Abstract
PURPOSE
We aimed to evaluate the success rate and complication occurrence of CT-guided localization of small pure ground-glass nodules (pGGNs) and mixed ground-glass nodules (mGGNs) with medical adhesive injection before video-assisted thoracoscopic surgery (VATS).
METHODS
From March 2015 to May 2017, 41 patients with 44 small pGGNs and mGGNs underwent CT-guided percutaneous localization with medical adhesive prior to wedge resection by VATS.
RESULTS
Localization with medical adhesive was successful in all patients (100%). The nodules (13 pGGNs, 31 mGGNs) had a mean maximal long-axis diameter of 9±4 mm and a mean distance of 10±7 mm from the most superficial edge of the nodule to the visceral pleura. The localization time was 16±8 minutes. There was a moderate inverse relationship between localization time and the nodule diameter (r= −0.42, P = 0.005). Thirty-three nodules with primary lung cancer were pathologically confirmed. There were 3 cases of pneumothorax (7%), 3 cases of parenchyma hemorrhage (7%) and 2 cases of irritable cough (5%), respectively. No conversion to thoracotomy was necessary in any patient.
CONCLUSION
CT-guided percutaneous localization with medical adhesive can label small pGGNs and mGGNs prior to VATS, with high success and low complication rates.
Screening low-dose computed tomography (CT) finds many pulmonary ground-glass nodules (GGNs), especially in the high-risk lung cancer population. For these nodules, video-assisted thoracoscopic surgery (VATS) has become the best choice for diagnostic and therapeutic purposes (1). However, detecting GGNs without preoperative localization can be challenging for surgeons. Similar problem is also encountered by pathologists for postoperative diagnosis. In VATS, solitary nodules less than 10 mm in diameter and located more than 5 mm deep from the pleural surface may be associated with a 63% chance of failure to be identified (2). The GGN, with its lower density than solitary nodule, is not easily detected in VATS, which leads to longer operation time spent looking for it. Therefore, preoperative localization is an important and necessary step for successful VATS. Compont® medical adhesive (the primary ingredient, n-butyl-cyanoacrylate) was certified by China Food and Drug Administration for human tissue adhesive closure. This agent provides rapid hemostasis and strong adherence to tissue, leading to fast wound closure at the lung puncture site. The aim of our study was to evaluate the efficacy and the safety of a new preoperative localization technique using Compont® medical adhesive.
Methods
This was a prospective cohort study. Patients who underwent CT-guided localization procedure applying Compont® medical adhesive prior to VATS at our institution were prospectively included from March 2015 to May 2017. Participants met the following criteria: presence of small pure GGNs (pGGNs) or mixed GGNs (mGGNs) in lung without any tumor history, with a recent increase in size or recently detected solid components requiring surgical removal; presence of stable pGGNs or mGGNs that are strongly requested to be removed based on suspicion of malignant transformation. All pGGNs and mGGNs were located in the periphery of the lobe with the maximal long-axis diameter ≤20 mm, the minimal long-axis diameter ≥5 mm and a maximum distance to the pleural surface of 30 mm. VATS was recommended for further treatment based on Fleischner criteria (2013). Preoperative localization procedure had to be done before VATS. The hospital institutional review board approved the study (20150104). Patient information and characteristics of pGGNs and mGGNs were collected.
Medical adhesive
Compont® medical adhesive is one of synthetic cyanoacrylates approved by China Food and Drug Administration, containing n-butyl-cyanoacrylate. It rapidly creates a thin elastic film of high tensile strength in the presence of anions in the blood, with a reticular structure that ensures firm adherence to tissues. It is applied not only to vascular embolization in digestive tract endoscopy treatment, interventional radiology and neuroradiology, but also to achieve hemostasis of diffuse bleeding in internal organs (3). The necessity, benefits and risks of preoperative localization procedure using the adhesive was explained to every patient. Different preoperative localization options were also discussed, including hook-wire placement. Each patient opted for the off-label use of medical adhesive and gave written informed consent.
Localization procedure
Localization procedure was performed with a multidetector (16-slice) CT helical scanner (GE lightspeed). The technical parameters were: 120 kV, 80 mAs, slice thickness 1.25 mm, reconstruction interval 1.25 mm. With the previous CT image used for guidance, patient was appropriately positioned, and a scaled marker with metal wires was used to determine if the puncture site was properly placed. Routine breathing instructions were not given during preliminary imaging or during localization procedure unless deemed necessary. An optimal puncture access route was designed by measuring the distance from the skin to the target localization point, envisioned as a circle 5–10 mm in diameter, with the GGN at the center (Figs. 1a and 2a). After sterilization of the skin and application of local anesthesia (1% lidocaine hydrochloride) at puncture entry site, the 21-gauge 150 mm long micropuncture needle (Cook Medical) was inserted percutaneously by a radiologist (Fig. 1b). Once the end of micropuncture needle was at the target localization point, the Compont® medical adhesive (0.3 mL; Beijing Compont Medical Devices Co.) was injected by hand at a flow rate of approximately 0.05 mL/s. The target localization point was not inside the nodule. The medical adhesive was injected in the region medial and posterior to the GGN, demonstrating a nearly 10 mm diameter round shape consolidation in the lung window of CT. There was no air in the injector. Any complication (i.e., pneumothorax, parenchymal hemorrhage) was confirmed by a final CT scan (Figs. 1c, 2b). The localization time was defined as duration in minutes from the beginning of first CT scan to the end of final CT scan. The localization procedure was scheduled 2–4 hours before VATS on the same day.
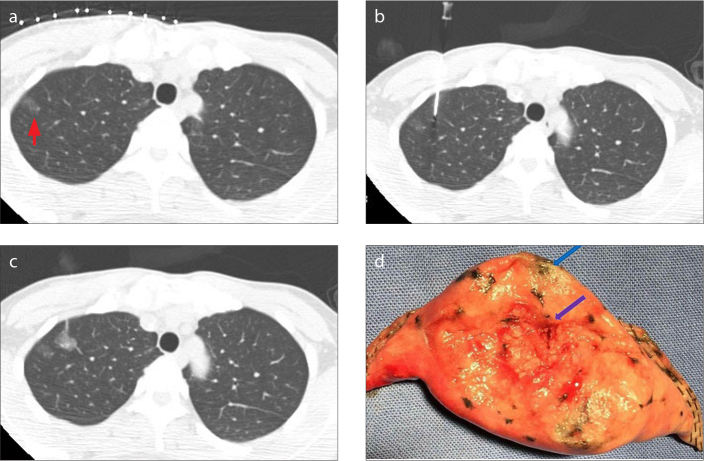
Figure 1 a–d.
A 42-year-old female had an 8 mm pulmonary GGN at 2 mm distance to the pleural surface in the right upper lobe. The patient had nodule localization by medical adhesive followed by wedge resection. Immediate frozen-resection histopathology revealed atypical adenomatous hyperplasia. There were no additional lobectomies. CT image (a) shows peripherally located GGN of the right upper lobe (red arrow). Image (b) shows CT-guided percutaneous localization and injection of Compont® medical adhesive using a 21-gauge micropuncture needle. Postprocedure CT image (c) shows the adhesive position marker near the target lesion. A gross specimen after wedge resection (d) shows the lesion (blue arrow) and the position marker (purple arrow).
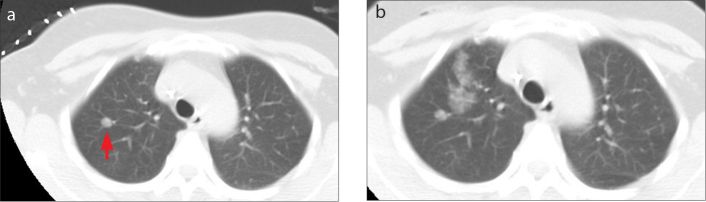
Figure 2 a, b.
A 44-year-old female had a 7 mm pulmonary mGGN at 22 mm distance to the pleural surface in the right upper lobe. The patient had nodule localization by medical adhesive. The procedure was complicated by parenchymal hemorrhage, but no intervention was needed. The histopathologic result was well-differentiated adenocarcinoma. CT image (a) shows deeply located pulmonary GNN of the right upper lobe (red arrow). Postprocedure CT image (b) shows parenchymal hemorrhage in the needle pathway which demonstrates slightly increased ground glass opacity.
Surgery
Wedge resection was performed according to standard surgical procedures. The target localization point injected with medical adhesive at lung parenchyma became a scar which made changes in both contour and color of visceral pleura (Fig. 1d). Finger palpation was applied to trace the scar when lung collapsed. The scar was pinched up and surrounding lung parenchyma was resected 10–30 mm down from the scar depending on measurement of the distance from GGNs to scar in the final CT image. Subsequent VATS lobectomy and mediastinal lymph node dissection were performed when the nodule was confirmed as malignant in pathology. Postoperative pathologic results were obtained for all nodules.
Statistical analysis
Patient and GGN characteristics were reported as mean ± standard deviation and percentages. Relationship between localization time, duration of VATS and GGN characteristics (i.e., diameter and distance to the pleural surface) were examined using Pearson’s correlation coefficient. P value less than 0.05 were considered statistically significant. All statistical analyses were performed with SPSS software v. 18 (SPSS Inc.).
Results
In total, 44 pulmonary GGNs (pure/mixed: 13/31) were identified in 41 patients (males/females: 12/29; mean age, 59±10 years). Thirty-eight patients had a single GGN, two patients had 2 GGNs in the ipsilateral lung and one patient had 2 GGNs in bilateral lungs; 64% of GGNs were in right lung. The GGNs had a mean diameter of 9±4 mm (range, 5–18 mm) with a mean distance to the pleural surface of 10±7 mm (range, 2–30 mm) (Table 1).
Table 1.
Patient, GGN, and localization procedure characteristics
| n (%) | |
|---|---|
| Gender | |
| Male | 12 (29) |
| Female | 29 (71) |
|
| |
| Age on day of procedure | |
| <45 years | 4 (10) |
| 45–60 years | 17 (41) |
| 61–70 years | 15 (37) |
| >70 years | 5 (12) |
|
| |
| Number of nodules | |
| 1 | 38 (93) |
| 2 | 3 (7) |
|
| |
| Location marked nodule | |
| Upper lobe left lung | 9 (20) |
| Lower lobe left lung | 7 (16) |
| Upper lobe right lung | 18 (41) |
| Middle lobe right lung | 2 (5) |
| Lower lobe right lung | 8 (18) |
|
| |
| Nodule type | |
| Pure | 13 (30) |
| Mixed | 31 (70) |
|
| |
| Nodule diameter on CT | |
| 0–5 mm | 7 (16) |
| 6–10 mm | 22 (50) |
| 11–15 mm | 12 (27) |
| 16–20 mm | 3 (7) |
|
| |
| Nodule distance to pleural surface | |
| 0–5 mm | 15 (34) |
| 6–10 mm | 13 (29) |
| 11–15 mm | 8 (18) |
| 16–20 mm | 3 (7) |
| 21–25 mm | 3 (7) |
| 25–30 mm | 2 (5) |
|
| |
| Localization time | |
| 10–15 min | 24 (59) |
| 15–20 min | 11 (27) |
| >20 min | 6 (14) |
|
| |
| Localization complications | |
| Pneumothorax | 3 (7) |
| Parenchymal hemorrhage | 3 (7) |
| Irritable cough | 2 (5) |
GGN, ground-glass nodule; CT, computed tomography.
All 44 GGNs were successfully localized with Compont® medical adhesive. The mean localization time was 16±8 min. In 6 patients procedure lasted more than 20 minutes, in part because of multiple GGNs in different lobes or different lungs. There was a moderate inverse relationship between localization time and diameter of GGN (r= −0.42, P = 0.005), but no relationship between localization time and distance of GGN to the pleural surface (r= −0.13, P = 0.394). Asymptomatic pneumothorax, parenchymal hemorrhage, and stimulus dry cough were observed in 3 patients (7%), 3 patients (7%), and 2 patients (5%), respectively. There was no other major complication such as hemopneumothorax or pulmonary embolism; also, there was no thoracotomy conversion to VATS surgery.
All 41 patients underwent the planned VATS. The mean duration of the VATS procedure was 147±58 min. There was no relationship between VATS duration and GGN diameter, distance of the nodule to the pleural surface and localization time (r= 0.23, P = 0.138; r= 0.10, P = 0.534; r= −0.14, P = 0.369; respectively). Pathologic examination revealed 33 primary lung cancers (Table 2). The proportions of malignant GGNs at different sizes were shown in Table 3.
Table 2.
Pathologic diagnosis of 44 GGNs
| n (%) | |
|---|---|
| NSCLC | |
| Moderately differentiated adenocarcinoma | 6 (13) |
| Well-differentiated adenocarcinoma | 13 (30) |
| MIA | 3 (7) |
| AIS | 11 (25) |
|
| |
| Benign | |
| AAH | 4 (9) |
| Inflammatory nodules | 3 (7) |
| Alveolar epithelial cell hyperplasia | 3 (7) |
| Intrapulmonary lymph node | 1 (2) |
GGN, ground-glass nodule; NSCLC, non-small cell lung carcinoma; MIA, minimally invasive adenocarcinoma; AIS, adenocarcinoma in situ; AAH, atypical adenomatous hyperplasia.
Table 3.
Malignancy rates in different size GGNs
| Diameter (mm) | n | Number of malignant nodules | Malignancy rate (%) |
|---|---|---|---|
| 0–5 | 7 | 4 | 57 |
| 6–10 | 22 | 16 | 73 |
| 11–15 | 12 | 11 | 92 |
| 16–20 | 3 | 2 | 67 |
GGN, ground-glass nodule.
Discussion
Following application of screening low-dose CT, an increasing number of GGNs have been found in otherwise healthy patients during routine checkups. The percentage of malignant lesions found in GGNs is higher than in solid pulmonary nodules. Most of these GGNs are adenocarcinomas, which was also demonstrated in our study (4). The 2011 international classification system of lung adenocarcinoma fully affirmed that these patients with adenocarcinoma in situ or minimally invasive adenocarcinoma are expected to have 100% or nearly 100% disease-specific survival following complete resections (5). However, GGNs are thoracoscopically invisible and impalpable due to their lower density, which hampers the development of VATS as an early intervention method. Effective preoperative localization techniques are helpful for successful VATS excision.
Dendo et al. (6) summarized preoperative localization techniques into three types: imaging modalities such as ultrasonography, injection of special agents, and hook-wire placement. Each technique has limitations that may prevent it from being widely adopted. Intraoperative ultrasonography has operator-dependence and lack of ability to detect lesions in emphysema lung (7). Lipiodol, as a water-insoluble contrast medium, can be injected by CT-guided puncture needle. However, it requires fluoroscopic guidance during VATS surgery and increases radiation exposure for operators (8). A failure rate of around 13% for methylene blue injection alone has been reported due to either an excess of liquid injected or an error in nodule localization (9). Hook-wire has been used for its low complication rate, but its dislodgement rate is rather high, up to 16% in some reports (10, 11). In addition, many lesions located close to the scapula or hilum are not suitable for hook-wire localization.
In 1995, Petsas, et al. (12) introduced fibrin glue as an agent to seal the track in fine-needle percutaneous lung biopsy. The severity of pneumothorax decreased in patients with percutaneous lung biopsy. Recently, medical adhesives have been widely used for rapid solidification of blood (13). They are nontoxic to human body and have no mutagenic, teratogenic, or carcinogenic impacts. It is harder to fall off on action of breathing than fibrin glue. With these strengths, we applied a medical adhesive in the current study for preoperative localization of GGNs. An artificial wound created in the lung by percutaneous puncture was patched by medical adhesive immediately, eventually forming a scar. Surgeons palpated the scar with the help of probe or finger and resected corresponding parenchyma in VATS procedure. In most cases, localization time was less than 20 minutes, similar to other localization studies (11).
Although the use of the medical adhesive in our study was off-label, the procedure was technically feasible and safe. All nodules were successfully localized without major complications. The conversion rate of VATS to thoracotomy was lower than other studies (11). In our study, minor hemorrhage, indicated by small areas of consolidation in the lung window on final CT scan, was found in 3 patients, with no cases of hemoptysis. Meanwhile, the incident rate of pneumothorax was also lower compared with other methods (14). No thoracic closed drainage or thoracic puncture procedures were required. A special complication in our study was irritable cough. Medical adhesive has a certain irritating odor, which brought out cough symptom when the injection rate was faster than 0.05 mL/s. Patients under this condition were suggested to take one piece of liquorice lozange and were relieved in a short time. The adhesive injected also did not raise the risk of severe adhesive, which was the usual main cause of long operative time. In our study, there was no difference in VATS duration even though localization was faster. The advantages of our new preoperative localization technique can be summarized as: 1) easy to operate and master for operators with CT-guided percutaneous lung puncture experience; 2) reduced incidence rate of complications; 3) ease of lesion detection during VATS without additional equipment and in specimen.
There are some limitations to our study. It was a single-center study and the sample size was relatively small. A randomized prospective multicenter study with large sample size should be conducted in the future.
In conclusion, our novel technique of using medical adhesive for preoperative localization of pulmonary GGNs has shown a high success and low complication rate. Pending the results of larger studies, this technique may be advocated as an option to label small pulmonary GGNs prior to VATS.
Main points.
Small pure and mixed ground-glass pulmonary nodules in lung can be localized successfully by medical adhesive before video-assisted thoracoscopic surgery.
CT-guided percutaneous localization with medical adhesive has low complication frequency.
This new preoperative localization technique using medical adhesive may be advocated as an option to label small pure and mixed ground-glass pulmonary nodules prior to video-assisted thoracoscopic surgery.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nor small cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265:431–437. doi: 10.1097/SLA.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115:563–568. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Zeng XQ, Ma ll, et al. Long-term efficacy of endoscopic ligation plus cyanoacrylate injection with or without sclerotherapy for variceal bleeding. J Dig Disease. 2016;17:252–259. doi: 10.1111/1751-2980.12331. [DOI] [PubMed] [Google Scholar]
- 4.Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol. 2002;178:1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002;225:511–518. doi: 10.1148/radiol.2252011025. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S, Hirata T, Ogawa E, et al. Ultrasonographic evaluation of small nodules in the peripheral lung during video-assisted thoracic surgery (VATS) Eur J Cardiothorac Surg. 2004;26:469–473. doi: 10.1016/j.ejcts.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg. 2006;132:320–324. doi: 10.1016/j.jtcvs.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Vandoni R, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labeling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14:265–270. doi: 10.1016/S1010-7940(98)00160-2. [DOI] [PubMed] [Google Scholar]
- 10.Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg. 2007;32:843–847. doi: 10.1016/j.ejcts.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Klinkenbery TJ, Dinjens L, Wolf RFE, et al. CT-guided percutaneous hookwire localization increases the efficacy and safety of VATS for pulmonary nodules. J Surg Oncol. 2017;115:898–904. doi: 10.1002/jso.24589. [DOI] [PubMed] [Google Scholar]
- 12.Petsas T, Siamblis D, Giannakenas C, et al. Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: part II--Clinical study. Cardiovasc Intervent Radiol. 1995;18:378–382. doi: 10.1007/BF00338305. [DOI] [PubMed] [Google Scholar]
- 13.Bhat YM. Tissue adhesives for endoscopic use. Gastroenterol Hepatol. 2014;10:251–253. [PMC free article] [PubMed] [Google Scholar]
- 14.Park CH, Han K, Hur J, et al. Comparative effectiveness and safety of preoperative lung localization for pulmonary nodules: a systematic review and meta-analysis. Chest. 2017;151:316–328. doi: 10.1016/j.chest.2016.09.017. [DOI] [PubMed] [Google Scholar]