Abstract
PURPOSE
We aimed to analyze computed tomography (CT) findings and medical records of patients diagnosed with median arcuate ligament syndrome (MALS) and evaluate possible risk factors associated with vascular complications that develop in patients with MALS.
METHODS
This retrospective study was approved by the institutional review board, and the requirement to obtain informed consent was waived. A total of 37 consecutive patients were diagnosed with MALS using both axial and sagittal CT reconstruction imaging at a single institution over a 7-year period. Dynamic contrast-enhanced CT data, medical records, and angiography results were reviewed.
RESULTS
Thirty-two (86.5%) patients were asymptomatic and incidentally diagnosed with MALS using CT. Seventeen (45.9%) patients exhibited significant arterial collateral circulations and nine (24.3%) were found to have splanchnic artery aneurysms, including one (2.7%) with acute bleeding secondary to aneurysm rupture. Peripancreatic vascular network including pancreaticoduodenal arcades and dorsal pancreatic artery was the most common site for development of both collateral circulations (16/22, 72.7%) and aneurysms (9/16, 56.3%). Splanchnic artery aneurysms were significantly more common in patients with collateral circulations (8/17, 47.1%) compared with those without collateral circulations (1/20, 5%) (P < 0.01). At least one peripancreatic vascular aneurysm was found in five of nine patients with splanchnic artery aneurysms (55.6%).
CONCLUSION
Splanchnic artery aneurysms are not uncommon in asymptomatic patients with collateral circulations caused by significant celiac trunk stenosis or obstruction due to median arcuate ligament. Therefore, careful imaging evaluation is necessary in patients with peripancreatic collateral circulations associated with MALS and regular follow-up is recommended for possibility of aneurysm development and rupture. Prophylactic endovascular treatment should be specifically performed in patients with pancreaticoduodenal arcade aneurysms to prevent life-threatening aneurysm rupture regardless of size.
Median arcuate ligament syndrome (MALS) is a clinically rare disease resulting from extrinsic compression of the celiac trunk by fibrous attachments of the diaphragmatic crura, median arcuate ligament, which results in various clinical symptoms including postprandial abdominal pain, vomiting, and/or weight loss. Although MALS was first reported in 1963 by Harjola (1), this disease entity remains challenging and controversial because a considerable number of patients who have celiac trunk compression by the median arcuate ligament (MAL) are asymptomatic and the degree of celiac trunk compression changes during the patient’s respiration (2–4). To date, there is no consensus as to whether asymptomatic patients should be included in MALS. Consequently, there are no clear guidelines for the treatment of MALS. Although patients have symptoms suspicious for MALS, this disease entity usually has a diagnosis of exclusion after ruling out other more common causes of symptoms (4). Most cases of MALS tend to be incidentally detected in patients who undergo CT or magnetic resonance imaging work-up for other clinical symptoms irrespective of MALS (5). However, MALS plays a major role in the development of celiac trunk stenosis or obstruction, which can cause changes in hemodynamic flow, and then, result in serious complications such as splanchnic artery aneurysms with their propensity to rupture (6–12).
In this retrospective study, we present our 7-year experience with MALS in a single institution and examine the risk factors associated with the development of splanchnic artery aneurysms in patients with MALS.
Methods
Patients
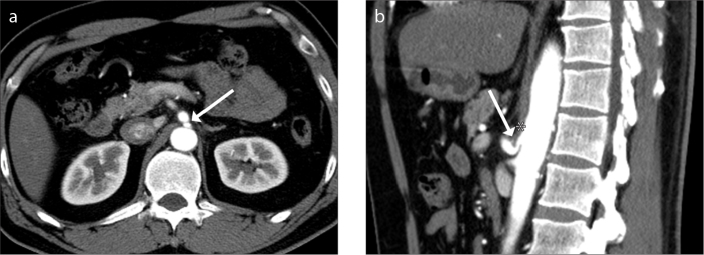
This retrospective study was approved by the authors’ institutional review board (protocol no. AJIRB-MED-MDB-17-264) and the requirement for informed consent from patients was waived. A keyword search of the electronic medical records and radiology reports in the picture archiving and communication system between August 2010 and July 2017 was performed using the terms “median arcuate ligament (MAL) or “celiac trunk compression”. Sixty-four patients were identified with MALS in either the electronic medical records or radiology reports. CT imaging performed in these patients was reviewed to confirm the radiologic diagnosis of MALS. The diagnostic CT criteria for MALS included the following: celiac trunk obstruction or stenosis ≥50%; hooked or U-shaped configuration of stenotic or expected course of the celiac trunk due to superior indentation by a thickened MAL using both axial and sagittal reconstructed CT images (Fig. 1) (6, 13). Patients were excluded if they had the following: 1) sagittal reconstructed imaging not available (n=12); 2) celiac trunk stenosis <50% (n=8); 3) celiac trunk stenosis due to reasons other than MALS (n=7). Hence, a total of 37 patients were finally included in this analysis.
Figure 1 a, b.
Representative case of a 48-year-old man diagnosed with median arcuate ligament syndrome (MALS). Contrast-enhanced arterial phase axial CT image (a) shows abrupt slit-like luminal narrowing (arrow) in the proximal celiac trunk at the fibrous attachment site of the diaphragmatic crura. Sagittal reconstructed arterial phase CT image (b) reveals hooked appearance of the proximal celiac trunk (arrow) due to MAL (asterisk) indenting the superior border of the celiac trunk.
Information regarding patient demographics, clinical characteristics, and clinical outcomes in cases requiring intervention were collected and reviewed from the electronic medical records.
All 37 patients underwent CT examinations using either a 16-channel (n=14) or 64-channel (n=23) scanner (Brilliance 16 or Ingenuity 64, Philips Medical Systems). CT scans were performed immediately after breath holding at end inspiration state in all patients. Therefore, there was a low risk for false-positive results or of exaggerated findings of MALS (14). After obtaining unenhanced CT images, two or three phased contrast-enhanced CT scans were performed with intravenous administration of nonionic contrast medium (Iopamiro 300, Bracco Imaging). Arterial phase images were obtained by adding 15 s to the time of peak abdominal aortic enhancement calculated at the hepatic hilum, and portal and delayed phase images were acquired 80 s and 150 s after contrast injection, respectively. The scan parameters for the 16- and 64-channel scanners were as follows: beam collimation, 0.75 mm and 0.625 mm; slice thickness, 5 mm and 3 mm; reconstruction interval, 5 mm and 3 mm; rotation time, 0.5 s; effective tube current-time charge, 200 mAs; and voltage, 120 kVp. Arterial phase images were obtained using axial and sagittal reconstruction, portal phase images with axial and coronal reconstruction, and unenhanced CT images with axial reconstruction.
Imaging analysis
Two board-certified abdominal radiologists with 12 and 5 years of experience retrospectively reviewed the CT images in consensus. Relevant imaging features, including celiac trunk obstruction (presence or absence) or stenosis (defined as diameter ≤4 mm) (13), the degree of celiac trunk stenosis (the ratio of the diameter of the post-stenotic dilated celiac trunk to the diameter of the most narrowed celiac trunk by MAL), presence (defined as diffuse engorged vascular structure compared with adjacent normal vascular diameter) and location of collateral circulation, presence, location, size (largest diameter), and number of splanchnic artery aneurysms, and findings of aneurysm rupture (defined as contrast extravasation or abdominal hematoma associated with aneurysm) were reviewed. Splanchnic artery aneurysm was defined as localized dilatation (>1.5 times the expected normal vascular diameter) of an artery according to Pasha et al. (8). Follow-up CT imaging was reviewed for comparison with the initial CT scan to evaluate any changes in the formerly evaluated CT imaging features. Therapeutic interventions were also reviewed including surgery and angiography with embolization.
Statistical analysis
All statistical analyses were performed by the institution’s statistician using R software (version 3.4.1.; R Foundation for Statistical Computing). Categorical values were compared by using Fisher’s exact test or chi-square test. Logistic regression analyses were used to identify factors that were associated with the development of collateral circulations and aneurysms. All reported P values were two sided, and P < 0.05 was considered to be statistically significant.
Results
Clinical and radiologic characteristics of the patients are presented in the Table. A total of 37 patients were radiologically diagnosed with MALS in the present study. The median age of the patients was 56 years (range, 24–84 years) with a male-to-female ratio of 22:15. Thirty-two patients (86.5%) had no typical symptoms related to MALS, such as postprandial abdominal pain, nausea, or vomiting, and they underwent CT for other clinical symptoms unrelated to MALS (n=19), cancer evaluation (n=8), or routine screening (n=5). Five patients (13.5%) had abdominal pain related to MALS, including one patient (2.7%) with bleeding due to aneurysm rupture. One patient also presented with weight loss of 9 kg during one-year period. Regarding medical history of hypertension or diabetes mellitus, body mass index, and the social habitus of alcohol consumption or cigarette smoking, there was no significant correlation with the development of collateral circulation or aneurysm formation (P ≥ 0.05).
Table.
Clinical and radiologic characteristics of the full cohort (n=37)
| Characteristics | Total |
|---|---|
| Age (years) | 56.1±13.8 |
|
| |
| Male gender | 22 (59.5) |
|
| |
| BMI (kg/m2) | 23.7±3.7 |
|
| |
| Associated factors | |
| Diabetes | 4 (26.5) |
| Hypertension | 12 (32.4) |
| Current smoker | 11 (29.7) |
| Alcohol consumption | 16 (43.2) |
|
| |
| Symptomatic | 5 (13.5) |
|
| |
| Radiologic findings of MALS | |
| Celiac trunk | |
| Stenosis | 30 (81.1) |
| Obstruction | 7 (18.9) |
| Collateral circulation | 17 (45.9) |
| Peripancreatic network | 14 (82.4) |
| Non-peripancreatic | 3 (17.6) |
| Aneurysm | 9 (24.3) |
| Peripancreatic network | 5 (55.6) |
| Non-peripancreatic | 4 (44.4) |
|
| |
| Treatments | 2 (5.4) |
| Curative surgery | 1 (50) |
| Coil embolization for bleeding | 1 (50) |
|
| |
| Follow-up period (months) | 23.3±28.4 |
|
| |
| Follow-up CT | 23 (62.2) |
Data are presented as n (%) or mean±standard deviation.
BMI, body mass index; MALS, median arcuate ligament syndrome.
Thirty patients (81.1%) had celiac trunk stenosis greater than 50%, while 7 patients (18.9%) exhibited complete celiac trunk obstruction. The average ratio of poststenotic dilated to narrowed celiac trunk was 2.8 (range, 1.6–7.4) in 30 patients with celiac trunk stenosis. In these patients, there was no significant association between the degree of celiac trunk stenosis (post-stenotic dilated to narrowed celiac trunk) and the development of collateral circulation and aneurysms (P > 0.05). Seventeen of 37 patients (45.9%) had 22 collateral circulations due to celiac trunk stenosis (n=14) or obstruction (n=8) due to MAL. All 7 patients with celiac trunk obstruction exhibited collateral circulations. The most common pathway for the development of collateral circulations was the peripancreatic network (16/22, 72.7%) including pancreaticoduodenal arcades (14/22, 63.6%) and dorsal pancreatic artery (2/22, 9.1%). The other 6 collateral circulations (6/22, 27.3%) were found in the arc of Buhler, splenic artery, jejunal artery, omental artery, hepatic artery, and left gastric artery.
Nine of 37 patients (24.3%) had 16 splanchnic artery aneurysms with a median size of 9.5 mm (range, 4.8–31.4 mm). Two patients had multiple aneurysms (7 in one patient, 2 in the other patient). The most common location for aneurysms was the pancreaticoduodenal arcades (9/16, 56.3%) followed by the common hepatic artery (2/16, 12.5%) and splenic artery (2/16, 12.5%). Dorsal pancreatic artery (1/16, 6.3%), jejunal artery (1/16, 6.3%), and left gastric artery (1/16, 6.3%) were found to have one aneurysm each (Figs. 2 and 3).
Figure 2 a, b.
A 48-year-old woman incidentally diagnosed with MALS at screening CT. Sagittal reconstructed arterial phase CT image (a) shows complete obstruction of the celiac trunk due to thickened MAL (asterisk) and the U-shaped configuration of the expected celiac trunk (dotted line). Coronal maximum intensity projection CT image (b) reveals collateral circulations via gastroduodenal artery (white arrowhead), pancreaticoduodenal arcades (black arrowheads), and dorsal pancreatic artery with aneurysms (arrows).
Figure 3 a, b.
A 40-year-old man presented with epigastric pain. Sagittal reconstructed arterial phase CT image (a) shows complete obstruction of the celiac trunk due to thickened MAL (asterisk) and the U-shaped configuration of the expected celiac trunk (dotted line), together with a large thrombosed aneurysm (arrowhead) and minimal narrowed superior mesenteric artery orifice. Superior mesenteric angiography (b) reveals collateral circulation and multiple aneurysms (arrowheads) in the pancreaticoduodenal arcades and retrograde contrast filling of the celiac trunk branch vessels with a synchronous splenic artery aneurysm (arrow).
Splanchnic artery aneurysms were more commonly found in patients with collateral circulations (8/17, 47.1%) compared with those without collateral circulations (1/20, 5%) (P < 0.01). Splenic artery (5.8 mm) aneurysm was discovered in only one patient without collateral circulations.
One patient presented with rupture of a pancreaticoduodenal arcade aneurysm measuring 6.6 mm together with collateral circulation of the pancreaticoduodenal arcade, causing a large amount of contrast extravasation, indicating acute hematoma in the abdominopelvic cavity (Fig. 4).
Figure 4 a–c.
A 52-year-old woman with sudden abdominal pain. Contrast-enhanced arterial phase axial CT image (a) shows an enhancing nodular structure suggesting aneurysm (arrow) in the peripancreatic area, and contrast extravasation indicating active bleeding (arrowheads) along the duodenum and mesentery with large amount of acute hematomas in the abdomen. Coronal maximum intensity projection image (b) reveals the ruptured aneurysm and contrast extravasation from the inferior pancreaticoduodenal arcade. Sagittal reconstructed arterial phase CT image (c) shows significant celiac trunk stenosis due to MAL (arrow) with large amount of mesenteric and retroperitoneal hematomas (asterisks).
The follow-up period between the CT examination and the last clinical follow-up or last CT examination ranged from 0 days to 56.4 months; three patients were lost to follow-up. Four of nine patients with aneurysms underwent follow-up CT and demonstrated neither size increase nor rupture in aneurysms during the follow-up CT, except for one presenting with a ruptured aneurysm. In 18 of 28 patients without aneurysm, there was no additionally developed collateral circulation or aneurysm formation during the follow-up CT examinations.
Two patients underwent angiography. One patient with active bleeding detected at CT (Fig. 4) underwent selective angiography of the celiac trunk and the superior mesentery artery. Celiac trunk stenosis with contrast extravasation of contrast material from the ruptured aneurysm arising in the anterior inferior pancreaticoduodenal artery was found. Selective embolization was performed for the anterior superior and inferior pancreaticoduodenal arteries using n-butyl cyanoacrylate glue and coil. The other patient underwent selective angiography of the superior mesentery artery. Retrograde filling of the completely obstructed celiac trunk from markedly hypertrophied pancreaticoduodenal arcades suggesting collateral circulation and multiple aneurysms up to 7 in number were found (Fig. 3b). Endovascular celiac recanalization or stenting failed because the wire could not pass the completely obstructed celiac trunk. Follow-up angiography performed after laparoscopic median arcuate ligament release revealed persistent complete celiac trunk obstruction. None of the remaining 35 patients underwent surgical or endovascular treatment.
Discussion
The results of our study demonstrated that collateral circulation occurred in nearly one-half of the patients who exhibited celiac trunk obstruction or significant stenosis due to MAL. Peripancreatic vascular network was the most common site for development of collateral circulation in addition to synchronous aneurysm occurrence.
Significant celiac trunk stenosis or obstruction should inevitably elicit the formation of the collateral circulation to maintain survival of the organs that its major branch vessels supply. Peripancreatic vascular network including the pancreaticoduodenal arcades and dorsal pancreatic artery becomes the best candidate via the superior mesenteric artery among the collateral pathways and provides the retrograde flow to the celiac trunk and its branch vessels (7, 9, 15). This abundant collateral circulation can prevent foregut ischemic pain, which is among the typical clinical manifestations that can occur in patients with MALS (16, 17, 18). Therefore, it is not surprising that only a small percentage of patients with MALS experience abdominal discomfort or pain, even though celiac ganglion compression by MAL or vascular steal phenomenon by collateral circulation are also associated with patient symptoms (19, 20).
Previous studies have reported that various degrees of celiac trunk compression can occur in 10% to 50% of the general population. This has led to a debate over whether this is a physiologic phenomenon or a pathologic disease process in attempting to diagnose MALS (4, 12). However, another study using angiography showed a MALS prevalence of only 7% in asymptomatic patients when including patients with hemodynamically significant celiac trunk stenosis greater than 50% (6). Accordingly, our study used the same diagnostic criteria as did previous studies (6, 11), but used CT imaging instead of angiography to diagnose MALS. We found only 37 patients with MALS in our institution during a recent 7-year period. This relatively low incidence of MALS may be due to the fact that it is generally difficult to detect celiac trunk stenosis with routine axial and coronal reconstructed CT images if there is no coexisting prominent and engorged collateral circulations for visual perception. Radiologists also tend not to report celiac axis stenosis with only collateral circulations unless accompanied by aneurysms in asymptomatic patients because they are not familiar with the clinical significance of this disease entity. Moreover, we only included patients with sagittal reconstructed CT images definitely demonstrating both acute downward angulation and hemodynamically significant stenosis of the celiac trunk by the MAL (14).
Our study found that splanchnic artery aneurysms more commonly occurred in patients with collateral circulations than without, and the pancreaticoduodenal arcade and dorsal pancreatic aneurysms accounted for approximately 56% of aneurysms. This result was consistent with a recent study by Nasr et al. (12). Once a collateral circulation through the smaller branches occurs, high blood flow rate continuously increases the shear stress on the arterial intimal layer, changes the medial layer responsible for the integrity and elasticity of vessels, weakens and dilates the arterial wall, which ultimately leads to the formation of a true aneurysm (21, 22). True pancreaticoduodenal arcade aneurysms are very rare and only constitute 2% of the splanchnic artery aneurysms (23). However, unlike other splanchnic artery aneurysms, pancreaticoduodenal arcade aneurysms are clinically important because of the high potential for spontaneous rupture, regardless of size (11, 12, 24). A recent study revealed that pancreaticoduodenal artery aneurysms with diameter as small as 2 mm can spontaneously rupture (11). This is in contrast to the other splanchnic artery aneurysms, which are associated with an increased risk for rupture in cases involving aneurysms greater than 2 to 2.5 cm in diameter (8). Furthermore, accumulating evidence revealed that 50% to 80% of the true pancreaticoduodenal arcade aneurysms are associated with celiac trunk stenosis or obstruction (22). MALS constitutes a major cause of celiac trunk stenosis or obstruction (25), whereas other conditions such as atherosclerosis or other connective tissue diseases are a minor cause. Therefore, if asymptomatic patients exhibit suspected celiac trunk stenosis or obstruction due to MAL at axial CT imaging, sagittal reconstructed CT imaging should be verified to confirm the diagnosis. At the same time, we should devote attention to the presence or absence of collateral circulations or aneurysms, and perform prophylactic endovascular treatment, such as coiling and/or stent placement to prevent rupture if pancreaticoduodenal arcade aneurysms, which are associated with mortality rate of 30% to 50%, are detected (21, 22, 24).
We hypothesized that post-stenotic dilatation of the celiac trunk already observed in patients with hemodynamically significant celiac trunk stenosis can be a predictor of collateral circulations or aneurysms. Accordingly, we used the ratio of the post-stenotic dilated to narrowed celiac trunk as an example of vascular remodeling. However, there was no significant association between the development of collateral circulations or aneurysms and the degree of celiac trunk stenosis in our study. In parallel with social habits including smoking and alcohol, a medical history of diabetes or hypertension, and body mass index, there was no significant correlation with the prevalence of collateral circulations or aneurysms. Celiac trunk stenosis by extrinsic indentation of the MAL is completely separate from the intrinsic vascular disease such as internal carotid or coronary arterial stenosis. Different pathophysiology may have an influence on the study results and the relatively small number of patients may hamper the investigation of association with previously predescribed risk factors.
This study has several limitations. First, we could not exclude the selection bias due to retrospective nature of the study. A significant number of asymptomatic patients with MALS, in whom there was no mention of MALS or celiac trunk compression syndrome in the radiology reports or medical records, could be omitted. Second, our study included only a small number of patients, which we believe was directly caused by selection bias. Additionally, we limited the diagnostic criteria of MALS to significant celiac trunk stenosis more than 50% or obstruction, and only included patients who underwent sagittal reconstructed CT imaging. Third, the long-term clinical and radiologic outcomes in many of our patients were not available. Additional long-term follow-up to evaluate risk stratification in developing collateral circulations or aneurysms in patients diagnosed with MALS is needed.
In conclusion, collateral circulations or aneurysms in patients with significant celiac trunk stenosis or obstruction due to MAL are not uncommon. A major proportion of patients with MALS are incidentally detected in asymptomatic patients at CT. Therefore, careful imaging evaluation is necessary in patients with peripancreatic collateral circulations associated with MALS and regular follow-up is recommended for possible aneurysm development and rupture. Prophylactic endovascular treatment should be performed in patients with pancreaticoduodenal arcade aneurysms due to the high potential for rupture, regardless of size.
Main points.
Of patients with median arcuate ligament syndrome (MALS), 87% were asymptomatic and incidentally diagnosed at CT.
Of patients with MALS, 46% exhibited significant arterial collateral circulations and 24% were found to have splanchnic artery aneurysms.
Pancreaticoduodenal arcades were the most common sites for the development of collateral circulation, as well as synchronous aneurysm occurrence.
Prophylactic endovascular treatment should be performed in patients with pancreaticoduodenal arcade aneurysms due to the high potential for spontaneous rupture, regardless of size.
Acknowledgements
The authors wish to acknowledge M.S. Soo Jin Kim for assistance in statistical analysis of this paper.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Harjola PT. A rare obstruction of the coeliac artery. Report of a case. Ann Chir Gynaecol Fenn. 1963;52:547–550. [PubMed] [Google Scholar]
- 2.Bron KM, Redman HC. Splanchnic artery stenosis and occlusion. Incidence; arteriographic and clinical manifestations. Radiology. 1969;92:323–328. doi: 10.1148/92.2.323. [DOI] [PubMed] [Google Scholar]
- 3.Szilagyi DE, Rian RL, Elliott JP, Smith RF. The celiac artery compression syndrome: does it exist? Surgery. 1972;72:849–863. [PubMed] [Google Scholar]
- 4.Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median arcuate ligament syndrome-review of this rare disease. JAMA Surgery. 2016;151:471–477. doi: 10.1001/jamasurg.2016.0002. [DOI] [PubMed] [Google Scholar]
- 5.Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177–1182. doi: 10.1148/rg.255055001. [DOI] [PubMed] [Google Scholar]
- 6.Park CM, Chung JW, Kim HB, Shin SJ, Park JH. Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol. 2001;2:8–13. doi: 10.3348/kjr.2001.2.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song SY, Chung JW, Kwon JW, et al. Collateral pathways in patients with celiac axis stenosis: angiographic–spiral CT correlation. Radiographics. 2002;22:881–893. doi: 10.1148/radiographics.22.4.g02jl13881. [DOI] [PubMed] [Google Scholar]
- 8.Pasha SF, Gloviczki P, Stanson AW, Kamath PS. Splanchnic artery aneurysms. Mayo Clin Proc. 2007;82:472–479. doi: 10.4065/82.4.472. [DOI] [PubMed] [Google Scholar]
- 9.Flood K, Nicholson AA. Inferior pancreaticoduodenal artery aneurysms associated with occlusive lesions of the celiac axis: diagnosis, treatment options, outcomes, and review of the literature. Cardiovasc Intervent Radiol. 2013;36:578–587. doi: 10.1007/s00270-012-0473-2. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong MB, Stadtlander KS, Grove MK. Pancreaticoduodenal artery aneurysm associated with median arcuate ligament syndrome. Ann Vasc Surg. 2014;28:741.e741–e745. doi: 10.1016/j.avsg.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Chivot C, Rebibo L, Robert B, Regimbeau J-M, Yzet T. Ruptured pancreaticoduodenal artery aneurysms associated with celiac stenosis caused by the median arcuate ligament: a poorly known etiology of acute abdominal pain. Eur J Vasc Endovasc Surg. 2016;51:295–301. doi: 10.1016/j.ejvs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Nasr LA, Faraj WG, Al-Kutoubi A, et al. Median arcuate ligament syndrome: a single-center experience with 23 Patients. Cardiovasc Intervent Radiol. 2017;40:664–670. doi: 10.1007/s00270-016-1560-6. [DOI] [PubMed] [Google Scholar]
- 13.Silveira LA, Silveira FB, Fazan VP. Arterial diameter of the celiac trunk and its branches. Anatomical study. Acta Cir Bras. 2009;24:43–47. doi: 10.1590/S0102-86502009000100009. [DOI] [PubMed] [Google Scholar]
- 14.Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34:93–115. doi: 10.1148/rg.341125010. http://[CrossRef/ [DOI] [PubMed] [Google Scholar]
- 15.Sutton D, Lawton G. Coeliac stenosis or occlusion with aneurysm of the collateral supply. Clin Radiol. 1973;24:49–53. doi: 10.1016/S0009-9260(73)80150-3. [DOI] [PubMed] [Google Scholar]
- 16.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Radium Ther Nucl Med. 1965;95:731–744. doi: 10.2214/ajr.95.3.731. [DOI] [PubMed] [Google Scholar]
- 17.Chou JW, Lin CM, Feng CL, Ting CF, Cheng KS, Chen YF. Celiac artery compression syndrome: an experience in a single institution in Taiwan. Gastroenterol Res Pract. 2012;2012:935721. doi: 10.1155/2012/935721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arazińska A, Polguj M, Wojciechowski A, Trębiński Ł, Stefańczyk L. Median arcuate ligament syndrome: predictor of ischemic complications? Clin Anat. 2016;29:1025–1030. doi: 10.1002/ca.22773. [DOI] [PubMed] [Google Scholar]
- 19.Bech FR. Celiac artery compression syndromes. Surgical Clin North Am. 1997;77:409–424. doi: 10.1016/S0039-6109(05)70558-2. [DOI] [PubMed] [Google Scholar]
- 20.Loukas M, Pinyard J, Vaid S, Kinsella C, Tariq A, Tubbs RS. Clinical anatomy of celiac artery compression syndrome: a review. Clin Anat. 2007;20:612–617. doi: 10.1002/ca.20473. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, Tachi Y, Ito S, et al. Endovascular management of ruptured pancreaticoduodenal artery aneurysms associated with celiac axis stenosis. Cardiovasc Intervent Radiol. 2008;31:1082–1087. doi: 10.1007/s00270-008-9343-3. [DOI] [PubMed] [Google Scholar]
- 22.Katsura M, Gushimiyagi M, Takara H, Mototake H. True aneurysm of the pancreaticoduodenal arteries: a single institution experience. J Gastrointest Surg. 2010;14:1409–1413. doi: 10.1007/s11605-010-1257-0. [DOI] [PubMed] [Google Scholar]
- 23.Kalva SP, Athanasoulis CA, Greenfield AJ, et al. Inferior pancreaticoduodenal artery aneurysms in association with celiac axis stenosis or occlusion. Eur J Vasc Endovasc Surg. 2007;33:670–675. doi: 10.1016/j.ejvs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 24.de Perrot M, Berney T, Deleaval J, Buhler L, Mentha G, Morel P. Management of true aneurysms of the pancreaticoduodenal arteries. Ann Surg. 1999;229:416–420. doi: 10.1097/00000658-199903000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mano Y, Takehara Y, Sakaguchi T, et al. Hemodynamic assessment of celiaco-mesenteric anastomosis in patients with pancreaticoduodenal artery aneurysm concomitant with celiac artery occlusion using flow-sensitive four-dimensional magnetic resonance imaging. Eur J Vasc Endovasc Surg. 2013;46:321–328. doi: 10.1016/j.ejvs.2013.06.011. [DOI] [PubMed] [Google Scholar]