Abstract
Context
No known published study has focused on a plant-based diet (PBD) in the treatment of ulcerative colitis (UC).
Objective
To investigate relapse prevention in UC after consumption of a PBD during educational hospitalization in Japan.
Design
Prospective study of patients with mild UC or UC in remission who did not need immediate treatment. A PBD and dietary guidance were provided during a two-week hospitalization.
Main Outcome Measures
The primary end point was relapse (a flare-up that required more aggressive treatment) during the follow-up period. Kaplan-Meier analysis was used to calculate the cumulative relapse rate. Secondary end points were immediate improvement in symptoms or laboratory data during hospitalization and a chronologic change in the PBD score, which evaluated adherence to the PBD.
Results
Sixty cases were studied: 29 initial episode cases and 31 relapse cases. Of these, 31 involved proctitis; 7, left-sided colitis; and 22, extensive colitis. Thirty-seven patients were receiving medication; 23 were not. The median age was 34 years; median follow-up was 3 years 6 months. Eight cases relapsed during follow-up. The cumulative relapse rates at 1, 2, 3, 4, and 5 years of follow-up were 2%, 4%, 7%, 19%, and 19%, respectively. Most patients (77%) experienced some improvement such as disappearance or decrease of bloody stool during hospitalization. The short- and long-term PBD scores after the hospitalization were higher than baseline PBD scores.
Conclusion
Relapse rates after educational hospitalization providing a PBD were far lower than those reported with medication. Educational hospitalization is effective at inducing habitual dietary changes.
INTRODUCTION
Ulcerative colitis (UC) and Crohn disease have a common etiopathogenesis and features, and they fall under the collective term inflammatory bowel disease (IBD).1 No longer a disease mainly seen in Europe and North America, IBD is now a global disease.2 Despite the recognition of westernization of lifestyle as a major driver of the growing incidence of IBD,3,4 no countermeasures against such lifestyle changes have been recommended, except that patients with Crohn disease should not smoke.5 Dysbiosis (imbalance) of the gut microflora has been observed in IBD,6 and it is apparent now that gut microflora is influenced by our diet.7,8 Thus, it seems critical to maintain gut symbiosis for the suppression of gut inflammation by consuming a suitable diet. With a suitable diet, substantial improvement in the prognosis of IBD can be expected. We consider that the lack of a suitable diet is the biggest issue faced in current treatment of IBD.9–11
We regard IBD as a lifestyle disease caused mainly by our omnivorous (Western) diet.9–12 We have been providing a plant-based diet (PBD) to all patients with IBD since 2003.10 By incorporating a PBD in treatment, we have achieved and published far better outcomes in both the active stage and quiescent stage in Crohn disease9,11 than those reported previously.
If IBD is accepted to be a lifestyle disease mainly caused by a westernized diet, then current practice must change. Current practice recommends lifelong medication for relapse prevention in IBD.13–15 Diet, however, is critically important. Although medication is needed in the active phase of IBD, diet is generally more important than medication to maintain remission in the quiescent phase.9 If a suitable diet is established as part of a changing lifestyle, medication ultimately may not be needed to maintain remission.9
The Japanese diet has become westernized and is now far from a PBD.10 With increasing affluence in Japan, replacement of our diet with a PBD is not easy. This replacement can, however, be achieved by a short period of educational hospitalization. We started educational hospitalizations in 2003. The percentage of patients with UC admitted for educational hospitalization was 30% of all admitted patients with UC.10
Our goal is the prevention of a relapse during the follow-up period after educational hospitalization. We hypothesized that educational hospitalization will decrease the relapse rate, and, eventually, remission will be maintained in most UC-affected patients not with medication but with a PBD.
METHODS
Design, Settings, and Patients
We designed a single-group trial (study number UMIN000019061), which was conducted at 2 tertiary care hospitals in Akita in northern Japan: Nakadori General Hospital and Akita City Hospital. The first author (MC) worked for Nakadori General Hospital between 2003 and 2012 and has been working for Akita City Hospital since 2013. This study was approved by the ethical committees of Nakadori General Hospital and Akita City Hospital (Protocol Numbers 19-2003 and 15-2015). Written informed consent was obtained from all patients.
Patients with UC who did not need immediate treatment and were able and willing to be admitted for about two weeks were included in the study. Cases comprised both initial episodes of UC and cases of disease relapse. Patients who received a diagnosis of UC through a health checkup but never had symptoms were excluded.
Educational Hospitalization
A lacto-ovo vegetarian diet (about 30 kcal/kg of standard body weight) with fish once a week and meat once every 2 weeks (ie, a semivegetarian diet) was provided during hospitalization. Details of the semivegetarian diet have been described previously.9 During hospitalization, food other than the meal service was discouraged. The plant-based diet score (PBDS), which evaluated adherence to the PBD, was 35 during hospitalization.10
On the first day of admission, for a patient who did not have bloody stool, a fecal occult blood test (OC-Auto III Latex Reagent, Eiken Chemical Co Ltd, Tokyo, Japan; normal range ≤ 50 ng/mL)16 was performed. Patients were provided with educational material on lifestyle diseases, healthy lifestyle habits,17 pathogenesis of IBD, and information on the PBD. During hospitalization, patients were provided with answers to any questions they had. A registered dietitian also visited the patients and talked to them about the PBD and helped them get used to it. At the end of the hospitalization, a qualified dietitian gave dietary guidance to the patients and the person who prepared the patient’s meals.9 Laboratory tests, including any with previously abnormal results, were repeated. Patients were advised to continue consuming the PBD after discharge.
Medication before and during Educational Hospitalization
Medication already prescribed by a physician was maintained before and during hospitalization irrespective of whether it was an initial case or a relapse case. When a patient was referred without a prescription of medication, no medication was administered before and during hospitalization. However, when there was no improvement observed during the first seven to ten days after hospitalization, medication was initiated according to guidelines.18
Food-Frequency Questionnaire and Plant-based Diet Score
A questionnaire of dietary habits and lifestyle behaviors before onset or relapse of the disease was given to patients when the educational hospitalization was scheduled. This food-frequency questionnaire included 45 questions that covered almost all foods or food groups in Japan.9 The questionnaire was obtained immediately after admission, before the patient received information about the PBD. On the basis of the questionnaire, a table was created that summarized a patient’s current and future recommended lifestyle and dietary habits.9 A representative table for a 22-year-old patient experiencing an initial episode of UC is shown in Tables 1a and 1b. The recommended dietary habits in Tables 1a and 1b are consistent with the PBD. These tables were given to the patient during hospitalization and was used by the dietitian when giving dietary guidance.
Table 1a.
Examples of a lifestyle habits summary provided to a 22-year-old patient at initial episode of ulcerative colitisa
| Habits | Frequency, amount, or type | ||||
|---|---|---|---|---|---|
| Smoking (no. of cigarettes/d) | (more than 20) | (6–19) | (1–5) | Rare | None |
| Regular exerciseb | Every day | 3–5 d/wk | 1–2 d/wk | Rare | None |
| Alcoholb | Every day | 3–5 d/wk | 1–2 d/wk | Rare | None |
| Eating between mealsb | Every day | 3–5 d/wk | 1–2 d/wk | Rare | None |
| Sugar in tea or coffeeb | Large amount | Average amount | Small amount | Rare | None |
| Type of dietb | Semivegetarian | Japanese | Pro-Japanese | Standard/mixed | Pro-Western |
Gray shading represents your present habit (style). Black shading indicates habits that need to change and represents the recommended habit (style); lack of a black-shaded box in a row indicates that no change is needed.
Item in boldface represents recommendation for drastic alteration in habit.
Table 1b.
Examples of a dietary habits summary provided to a 22-year-old patient at initial episode of ulcerative colitisa
| Food | Daily | 3–5 servings/wkb | 1–2 servings/wkb | Rarely | None |
|---|---|---|---|---|---|
| Rice | |||||
| Miso soup | |||||
| Pulses (beans, soybeans, peas, etc) | |||||
| Vegetables | |||||
| Udon/soba (Japanese noodles) | |||||
| Ramen (Chinese noodles) | |||||
| Bread | |||||
| Tea, coffee | |||||
| Juice | |||||
| Cola/soda | |||||
| Beefc | |||||
| Pork/chickenc | |||||
| Minced or processed meatc | |||||
| Fish | |||||
| Cheese/butter/margarine | |||||
| Sweets | |||||
| Ice cream/milk shake | |||||
| Yogurt (plain)c | |||||
| Green tea | |||||
| Potatoes/starchesc | |||||
| Fruitsc |
Gray shading represents your present habit (style). Black shading indicates habits that need to change and represents the recommended habit (style); lack of a black-shaded box in a row indicates that no change is needed.
Servings are spread over a week.
Item in boldface represents recommendation for drastic alteration in habit.
A PBDS was calculated from the questionnaire responses. The method for how the PBDS was calculated has been described previously.10 In brief summary, 8 items considered to be preventive factors for IBD had a positive score, and 8 items considered to be IBD risk factors had a negative score. The PBDS was calculated as the sum of the positive scores (PBDS+) and negative scores (PBDS−). A higher PBDS indicated greater adherence to the PBD.10 The PBDS for the same 22-year-old patient in Tables 1a and 1b is presented in Table 2.
Table 2.
Plant-based diet score (PBDS) for a 22-year-old Japanese patient with inflammatory bowel disease
| Food group | Scoring by frequency of consumption | Example: 22-year-old at initial episode of ulcerative colitis | |||||
|---|---|---|---|---|---|---|---|
| Daily | 3–5 servings/wka | 1–2 servings/wka | Rarely | Baseline (before hospitalization) | PBD during educational hospitalization | 19 mo after discharge | |
| Positive score | |||||||
| Vegetables | 5 | 3 | 1 | 0 | 3 | 5 | 3 |
| Fruits | 5 | 3 | 1 | 0 | 0 | 5 | 3 |
| Pulses (beans, soybeans, peas, etc) | 5 | 3 | 1 | 0 | 5 | 5 | 5 |
| Potatoes/starches | 5 | 3 | 1 | 0 | 0 | 5 | 1 |
| Rice | 5 | 3 | 1 | 0 | 5 | 5 | 5 |
| Miso soup | 5 | 3 | 1 | 0 | 5 | 5 | 5 |
| Green teab | 5 | 3 | 1 | 0 | 5 | 0a | 5 |
| Yogurt (plain) | 5 | 3 | 1 | 0 | 0 | 5 | 5 |
| Negative score | |||||||
| Meat | −5 | −3 | −1 | 0 | −3 | 0 | −3 |
| Minced or processed meat | −5 | −3 | −1 | 0 | −3 | 0 | −3 |
| Cheese/butter/margarine | −5 | −3 | −1 | 0 | 0 | 0 | 0 |
| Sweets/ice cream/milk shake | −5 | −3 | −1 | 0 | 0 | 0 | 0 |
| Soft drinks (cola/carbonated beverages/juice) | −5 | −3 | −1 | 0 | 0 | 0 | 0 |
| Alcohol | −5 | −3 | −1 | 0 | −5 | 0 | 0 |
| Bread | −5 | −3 | −1 | 0 | 0 | 0 | 0 |
| Fish | −2 | −1 | 0 | 0 | −1 | 0 | −1 |
| PBDS | 11 | 35 | 25 | ||||
Servings are spread over a week.
Green tea is recommended to drink at home but is not provided at the hospital.
PBD = plant-based diet.
Follow-up
Follow-up was continued as long as possible. The interval between the educational hospitalization and initial follow-up visit to the Outpatient Department after discharge varied depending on the stability of the patient’s condition. For a patient who started receiving medication at the end of hospitalization, the interval to initial follow-up was three to four weeks. For a patient who was in unstable remission, the interval was four to six weeks. For a patient who was in stable remission, the interval was eight weeks. For a patient who was in remission for more than a few years without medication, the interval was three to six months.
Assessment of Efficacy
The primary end point was relapse during the follow-up period after educational hospitalization. Relapse was defined as a change in the clinical status of the patient that required more aggressive medical treatment.15,19–22 Reappearance of streaks of blood, a small volume of blood, or bloody stool was not counted as relapse if blood disappeared or was controlled with previous medication and/or with modification of the diet or a lifestyle behavior.
The secondary end point was immediate improvement during educational hospitalization. Patients with UC recruited for educational admission comprised 3 groups: mild (disease) activity23; remission23 with abnormal laboratory test results, including fecal occult blood tests; and remission with normal laboratory test results. For the first 2 groups, improvement was defined as the disappearance of bloody stool (clinical remission),24 a decrease in the volume of blood, normalization of the fecal occult blood test result or a decreased volume of fecal occult blood, and other improvements. Otherwise, the immediate outcome was defined as unchanged. Short-term (≤ 2 years) and long-term (> 2 years) chronologic changes in the PBDS were also studied.
Safety Evaluations
Safety assessments included vital signs, patient complaints, findings during daily practitioner rounds, and physical examinations.
Statistical Analysis
Demographic parameters are expressed as mean and standard deviation (SD) and/or median (interquartile range), as appropriate. The frequency of categorical variables between initial episode cases and relapse cases was assessed using the χ2 test. Chronologic changes in PBDS+, PBDS-, and total scores in identical patients were compared using the paired t-test or Wilcoxon test. Kaplan-Meier survival analysis was used to calculate the cumulative proportion of patients who had a relapse. Comparison of cumulative relapse rates between patients with an initial episode and those with a relapse, or between patients on and without a medication regimen, was tested using the log-rank test. All directional tests were 2-tailed. A p value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using JMP 8 software (SAS Institute Inc, Cary, NC).
RESULTS
Patient Characteristics
By extent of disease, E1 (proctitis)23 was most frequent (31 cases, 52%), followed by E3 (extensive colitis; 22 cases, 37%), and E2 (left-sided colitis; 7 cases, 12%). Of 60 cases, 48 (80%) were mild,23 11 (18%) were moderate, and 1 (2%) was severe by maximum severity (Table 3). The difference between mean disease duration (7 months) for initial episode cases compared with mean disease duration (53 months) for relapse cases was statistically significant (p < 0.0001; Table 3). Medication was not provided during the hospitalization in 23 cases (38%). There were 2 cases in which immunomodulators (systemic prednisolone or azathioprine, or both) were used for initial episode cases and 5 for relapse cases. There were 2 relapse cases with steroid dependence. None of the 60 cases had previous surgical resection of the large bowel (Table 3). All patients ingested the PBD during the hospitalization.
Table 3.
Demographics of study patients with ulcerative colitis
| Characteristic | Total (N = 60) | Initial episode cases (n = 29) | Relapse cases (n = 31) | p valuea |
|---|---|---|---|---|
| Male/female (%) | 35/25 (58/42) | 18/11 (62/38) | 17/14 (55/45) | 0.5700 |
| Age (years), range | 16–79 | 16–79 | 17–79 | 0.4053 |
| Mean (SD) | 39 (18) | 38 (3) | 41 (3) | |
| Median (IQR) | 34 (22–54) | 34 (22–51) | 34 (25–58) | |
| Extent of ulcerative colitis, no. (%) | 0.9470 | |||
| E1: Proctitis | 31 (52) | 15 (52) | 16 (52) | |
| E2: Left-sided colitis | 7 (12) | 3 (10) | 4 (13) | |
| E3: Extensive colitis | 22 (37) | 11 (38) | 11 (35) | |
| Severity: maximum, no. (%) | 0.4968 | |||
| S1: Mild | 48 (80) | 24 (83) | 26 (84) | |
| S2: Moderate | 11 (18) | 5 (17) | 4 (13) | |
| S3: Severe | 1 (2) | 0 (0) | 1 (3) | |
| Severity on educational admission, no. (%) | 0.2000 | |||
| S0: Remission with normal FOB | 20 (33) | 12 (41) | 8 (26) | |
| S1: Mild or S0 Remission with abnormal FOB | 40 (67) | 17 (59) | 23 (74) | |
| Disease duration: range (months) | 1–204 | 1–60 | 2–204 | |
| Mean (SD) | 31 (45) | 7 (12) | 53 (53) | < 0.0001 |
| Median (IQR) | 8 (2–40) | 3 (1–6) | 33 (11–72) | |
| Case referral status, no. (%) | 0.7341 | |||
| Referred | 38 (63) | 19 (66) | 19 (61) | |
| Nonreferred | 22 (37) | 10 (34) | 12 (39) | |
| Medication during hospitalization, no. (%) | 0.1066 | |||
| None | 23 (38) | 13 (45) | 10 (32) | |
| Local (suppository, enema): 5-ASA and/or SH | 9 (15) | 6 (21) | 3 (10) | |
| Oral 5-ASA | 16 (27) | 8 (27) | 8 (26) | |
| Both local medication and oral 5-ASA | 5 (8) | 0 (0) | 5 (16) | |
| Immunomodulator | 7 (12) | 2 (7) | 5 (16) | |
| Oral PS and oral 5-ASA | 3 (5) | 1 (3) | 2 (6) | |
| PS, AZA, and local medication or oral 5-ASA | 3 (5) | 1 (3) | 2 (6) | |
| AZA, local medication, and oral 5-ASA | 1 (2) | 0 (0) | 1 (3) | |
| Corticosteroid dependent | 2 (3) | 0 (0) | 2 (6) | |
| Previous proctocolectomy | 0 (0) | 0 (0) | 0 (0) | |
| Days of hospitalization, range | 5–30 | 5–23 | 7–30 | |
| Mean (SD) | 14 (5) | 13 (1) | 14 (1) | 0.4184 |
| Median (IQR) | 13 (11–16) | 13 (11–16) | 14 (11–16) | |
| F/U after educational hospitalizationb | (n = 57) | (n = 28) | (n = 29) | |
| Mean (SD), months | 46 (39) | 45 (35) | 47 (44) | 0.8146 |
| Median (IQR), months | 36 (17–59) | 38 (14–65) | 36 (18–58) | |
Comparison between initial episode cases and relapse cases (χ2 test).
This section has different n values than the main column headers for this table.
5-ASA = 5-aminosalicylic acid; AZA = azathioprine; FOB, fecal occult blood test result; F/U = follow-up; IQR = interquartile range; PS = prednisolone; SD = standard deviation; SH = steroid hormone.
Twenty-two of 60 cases were admitted during their school or company’s seasonal holidays. Although a 2-week period was recommended for educational hospitalization, the period differed among patients. Most ranged between 11 and 16 days, with a peak at 14 days. One patient had a psychiatric illness and was discharged earlier than planned, on Day 5, because of anxiety. In this case, dietary guidance was provided at the Outpatient Department.
Three of 60 patients were transferred to physicians immediately after discharge from the educational hospitalization for unavoidable reasons. Of the remaining 57 patients, 34 were followed-up while they received medication, whereas 23 cases received no medication during follow-up. All patients who did not achieve clinical remission during educational hospitalization achieved remission soon after discharge to the Outpatient Department. During the follow-up period, 17 patients moved out of Akita and were transferred to other physicians. Ten patients stopped attending follow-up sessions. The remaining 30 patients attended follow-up sessions to the end of July 2017. The mean follow-up period after educational hospitalization was 3 years 10 months (median = 3 years; Table 3).
Efficacy
Primary End Point: Relapse Rate
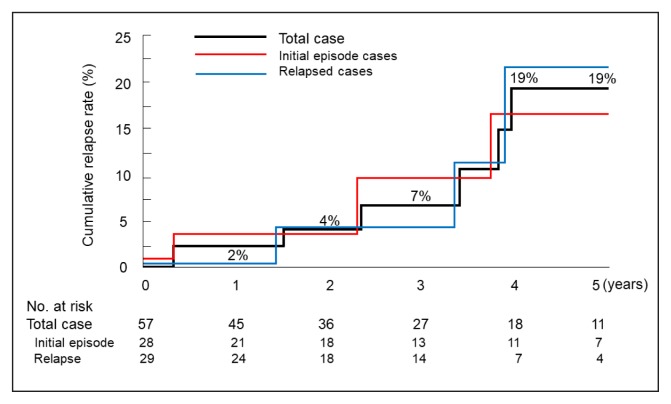
Of 57 cases, 8 (4 of 28 initial episode cases and 4 of 29 relapse cases; 4 cases each on and without a medication regimen) relapsed during the follow-up period (Table 4). Cumulative relapse rates at 1, 2, 3, 4, and 5 years were 2%, 4%, 7%, 19%, and 19%, respectively (Figure 1). There were no differences in cumulative relapse rates between initial episode cases and relapse cases (p = 0.9651; Figure 1). Mean time to relapse was 7 years 3 months (6 years 7 months for initial episode cases and 7 years 6 months for relapse cases). Similarly, there were no differences in cumulative relapse rates between cases on (n = 34) and without (n = 23) a medication regimen (p = 0.9644). In 2 of 8 relapse cases, a colectomy was eventually performed because of corticosteroid dependency (Table 4). Biologic agents were not administered for these 2 cases because of unavailability at the time.
Table 4.
Relapsed and surgically treated cases in the follow-up after educational hospitalization
| No. | Sex | Age, y | Educational hospitalization | Follow-up | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extenta | Clinical case | Disease duration | Medication | Efficacy | Medication | First relapse | Surgical therapy | ||||||
| Duration after EH | Medication | Duration after EH | Indication | ||||||||||
| 1 | M | 16 | E2 | IE | 1 mo | 5-ASA, PS | NA because of remission | 5-ASA | 3.5 mo | Addition of PS, AZA | None | ||
| 2 | F | 28 | E3 | IE | 3 mo | None | NA because of remission | 5-ASA | 2 y 2 mo | Addition of PS | 3 y 5 mo | Steroid dependency | Eosinophilia 12%, thrombocytosis (57.9 × 104/mm3) |
| 3 | M | 59 | E3 | IE | 3 mo | None | Unchanged | None | 3 y 11 mo | Local | None | Erythema nodosum on relapse | |
| 4 | M | 18 | E3 | IE | 1 mo | None | Improved | None | 6 y 6 mo | PS, IFX, AZA | None | CRMO of left tibia preceded 18 mo before relapse | |
| 5 | M | 20 | E3 | Relapse | 6 y | Local, 5-ASA, PS, AZA | NA because of remission | Local, 5-ASA, AZA | 1 y 5 mo | Addition of PS | 2 y 12 mo | Steroid dependency | Bronchial asthma, atopic dermatitis, BMI of 27.2 kg/m2 |
| 6 | F | 19 | E1 | Relapse | 9 mo | Local | Improved | None | 3 y 4 mo | IFX | None | Extension of lesion: E1 to E3a | |
| 7 | M | 61 | E3 | Relapse | 10 y | Local | Improved | Local as per occasional demands | 3 y 11 mo | PS | None | Distress with 5-ASA | |
| 8 | F | 34 | E3 | Relapse | 6 y | Local, 5-ASA | Improved | None | 8 y 9 mo | 5-ASA | None | ||
E1 = proctitis; E2 = left-sided colitis; and E3 = extensive colitis.
5-ASA = 5-aminosalicylic acids (orally); AZA = azathioprine; BMI = body mass index; CRMO = chronic recurrent multifocal osteomyelitis; EH = educational hospitalization; F = female; IE = initial episode of ulcerative colitis; IFX = infliximab; local = suppository, enema; M = male; mo = months; NA = not applicable; PS = prednisolone (orally); y = years.
Figure 1.
Cumulative relapse rates during follow-up after discharge from educational hospitalization for patients with ulcerative colitis.
Log-rank test between initial episode cases and relapse cases (p = 0.9001).
Secondary End Points
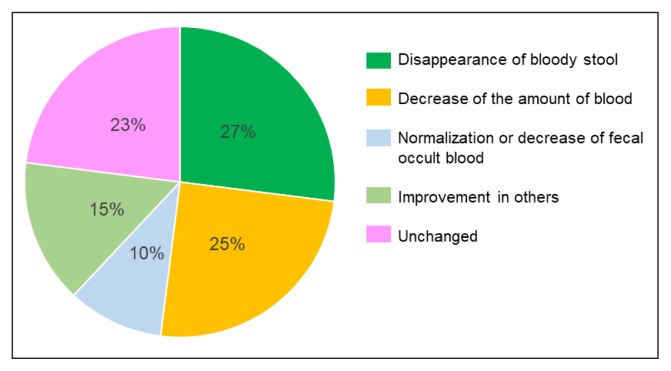
Twenty of 60 patients had no symptoms, and their fecal occult blood tests were negative. However, the remaining 40 patients had either mild activity23 with symptoms or were in remission23 with some abnormality in fecal occult blood tests or serum C-reactive protein concentration (Table 3). In these 40 cases, the immediate effects of hospitalization were assessed. Disappearance of bloody stool (clinical remission) occurred in 11 cases (27%); 4 of these patients were not receiving medication. A decrease in the volume of blood was observed in 10 cases (25%). Normalization of fecal occult blood or a decrease in the volume of fecal occult blood was observed in 4 cases (10%). Other improvements, such as in serum C-reactive protein concentration, bowel movements, or body temperature, were observed in 6 cases (15%). No improvement was observed in 9 cases (23%; Figure 2). In 2 of these 9 cases, some medication was added.
Figure 2.
Outcome at discharge from educational hospitalization (n = 40).
One patient was mistakenly not asked to respond to the food-frequency questionnaire. Therefore, baseline PBDS was determined from 59 patients. Mean (SD) baseline PBDS+, PBDS−, and PBDS were 23.2 (8.4), 13.2 (5.8), and 9.8 (9.8), respectively (Table 5). For 23 patients, at a median follow-up period of 1 year 2 months, respective scores were 28.4 (8.0), 6.8 (5.9), and 21.6 (10.6). These 3 values were significantly better than those at baseline (p < 0.0001 or p = 0.0001; Table 5). In the other 16 patients, at a median follow-up period of 3 years 11 months, respective scores were 27.5 (6.8), 8.8 (7.3), and 18.7 (9.8). These 3 values were better than those at baseline: The last 2 were statistically significant (p = 0.0461, p = 0.0340; Table 5).
Table 5.
Chronologic change of plant-based diet score (PBDS)
| Base | n | Follow-up period (months) | PBDS+ | PBDS- | PBDS | ||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| 59 | 23.2 (8.4) | 23.0 (17.0–31.0) | 13.2 (5.8) | 12.0 (9.0–19.0) | 9.8 (9.8) | 12.0 (3.0–15.0) | |||
| 23 | 20.9 (8.3) | 19.0 (16.0–27.0) | 13.6 (6.3) | 13.0 (9.0–19.0) | 7.3 (9.7) | 12.0 (0–15.0) | |||
| 16 | 25.1 (9.1) | 24.0 (18.3–33.5) | 12.8 (5.4) | 12.5 (9.0–17.8) | 12.3 (8.9) | 15.0 (3.8–17.0) | |||
| Follow-up (F/U) | |||||||||
| Short-term | 23 | 15.2 (5.9) | 14.0 (12.0–22.0) | 28.4 (8.0) | 30.0 (23.0–36.0) | 6.8 (5.9) | 5.0 (2.0–11.0) | 21.6 (10.6) | 20.0 (13.0–31.0) |
| Long-term | 16 | 59.5 (32.7) | 46.5 (39.0–54.0) | 27.5 (6.8) | 28.5 (24.0–32.0) | 8.8 (7.3) | 8.5 (2.3–11.8) | 18.7 (9.8) | 23.0 (8.8–27.8) |
| p value (paired t-test or Wilcoxon test) | |||||||||
| Base vs short-term F/U | 23 | < 0.0001 | 0.0001a | < 0.0001a | |||||
| Base vs long-term F/U | 16 | 0.1307 | 0.0461a | 0.0340a | |||||
Safety
All patients ate the PBD, and none experienced an adverse effect. There was no serious adverse event because of 5-aminosalicylic acid or local use of corticosteroid hormone.
DISCUSSION
To our knowledge, this is the first published study of prevention of relapse of UC by means of a PBD in patients guided through a short educational hospitalization. Cumulative relapse rates at 1, 2, 3, 4, and 5 years during follow-up after educational hospitalization were 2%, 4%, 7%, 19%, and 19%, respectively. These relapse rates are far better than those previously reported.15,19–22,25–27
The definitions of relapse and remission for UC vary according to the perspective of clinical trials, guidelines, clinical practice, and patients.28–30 The symptom, streaks of blood in the stool, is determined as relapse by Mayo scoring28 and Montreal classification,23 but not by a simple clinical colitis activity index.29 The definition of these terms influences relapse and remission rates.31,32 In the current study, we followed the Inflammatory Bowel Southeastern Norway (IBSEN) Study Group’s definition of relapse: A change in clinical status of the patient that requires more aggressive medical treatment.19–22 This definition seems adequate for clinical practice.
On the basis of inception cohort studies, it has been determined that extensive colitis or severe systemic symptoms at diagnosis are not associated with increased relapse rates.19–22,33,34 This means that relapse occurs irrespective of the extent of the disease and the severity. Few articles have described relapse rates at 1 year for initial episode cases.19,21,27 The rate is reported as 50% by the IBSEN Study Group,19 and estimated to be 28% from a Kaplan-Meier plot provided by the European Collaborative Study Group of Inflammatory Bowel Disease.21 It is 68.1% according to a Japanese study in which the definition of relapse was based on a Disease Activity Index28 of 2 or greater.27 After the first year, disease activity decreases over time.19,26,34 Cumulative relapse rates are 57% to 78% at 5 years and 67% to 83% at 10 years.20–22,26 The participants in the current study comprised almost half each of initial episode cases and relapse cases. Although the median disease duration was short for initial episode cases compared with relapse cases (3 months vs 33 months; Table 3), a higher relapse rate was not observed for initial episode cases compared with relapse cases (Figure 1).
To date, adherence to 5-aminosalicylic acid in the quiescent stage has been advocated to prevent a relapse.13–15,18 Kawakami et al15 used the same definition of relapse that we did in a study of Japanese patients who were in remission for more than 6 months, and they reported a relapse rate at 1 year of 41% for nonmedication-adherent patients and 16% for medication-adherent patients. Reports of this kind have been the basis for lifelong maintenance medication in UC.13–15
Our relapse rate of 2% at 1 year is far better than that previously reported.15,19,21,26,27,35 Whether a similar low relapse rate is found in ordinary patients with UC who have not undergone educational hospitalization needs to be elucidated. Our educational protocol resulted in patients voluntarily moderating their meat, processed meat, and alcohol intake, which are reported to be dietary risk factors for relapse in UC.35 We believe that IBD is a lifestyle disease mediated mainly by a westernized diet. It is suggested that patients can stop medication when they feel confident after a few years of remission using the PBD. This may go some way to relieving a patient’s fear about the disease, especially compared with being told that they may need to receive medication for life.13–15
In the current study, the majority (77%) of patients experienced improvements in symptoms and/or laboratory data during hospitalization (Figure 2). We can attribute this to some extent to the patients’ appreciation of the importance of diet. The mean baseline PBDS (9.8 [SD = 9.8] from 59 patients) in this study was comparable with the score (10.9 [9.5] from 159 patients with UC) in a previous study.10 The significantly high PBDS at the short-term follow-up (median = 14.0 months) compared with the baseline score (p < 0.0001; Table 5) indicated that patients altered their dietary habits in favor of the PBD. Dietary adherence to the PBD for more than 1 year might change gut microbial enterotypes,8 resulting in relapse prevention. In this study, a high relapse rate in initial episode cases compared with relapse cases19,26,34 was not observed. This indicates that the high relapse rate in the first year for initial episode cases might be suppressed with dietary intervention.
Although sustained dietary change is desired, a decrease in PBDS was observed during the long term (median duration = 3 years and 11 months). Most patients tended to lose their determination to adhere to the PBD once they had been in remission for a few years. However, they still consumed more of the recommended food and consumed less of the food that was discouraged compared with baseline (Table 5). Consequently, the PBDS was higher compared with baseline (p = 0.0340; Table 5). Patients appeared to manage the level of PBD by themselves according to their condition, suggesting that educational hospitalization enhanced their self-management skills.
A PBD was previously shown to be effective in both the active and quiescent stages of Crohn disease.9,11 The current study has shown that a PBD is effective in both the active and quiescent stages of UC as well. Of note, four patients with mild activity of UC achieved remission without medication during the educational hospitalization. Except for our case report,36 this is the first reported successful induction of remission by dietary manipulation without medication among published dietary trials.37–40 A reduction in the incidence of relapse by means of educational hospitalization will contribute not only to personal benefits to the patients themselves but also to health care savings.
Research on gut microflora has advanced our understanding about the key role of the gut microflora in health and disease.6–8,41–46 It is not limited to gut homeostasis but extends to individual health.41,43,44 Microbial diversity plays an important role in gut homeostasis.6,41–46 Reduced microbial diversity (dysbiosis) is commonly observed in a variety of chronic diseases.41,44 Recently, the relationship between diet and microbial diversity has been elucidated.6,41–46 A diet that is high in fat and sugar and low in dietary fiber tends to reduce microbial diversity, resulting in poor production of microbial metabolites such as short-chain fatty acids, which have diverse effects in maintaining homeostasis. In contrast, a PBD rich in dietary fiber increases microbial diversity and produces beneficial microbial metabolites.6,41–46 This observation might partly explain why a PBD prevents a variety of chronic diseases.47–50 Indeed, the same explanation applies to IBD,6,41,43–45 indicating that replacing an omnivorous diet with a PBD in IBD is the right approach.
Comprehensive lifestyle changes are fundamental for treating chronic diseases.51,52 However, changes in lifestyle, including dietary habits, are not easy.53 Our study indicates that educational hospitalization is an effective method for the replacement of an omnivorous diet with a PBD. Educational hospitalization is seldom seen in the literature,53 but it is common for diabetes mellitus in Japan.54 It is suggested that this modality will be effective for a variety of chronic diseases.
Our study had some limitations. The PBDS was developed at the late stage of the study.10 Therefore, short- and long-term chronologic changes in PBDS were not obtained from the same patients. Patients with short-term PBDS (n = 23) and those with long-term PBDS (n = 16) were different: They did not overlap (Table 5). Comparison of PBDS at 3 time points (ie, baseline, short-term, and long-term) from the same patients would have been more appropriate. Our study was also limited in that there was no control group and the sample size was small. Larger controlled studies are needed to validate the results. Additionally, further studies are needed to elucidate how educational hospitalization can alter the natural history of UC.
CONCLUSION
Relapse rates after educational hospitalization providing a PBD experience are far lower than those reported with medication. Educational hospitalization is effective at inducing habitual dietary changes.
Good Advice
We [now] devote more attention to the patient’s diet and habits, and more often send him away with good advice than with hastily written prescriptions.
— Robert Hall Babcock, MD, LLD, 1851–1930, blind American physician
Acknowledgments
We thank Marcin J Schroeder, PhD, Professor of Mathematics at Akita International University, for the statistical review.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: Current state of the art. Nat Rev Gastroenterol Hepatol. 2016 Jan;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, Soon IS, Rabi DM, et al. Increased incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: Reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008 Sep;57(9):1185–91. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 4.Hold GL. Western lifestyle: A ‘master’ manipulator of the intestinal microbiota? Gut. 2014 Jan;63(1):5–6. doi: 10.1136/gutjnl-2013-304969. [DOI] [PubMed] [Google Scholar]
- 5.Mowat C, Cole A, Windsor A, et al. IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011 May;60(5):571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 6.Gong D, Gong X, Wang L, Yu X, Dong Q. Involvement of reduced microbial diversity in inflammatory bowel disease. Gastroenterol Res Pract. 2016;2016:6951091. doi: 10.1155/2016/6951091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011 Oct 7;334(6052):105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May 28;16(20):2484–95. doi: 10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J. 2016 Fall;20(4):62–8. doi: 10.7812/TPP/16-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and plant-based diet as first-line (IPF) therapy for Crohn disease: A single-group trial. Perm J. 2017;21:17–009. doi: 10.7812/TPP/17-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiba M, Tsuda H, Abe T, Sugawara T, Morikawa Y. Missing environmental factor in inflammatory bowel disease: Diet-associated gut microflora. Inflamm Bowel Dis. 2011 Aug;17(8):E82–3. doi: 10.1002/ibd.21745. [DOI] [PubMed] [Google Scholar]
- 13.Kane SV. Overcoming adherence issues in ulcerative colitis. Gastroenterol Hepatol (N Y) 2007 Oct;3(10):795–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson CA, Clatworthy J, Robinson A, Horne R. Factors associated non-adherence to oral medication for inflammatory bowel disease: A systematic review. Am J Gastroenterol. 2010 Mar;105(3):525–39. doi: 10.1038/ajg.2009.685. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami A, Tanaka M, Nishigaki M, et al. Relationship between non-adherence to aminosalicylate medication and the risk of clinical relapse among Japanese patients with ulcerative colitis in clinical remission: A prospective cohort study. J Gastroenterol. 2013 Sep;48(9):1006–15. doi: 10.1007/s00535-012-0721-x. [DOI] [PubMed] [Google Scholar]
- 16.Hiraoka S, Kato J, Nakarai A, et al. Consecutive measurements by faecal immunochemical test in quiescent ulcerative colitis patients can detect clinical relapse. J Crohns Colitis. 2016 Jun;10(6):687–94. doi: 10.1093/ecco-jcc/jjw025. [DOI] [PubMed] [Google Scholar]
- 17.Breslow L, Enstrom JE. Persistence of health habits and their relationship to mortality. Prev Med. 1980 Jul;9(4):469–83. doi: 10.1016/0091-7435(80)90042-0. [DOI] [PubMed] [Google Scholar]
- 18.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010 Mar;105(3):501–23. doi: 10.1038/ajg.2009.727. doi: 10.1038/ajg.2009.727. . Erratum in: Am J Gastroenterol 2010 Mar;105(3):500. DOI: https://doi.org/10.1038/ajg.2010.52. [DOI] [PubMed] [Google Scholar]
- 19.Moum B, Ekbom A, Vatn MH, et al. Clinical course during the 1st year after diagnosis in ulcerative colitis and Crohn’s disease. Results of a large, prospective population-based study in southeastern Norway, 1990–93. Scand J Gastroenterol. 1997 Oct;32(10):1005–12. doi: 10.3109/00365529709011217. [DOI] [PubMed] [Google Scholar]
- 20.Henriksen M, Jahnsen J, Lygren I, et al. IBSEN Study Group. Ulcerative colitis and clinical course: Result of a 5-year population-based follow-up study (the IBSEN study) Inflamm Bowel Dis. 2006 Jul;12(7):543–50. doi: 10.1097/01.mib.0000225339.91484.fc. [DOI] [PubMed] [Google Scholar]
- 21.Höie O, Wolters F, Riis L, et al. European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) Ulcerative colitis: Patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007 Aug;102(8):1692–701. doi: 10.1111/j.1572-0241.2007.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Solberg IC, Lygren I, Jahnsen J, et al. IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: Results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44(4):431–40. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 23.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut. 2006 Jun;55(6):749–53. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bebb JR, Scott BB. How effective are the usual treatments for ulcerative colitis? Aliment Pharmacol Ther. 2004 Jul;20(2):143–9. doi: 10.1111/j.1365-2036.2004.02018.x. [DOI] [PubMed] [Google Scholar]
- 26.Magro F, Rodrigues A, Vieira AL, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012 Mar;18(3):573–83. doi: 10.1002/ibd.21815. [DOI] [PubMed] [Google Scholar]
- 27.Kitano A, Okawa K, Nakamura S, Komeda Y, Ochiai K, Matsumoto T. [The long-term assessment of the patients with ulcerative colitis (> 10 years follow-up, mean follow-up 21.7 years)]. [Article in Japanese; abstract in English] Journal of New Remedies & Clinics. 2011;60(7):1347–55. [Google Scholar]
- 28.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987 Dec 24;317(26):1625–9. doi: 10.1056/nejm198712243172603. [DOI] [PubMed] [Google Scholar]
- 29.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical activity index. Gut. 1998 Jul;43(1):29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travis SP, Higgins PD, Orchard T, et al. Review article: Defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011 Jul;34(2):113–24. doi: 10.1111/j.1365-2036.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- 31.Meucci G, Fasoli R, Saibeni S, et al. IG-IBD. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: A prospective, multicenter study. Inflamm Bowel Dis. 2012 Jun;18(6):1006–10. doi: 10.1016/s1590-8658(06)80327-0. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg L, Lawlor GO, Zenlea T, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013 Mar-Apr;19(4):779–84. doi: 10.1097/mib.0b013e3182802b0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monstad I, Hovde O, Solberg IC, Moum B. Clinical course and prognosis in ulcerative colitis: Results from population-based and observational studies. Ann Gastroenterol. 2014;27(2):95–104. [PMC free article] [PubMed] [Google Scholar]
- 34.Prosberg MV, Vester-Andersen MK, Andersson M, et al. Long-term compliance with oral 5-aminosalicylic acid therapy and risk of disease recurrence in patients with ulcerative colitis: A population-based cohort study. Inflamm Bowel Dis. 2016 Apr;22(4):925–32. doi: 10.1097/mib.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 35.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut. 2004 Oct;53(10):1479–84. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiba M, Tsuda S, Komatsu M, Tozawa H, Takayama Y. Onset of ulcerative colitis during a low-carbohydrate weight-loss diet and its treatment with a plant-based diet: A case report. Perm J. 2016 Winter;20(1):80–4. doi: 10.7812/TPP/15-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr J. 2014 Jan 16;13:5. doi: 10.1186/1475-2891-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obih C, Wahbeh G, Lee D, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016 Apr;32(4):418–25. doi: 10.1016/j.nut.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Prince AC, Myers CE, Joyce T, Irving P, Lomer M, Whelan K. Fermentable carbohydrate restriction (low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016 May;22(5):1129–36. doi: 10.1097/mib.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 40.Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology. 2017 Feb;152(2):398–414. doi: 10.1053/j.gastro.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Sommer F, Rühlemann MC, Bang C, et al. Microbiomarkers in inflammatory bowel diseases: Caveats come with caviar. Gut. 2017 Oct;66(10):1734–8. doi: 10.1136/gutjnl-2016-313678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnorr SL, Candela M, Rampelli S, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014 Apr 15;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: The deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014 Nov 4;20(5):779–86. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson HL, Campbell BJ. Review article: Dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015 Jul;42(2):158–79. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai MS, Seekatz AM, Koropatkin NM, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016 Nov 17;167(5):1339–53.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Cancer Research Fund; American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: A global perspective [Internet] Washington, DC: American Institute for Cancer Research; 2007. [cited 2018 Feb 14]. Available from: www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf. [Google Scholar]
- 48.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: A review. Public Health Nutr. 2012 Dec;15(12):2287–94. doi: 10.1017/s1368980012000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: Plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013 Jul 8;173(13):1230–8. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998 Dec 16;280(23):2001–7. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- 52.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1097/01.ogx.0000055759.75837.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S. Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev. 2013 Feb;28(2):CD008722. doi: 10.1002/14651858.cd008722.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimizu K, Koyama A, Ogihara C, et al. [Educational hospitalization programs for diabetes patients by Sapporo General Hospital: Effectiveness of short-term physical exercise for type 2 diabetes mellitus]. [Article in Japanese; abstract in English] Hokkaido Journal of Physical Therapy. 2001 Apr 20;18:33–9. [Google Scholar]