Abstract
PURPOSE
We aimed to compare the clinical effectiveness of combination therapy of transarterial chemoembolization (TACE) and microwave ablation (MWA) with TACE monotherapy in BCLC stage B HCC patients with tumor size ≤7 cm and tumor number ≤5.
METHODS
We retrospectively reviewed 150 BCLC stage B HCC patients who had received TACE monotherapy or TACE-MWA combination therapy in our hospital from March 2007 to April 2016. The patients were matched by propensity score at the ratio of 1:2 by optimal method. The median follow-up period was 16 months. The overall survival, tumor response and progression-free survival were compared between the two groups by Kaplan–Meier method and Log rank test.
RESULTS
Tumor response (complete or partial response or stable disease) rates at 6, 12, 18, 24 months were 55.5%, 37.3%, 21.3%, 15.8% for TACE group, and 74%, 47.8%, 35%, 31.8% for TACE-MWA group, respectively. The survival rates at 1, 3, 5 years were 77.5%, 42.1%, 21% for TACE group and 93.1%, 79%, 67.7% for TACE-MWA group, respectively. Compared with TACE group, the TACE-MWA group had significantly improved progression-free survival (P = 0.044) and overall survival (P = 0.002).
CONCLUSION
TACE-MWA combination therapy has better clinical effectiveness than TACE monotherapy in BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5.
Hepatocellular carcinoma (HCC) ranks third as the most deadly cancer and sixth as the most prevalent cancer (1). The Barcelona Clinic Liver Cancer (BCLC) staging includes four stages, among which BCLC stage B patients are defined as asymptomatic multinodular HCC patients without vascular invasion or metastasis (2). EASL-EORTC Clinical Practice Guidelines, widely used for the management of hepatocellular carcinoma, recommends transarterial chemoembolization (TACE) as the standard treatment for BCLC stage B patients (3).
The TACE procedure involves the infusion of chemotherapy agents and lipiodol, followed by the embolization of the tumor’s supplying artery. As a result, TACE produces both cytotoxic and ischemia effects within the tumor tissue (4). However, the effect of TACE monotherapy for BCLC stage B patients is less than satisfactory with frequent progression of tumors. Furthermore, a Cochrane systemic review challenged the clinical effectiveness of TACE monotherapy in unresectable HCC, citing insufficient evidence (5).
Microwave ablation (MWA), a thermal ablation using electromagnetic waves, can be used for the treatment of HCC. As the tumor is fully covered by the coagulation zone with an acceptable safety margin, MWA may act as a potential curative substitute to surgery for patients with unresectable HCC.
A consensus made by an International Expert Panel on Interventions in Hepatocellular Carcinoma in Hong Kong in September 2011 stated that in order to prolong survival and improve outcomes for the BCLC stage B HCC patients, combination therapy including TACE therapy should be used (6). Two previous retrospective studies found that combination therapy of TACE and MWA led to better outcome than TACE monotherapy for specific HCC patients (7, 8), but their studies did not specify the BCLC stage of included patients. Currently, no clinical guideline clearly defines the indication of combination therapy in BCLC stage B patients.
We compared the tumor response, progression-free survival and overall survival of TACE monotherapy with TACE-MWA combination therapy using propensity score matching. Subgroup analysis was also performed based on tumor size and number. The primary aim of our study was to compare the clinical effectiveness of TACE-MWA combination therapy with TACE monotherapy in BCLC stage B HCC patients with tumor size ≤7 cm and tumor number ≤5.
Methods
Study design and selection criteria
The study was reviewed and approved by the institutional review board of our hospital and informed consent was obtained from all included patients. We retrospectively reviewed BCLC stage B patients’ data from March 2007 to April 2016. The inclusion criteria were: a) BCLC stage B HCC diagnosed by EASL guideline (2); b) TACE as initial treatment; c) potential candidates for MWA evaluated by two interventional radiologists with more than 10 years’ experience 1 month after the initial TACE treatment; d) Child-Pugh A or B. Exclusion criteria were: a) tumor size >7 cm and tumor number >5 before the initial TACE treatment; b) lost to follow-up 1 month after the initial TACE treatment.
TACE-MWA group included patients who received MWA anytime between the second and fourth treatment sessions after confirmed diagnosis. The patients who received only TACE were included in the TACE group. The treatment decision following the initial TACE was made jointly by doctors and patients. In our study, each case was fully discussed by a multidisciplinary tumor board including interventional radiologists, oncologists and hepatic surgeons. After the discussion, treatment options based on tumors size, number, histologic analysis, and hepatic function were presented to the patient. The treatment decision was finally made after the informed consent was signed by the patient.
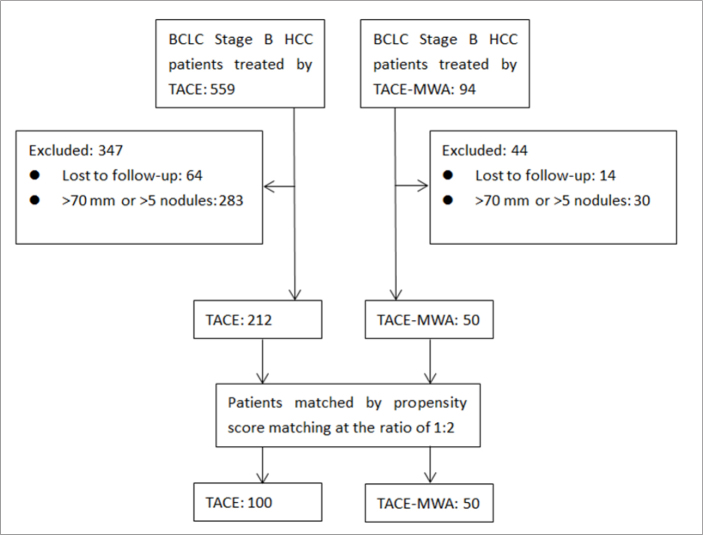
Propensity score matching was performed using optimal matching method at the ratio of 1:2 (9), and according to the study design and selection criteria described above, 100 patients were included in TACE group and 50 patients were included in TACE-MWA group (Fig. 1).
Figure 1.
Patient selection procedure.
Transarterial chemoembolization
A 5F catheter was introduced through the femoral artery by Seldinger technique and the angiogram of abdominal vessels was performed to visualize the arterial supply of the tumor. Depending on the tumor size, location, number, and vascular supply, the super-selective microcatheter was inserted into the supplying artery of the tumor. Then a combination of lipiodol (5–10 mL), lobaplatin (50 mg) and pirarubicin (30 mg) was introduced into the tumor followed by gelatin sponge particles embolization. An additional angiogram was performed at the end of the procedure to ensure full embolization of the supplying artery.
Microwave ablation
The MWA device used in the study was FORSEA (Qinghai Microwave Electronic Institute) with an MTC-3 microwave generator (FORSEA, 2450 MHz). At the beginning of the procedure, the patient was put into deep sedation and a contrast-enhanced computed tomography (CT) scan was performed at supine position to evaluate the tumor location, size, number and the optimal puncture point. After the local anesthesia of the puncture area, a 15G microwave antenna with water circulation cooling system was inserted into the tumor. The microwave was set at 50–100 W for 10–20 minutes. At the end of the procedure, an additional CT scan was performed to ensure full ablation of the residual tumor.
Follow-up
One month after the first TACE treatment session, contrast-enhanced CT or magnetic resonance imaging (MRI) was performed to evaluate treatment effect and detect residual viable tumor. Residual viable tumor was defined as uptake of contrast agent in the arterial phase of CT or MRI with a longest diameter no less than 1 cm according to modified Response Evaluation Criteria in Solid Tumors (mRECIST) assessment (10). If residual viable tumor was found, additional TACE or MWA treatment sessions were considered. The follow-up interval of the patients with satisfactory treatment effect ranged from 3 to 6 months and tumor response was evaluated according to mRECIST (10).
Statistical analysis
The R software package (version 3.4.2, The R Foundation for Statistical Computing) was used in propensity score matching. The propensity score model was determined by previous studies (8, 11), the independent variables used in the matching included gender, age, alpha-fetoprotein (AFP) level, Child-Pugh score, alanine aminotransferase (ALT), tumor size and tumor number. Propensity score matching was performed using optimal matching method at the ratio of 1:2 (9). The SPSS software package (version 20.0.0, SPSS Inc.) was used for the statistical analysis. Patients’ baseline characteristics were analyzed by Pearson chi-square tests or Fisher’s exact test (expected cell counts <5) before and after matching. The overall survival curves and the progression-free survival curves were plotted by Kaplan-Meier method and compared by Log rank test. The Cox regression model was used in the multivariate analysis of progression risk factors. In the comparison of complications between TACE and TACE-MWA group, Pearson chi-square tests or Fisher’s exact test (expected cell counts <5) were used. All statistical tests used in this study were two-sided, and the difference was considered as statistically significant for P < 0.05.
Results
Before propensity score matching, maximal tumor size and tumor number were significantly different between the two groups; however, after matching, no statistically significant difference was found on any covariate (Table 1).
Table 1.
Patients’ baseline characteristics before and after matching
| Before matching | Matched | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| TACE n=212 |
TACE-MWA n=50 |
P | TACE n=100 |
P | ||
| Gender | Male | 195 (92) | 43 (86) | 0.183a | 91 (91) | 0.350 |
| Female | 17 (8) | 7 (14) | 9 (9) | |||
|
| ||||||
| Age (years) | ≤55 | 83 (39) | 22 (44) | 0.529 | 42 (42) | 0.815 |
| >55 | 129 (61) | 28 (56) | 58 (58) | |||
|
| ||||||
| ALT (U/L) | ≤56 | 103 (49) | 27 (54) | 0.491 | 50 (50) | 0.644 |
| >56 | 109 (51) | 23 (46) | 50 (50) | |||
|
| ||||||
| AFP (ng/mL) | ≤25 | 78 (37) | 22 (44) | 0.345 | 41 (41) | 0.726 |
| >25 | 134 (63) | 28 (56) | 59 (59) | |||
|
| ||||||
| Child-Pugh score | A | 184 (87) | 46 (92) | 0.312 | 94 (94) | 0.732a |
| B | 28 (13) | 4 (8) | 6 (6) | |||
|
| ||||||
| Tumor size (mm) | ≤50 | 110 (52) | 36 (72) | 0.010 | 73 (73) | 0.897 |
| >50 | 102 (48) | 14 (28) | 27 (27) | |||
|
| ||||||
| Tumor number | ≤3 | 59 (28) | 23 (46) | 0.013 | 46 (46) | 1.000 |
| >3 | 153 (72) | 27 (54) | 54 (54) | |||
Data are presented as n (%).
TACE, transarterial chemoembolization; TACE-MWA, combination therapy of transarterial chemoembolization and microwave ablation; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
Fisher’s exact test was performed for expected cell count <5.b
The median follow-up period was 16.2 months for the whole population (TACE group: 16.1 months, TACE-MWA group: 17.5 months), ranging from 1 to 98.7 months (TACE group: 1.47–65.5 months, TACE-MWA group: 1–98.7 months). The median maximum tumor size was 42 mm in the whole population (TACE group: 44 mm, TACE-MWA group: 40 mm), while the range was 5–70 mm (TACE group: 5–70 mm, TACE-MWA group: 16–68 mm).
The median number of TACE treatment sessions was 2 (range, 1–5) in TACE group and 2 (range, 1–3) in TACE-MWA group, while the median number of MWA treatment sessions was 1 (range, 1–5) in TACE-MWA group.
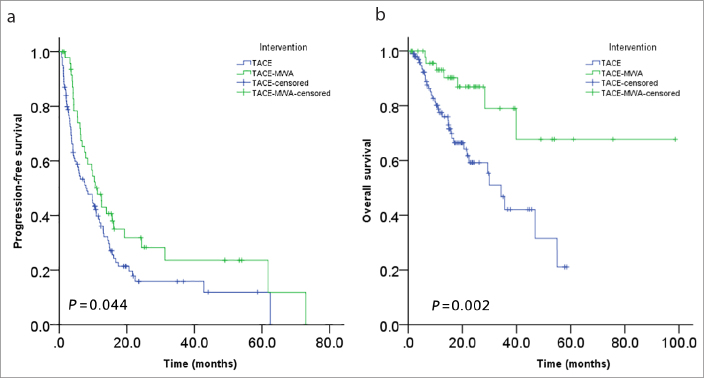
The median time of tumor progression was 6.16 months in TACE group and 10.14 months in TACE-MWA group. The tumor control rates (including complete and partial response and stable disease) at 6, 12, 18, 24 months were 55.5%, 37.3%, 21.3%, 15.8% for TACE group, while 74%, 47.8%, 35%, 31.8% for TACE-MWA group. Progression-free survival rate was significantly higher in TACE-MWA group than in TACE group (P = 0.044, Fig. 2a).
Figure 2 a, b.
Comparisons of the progression-free survival (a) and overall survival (b) of TACE and TACE-MWA groups.
Multivariate analysis using Cox regression showed that tumor size and intervention were independent prognostic factors associated with tumor progression (Table 2).
Table 2.
Multivariate analysis of progression risk factors using Cox regression model
| P | HR | 95% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.655 | 0.869 | 0.470 | 1.609 |
| Age | 0.347 | 1.213 | 0.811 | 1.815 |
| ALT | 0.504 | 1.147 | 0.767 | 1.716 |
| AFP | 0.073 | 0.676 | 0.440 | 1.037 |
| Child-Pugh score | 0.448 | 0.763 | 0.380 | 1.533 |
| Tumor size | 0.001 | 1.025 | 1.010 | 1.041 |
| Tumor number | 0.420 | 1.096 | 0.877 | 1.371 |
| Intervention | 0.049 | 1.518 | 1.002 | 2.299 |
CI, confidence interval; HR, hazard ratio; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
The median survival of TACE and TACE-MWA groups were 14.8 months and 18.5 months, respectively. Survival rates at 1, 3, 5 years were 77.5%, 42.1%, 21% for TACE group, while 93.1%, 79%, 67.7% for TACE-MWA group, respectively. The overall survival of TACE-MWA group was significantly higher than that of TACE group (P = 0.002, Fig. 2b). Multivariate analysis using Cox regression showed that AFP level, intervention, and tumor size were independent prognostic factors associated with overall survival (Table 3).
Table 3.
Multivariate analysis of overall survival risk factors using Cox regression risk model
| P | HR | 95% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.411 | 0.661 | 0.247 | 1.772 |
| Age | 0.865 | 1.060 | 0.541 | 2.077 |
| ALT | 0.297 | 1.439 | 0.726 | 2.851 |
| AFP | <0.001 | 0.206 | 0.092 | 0.459 |
| Child-Pugh score | 0.052 | 0.390 | 0.151 | 1.006 |
| Tumor size | <0.001 | 1.052 | 1.025 | 1.079 |
| Tumor number | 0.186 | 1.293 | 0.884 | 1.890 |
| Intervention | 0.002 | 4.171 | 1.719 | 10.116 |
CI, confidence interval; HR, hazard ratio; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
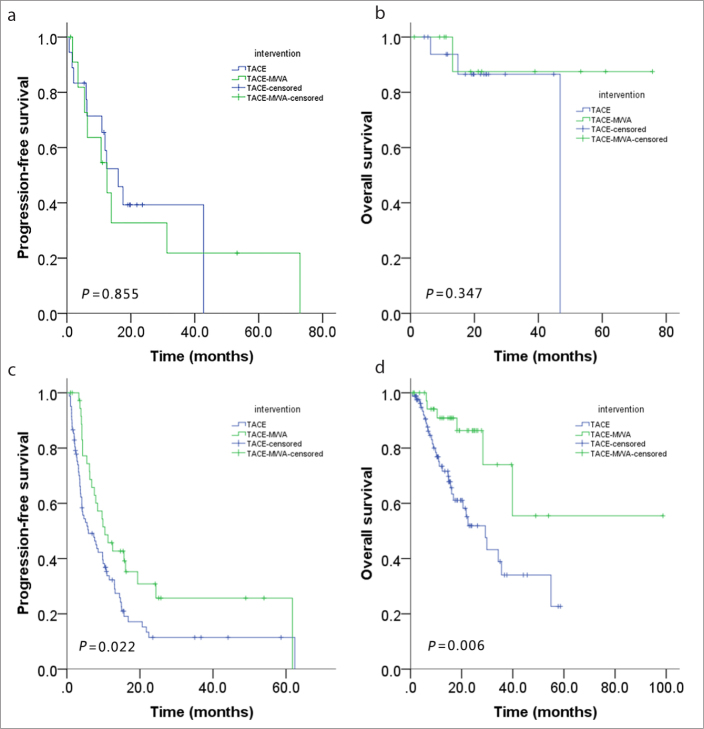
When tumor size was ≤3 cm, no significant statistical difference was found in progression-free survival (P = 0.855, Fig. 3a) or overall survival (P = 0.347, Fig. 3b) between the groups; but when tumor size was >3 cm and ≤7 cm, significant statistical difference existed in both progression-free survival (P = 0.022, Fig. 3c) and overall survival (P = 0.006, Fig. 3d).
Figure 3 a–d.
Subgroup analysis: progression-free survival of tumor size ≤3 cm (a), overall survival of tumor size ≤3 cm (b), progression-free survival of tumor size >3 cm (c), overall survival of tumor size >3 cm (d).
Neither tumor number ≤3 (P = 0.153) nor >3 (P = 0.313) showed significant statistical difference between the two groups.
We recorded adverse events according to Society of Interventional Radiology Clinical Practice Guidelines (12, 13). Minor complications included nausea, vomiting, fever, mild abdominal pain, and elevated liver enzymes. They were all transient and resolved within 1 week. Four patients suffered major complications in TACE group, including hypokalemia caused by severe vomiting, severe hepatic dysfunction and gastrointestinal bleeding. One patient in TACE-MWA group suffered pneumothorax due to the accidental puncture of the right lung during the MWA procedure. The major complications significantly prolonged hospitalization and required intensive care. However, all of them recovered in the end without any permanent adverse sequela. No statistically significant difference was observed between TACE and TACE-MWA groups in terms of complications (Table 4).
Table 4.
Complications analysis of TACE and TACE-MWA groups
| TACE group n=100 |
TACE-MWA group n=50 |
P | |
|---|---|---|---|
| Minor complications | 83 (83) | 43 (86) | 0.637 |
| Nausea, vomiting | 38 (38) | 13 (26) | 0.144 |
| Abdominal pain | 56 (56) | 35 (70) | 0.098 |
| Fever | 53 (53) | 22 (44) | 0.299 |
| Elevated liver enzyme | 25 (25) | 12 (24) | 0.893 |
|
| |||
| Major complications | 4 (4) | 1 (2) | 0.665a |
| Hypokalemia caused by severe vomiting | 1 (1) | 0 | 1.000a |
| Severe hepatic dysfunction | 2 (2) | 0 | 0.553a |
| Gastrointestinal bleeding | 1 (1) | 0 | 1.000a |
| Iatrogenic pneumothorax | 0 | 1 (0.5) | 0.333a |
Data are presented as n (%).
TACE, transarterial chemoembolization; TACE-MWA, combination therapy of transarterial chemoembolization and microwave ablation.
Fisher’s exact test was performed for expected cell count <5.
Discussion
Our study specifically focused on the comparison of clinical effectiveness between TACE-MWA combination therapy and TACE monotherapy in BCLC stage B HCC patients. We found that TACE-MWA combination therapy significantly improved tumor response, progression-free survival, and overall survival compared with TACE monotherapy in BCLC stage B HCC with tumor size ≤7 cm and tumor number ≤5.
According to EASL-EORTC Clinical Practice Guidelines, TACE monotherapy is the standard treatment for BCLC stage B patients (3). However, apart from the less than satisfactory clinical effectiveness of TACE monotherapy, some evidence has shown that TACE may not be the optimal choice for BCLC stage B patients (5, 14). A comment on Journal of Hepatology pointed out that the EASL-EORTC guideline recommendation might be based on the comparison of TACE to the best supportive therapy instead of other available treatments due to lack of robust evidence (15).
Insufficient evidence has been reported for BCLC stage B HCC patients on treatment selection, especially those who have larger tumor size and higher number of tumors. A consensus proposed combination therapy instead of TACE monotherapy (6), which differs from the recommendation of the widely used EASL-EORTC guideline (3). Previously, two retrospective studies demonstrated the superior treatment effectiveness of the TACE-MWA combination therapy compared with the TACE monotherapy for specific HCC patients (7, 8). However, their studies did not specify the BCLC stage and included HCC patients at BCLC stages A, B, and C. Our study focused mainly on BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5. We also performed subgroup analysis and showed that the statistical difference was mainly seen in the subgroup with tumor size of 3–7 cm, while the ≤3 cm subgroup shows no statistical difference. Our study suggests that the application of TACE-MWA combination therapy may extend to BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5.
In terms of radiofrequency ablation (RFA), a retrospective study in 2014 found that TACE and RFA combination therapy has better tumor progression survival and overall survival than TACE monotherapy in BCLC stage B HCC patients (16). Their study showed that tumor control rate at 6 months was 74.5% in TACE-RFA group while 54.5% in TACE monotherapy group, similar to our results (TACE-MWA: 74%; TACE: 55.5%). In contrast to the palliative effect of TACE, both MWA and RFA are potentially curative treatments. Compared with RFA, greater and faster energy can be generated by MWA within a larger ablation area. The improvement is mainly attributed to the reduction of heat-sink effect due to the vascular outflow of the liver (17). Furthermore, a randomized-controlled study in 2016 found that TACE-MWA combination therapy was more effective than TACE-RFA combination therapy in unresectable single-lesion HCC patients (18).
As for patients’ baseline characteristics, tumor size and number were significantly different between TACE and TACE-MWA groups before matching. By using propensity score matching, we managed to balance the covariate differences of the two groups and strengthen our causal argument.
For safety evaluation, the combination therapy of TACE and MWA did not significantly increase the risk of complications compared with TACE monotherapy (Table 4). Although minor complications were commonly seen in both TACE (83%) and TACE-MWA (86%) groups, they were transient with prompt recovery. Moreover, major complications were rare in both TACE (4%) and TACE-MWA (2%) groups. In general, both TACE monotherapy and TACE-MWA combination therapy are relatively safe treatments for BCLC stage B HCC with acceptable complications.
This study has several advantages: First, by applying propensity score matching, we managed to reduce the selection bias that is inherently present in retrospective studies. Second, we demonstrated that TACE-MWA group had better progression-free survival and overall survival than TACE group. Therefore, TACE-MWA combination therapy is recommended to BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5, especially for patients with tumor size >3 cm.
However, our study also has some limitations. First, this is a retrospective study without randomization, so we used propensity score matching to reduce the selection bias. Second, all treatments were performed in our hospital, which may lead to inevitable bias due to patients’ characteristics, doctors’ experience or equipment quality.
In conclusion, TACE-MWA combination therapy has better clinical effectiveness than TACE monotherapy in BCLC stage B patients with tumor size ≤7 cm and tumor number ≤5, especially for patients with tumor size >3 cm. However, further randomized controlled trials are needed for to confirm our findings.
Main points.
The progression-free survival of combined transarterial chemoembolization and microwave ablation therapy (TACE-MWA) was significantly higher than TACE monotherapy (P = 0.044).
The overall survival of combined TACE-MWA therapy was significantly higher than TACE monotherapy (P = 0.002).
Multivariate analysis using Cox regression showed that intervention (combined TACE-MWA or TACE monotherapy) was significantly associated with both tumor progression and overall survival.
Footnotes
The raw data in this paper has been successfully uploaded and locked onto Research Data Deposit (RDDA2018000564).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 3.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012;48:599–641. doi: 10.1016/j.ejca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–535. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 5.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011:D4787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JW, Amarapurkar D, Chao Y, et al. Consensus recommendations and review by an International Expert Panel on Interventions in Hepatocellular Carcinoma (EPOIHCC) Liver Int. 2013;33:327–337. doi: 10.1111/liv.12083. [DOI] [PubMed] [Google Scholar]
- 7.Xu LF, Sun HL, Chen YT, et al. Large primary hepatocellular carcinoma: transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-percutaneous microwave coagulation therapy. J Gastroenterol Hepatol. 2013;28:456–463. doi: 10.1111/jgh.12088. [DOI] [PubMed] [Google Scholar]
- 8.Chen QF, Jia ZY, Yang ZQ, Fan WL, Shi HB. Transarterial chemoembolization monotherapy versus combined transarterial chemoembolization-microwave ablation therapy for hepatocellular carcinoma tumors </=5 cm: A propensity analysis at a single center. Cardiovasc Intervent Radiol. 2017;40:1748–1755. doi: 10.1007/s00270-017-1736-8. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song MJ, Bae SH, Lee JS, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016;31:242–252. doi: 10.3904/kjim.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed M. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update: supplement to the consensus document. J Vasc Interv Radiol. 2014;25:1706–1708. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 13.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology Clinical Practice Guidelines. J Vasc Interv Radiol. 14:S199–S202. doi: 10.1097/01.RVI.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 14.Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82–88. doi: 10.1016/j.jhep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Roayaie S. TACE vs. surgical resection for BCLC stage B HCC. J Hepatol. 2014;61:3–4. doi: 10.1016/j.jhep.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer. 2014;14:849. doi: 10.1186/1471-2407-14-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Locoregional therapy of hepatocellular carcinoma. Clin Liver Dis. 2015;19:401–420. doi: 10.1016/j.cld.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28:1198–1203. doi: 10.1097/MEG.0000000000000688. [DOI] [PubMed] [Google Scholar]