerings, moving the class to a larger venue, reducing the length of the class by eliminating extraneous or repeated content, and preparing more staff to teach the class allowed our team to address the greatest bottleneck in the process by increasing our capacity to train patients. These improvements dramatically reduced the time from pump order to Pump Preparation class after the first 20 patients (Figure 3). Although we remained committed to a series of 3 classes, having a list of trusted trainers in remote locations facilitated training for rural patients. Some patients even completed both Saline Start and Insulin Start trainings remotely.
Streamlining scheduling and administrative tasks and cross-training staff dramatically reduced the burden even as we increased throughput. Although the effects of individual changes on lead time are difficult to measure, staff report greater satisfaction with the process, and the process has been less prone to breakdown when a member is out on leave. Defining metrics and designing a monitoring system have allowed the team to measure impact and maintain surveillance beyond the closure of this project.
Because of rare occurrences in which a patient stops using an insulin pump within the first few weeks and because the follow-up visit is aimed to support patients’ more advanced questions after using the pump at home, we agreed that lead time would best encompass this entire interval to ensure a successful transition before being deemed complete. Knowing there is inconsistency in timing of the follow-up visit that we recommend be 1 month after starting the pump, we therefore also chose to track time from the pump order to the Insulin Start class as a secondary measure. Indeed, the difference between both mean and median lead time and time to Insulin Start class were greater than 30 days in both groups, although smaller in the postintervention group.
We were surprised to find that 14% of patients who had made the decision to transition to an insulin pump still had not completed the process a year later. The fact that the new process, with decreased burden on the patient and family, did not affect the frequency of nonstarters reinforces our anecdotal experience that many people hesitate for reasons other than miscommunication and delays on the part of our team. The next steps are to understand how better to support these patients to make a successful transition or to anticipate when not to place an order for an expensive medical device that will not be used.
The findings that longer duration of diabetes was associated with both longer lead time and likelihood of being a nonstarter was unsurprising, because patients with more longstanding diabetes who have not already transitioned to a pump seem to be less in a hurry to do so.
One of the major hurdles we confronted in overhauling this homegrown system that had evolved over two decades was the sense of comfort and ownership that staff had developed. With a paucity of published best practices for insulin pump starts in a pediatric population, it was difficult to challenge the status quo. For example, although it was roundly discussed, nurses elected to maintain control over the scheduling process out of concern that the complicated sequence would be disrupted and patients would arrive unprepared. Our staff also hesitated to use outside pump trainers with whom we had not previously worked because those trainers may lack pediatric experience and the training content may not meet our standards.
Because most changes were in place around a single date, we chose to demonstrate the effects of our improvement project as a pre- and postintervention comparison (Figure 1). Figure 2 supports this rationale, showing a sharp change in lead time beginning in September, coinciding with implementation of the improvements. During the project we also maintained an updated dashboard showing the number of people currently at each stage of the process (not shown). This allowed real-time assessment but made it difficult to see temporal changes. Lead time is a sum of several components, and we therefore show Figure 3 as a stacked bar graph rather than a statistical process control chart.
After the project conclusion, we implemented two additional changes that took many months to complete and had many hurdles to overcome. Without funds for a professional build, the Pump Preparation class was redesigned by one of the authors (KV) as an online module with embedded content assessments that patients complete at home, reducing the total number of in-person classes from three to two. Families now going through the process report high satisfaction with the online training, and trainers have not reported a lower level of baseline knowledge about insulin pumps at the in-person classes. The second change was standardizing the insulin pump order process with an order in the electronic medical record. This simplified process has reduced miscommunication by prompting the clinician to provide all necessary information and to transmit the order without having to recall the frequently changing responsibilities of individual administrative staff members. A bonus is that we now can track pump orders and metrics without staff manually updating a spreadsheet. Because both these steps overlapped in the ordering process, we elected to wait until the electronic order was built and tested to launch them simultaneously.
Few other clinics have such high volume of insulin pump initiations and therefore may not confront similar administrative obstacles. Smaller teams with fewer patients may not find benefit in such an exhaustive dissection and rebuild of their program as we did. We did not include qualitative data from patient representatives, but that is a step that could have improved the outcome of the redesigned process.
CONCLUSION
Project goals were met. Inefficiency of the process and increasing demand were strong motivating factors for this initiative. Deconstructing and rebuilding the insulin pump initiation process required coordination from many stakeholders but ultimately resulted in a much more satisfactory state. Bringing stakeholders together to create a shared understanding of the entire process and collecting data to make objective assessments allowed our team to simplify communication and remove unnecessary obstacles. Patients are better served, and our work is much more efficient. We implemented further changes and set up a system to monitor the process. After our first team experience with the Define, Measure, Analyze, Improve, Control method, we are now using this approach to address other problems identified in the clinic, including the process to onboard patients to newer, more advanced insulin pumps.
A Melting Down
Diabetes is a wonderful affection, not very frequent among men, being a melting down of the flesh and limbs into urine … . The patients never stop making water, but the flow is incessant, as if from the opening of aqueducts. The nature of the disease, then is chronic, and it takes a long period to form; but the patient is short-lived, if the constitution of the disease be completely established; for the melting is rapid, the death speedy.
— Aretaeus the Cappadocian, c 1st century CE, celebrated Greek physician
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Wood JR, Miller KM, Maahs DM, et al. T1D Exchange Clinic Network. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013 Jul;36(7):2035–7. doi: 10.2337/dc12-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liker JK, Meier D. The Toyota way fieldbook: A practical guide for implementing Toyota’s 4Ps. New York, NY: McGraw-Hill Education; 2005. [Google Scholar]
- 3.George ML, Rowlands D, Kastle B. What is lean six sigma? New York, NY: McGraw-Hill Professional; 2005. [Google Scholar]
- 4.Womack JP. Gemba walks. 1st ed. Cambridge, MA: The Lean Enterprise Institute, Inc; 2011. [Google Scholar]
- 5.Rother M, Shook J. Learning to see: Value-stream mapping to create value and eliminate muda. 1st ed. Cambridge, MA: The Lean Enterprise Institute, Inc; 1998. [Google Scholar]
- 6.Ohno T, Bodek N. Toyota production system: Beyond large-scale production. New York, NY: Productivity Press; 1988. [Google Scholar]
- 7.McLean GN. Organization development: Principles, processes, performance. San Francisco, CA: Berrett-Koehler Publishers, Inc; 2005. [Google Scholar]