Abstract
Magnetic resonance imaging (MRI) of the scrotum represents a useful supplemental imaging technique in the characterization of scrotal masses, particularly recommended in cases of nondiagnostic ultrasonographic findings. An accurate characterization of the benign nature of scrotal masses, including both intratesticular and paratesticular ones may improve patient management and decrease the number of unnecessary radical surgical procedures. Alternative treatment strategies, including follow-up, lesion biopsy, tumor enucleation, or organ sparing surgery may be recommended. The aim of this pictorial review is to present how MRI helps in the characterization of sonographically indeterminate scrotal masses and to emphasize the key MRI features of benign scrotal masses.
Ultrasonography (US), including conventional gray-scale and color Doppler US represents the primary examination for the assessment of scrotal masses (1–3). However, a confident characterization of their nature is not always possible, based on the conventional US findings alone (4–23).
Magnetic resonance imaging (MRI) of the scrotum has emerged as an important supplemental diagnostic modality for the assessment of scrotal diseases (4–24). Because of the advantages of the technique, namely the wide field of view, the simultaneous assessment of testes, paratesticular spaces and spermatic cords, the absence of radiation exposure and the high contrast and spatial resolution, MRI of the scrotum may provide useful diagnostic data in the detection, localization, and characterization of scrotal masses (4–24). Based on the recommendations of the Scrotal and Penile Imaging Working Group of the European Society of Urogenital Radiology, MRI is recommended as an adjunct diagnostic tool for the characterization of both intratesticular and paratesticular masses, especially when sonographic findings are equivocal (24). MRI is also recommended in the rare cases when discrimination between intratesticular and paratesticular masses is impossible, based on the sonographic characteristics alone (24). The added information provided by MRI may often allow a more conservative therapeutic plan to be used, obviating the need for surgical explorations or radical orchiectomy. When sonographic features are indeterminate, MRI has been reported to reduce healthcare costs, improving patient management (4–24).
Recently, preliminary data on functional MRI techniques, including dynamic contrast-enhanced MRI (DCE-MRI) (25, 26), diffusion-weighted imaging (DWI) (27, 28), magnetization tensor imaging (29) and diffusion tensor imaging (30) have reported useful diagnostic information in the assessment of various scrotal diseases.
The MRI protocol of the scrotum should include transverse T1-weighted images, transverse and coronal T2-weighted images with thin sections, transverse diffusion-weighted images with at least three b values (0, 400–500, and 800–1000 s/mm2) and coronal subtracted DCE-MRI (24). Patients should be examined in the supine position, with the testes elevated by means of a towel placed between the thighs and the penis raised to the anterior abdominal wall. Patients should be placed in the MRI unit with the feet first and the use of surface coils is recommended (24).
Normal findings
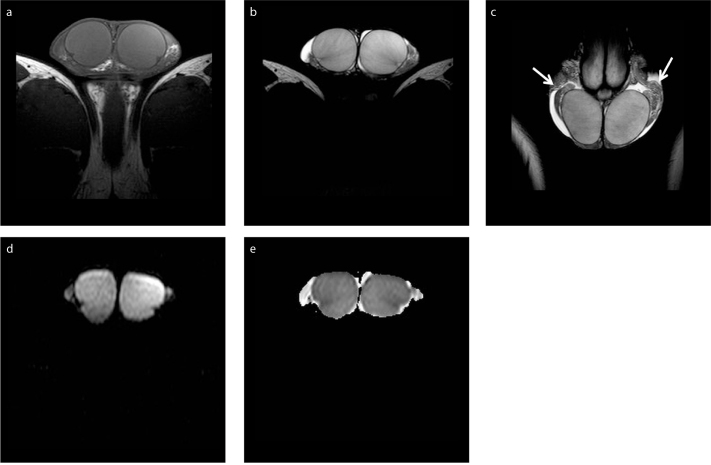
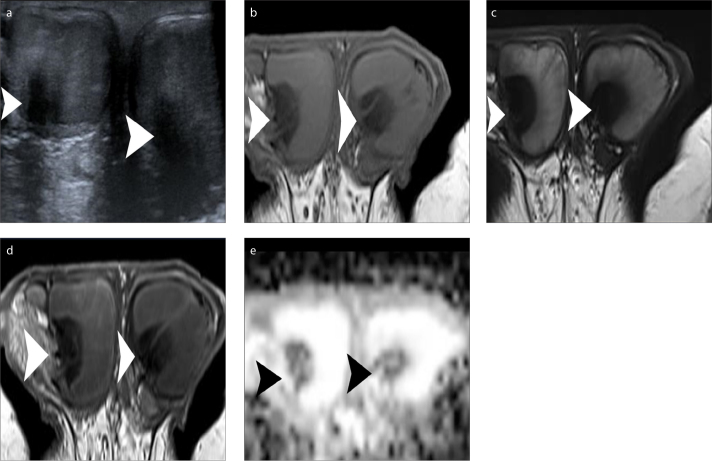
Normal testes appear as well-delineated, homogeneous structures, with T1 signal similar to that of skeletal muscles and high T2 signal (4, 9, 12, 14, 17). The internal architecture of the testis is nicely depicted on T2-weighted imaging. The tunica albuginea is seen around the testis as a thin, hypointense rim on both T1 and T2 pulse sequences, better defined on T2-weighted image (Fig. 1). Thin septa of low T2 signal are usually seen coursing through the testicular parenchyma toward the mediastinum testis, which is detected as a low signal intensity area in the posterior parts of the testis. Normal testes have high and slightly low signal on high b value DWI and apparent diffusion coefficient (ADC) maps, respectively, due to the histologic complexity of normal parenchyma (Fig. 1d, 1e) (27, 28). Normal testicular parenchyma enhances moderately and homogeneously (25, 26).
Figure 1 a–e.
Normal MRI findings in a 21-year-old man. Axial T1-weighted image (a) shows testes mainly isointense to the muscles. T2-weighted image in axial (b) and coronal (c) orientation depicts hyperintensity of normal testicular parenchyma. Thin, hypointense septa are seen traversing normal testes. The tunica albuginea is clearly depicted as a thin, hypointense halo surrounding the testes. Parts of the epididymis (arrows) are demonstrated bilaterally, slightly heterogeneous, mainly of low signal intensity. Small bilateral hydrocele is also seen, as a normal finding. Axial DWI (b=900 s/mm2) image (d) and the corresponding ADC map (e) show normal testes as hyperintense and slightly hypointense, respectively. The ADC (×10−3 mm2/s) of the right and left testis is 0.91 and 0.86, respectively.
The epididymis is slightly heterogeneous, with T1 signal similar to that of testis. It has lower signal intensity compared with that of the adjacent testicular parenchyma on T2-weighted imaging (Fig. 1c). The scrotal wall is typically of low signal intensity on both pulse sequences. The spermatic cords are detected mainly hyperintense, due of the presence of fat, with hypointense vessels coursing through them, better depicted on coronal T2-weighted imaging. A small hydrocele, often seen, represents a normal finding (4, 9, 12, 14, 17).
Intratesticular masses
Although the majority of intratesticular masses are malignant, a probable diagnosis of various benign intratesticular mass lesions, including lipoma, tubular ectasia of the rete testis (TERT), epidermoid cyst, hematoma, segmental testicular infarction, fibrosis and granulomatous orchitis based on imaging findings may improve patient management and reduce the number of unnecessary radical surgical explorations. Alternative treatment planning including follow-up, lesion biopsy, tumor enucleation, and testis sparing surgery may be acceptable (4–17). MRI is mainly recommended as a problem-solving modality when sonographic findings are indeterminate (4, 5, 7–17, 24). The technique might provide valuable diagnostic information in the characterization of benign intratesticular masses by detecting the presence of fat, fluid, hemorrhage, fibrous tissue, and contrast-enhancing elements (4–17).
There are several, albeit small series where the use of MRI has been proved to be superior to US in testicular mass lesion characterization, with alterations in patient management in 80%–90% of cases (5, 7, 8, 13). Cramer et al. (5) evaluated 200 patients with scrotal diseases on a 1.5 T system, including various benign scrotal entities: 17 cases of pseudotumor of the tunica albuginea, 56 cases of acute epididymitis/epididymoorchitis, two cases of tuberculous (TB) epididymitis, one case of orchitis with abscess, seven cases of testicular torsion, 11 cases of trauma, six cases of undescended testis, one lipoma, 20 cases of hydrocele/spermatocele, three cases of varicocele and four cases of scars from previous surgery. MRI correctly indicated the benign nature of the disease in 20 cases, clinically suspected of malignancy, all subsequently confirmed by surgical biopsy (5). MRI was used by Parenti et al. (13) as a second-line imaging examination in 48 out of 230 patients, referred with a variety of penoscrotal diseases, including a small number of benign intratesticular pathologies, namely four cases of acute epididymoorchitis, two cases of intratesticular cysts, one adrenal rest tissue, three cases of torsion, four cases of trauma, four cases of hydrocele and two inguinal hernias. Although, the authors reported difficulties in differentiating solid benign from malignant testicular masses, they concluded that MRI may be used as an adjunct tool when sonographic or clinical findings are indeterminate (13). Serra et al. (7) in a multicenter retrospective study, including 34 patients referred for scrotal MRI after inconclusive clinical and US examination, reported an accuracy of 91% in lesion characterization for MRI, higher than that of US (29%). Benign diagnoses in this study included those of orchitis (n=11), testicular infarct (n=6), rupture (n=3), torsion (n=2), post-radiation fibrosis (n=1), acute epididymitis (n=2), and epididymal abscess (n=2). Although the prevalence of inconclusive clinical and US examinations in this study was 1.4%, MRI improved the therapeutic decision of the general urologist in 19 cases, obviating surgery in nine patients and the management decision of the urologic oncologist in 17 cases, avoiding radical surgical explorations in seven patients (7). The authors concluded that MRI improves patient management, decreases the number of unnecessary surgical explorations and results in net cost savings (7).
In another study (8), including 26 out of 622 (5%) patients referred for MRI of the scrotum following inconclusive sonographic findings or a discrepancy between the clinical and US findings, MRI provided additional and correct diagnostic information in 23 cases (82.1%), when compared with sonography. Specifically, MRI yielded supplementary information in all trauma cases, including intratesticular hematoma and in all pseudotumoral lesions, including TERT, testicular fibrosis, and chronic inflammation (8).
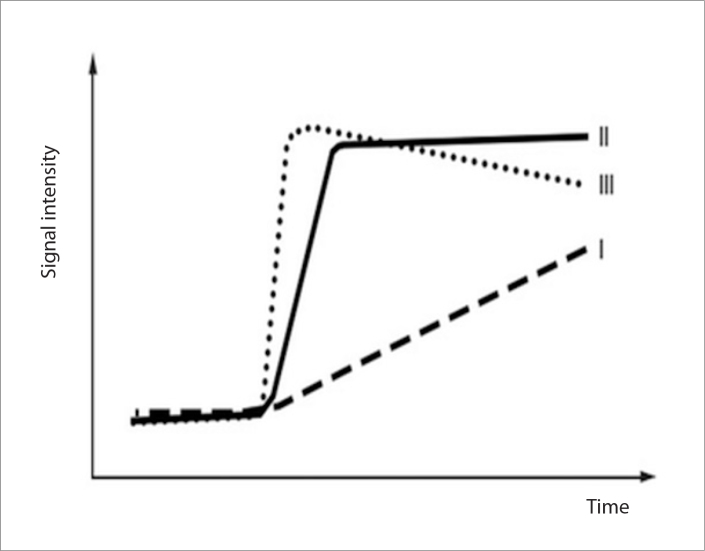
DCE-MRI has been reported useful in the characterization of scrotal mass lesions (25, 26). Watanabe et al. (25) in a study of 42 patients with various scrotal diseases, assessed the degree of enhancement (peak height) and the rate of enhancement (mean slope) for eight minutes after an intravenous bolus administration of contrast medium. The authors concluded that the relative percentages of peak height and mean slope based on time-signal intensity curves may be used to differentiate tumors from benign scrotal diseases (25). This study included 18 benign testicular diseases, namely seven cases of testicular torsion, three cases of testicular infarction, two cases of traumatic hemorrhagic necrosis, two epidermoid cysts, two cases of acute mumps orchitis and three undescended testes. Nineteen benign extratesticular disorders were assessed in the same report, including six cases of acute epididymitis, six cases of appendiceal torsion, six epididymal cysts, and one case of varicocele (25). The preliminary data of a retrospective review of 44 men with various testicular diseases (26) described three types of contrast material enhancement, based on the shape of the time-signal intensity curves: type I, a progressive, linear increase of contrast enhancement during the examination, corresponding to the enhancement patterns of normal testicular parenchyma; type II, an early strong enhancement, followed by either a plateau or a slight further increase, detected in benign intratesticular lesions, namely acute epididymoorchitis (n=4) and postbiopsy changes (n=1); and, type III, a vigorous early enhancement, followed by gradual washout of the contrast material, detected in testicular malignancies (Fig. 2) (26). In the same study, three intratesticular lesions, including hematoma, epidermoid cyst, and testicular hemorrhagic necrosis showed absence of enhancement (26). The authors reported that the relative percentages of maximum time to peak may be used as a discriminating factor in characterizing testicular mass lesions (26).
Figure 2.
Schematic drawing shows time-signal intensity curve types. Type I shows the progressive, linear increase of contrast enhancement of normal testicular parenchyma. Type II demonstrates an early, strong enhancement, followed by either a plateau or a slight further increase, detected in benign intratesticular lesions, whereas the type III corresponds to a vigorous, early enhancement, followed by gradual washout of the contrast material, detected in testicular malignancies.
DWI with measurement of ADC represents another useful, additional diagnostic tool in the characterization of intratesticular masses (27, 28). In a retrospective study of 23 testicular lesions (27), an accuracy of 91%, 87%, and 100% was reported for conventional MRI alone, DWI alone and DWI combined with conventional sequences, respectively, in lesion characterization. The diagnoses of benign intratesticular diseases in this report included four cases of acute epididymoorchitis, one case of postbiopsy changes, one intratesticular hematoma and three cases of TERT (27). The ADC of testicular malignancies has been reported lower than that of normal testis and various benign intratesticular lesions (27, 28). Algebally et al. (28) reported a sensitivity of 93.3%, specificity of 90%, positive predictive value of 87.5%, and negative predictive value of 94.7% for a cutoff ADC of less than 0.99 in the characterization of testicular lesions. This study assessed 20 benign testicular entities, including seven cases of epididymoorchitis, four cases of post-traumatic/postinfective changes, three testicular cysts, three cases of TERT and three testicular granulomas (28).
The preliminary data of a prospective study reported the efficacy of magnetization tensor imaging in differentiating various intratesticular lesions (29). The magnetization transfer ratio of testicular carcinomas was reported higher compared to that of benign testicular diseases, all cases of acute epididymoorchitis in this report (29). Recently, a preliminary study, including 10 cases of acute epididymoorchitis and one patient with segmental testicular infarction reported diffusion tensor imaging as an adjunct tool in differentiation from testicular germ cell neoplasms (30).
T1-weighted imaging, T2-weighted imaging, DWI, and subtracted DCE-MRI should be included in the MRI protocol when characterizing intratesticular masses (24).
Sonographically indeterminate intratesticular masses where MRI helps in characterization
Intratesticular fat
Lipoma
Intratesticular lipomas are uncommon, benign fat-containing tumors (14, 15). As it is characteristic with most fatty lesions, lipomas are often homogeneously hyperechoic on sonography, but this finding is neither sensitive nor specific (31). MRI findings are usually diagnostic, as lipomas have the signal intensity characteristics of fat on conventional images, with absence of enhancement (14) and are sufficient to obviate radical surgical procedures.
Lipomatosis
Testicular lipomatosis is a rare, newly reported entity seen in patients with Cowden disease, in which collections of fat cells are present within the testes (14, 32). MRI may confirm the diagnosis of this benign entity by showing multiple hyperintense intratesticular foci on T1-weighted imaging, in a patient with known Cowden disease (14, 32).
Cystic lesions
Testicular cyst
Simple testicular cysts are often impalpable and therefore incidentally detected. They are usually involving the area of the mediastinum testis and may be associated with TERT (9, 12). Sonographic diagnosis is typically straightforward. No treatment is required in cases of simple cysts (33). Rarely, differential diagnosis from cystic testicular neoplasms, such as cystic teratomas may be needed when testicular cyst shows features of complexity on US. MRI is helpful in these cases by showing absence of solid components and lack of contrast enhancement (9, 12).
Tubular ectasia of rete testis
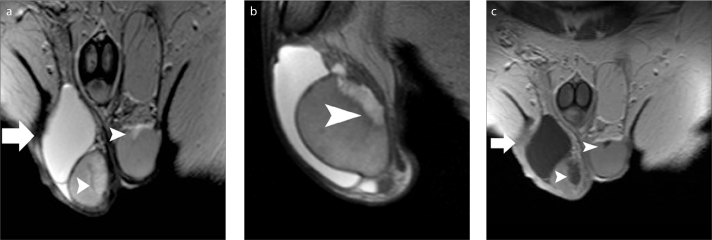
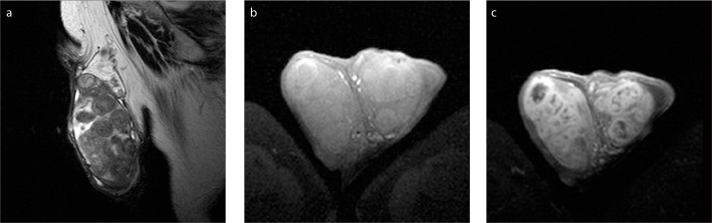
TERT is a benign condition, probably due to partial or complete obstruction of the efferent ducts (9, 12, 33, 34). It is usually bilateral, although asymmetrical and almost always associated with spermatocele. The US findings of TERT are often specific and should obviate unnecessary surgery or biopsy (33, 34). The need for MRI is rare in daily practice (34). However, it may be used to confirm the diagnosis and differentiate this entity from cystic malignant testicular tumors. Typical diagnostic criteria include the presence of tubular cystic structures, sometimes with branching pattern, characteristic location in the mediastinum testis and absence of contrast enhancement (Fig. 3) (9, 12, 34).
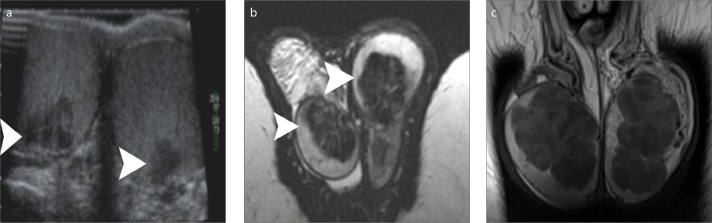
Figure 3 a–c.
A 58-year old man with bilateral TERT. T2-weighted images in coronal (a) and sagittal (b) orientation, and coronal contrast-enhanced T1-weighted image (c) show multicystic intratesticular lesions (arrowheads), within the mediastinum (larger on the right side). The lesions are hyperintense and hypointense on T2- and T1-weighted images, respectively and do not enhance after gadolinium administration. A large right spermatocele (arrow) and a moderate ipsilateral hydrocele are also depicted.
Benign solid tumors
Epidermoid cyst
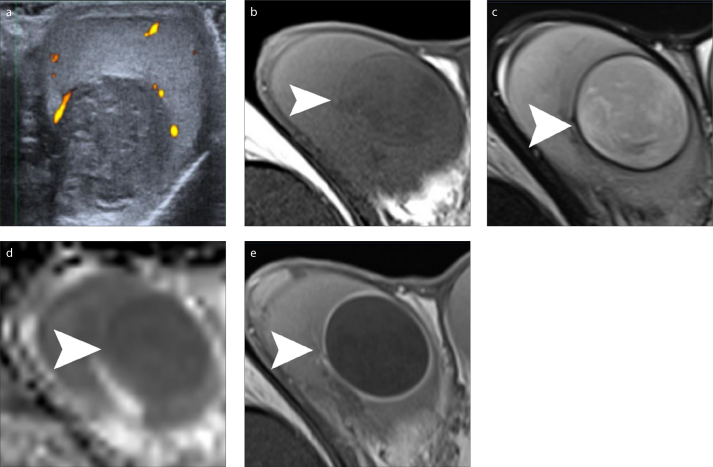
Epidermoid cyst is the most common benign intratesticular neoplasm, accounting for approximately 1%–2% of all resected testicular masses (35, 36). The ability of preoperative imaging modalities to suggest the diagnosis of an epidermoid cyst may prompt lesion enucleation or a testis sparing surgery, instead of an orchiectomy (9, 10, 12, 17, 35, 36). Although conventional US often shows highly suggestive findings, it may not be always diagnostic. In these cases, an onion skin appearance with alternating hyperintense and hypointense bands, a target appearance with a hypointense center, a hyperintense mid zone and a peripheral hypointense rim and absence of contrast enhancement on MRI (Fig. 4), might strengthen the preoperative diagnosis and guide the urologists to perform an organ sparing surgery (9, 10, 12, 17, 35, 36).
Figure 4 a–e.
Epidermoid cyst of the right testis. Power Doppler sonographic image (a) of the right testis in sagittal orientation depicts heterogeneous, mainly hypoechoic intratesticular mass, with absence of internal vascularity. Transverse T1-weighted image (b), T2-weighted image (c), ADC map (d) and fat-suppressed contrast-enhanced T1-weighted image (e) show inhomogeneous right intratesticular mass (arrowhead). The lesion is slightly hypointense and hyperintense on T1- and T2-weighted images, respectively, when compared to normal testicular parenchyma and is surrounded by a low signal intensity rim, best seen on T2-weighted images. Restricted diffusion within the mass is attributed to the presence of dense keratinous material. Peripheral lesion enhancement is seen after gadolinium administration.
Leydig’s cell hyperplasia
Testicular Leydig’s cell hyperplasia (LCH) is an uncommon benign entity, characterized by an increased number of Leydig cells and detected as small, multifocal, and often bilateral testicular nodules. Typically, these lesions are not palpable and are discovered incidentally (37, 38). Primary and secondary forms of this entity are recognized. In the primary form seen in children, LCH may present with precocious puberty. The secondary form usually seen in adults is often idiopathic. Identifiable causes of LCH include cryptorchidism, congenital adrenal hyperplasia, Klinefelter syndrome, production of human chorionic gonadotropin (hCG) by germ cell tumors, exogenous hCG administration, diseases of the pituitary gland, and antiandrogen therapy for prostatic carcinoma. LCH also has been associated with cachexia and chronic diseases, including TB, syphilis, pernicious anemia and alcoholism, as well as local diseases, including chronic spermatic cord compression, chronic diseases of the urinary bladder and/or the prostate and strictures of the vas deferens (37, 38). The clinical presentation of the secondary form may include signs of adult feminization, as painful gynecomastia and decreased libido, scrotal pain, swelling or infertility. Serum follicle-stimulating hormone levels may be slightly increased and testosterone levels may be elevated or decreased (38).
On MRI, multiple, bilateral intratesticular mass lesions are detected, ranging in size from 1 to 6 mm, hypointense on T2-weighted imaging, with mild contrast enhancement (Fig. 5) (9, 37). Compared with US, MRI may definitively identify many more lesions and also confirm bilaterality (9, 37). The presence of multiple, small bilateral testicular lesions on imaging studies that are clinically nonpalpable should strongly suggest the diagnosis of LCH. If clinical history and laboratory data reveal a cause for LCH, medical treatment may be feasible, avoiding unnecessary surgical interventions (37).
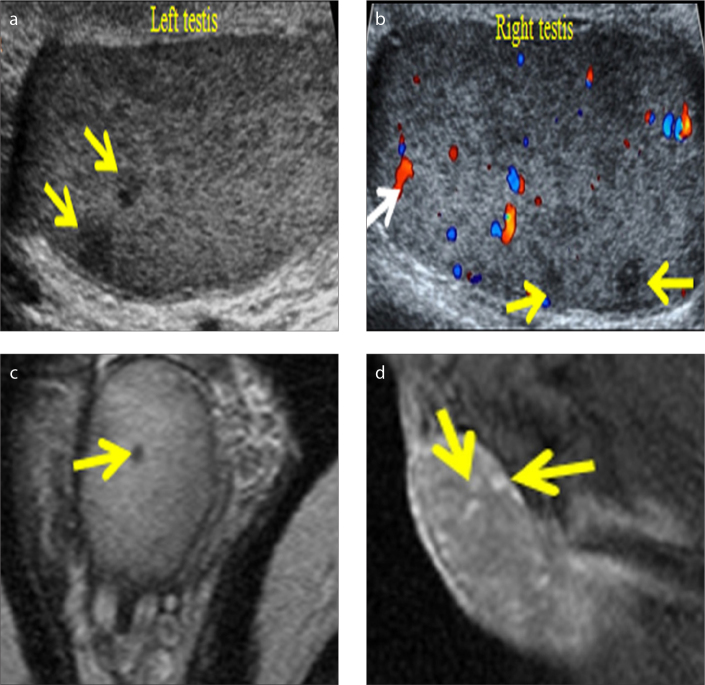
Figure 5 a–d.
LCH in a 34-year-old man referred with mild, intermittent testicular pain, no palpable mass and negative serum tumoral markers. Sagittal US image (a) of the left testis reveals sub-centimeter-sized hypoechoic intratesticular nodules (arrows). Sagittal color Doppler US image (b) of the right testis shows multiple hypoechoic lesions (yellow arrows) of few mm in diameter, some with vascularity (white arrow). Axial T2-weighted image (c) depicts hypointensity of the testicular nodule (arrow). Sagittal contrast-enhanced T1-weighted image (d) shows lesion enhancement (arrows).
Testicular adrenal rest tumors
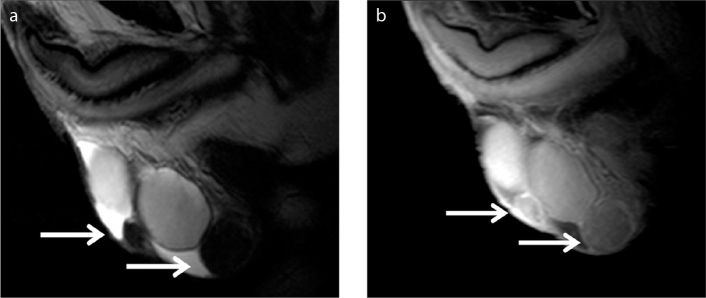
Testicular adrenal rest tumors often develop in men with congenital adrenal hyperplasia due to overstimulation of aberrant adrenal cells within the testes or rarely in men with Cushing syndrome (9, 39, 40). These tumors are benign and typically multiple, bilateral, and eccentrically located. The correct characterization of this benign entity is significant because the appropriate medical treatment may be planned. US is considered the examination of choice for the detection and follow-up of testicular adrenal rest tumors (9, 39, 40). Lesion shrinkage after therapy supports the diagnosis. If the lesions are unresponsive to therapy, testis sparing surgery may be indicated. In these cases, MRI is recommended. Compared with US, MRI provides more accurate information regarding size and lesion margins, providing therefore an accurate mapping of the extent of the disease, prior to surgery. The location in the mediastinum testis and bilaterality are helpful findings to distinguish between adrenal rest tumors and other entities. At MRI, they are hypointense on T2-weighted imaging, often enhancing after gadolinium administration (Fig. 6) (9, 39, 40).
Figure 6 a–c.
Testicular adrenal rest tumors in a young man with congenital adrenal hyperplasia. Transverse sonographic image (a) including both testes demonstrates small, bilateral, mainly hypoechoic intratesticular mass lesions (arrowheads). T2-weighted images in transverse (b) and coronal (c) planes show multiple, bilateral intratesticular masses of low signal intensity (arrowheads).
Vascular pathologies
Intratesticular hematoma
Intratesticular hemorrhage or hematomas are usually seen in patients with scrotal trauma. The US appearance may be variable and depends on the age of hematoma, often simulating malignancy. When prompt diagnosis is needed, MRI is recommended. On MRI, subacute blood appears hyperintense on T1-weighted imaging, with variable signal on T2-weighted imaging. Old hematomas may have a hypointense rim on T2-weighted image due to hemosiderin deposition. Absence of contrast enhancement confirms the benign nature in these cases (Fig. 7).
Figure 7 a–d.
Left traumatic intratesticular hematoma in a 54-year-old man. Transverse T2-weighted image (a) depicts slightly hyperintense left testicular lesion (arrow), surrounded by a low signal intensity rim. The mass (arrow) appears hyperintense on both T1-weighted (b) and fat-suppressed T1-weighted (c) images. Absence of contrast enhancement on fat-saturated contrast-enhanced T1-weighted image (d) confirms benign nature of the lesion (arrow).
Segmental testicular infarction
Segmental testicular infarction is a rare testicular disorder, often seen in patients with predisposing factors for small vessel ischemic disease, such as vasculitis, sickle cell disease, and hypercoagulable states. It also represents a rare complication of acute epididymoorchitis, trauma, and inguinal hernia surgical repair (12, 41, 42). Color Doppler US is often diagnostic, showing an avascular, wedge-shaped mass lesion. A more rounded and less well-defined lesion may be confused with an intratesticular tumor (4, 12, 42). MRI is excellent for establishing the diagnosis, by showing an avascular, triangular-shaped lesion, with the vertex directed toward the rete testis, mainly of low T2 signal, and a markedly enhanced halo surrounding the avascular area (Fig. 8). Some segmental testicular infarctions may become hemorrhagic, with hyperintense foci on T1-weighted imaging (12, 41, 42). These characteristics, combined with negative serum tumoral markers and follow-up, may allow a confident diagnosis, thus avoiding radical surgical interventions.
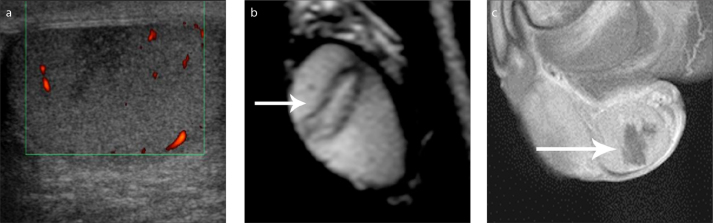
Figure 8 a–c.
MRI findings suggestive for the diagnosis of segmental testicular infarction. Sagittal grayscale sonographic image (a) depicts an ill-defined, hypoechoic intratesticular mass lesion. Sagittal T2-weighted (b) and contrast-enhanced T1-weighted (c) images show triangular lesion shape and absence of contrast enhancement (long arrow).
Testicular fibrosis
Testicular fibrosis may be seen as sequelae of trauma, inflammation, incomplete testicular torsion, post-radiation therapy, or biopsy. US findings are often inconclusive and mimic malignancy. MRI may suggest the benign nature of this condition, by showing an intratesticular mass with low T1 and very low T2 signal and absence of contrast enhancement (Fig. 9) (7, 8, 17), and therefore prevent unnecessary explorations.
Figure 9 a–e.
Bilateral testicular fibrosis. Transverse sonographic image (a) including both testes demonstrates bilateral hypoechoic intratesticular masses, with ill-defined margins (arrowheads). Transverse T1-weighted (b), T2-weighted (c), contrast-enhanced T1-weighted (d) images and ADC map (e). The lesions have very low signal intensity on both T1-weighted and T2-weighted images and do not enhance after gadolinium administration, findings suggestive for the presence of fibrous tissue. Restricted diffusion within the masses is also related to the presence of fibrous component.
Inflammatory conditions
Granulomatous orchitis
Granulomatous orchitis may mimic neoplasia both on clinical and US examination. Multiple pathogens can cause granulomatous orchitis, including TB, brucellosis, leprosy, syphilis, fungi, and parasites (12, 14).
The incidence of TB has increased worldwide, with an increase in extrapulmonary manifestations (43). The genitourinary tract represents the commonest site of extrapulmonary TB, affecting the kidneys, ureters, urinary bladder, epididymis, and testis. MRI findings, although nonspecific, include the presence of intratesticular lesions of high T1 and low T2 signal, strongly enhancing after contrast medium administration (Fig. 10) (43). The coexistence of scrotal calcifications, sinus tracts, poor response to conventional antibiotics, findings of pulmonary and extrapulmonary TB manifestations may strongly suggest the diagnosis of TB orchitis.
Figure 10 a–c.
Bilateral TB epididymo-orchitis in a 70-year-old man. Sagittal T2-weighted image (a) depicts testicular enlargement and multiple, nodular, mainly hypointense mass lesions, involving both the testis and the head of the epididymis. On transverse T1-weighted (b) and contrast-enhanced T1-weighted (c) images, lesions have signal intensity slightly higher than that of normal testis and enhance strongly and heterogeneously.
Isolated granulomatous orchitis
Isolated granulomatous orchitis is an uncommon inflammatory condition of the testis, with unknown etiology. Possible causative factors include trauma, autoimmune disease, vascular insufficiency, reaction to extravasated sperm and urinary tract infections with retrograde spread through the vas deferens (44, 45). This benign entity is often confirmed after radical orchiectomy, since characterization based on clinical and conventional US findings is not possible (44, 45). Pekindil et al. (45) in a report of a 60-year-old man with synchronous bilateral granulomatous orchitis, histologically confirmed after bilateral orchiectomy, concluded that the presence of diffuse hypoechoic testis infiltration, with peripheral low-resistance flow on color Doppler US should suggest the diagnosis of this disease (45). Although it is also difficult to characterize this entity on MRI, absence of contrast enhancement represents a sensitive feature for predicting the benign nature of intratesticular masses (9,46), including isolated granulomatous orchitis (44).
Sarcoidosis
Sarcoidosis rarely involves the genitourinary tract, affecting more commonly the epididymis than the testis. Testicular sarcoidosis is more frequent in African-American men, compared with other racial groups. Testicular lesions are more often multiple and bilateral (9, 12, 14). Differentiation from malignant tumors may be difficult based on imaging findings. At MRI, the lesions are hypointense on T2-weighted imaging, with contrast enhancement. Sarcoidosis should be considered in the differential diagnosis of a testicular mass detected in an African-American patient (9, 12, 14).
Table 1 summarizes benign intratesticular masses for which MRI is helpful in diagnosis.
Table 1.
Sonographically indeterminate intratesticular masses for which MRI helps in characterization
| Intratesticular masses | Is MRI necessary? | MRI appearance |
|---|---|---|
| Intratesticular fat | ||
| • Lipoma | Recommended. It provides a definite diagnosis | Signal characteristics of fat, absence of enhancement |
|
| ||
| Cystic lesions | ||
| • Simple cyst | Not routinely recommended. Rarely, MRI may be used to differentiate complex intratesticular cysts from cystic testicular tumors. It is strongly suggestive of the diagnosis | Absence of solid components and enhancement |
| • Tubular ectasia of rete testes | Not routinely recommended. Rarely, MRI may be used to differentiate from cystic malignant neoplasms. It provides a definite diagnosis | Multiple, tubular cystic structures in the mediastinum testis, signal characteristics of fluid on all pulse sequences, no internal enhancement |
|
| ||
| Benign solid tumors | ||
| • Epidermoid cyst | Recommended in cases of indeterminate sonographic findings. It is strongly suggestive of the diagnosis | Sharply-demarcated, surrounded by a hypointense rim on T2WI, onion skin or target appearance, absence of enhancement |
| • Leydig’s cells hyperplasia | MRI detects more lesions than US and confirms bilaterality. It is strongly suggestive of the diagnosis | Multiple, bilateral intratesticular lesions, 1–6 mm in diameter, low T2 signal, mild enhancement |
| • Adrenal rest tumors | Recommended in candidates for partial orchiectomy. It is more accurate than US regarding number, size and lesion margins. It is strongly suggestive of the diagnosis | Multiple, bilateral eccentrically located lesions, low T2 signal, contrast enhancement |
|
| ||
| Vascular pathologies | ||
| • Hematoma | Recommended. It is strongly suggestive of the diagnosis | Hyperintense on T1WI (subacute hematoma), surrounded by hypointense rim on T2WI (chronic hematoma), no enhancement |
| • Segmental testicular infarction | In cases of indeterminate conventional US findings (nodular mass, ill-defined margins). It is strongly suggestive of the diagnosis | Triangular morphology, vertex directed toward rete testis, low T2 signal, avascular mass with intensely enhancing rim. May show hemorrhagic, hyperintense foci on T1WI |
| • Fibrosis | Recommended. It is strongly suggestive of the diagnosis | Very low T2 signal, no enhancement |
|
| ||
| Inflammatory conditions | ||
| • Granulomatous orchitis | May mimic neoplasia on US. It is difficult to characterize on MRI | TB orchitis: high T1 and low T2 signal, strong enhancement, appropriate clinical setting |
|
| ||
| • Isolated granulomatous orchitis | Often resembles malignancy on US. It is difficult to characterize on MRI | Absence of contrast enhancement suggests benign nature |
| • Sarcoidosis | Presence of intratesticular mass in an African-American man may suggest the diagnosis | Low T2 signal, contrast enhancement |
MRI, magnetic resonance imaging; US, ultrasonography; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; TB, tuberculosis.
Paratesticular masses
The majority of paratesticular masses are benign epididymal lesions such as cysts or spermatoceles, fluid collections such as hydroceles and pyoceles, inflammatory conditions, and hernias (22). Primary solid paratesticular tumors are not common, although the incidence on US studies varies from 3%–15% (22, 23). The most common benign paratesticular neoplasms are lipomas, adenomatoid tumors, and leiomyomas. Other uncommon histologic subtypes are papillary cystadenomas, fibromas, hemangiomas, and neurofibromas (22). An accurate characterization of their benign nature may obviate unnecessary radical surgical procedures. Alternative treatments, including lesion excision, biopsy or follow-up may be recommended.
US findings of many solid paratesticular masses are indeterminate, precluding an accurate diagnosis in most cases (22, 23). Conventional MRI features may represent a useful supplemental diagnostic tool in the pretreatment work-up of paratesticular masses (5, 11, 12, 14, 18, 22, 23, 47–52).
There are limited data regarding the DWI characteristics of paratesticular mass lesions (27, 28, 50). In a retrospective review of eight benign paratesticular masses (27), including five cases of acute epididymitis, two spermatoceles and one adenomatoid tumor of the tunica albuginea, the ADC was reported higher compared with that of the normal epididymis. These results were not confirmed by Algebally et al. (28). The authors reported no significant differences between the ADC of 15 benign paratesticular lesions (including three cases of acute epididymitis, two cases of chronic granulomatous epididymitis, two epididymal hematomas, two spermatoceles, two cases of fibrous pseudotumor of the epididymis, and four cases of adenomatoid tumors of the epididymis and the testicular tunica) and that of the normal epididymis (28).
The MRI protocol for investigating paratesticular masses should include T1-weighted imaging, T2-weighted imaging in three planes, DWI, and subtracted DCE-MRI (24).
Sonographically indeterminate paratesticular masses where MRI is useful
Lipoma
Lipomas are the commonest paratesticular neoplasms, mostly originating from the spermatic cord (11, 12, 14, 22, 23). Their hyperechoic appearance on US is neither sensitive nor specific. Other paratesticular lesions, including hernias and sarcomas may be echogenic. An atypical lipoma may appear hypoechoic. MRI is helpful for confirming the diagnosis and guiding surgeon to lesion enucleation when required, rather that radical surgery. The tumor is detected with fat signal intensity on all sequences, including fat-saturated images and absence of contrast enhancement (11, 12, 14, 22, 23, 47, 48).
Adenomatoid tumor
Adenomatoid tumors represent the most common tumors of the epididymis, although they may arise from the testicular tunica, the testis, and the spermatic cord (11, 12, 14, 18, 22, 23, 47–49, 52, 53). MRI is helpful in confirming the paratesticular origin of this lesion and in discriminating adenomatoid tumor from an intratesticular mass lesion located in the periphery of the testicular parenchyma (12, 14, 18, 24). The detection of a hypointense rim between the mass and the adjacent testis, corresponding to the tunica albuginea, helps to show the origin of the tumor.
US findings are often nondiagnostic in characterizing the nature of this neoplasm. On MRI, the tumor often appears hypointense on T2-weighted imaging, enhancing after gadolinium administration (Fig. 11). However, these features are also nonspecific. Tumoral slow or decreased contrast enhancement compared with normal testis may indicate the benign nature of this neoplasm, and may allow conservative management (12, 18).
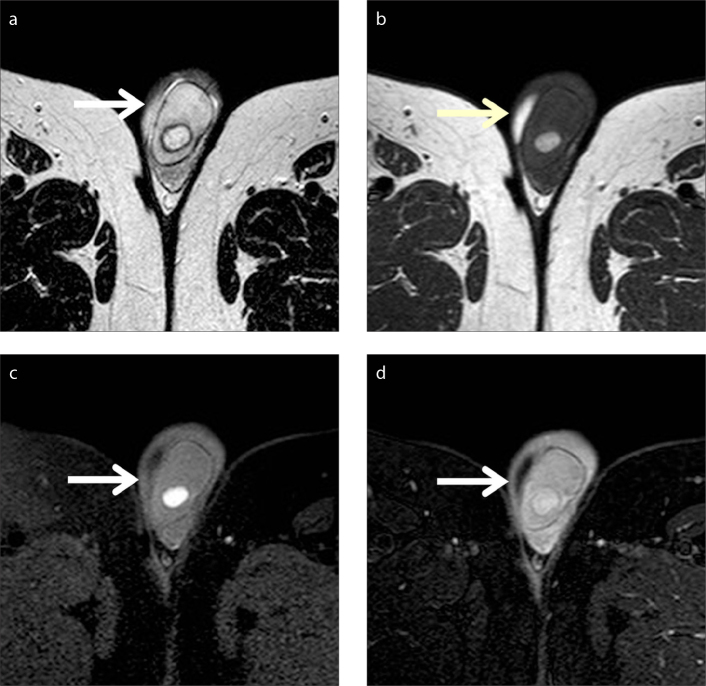
Figure 11.
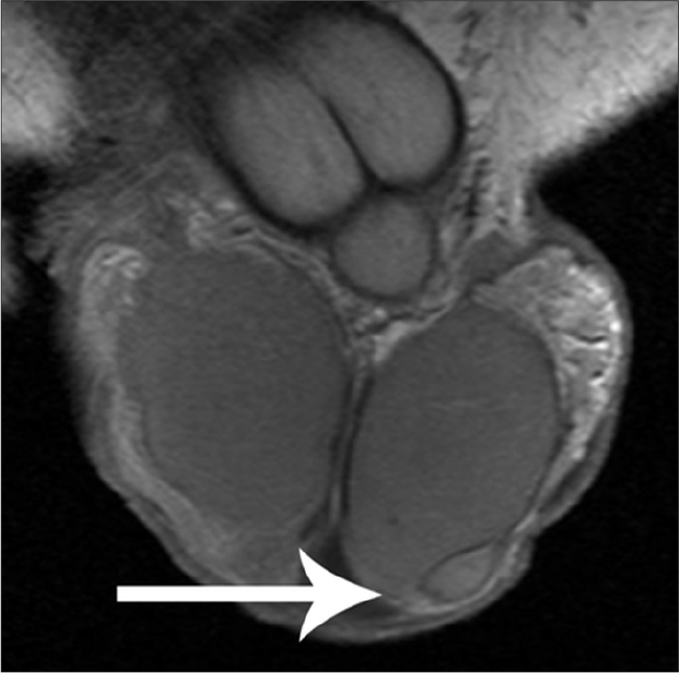
Adenomatoid tumor arising from the tail of the left epididymis. Coronal contrast-enhanced T1-weighted image depicts a small, sharply demarcated left paratesticular mass (arrow). The lesion enhances less than the normal testes. A small amount of hydrocele (arrowhead) is detected ipsilaterally.
Leiomyoma
Leiomyoma represents the second most common tumor of the epididymis (22, 47). The neoplasm has variable US findings. No adequate data on the MRI appearance of this tumor (Fig. 12) exist in the literature.
Figure 12 a, b.
Bilateral paratesticular leiomyomas in a 60-year-old man, confirmed pathologically after tumor enucleation. Sagittal T2-weighted (a) and contrast-enhanced T1-weighted (b) images depict bilateral paratesticular masses (long arrows). The lesions are well-delineated, hypointense on T2-weighted image, with mild, delayed contrast enhancement.
Fibrous pseudotumor
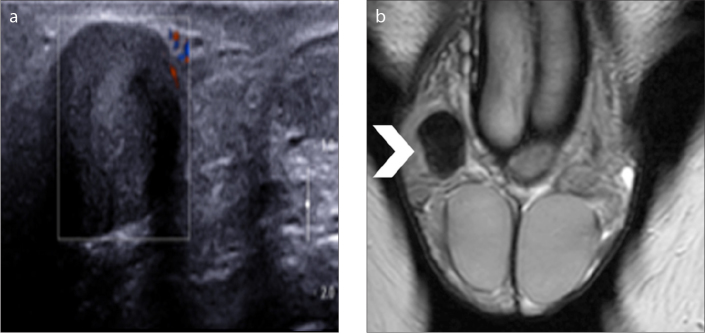
Fibrous pseudotumor is the third most common paratesticular mass, after lipoma and adenomatoid tumor. It is not a true neoplasm but represents a benign reactive fibrous proliferation resulting from one or several paratesticular elements, more often from the tunica vaginalis (12, 14, 22, 23, 47, 48, 54, 55). These lesions may mimic a neoplasm both on physical and US examination. MRI is highly specific in characterizing the benign nature of this tumor. At MRI, the lesion demonstrates uniformly low T1 and very low T2 signal, respectively, with slow but persistent contrast enhancement due to the presence of fibrosis (Fig. 13) (12, 14, 22, 23, 47, 48, 54, 55). Accurate characterization of this entity may allow a more conservative scrotal exploration rather than a radical orchiectomy.
Figure 13 a, b.
Fibrous pseudotumor. Color Doppler US image (a) shows a hypoechoic right paratesticular mass, without detectable vascularity. Coronal T2-weighted image (b) depicts paratesticular mass lesion (arrowhead), with very low signal intensity, suggestive for the presence of fibrous tissue.
Congenital anomalies
Polyorchidism is a very rare developmental anomaly (14, 22, 23, 56, 57). Although presence of three testes is the most common form, as many as five have been reported (22). The supernumerary testes are intrascrotal in approximately 75% of cases, inguinal in 20% and retroperitoneal in 5% of cases. Usually the diagnosis is based on US findings.
MRI may be used if US findings are inconclusive (14, 22, 23, 56, 57). MRI depicts a round or oval structure, with signal characteristics typical of testicular tissue. The detection of a hypointense rim surrounding the extra-testis, corresponding to the tunica albuginea is an ancillary finding, confirming the diagnosis. Sometimes, the mediastinum testis, supernumerary epididymis and bridging vessels between the normal testis and the supernumerary testis also may be recognized. (14). The management of these men is generally conservative, including close follow-up imaging, due to the slightly increased risk of malignancy for the accessory testis. However, in cases of cryptorchidism, torsion, or neoplasia, surgery is indicated (14, 22, 23, 56, 57).
Table 2 summarizes sonographically indeterminate paratesticular masses for which MRI helps in diagnosis.
Table 2.
Sonographically indeterminate paratesticular masses for which MRI helps in characterization
| Paratesticular masses | Is MRI necessary? | MRI appearance |
|---|---|---|
| • Lipoma | Recommended. It provides a definite diagnosis | Signal characteristics of fat, absence of enhancement |
|
| ||
| • Adenomatoid tumor | It can differentiate adenomatoid tumor from peripherally located intratesticular mass. It provides accurate lesion localization. But, MRI findings are nonspecific | Low T2 signal, contrast enhancement (may be slow and decreased) |
|
| ||
| • Leiomyoma | It can differentiate tumor from peripherally located intratesticular mass. It provides accurate lesion localization | No data exist |
|
| ||
| • Fibrous pseudotumor | Recommended. It is strongly suggestive of the diagnosis | Very low T2 signal, slow, persistent contrast enhancement |
|
| ||
| Congenital anomalies | ||
| • Polyorchidism | In cases of indeterminate US findings, it provides a definite diagnosis | Round or oval structure, signal characteristics similar to normal testis, surrounded by hypointense rim (tunica albuginea). Infrequently also detected: mediastinum testis, bridging vessels, supernumerary epididymis |
MRI, magnetic resonance imaging; US, ultrasonography.
Conclusion
Imaging has a central role in the assessment of scrotal masses. Although US represents the examination of choice, it does not always provide an accurate characterization of the nature of scrotal mass lesions. Benign scrotal masses, including both intratesticular and paratesticular ones are not common. However, a pretreatment diagnosis of their benign nature based on imaging findings may obviate unnecessary radical orchiectomy, improving patient management. MRI of the scrotum represents a useful complimentary imaging technique, which may provide important information in the characterization of the histologic nature of various sonographically indeterminate scrotal masses. The technique performs well in the morphologic assessment and tissue characterization. A preoperative diagnosis of benign nature based on imaging findings could probably save some testes.
Main points.
Ultrasonography (US), including conventional gray-scale and color Doppler US, represents the primary examination for the assessment of scrotal masses. However, a confident characterization of their nature is not always possible based on the US findings alone.
MRI of the scrotum has emerged as an important supplemental diagnostic imaging modality in the assessment of scrotal masses, especially recommended when sonographic findings are indeterminate.
Although the majority of intratesticular masses are malignant, a possible diagnosis of various benign intratesticular entities, including lipoma, tubular ectasia of the rete testis, epidermoid cyst, hematoma, segmental testicular infarction, fibrosis and granulomatous orchitis, based on imaging features may improve patient management and decrease the number of unnecessary radical surgical explorations.
MRI is helpful in characterization of sonographically indeterminate scrotal masses by showing the presence of fat, fluid, hemorrhage, fibrous tissue, and contrast-enhancing elements. The technique performs well in the morphologic assessment and tissue characterization.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Appelbaum L, Gaitini D, Dogra VS. Scrotal ultrasound in adults. Semin Ultrasound CT MR. 2013;34:257–273. doi: 10.1053/j.sult.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Dogra VS, Gottlieb RH, Oka M, Rubens DJ. Sonography of the scrotum. Radiology. 2003;227:18–36. doi: 10.1148/radiol.2271001744. [DOI] [PubMed] [Google Scholar]
- 3.Basu S, Howlett DC. High-resolution ultrasound in the evaluation of the non acute testis. Abdom Imaging. 2001;26:425–432. doi: 10.1007/s002610000169. [DOI] [PubMed] [Google Scholar]
- 4.Baker LL, Hajek PC, Burkhard TK. MR imaging of the scrotum: pathologic conditions. Radiology. 1987;163:93–98. doi: 10.1148/radiology.163.1.3823465. [DOI] [PubMed] [Google Scholar]
- 5.Cramer BM, Schlegel EA, Thueroff JW. MR imaging in the differential diagnosis of scrotal and testicular disease. Radiographics. 1991;11:9–21. doi: 10.1148/radiographics.11.1.1996400. [DOI] [PubMed] [Google Scholar]
- 6.Sica GT, Teeger S. MR imaging of scrotal, testicular, and penile diseases. Magn Reson Imaging Clin N Am. 1996;4:545–563. [PubMed] [Google Scholar]
- 7.Serra AD, Hricak H, Coakley FV, et al. Inconclusive clinical and ultrasound evaluation of the scrotum: impact of magnetic resonance imaging on patient management and cost. Urology. 1998;51:1018–1021. doi: 10.1016/S0090-4295(98)00097-1. [DOI] [PubMed] [Google Scholar]
- 8.Muglia V, Tucci S, Jr, Elias J, Jr, Trad CS, Bilbey I, Cooperberg PL. Magnetic resonance imaging of scrotal diseases: when it makes the difference. Urology. 2002;59:419–423. doi: 10.1016/S0090-4295(01)01579-5. [DOI] [PubMed] [Google Scholar]
- 9.Woodward PJ, Sohaey R, O’Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics. 2002;22:189–216. doi: 10.1148/radiographics.22.1.g02ja14189. [DOI] [PubMed] [Google Scholar]
- 10.Andipa E, Liberopoulos K, Asvestis C. Magnetic resonance imaging and ultrasound evaluation of penile and testicular masses. World J Urol. 2004;22:382–391. doi: 10.1007/s00345-004-0425-9. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R, Alobaidi M, Jafri SZ, Bis K, Amendola M. Correlation of US and MRI Findings of Intratesticular and Paratesticular Lesions: From Infants to Adults. Curr Prob Diagn Radiol. 2005;34:35–45. doi: 10.1067/j.cpradiol.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Rosen MA, Langer JE, Banner MP, Siegelman ES, Ramchandani P. US MR imaging correlation in pathologic conditions of the scrotum. Radiographics. 2007;27:1239–1253. doi: 10.1148/rg.275065172. [DOI] [PubMed] [Google Scholar]
- 13.Parenti GC, Feletti F, Brandini F, et al. Imaging of the scrotum: role of MRI. Radiol Med. 2009;114:414–424. doi: 10.1007/s11547-009-0377-7. [DOI] [PubMed] [Google Scholar]
- 14.Cassidy FH, Ishioka KM, McMahon CJ. MR imaging of scrotal tumors and pseudotumors. Radiographics. 2010;30:665–683. doi: 10.1148/rg.303095049. [DOI] [PubMed] [Google Scholar]
- 15.Philips S, Nagar A, Dighe M, Vikram R, Sunnapwar A, Prasad S. Benign non-cystic scrotal tumors and pseudotumors. Acta Radiol. 2012;53:102–111. doi: 10.1258/ar.2011.110185. [DOI] [PubMed] [Google Scholar]
- 16.Mohrs OK, Thoms H, Egner T, et al. MRI of patients with suspected scrotal or testicular lesions: diagnostic value in daily practice. AJR Am J Roentgenol. 2012;199:609–615. doi: 10.2214/AJR.11.7349. [DOI] [PubMed] [Google Scholar]
- 17.Tsili AC, Giannakis D, Sylakos A, Ntorkou A, Sofikitis N, Argyropoulou MI. MR imaging of scrotum. Magn Reson Imaging Clin N Am. 2014;22:217–238. doi: 10.1016/j.mric.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Park SB, Lee WC, Kim JK, et al. Imaging features of benign solid testicular and paratesticular lesions. Eur Radiol. 2011;21:2226–2234. doi: 10.1007/s00330-011-2155-x. [DOI] [PubMed] [Google Scholar]
- 19.Aganovic L, Cassidy F. Imaging of the scrotum. Radiol Clin North Am. 2012;50:1145–1165. doi: 10.1016/j.rcl.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Woldrich JM, Im RD, Hughes-Cassidy FM, Aganovic L, Sakamoto K. Magnetic resonance imaging for intratesticular and extratesticular scrotal lesions. Can J Urol. 2013;20:6855–6859. [PubMed] [Google Scholar]
- 21.Parker RA, 3rd, Menias CO, Quazi R, et al. MR imaging of the penis and scrotum. Radiographics. 2015;35:1033–1050. doi: 10.1148/rg.2015140161. [DOI] [PubMed] [Google Scholar]
- 22.Woodward PJ, Schwab CM, Sesterhenn IA. From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation. Radiographics. 2003;23:215–240. doi: 10.1148/rg.231025133. [DOI] [PubMed] [Google Scholar]
- 23.Akbar SA, Sayyed TA, Jafri SZ, Hasteh F, Neill JS. Multimodality imaging of paratesticular neoplasms and their rare mimics. Radiographics. 2003;23:1461–1476. doi: 10.1148/rg.236025174. [DOI] [PubMed] [Google Scholar]
- 24.Tsili A, Bertolotto M, Turgut AT, et al. MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur Radiol. 2018;28:31–43. doi: 10.1007/s00330-017-4944-3. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe Y, Dohke M, Ohkubo K, et al. Scrotal disorders: evaluation of testicular enhancement patterns at dynamic contrast-enhanced subtraction MR imaging. Radiology. 2000;217:219–227. doi: 10.1148/radiology.217.1.r00oc41219. [DOI] [PubMed] [Google Scholar]
- 26.Tsili AC, Argyropoulou MI, Astrakas LG, et al. Dynamic contrast-enhanced subtraction MRI for characterizing intratesticular mass lesions. AJR Am J Roentgenol. 2013;200:578–585. doi: 10.2214/AJR.12.9064. [DOI] [PubMed] [Google Scholar]
- 27.Tsili AC, Argyropoulou MI, Giannakis D, Tsampalas S, Sofikitis N, Tsampoulas K. Diffusion-weighted MR imaging of normal and abnormal scrotum: preliminary results. Asian J Androl. 2012;14:649–654. doi: 10.1038/aja.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algebally AM, Tantawy HI, Yousef RR, Szmigielski W, Darweesh A. Advantage of adding diffusion weighted imaging to routine mri examinations in the diagnostics scrotal lesions. Pol J Radiol. 2015;80:442–449. doi: 10.12659/PJR.894399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsili AC, Ntorkou A, Baltogiannis D, et al. Magnetization transfer imaging of normal and abnormal testis: preliminary results. Eur Radiol. 2016;26:613–621. doi: 10.1007/s00330-015-3867-0. [DOI] [PubMed] [Google Scholar]
- 30.Tsili AC, Ntorkou A, Astrakas L, et al. Magnetic resonance diffusion tensor imaging of the testis: Preliminary observations. Eur J Radiol. 2017;95:265–270. doi: 10.1016/j.ejrad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Hertzberg BS, Mahony BS, Bowie JD, Anderson EE. Sonography of an intratesticular lipoma. J Ultrasound Med. 2015;4:619–621. doi: 10.7863/jum.1985.4.11.619. [DOI] [PubMed] [Google Scholar]
- 32.Woodhouse J, Ferguson MM. Multiple hyperechoic testicular lesions are a common finding on ultrasound in Cowden disease and represent lipomatosis of the testis. Br J Radiol. 2006;79:801–803. doi: 10.1259/bjr/50628431. [DOI] [PubMed] [Google Scholar]
- 33.Dogra VS, Gottlieb RH, Rubens DJ, Liao L. Benign intratesticular cystic lesions: US features. Radiographics. 2001;21:S273–281. doi: 10.1148/radiographics.21.suppl_1.g01oc15s273. [DOI] [PubMed] [Google Scholar]
- 34.Rouvière O, Bouvier R, Pangaud C, Jeune C, Dawahra M, Lyonnet D. Tubular ectasia of the rete testis: a potential pitfall in scrotal imaging. Eur Radiol. 1999;9:1862–1868. doi: 10.1007/s003300050936. [DOI] [PubMed] [Google Scholar]
- 35.Langer JE, Ramchandani P, Siegelman ES, Banner MP. Epidermoid cysts of the testicle: sonographic and MR imaging features. AJR Am J Roentgenol. 1999;173:1295–1299. doi: 10.2214/ajr.173.5.10541108. [DOI] [PubMed] [Google Scholar]
- 36.Cho JH, Chang JC, Park BH, Lee JG, Son CH. Sonographic and MR imaging findings of testicular epidermoid cysts. AJR Am J Roentgenol. 2002;178:743–748. doi: 10.2214/ajr.178.3.1780743. [DOI] [PubMed] [Google Scholar]
- 37.Carucci LR, Tirkes AT, Pretorius ES, Genega EM, Weinstein SP. Testicular Leydig’s cell hyperplasia: MR imaging and sonographic findings. AJR Am J Roentgenol. 2003;180:501–503. doi: 10.2214/ajr.180.2.1800501. [DOI] [PubMed] [Google Scholar]
- 38.Naughton CK, Nadler RB, Basler JW, Humphrey PA. Leydig cell hyperplasia. Br J Urol. 1998;81:282–289. doi: 10.1046/j.1464-410X.1998.00503.x. [DOI] [PubMed] [Google Scholar]
- 39.Avila NA, Premkumar A, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. AJR Am J Roentgenol. 1999;172:1003–1006. doi: 10.2214/ajr.172.5.10227495. [DOI] [PubMed] [Google Scholar]
- 40.Stikkelbroeck NM, Suliman HM, Otten BJ, Hermus AR, Blickman JG, Jager GJ. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: sonographic and MR features. Eur Radiol. 2003;13:1597–1603. doi: 10.1007/s00330-002-1786-3. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Perez GC, Tardaguila FM, Velasco M, Rivas C, Dos Santos J, Cambronero J, Trinidad C, San Miguel P. Radiologic findings of segmental testicular infarction. AJR Am Roentgenol. 2005;184:1587–1593. doi: 10.2214/ajr.184.5.01841587. [DOI] [PubMed] [Google Scholar]
- 42.Parenti GC, Sartoni M, Gaddoni E, Zago S, Campioni P, Mannella P. Imaging of segmental testicular infarction: our experience and literature review. Radiol Med. 2012;117:1161–1175. doi: 10.1007/s11547-012-0798-6. [DOI] [PubMed] [Google Scholar]
- 43.Tsili AC, Tsampoulas C, Giannakis D. Case report. Tuberculous epididymo-orchitis: MRI findings. Br J Radiol. 2008;81:e166–169. doi: 10.1259/bjr/16348966. [DOI] [PubMed] [Google Scholar]
- 44.Tsili AC, Argyropoulou MI, Giannakis D, Zioga E, Koukos S, Sofikitis N, Tsampoulas K. Isolated granulomatous orchitis: MR imaging findings. Eur J Radiol Extra. 2011;79:e81–e83. doi: 10.1016/j.ejrex.2011.05.002. [DOI] [Google Scholar]
- 45.Pekindil G, Hüseyin Atakan I, Kaya E, Bilgi S, Inci O. Bilateral synchronous granulomatous orchitis: gray-scale and colour Doppler sonographic findings. Eur J Radiol. 1999;31:201–203. doi: 10.1016/S0720-048X(98)00122-3. [DOI] [PubMed] [Google Scholar]
- 46.Tsili AC, Argyropoulou MI, Giannakis D, Sofikitis N, Tsampoulas K. MRI in the characterization and local staging of testicular neoplasms. AJR Am J Roentgenol. 2010;194:682–689. doi: 10.2214/AJR.09.3256. [DOI] [PubMed] [Google Scholar]
- 47.Secil M, Bertolotto M, Rocher L, et al. Imaging features of paratesticular masses. J Ultrasound Med. 2017;36:1487–1509. doi: 10.7863/ultra.16.07015. [DOI] [PubMed] [Google Scholar]
- 48.Nicola R, Menias CO, Dahiya N, Robinson K, Hara AK, Siegel CL. Review of paratesticular pathology: findings on ultrasound and MRI. Abdom Radiol (NY) 2017;42:585–601. doi: 10.1007/s00261-016-0870-0. [DOI] [PubMed] [Google Scholar]
- 49.Wolfman DJ, Marko J, Gould CF, Sesterhenn IA, Lattin GE., Jr Mesenchymal extratesticular tumors and tumorlike conditions: from the radiologic pathology archives. Radiographics. 2015;35:1943–1954. doi: 10.1148/rg.2015150179. [DOI] [PubMed] [Google Scholar]
- 50.Tsili ACh, Tsampoulas C, Giannakopoulos X, et al. Solitary fibrous tumour of the epididymis: MRI features. Br J Radiol. 2005;78:565–568. doi: 10.1259/bjr/31560902. [DOI] [PubMed] [Google Scholar]
- 51.Hashiguchi Y, Matsuo Y, Torri Y, et al. Polyarteritis nodosa of the epididymis. Abdom Imag. 2001;26:102–104. doi: 10.1007/s002610000097. [DOI] [PubMed] [Google Scholar]
- 52.Tsili AC, Argyropoulou MI, Giannakis D, Sofikitis N, Tsampoulas K. Conventional and diffusion-weighted magnetic resonance imaging findings of benign fibromatous paratesticular tumor: a case report. J Med Case Rep. 2011;5:169. doi: 10.1186/1752-1947-5-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel MD, Silva AC. MRI of an Adenomatoid tumor of the tunica albuginea. AJR Am J Roentgenol. 2004;182:415–417. doi: 10.2214/ajr.182.2.1820415. [DOI] [PubMed] [Google Scholar]
- 54.Saginoya T, Yamaguchi K, Toda T, Kiyuna M. Fibrous pseudotumor of the scrotum: MR imaging findings. AJR Am J Roentgenol. 1996;167:285–286. doi: 10.2214/ajr.167.1.8659413. [DOI] [PubMed] [Google Scholar]
- 55.Grebenc ML, Gorman JD, Sumida FK. Fibrous pseudotumor of the tunica vaginalis testis: imaging appearance. Abdom Imaging. 1995;20:379–380. doi: 10.1007/BF00203377. [DOI] [PubMed] [Google Scholar]
- 56.Oner AY, Sahin C, Pocan S, Kizilkaya E. Polyorchidism: sonographic and magnetic resonance image findings. Acta Radiol. 2005;46:769–771. doi: 10.1080/02841850500216293. [DOI] [PubMed] [Google Scholar]
- 57.Figler TJ, Olson MC, Kinzler GJ. Polyorchidism and rete testis adenoma: ultrasound and MR findings. Abdom Imaging. 1996;21:470–472. doi: 10.1007/s002619900108. [DOI] [PubMed] [Google Scholar]