Abstract
Physalis alkekengi var. francheti is an indigenous herb well known for its anti-inflammatory, sedative, antipyretic, and expectorant properties. However, the information regarding the impacts of P. alkekengi fruits (PAF) in modulation of oxidative stress and learning memory are still unknown. This study therefore evaluated the antioxidant properties of ethyl acetate (EA) fraction of PAF and its impacts on learning and memory. The antioxidant activities of PAF were evaluated in LPS-induced BV2 microglial cells. The potent EA fraction then investigated and confirmed for its involvement of HO-1 pathway using hemin (HO-1 inducer) and ZnPP (HO-1 inhibitor) through Western blotting, DCFH-DA, and/or Griess assay. The involvements of PI3K/Akt, MEK, and p38 MAPK also investigated. Furthermore, we applied EA fraction to the animals at 100 and 200 mg/kg doses to check if the extract could improve scopolamine-induced memory deficits in passive avoidance and elevated plus maze tests. Our results demonstrated that the fractions from PAF significantly inhibited the generation of intracellular reactive oxygen species (ROS) induced by LPS in concentration-dependent manners. In comparison to other fractions, the EA fraction exhibited potent effect in suppressing intracellular ROS generation. Besides, EA fraction also induced the expression of HO-1 in time- and concentration-dependent manners. ZnPP significantly reversed the suppressive effect of EA fraction on LPS-induced ROS generation and NO production, which confirm the involvement of HO-1 signaling in EA-fraction-mediated antioxidant activities. Consistently, blocking of PI3K/Akt, MEK, and p38 MAPK pathways by PAF-EA suppressed the production of intracellular ROS, indicating their potential participation. In addition, one of the major constituents of EA fraction, luteolin-7-O-β-D-glucoside, also demonstrated HO-1-dependent antioxidant effects in BV2 cells. Further, the EA fraction significantly (p < 0.05) improves scopolamine-induced memory deficits in mice. Taken together, our findings highlight the antioxidant effects of EA fraction of PAF which may be beneficial in treatment of different neurodegenerative diseases associated with free radicals.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0887-0) contains supplementary material, which is available to authorized users.
Keywords: Physalis alkekengi, Solanaceae, Luteolin-7-O-β-D-glucoside, Antioxidant, HO-1 (Hsp-32), Scopolamine, Memory deficit
Introduction
Dementia is a global problem characterized by a progressive decline in cognition, which especially affects elderly peoples in their regular activities including memory as well as aging (Terry et al. 2011). Substantial scientific evidence revealed that aging and loss of memory are associated with excessive neuronal death due to the destruction of synapses, oxidative stress, and neuroinflammation. These are the most common features observed in the brain of Alzheimer’s disease (AD) patients, thought to result from the functional alterations of the non-neuronal cells including astrocytes and microglia (Morrison and Hof 1997; Reiter 1995; Nie et al. 2009). Microglia are the brain macrophages play critical role in the immune system of the central nervous system (CNS). In response to different pro-inflammatory stimuli generated by neuronal injury, environmental factors (lipopolysaccharide (LPS), rotenone, paraquat), and endogenous peptides (amyloid beta, alpha-synuclein), microglia undergo morphological changes and consistently generate reactive oxygen species (ROS) including hydroxyl radical, superoxide anion, peroxyl, nitric oxide, and hydrogen peroxide. Excessive production of these radical species further triggers neurotoxicity leading to a self-propagating cycle of neuronal death (Barron 1995; Block and Hong 2005; Balmus et al. 2016).
The inducible heme oxygenase-1 (HO-1) also known as heat shock protein-32 (Hsp-32) (Ryter et al. 2002) is a phase 2 antioxidant enzyme, gets upregulated in response to cellular injury, oxidative stress, and diseases. Following stress, HO-1 gets dissociated and induces the conversion of cellular heme into carbon monoxide, biliverdin, and free iron. These three by-products act as antioxidant and anti-inflammatory enzymes and reported alleviating extent of oxidative stress and related disorders (Otterbein et al. 2000; Ryter et al. 2006; Syapin 2008). In support, Kim et al. found that induction of HO-1 can protect primary cultured rat cortical cells from oxidative stress induced by hydrogen per oxide (H2O2) and xanthine/xanthine oxidase (Kim et al. 2017). Therefore, HO-1 emerged as one of the prime targets to treat oxidative and cellular stress as well as its associated neurological disorders.
Physalis alkekengi L. var. franchetii (PA; Family: Solanaceae) also known as Chinese lantern or winter cherry is a very popular medicinal herb in different Asian and European countries. All parts of this plant, especially its fruits, are widely used in the traditional medicine of these countries in treatment of insomnia, inflammation, rheumatism, toothache, sore throat, fever, heat and cold, fungal infection, and diabetes (Kranjc et al. 2016; Moniruzzaman et al. 2016; Li et al. 2018). Accordingly, researchers have validated the beneficial impacts of PA fruits on gastric ulcer, wound, and immune system (Asilbekova et al. 2016; Yang et al. 2014). Previously, we have shown that the ethyl acetate fraction from P. alkekengi fruit (PAF) inhibits LPS-induced pro-inflammatory mediators in BV2 microglial cells, which involves suppression of Akt and MAPK phosphorylations as well as inhibition of nuclear translocation of Nf-κB. These events also produced a positive impact on the anti-inflammatory effects of this extract in different inflammatory pain models in mice (Moniruzzaman et al. 2016). In the present study, we investigated the antioxidant effects of P. alkekengi fruits and tried to elucidate possible involvement of HO-1, Akt, and MAPKs as underlying mechanisms. We also justified the effectiveness of PAF against scopolamine-induced memory impairment in mice.
Materials and methods
Chemicals and reagents
LPS (E. coli, serotype 0111:B4), 2′,7′-dichloro dihydro fluorescein diacetate (DCFH-DA), 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and anti-β-actin, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G, LY294002, and U0126 were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti HO-1 antibody was obtained from Enzo life sciences (Seoul, South Korea). SB203580 was purchased from Calbiochem (Darmstadt, Germany). Dulbecco’s modified eagle’s medium (DMEM) and 0.05% trypsin-EDTA were procured from Thermo Scientific (Rockford, IL, USA). Fetal bovine serum (FBS) and antibiotic-antimycotic agents were purchased from Gibco BRL (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) was obtained from Junsei Chemical Co. Ltd. (Tokyo, Japan).
Sample collection and extraction
The fruits of Physalis alkekengi were obtained from a traditional herb store of the Republic of Korea and identified by Professor Young-Won Chin from Dongguk University. A voucher specimen (No. CYWDU-KP0006) has been deposited in the Pharmacognosy Lab, College of Pharmacy, Dongguk University-Seoul for further reference. Dried fruits of 1.9 kg was macerated with 2 L of methanol, which yields 564 g of crude extract after in vacuo evaporation. Then, the crude extract was dissolved in water and fractionated with n.hexane, chloroform, ethyl acetate (EA), and n.butanol, respectively. The fridge-dried fractions were then stored at − 20 °C for further use.
Cell culture and measurement of cell viability
BV2 cells were maintained in DMEM containing heat-inactivated 10% FBS and 1% antibiotic-antimycotic agents. Cells were grown in a humidified atmosphere containing 5% CO2 and having a constant temperature of 37 °C. The cells were plated in a 24-well plate and incubated for 24 h to achieve at least 70% confluence. Following serum starvation for 4 h, the cells were treated with different fractions of PAF at the indicated concentrations or luteolin-7-O-β-D-glucoside (10 μM) in absence or presence of LPS (1 μg/ml) and incubated for 24 h. Then, the cell viability was determined using MTT assay as described previously (Kim et al. 2014).
Measurements of intracellular ROS
DCFH-DA assay was employed to determine the intracellular ROS. Briefly, cells in 24-well plate were pretreated with PAF fractions, PAF-EA (100 μg/ml) + ZnPP (2 μM), luteolin-7-O-β-D-glucoside (10 μM) + ZnPP, and/or signaling inhibitors (10 μM) for 1 h and then incubated for 24 h in absence or presence of LPS. Following gentle washing with phosphate-buffered saline (PBS), the cells were treated with 10 μM DCFH-DA for 30 min at 37 °C. The generation of intracellular ROS was then determined through the fluorescence detection of microplate reader (Spectra Max M2e, Molecular Devices) with excitation and emission wavelengths of 490 and 520 nm (Kim et al. 2014).
Measurement of nitric oxide (NO)
Following 24-h treatments, the level of NO in the culture medium was determined using Griess reagent from Promega Corporation (Madison, USA) following manufacturer’s instructions (Lin et al. 2008).
Western blot analysis
To investigate HO-1 expression, the cells were lysed with normal lysis buffer containing protease and phosphatase inhibitors as described previously. An equal amount of proteins was resolved in SDS-PAGE and electrophoretically transferred to nitrocellulose membrane (GE Healthcare UK Ltd., Buckinghamshire, UK). Following blocking with 5% skimmed milk, the membranes were incubated with primary antibodies for overnight at 4 °C. Membranes were then washed thoroughly and incubated with HRP-conjugated secondary antibody for 90 min. After washing and application of enhanced chemiluminescence reagents (Bio-Rad, Hercules, CA, USA), the membranes were scanned using Bio-Rad ChemiDoc XRS imaging system (Kim et al. 2014).
Animals
Male ICR mice weighing 28–32 g were purchased from Daehan Biolink (Chungbuk, Korea). The mice were kept under standard laboratory conditions at a temperature of 22 ± 2 °C and relative humidity (55–60%) with a 12-h light-dark cycle. The animals were allowed to have free access to food (standard chow diet) and water ad libitum. Mice were acclimatized with laboratory environment at least for 1 week before experiments.
Passive avoidance test
The passive avoidance test instrument is a rectangular box consisting of two identical chambers (20 × 20 × 20 cm3), one chamber illuminated with a 50-W bulb. The two chambers were separated by a guillotine door (5 × 5 cm2). The floor of the non-illuminated compartment was composed of 2-mm stainless steel rods spaced 1 cm apart and connected with an electric grid as described previously (Jung et al. 2016). Mice were administered with PAF-EA (100 or 200 mg/kg, p.o.) or donepezil (10 mg/kg, p.o.) 1 h before an acquisition trial. The control group received 10% PEG in saline instead of PAF-EA. Mice were treated with scopolamine (3 mg/kg, i.p.) or 0.9% saline 30 min before the acquisition trial. Following desired treatments, animals were placed in the illuminated compartment and allowed them to explore. The guillotine door was opened 10 s later and closed automatically when the animals entered into the dark compartment. An electrical foot shock (0.5 mA) was then delivered to the animals for 5 s through the stainless steel rods. The retention trial was conducted 24 h after the acquisition trial by returning the individual mice to the illuminated compartment. In both trials, latency was defined as the time it took for the mouse to enter the dark compartment after the door was opened. During the acquisition trial, the mice that did not enter the non-illuminated compartment within 60 s of the door opening the door were gently introduced into the dark chamber, and the latency was recorded as 60 s. Latencies were recorded for up to 300 s during the retention trial.
Elevated plus maze test
The elevated plus maze (EPM) consists of two closed arms (15 × 5 × 5 cm3) and two open arms (15 × 5 cm2) extended from the central platform (5 × 5 cm2). The plus maze was placed in a stand 50 cm above the ground. Thirty minutes after the oral administered with PAF-EA (100 or 200 mg/kg) or 10% PEG in saline (0.1 ml/mouse) or donepezil (10 mg/kg), animals received scopolamine (3 mg/kg in saline; i.p.). Then, the animals were placed on the open arm of EPM following 30 min of scopolamine injection, and the latency to enter into the closed arm was recorded. The animals were allowed to explore more 2 min and return them to the home cage. After 24 h of the first reading (acquisition trial), the transfer latency was observed again as an index of retrieval (Sharma and Kulkarni 1992; Deb et al. 2015).
HPLC-NMR analysis of PAF-EA fraction
HPLC analysis of PAF-EA was conducted to identify luteolin-7-O-β-D-glucoside as a marker compound (Qiu et al. 2008). This compound was obtained from the precipitate of PAF-EA fraction and confirmed using NMR spectroscopy. One milligram of the tested sample as well as the standard luteolin-7-O-β-D-glucoside were dissolved in methanol to prepare the stocks. The standard was then serially diluted to 5, 10, 50, and 100 μg/ml concentrations to make the calibration solutions. All solutions were then filtered using a 0.45-μm hydrophobic PTFE filter, and 20 μl of each was injected through an INNO C18 column (4.6 × 250 mm, 5 μm; Innopia, Seongnam, South Korea) in the HPLC (Gilson, USA). Formic acid of 0.1% v/v in acetonitrile (A) and 0.1% (v/v) formic acid in water (B) were used as the mobile phases with a flow rate of 1 ml/min as follows: 10% (A) isocratic for 10 min, 10–20% (A) for 20 min, 20% (A) isocratic for 30 min for post-run column reconditioning.
Statistical analysis
Data are presented as mean ± SEM and analyzed using one-way ANOVA followed by Dunnett’s post hoc test using SPSS software. Calculations were done using Microsoft Excel, and figures were drawn using GraphpadPrism softwares.
Results and discussion
This study evaluated the antioxidant potential of PAF in BV2 microglial cells as well as its impacts on learning memory in mice. BV2 cells are the widely used in vitro system originally derived from immortalized neonatal microglia and behave as the primary microglial culture (Moniruzzaman et al. 2016). However, before going to the main experiments, we have tested different fractions of PAF in the BV2 microglial cells if they produce cytotoxicity to the cells. The results demonstrated that the n.hexane and chloroform fractions produced significant toxicity of the BV2 cells where the total extract only at higher concentration reduced cell viability (Fig. 1a). Interestingly, the ethyl acetate and butanol fraction did not affect the viability, and therefore, we used these two non-toxic fractions in our next experiments. Moreover, we also included the total extract in our experiments to compare the effects. From the DCFH-DA experiment, it is clear that LPS significantly (p < 0.05) induced the production of intracellular ROS in BV2 cells which contributes to the oxidative stress accompanying the inflammatory process (Block and Hong 2005). Although all fractions at all concentrations (10, 30, and 100 μg/ml) showed significant (p < 0.05) suppression, the EA fraction produced maximum inhibition of LPS-induced ROS in BV2 cells (Fig. 1b). Therefore, we checked whether these changes are due to the cytotoxicity of cells induced by co-treatments with LPS and extracts. We found that LPS produced significant (p < 0.05) toxicity to the cells which has been protected by the extracts (except total extract at 100 μg/ml) in a concentration-dependent manner. Although LPS markedly reduced cell viability, the levels of intracellular ROS were significantly higher (Fig. 1b, c). Moreover, we found that PAF-EA is also able to suppress H2O2 and Aβ(25–35)-induced intracellular ROS in BV2 cells (data not shown). These, therefore, influenced us to elucidate the underlying mechanisms behind EA fraction-mediated antioxidant effects.
Fig. 1.
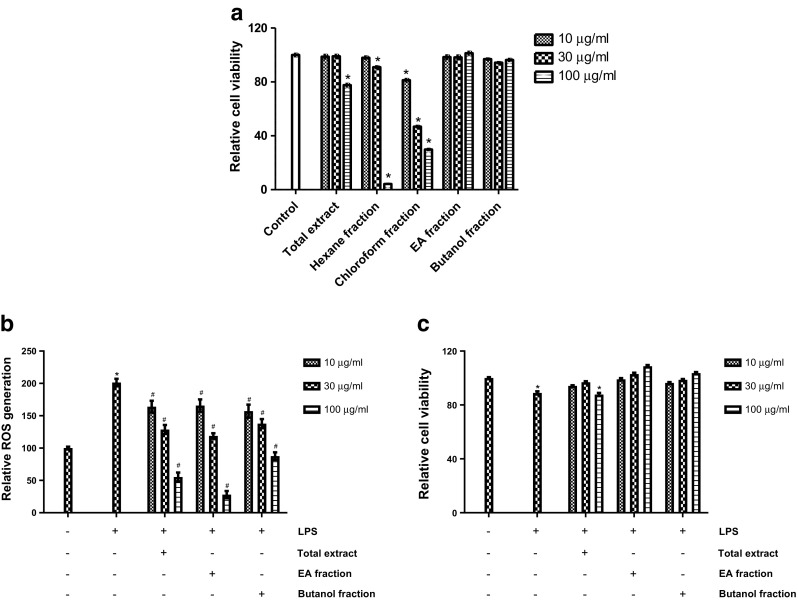
The ethyl acetate (EA) fraction from PAF is potentially non-toxic and has highest potential to suppress LPS-induced production of intracellular ROS. Cells were treated with different fractions of PAF (10, 30, and 100 μg/ml) for 24 h, and then, viability was checked using MTT assay (a). Cells were pretreated with total, EA, or butanol fractions at indicated concentrations for 1 h and then incubated in absence or presence of LPS (1 μg/ml) for 24 h. Then, the intracellular ROS was determined using DCFH-DA as a fluorescent probe (b) and cell viability using MTT assay (c). Data represent the mean ± SEM of at least three independent experiments (*p < 0.05 vs. control group; #p < 0.05 vs. LPS group)
Several studies have been suggested that HO-1 plays a pivotal protective role in inflammatory responses because of its capability to inhibit pro-inflammatory mediators such as NO (Takagi et al. 2010; Wu et al. 2011). Other studies revealed that the HO-1 gene is regulated by the nuclear transcription factor Nrf2 which is necessary for HO-1 in diminishing oxidative insults, thus offering protection against inflammatory diseases due to oxidative stress (Ishii et al. 2000). Therefore, it would be worth to focus on HO-1 as a potential therapeutic target in treatment of different inflammatory neurodegenerative diseases. It has been asserted that the inhibition or knockdown of HO-1 reverses the inhibitory effects of negative regulators on LPS-induced NO production (Chien et al. 2012). This implies that up-regulation of HO-1 expression would significantly decrease NO production. To confirm the HO-1 expression-mediated regulation of NO production and generation of intracellular ROS, we investigated whether EA fraction can induce the expression of HO-1 in BV2 microglial cells. Treatment of BV2 microglia with 100 μg/ml of EA fraction resulted in a significant increase in HO-1 protein expression in a time-dependent manner, and its maximal expression was found at 24 h (Fig. 2a). Moreover, Western blot analysis showed that this fraction increased HO-1 expression in a concentration-dependent manner where EA fraction only at 100 μg/ml significantly (p < 0.05) increase the level of HO-1 protein. In contrast, the lower concentrations possessed moderate effect, compared to that untreated control group (Fig. 2b). To further scrutinize the functional activity of HO-1, we used the HO-1 inducer, hemin, and evaluated whether it can affect the regulation of LPS-stimulated NO production. We found that pretreatment with Hemin significantly suppressed LPS-induced production of NO, which suggest that the expression HO-1 can decrease NO production (Fig. 2c). On the other hand, the HO-1 inhibitor (ZnPP) reversed EA fraction-mediated inhibition of NO and intracellular ROS in the LPS-activated BV2 microglia (Fig. 2d, e), implying that inhibition of HO-1 expression sensitized LPS-induced NO production and generation of ROS. These findings indicate that EA fraction-induced HO-1 expression is intimately associated with the down-regulation of LPS-induced NO and ROS production.
Fig. 2.
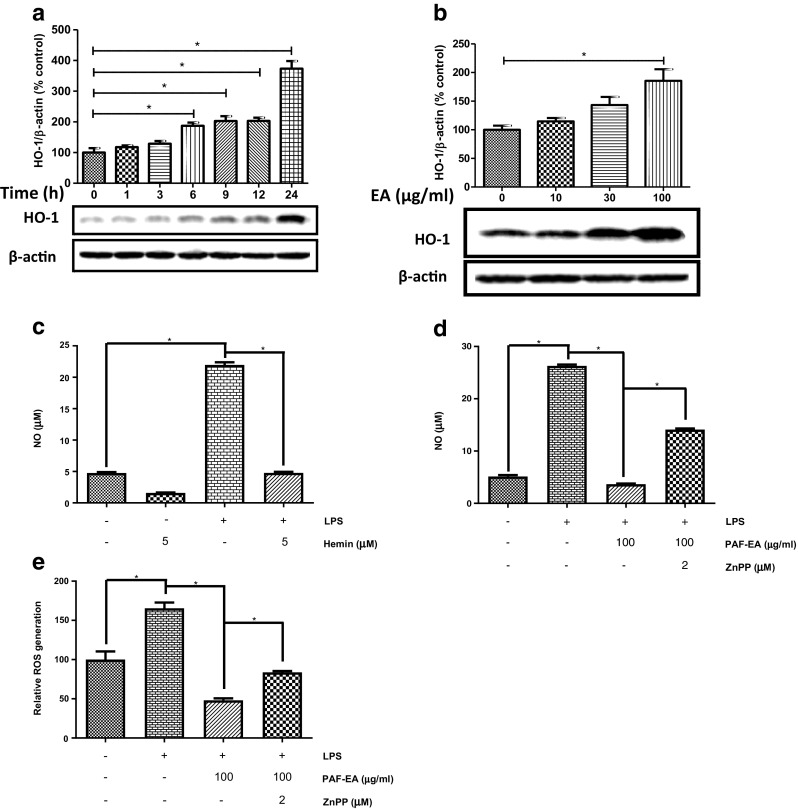
PAF-EA exhibited antioxidant activity potentially through HO-1 pathway. Cells were incubated with EA fraction (100 μg/ml) for 0–24 h. Cell lysates were prepared and then Western blot was conducted using antibody specific for HO-1 (a). Cells were treated with different concentrations of EA fraction for 24 h. Western blot analysis was executed for cell lysates using HO-1 specific antibody (b). Cells were pretreated with 5 μM Hemin for 1 h and then incubated for 24 h in absence or presence of LPS (1 μg/ml) (c). Cells were pretreated with 100 μg/ml of EA fraction in the absence or presence of 2 μM ZnPP for 1 h and then incubated with 1 μg/ml LPS for 24 h. The amount of NO production in the medium was measured using the Griess reaction (d) and ROS was determined using DCFH-DA as a fluorescent probe (e). Data represent as the mean ± SEM of at least three independent experiments (*p < 0.05)
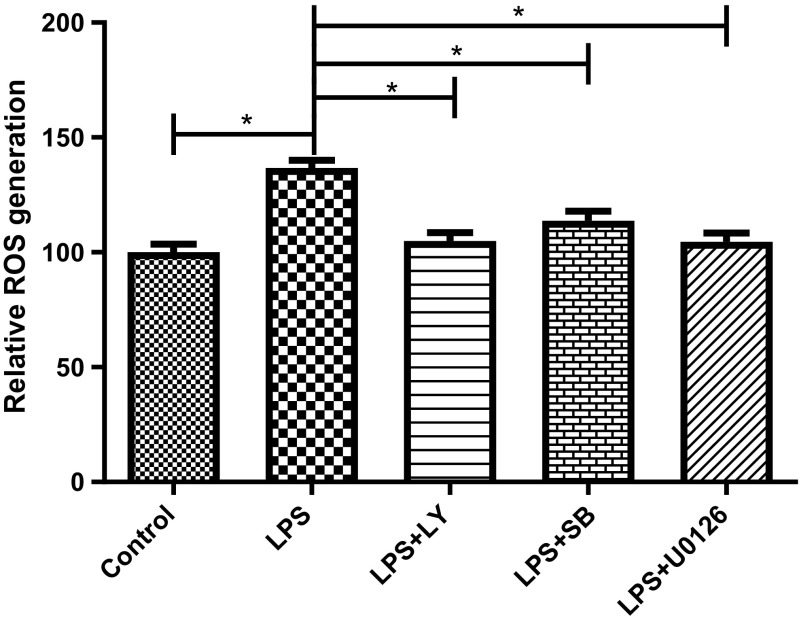
Previously, we have reported that LPS induces the activation of Akt, MEK1/2, ERK1/2, and p38 MAPK in BV2 cells, where the pretreatment with PAF-EA results in a subsequent suppression of these signaling pathways to show its anti-inflammatory effects (Moniruzzaman et al. 2016). Therefore, we sought to understand whether inhibition of these signaling pathways also contribute in the antioxidant effects of the EA fraction. Our results demonstrated that inhibition of Akt, MEK1/2, ERK1/2, and p38 MAPK pathways using their specific inhibitors LY294002, SB203580, or U0126 significantly (p < 0.05) suppressed LPS-induced generation of intracellular ROS (Fig. 3) and revealed possible participation of these signaling pathways in EA fraction-mediated antioxidant effects.
Fig. 3.
PAF-EA exhibited antioxidant activity potentially through Akt and MAPK pathways. Cells were pretreated with 10 μM of LY294002 (PI3K inhibitor), SB203580 (p38 MAPK inhibitor), or U0126 (MEK inhibitor) for 1 h and then incubated with LPS (1 μg/ml) for 24 h and DCFH-DA assay was performed. Data represent the mean ± SEM of at least three independent experiments (*p < 0.05)
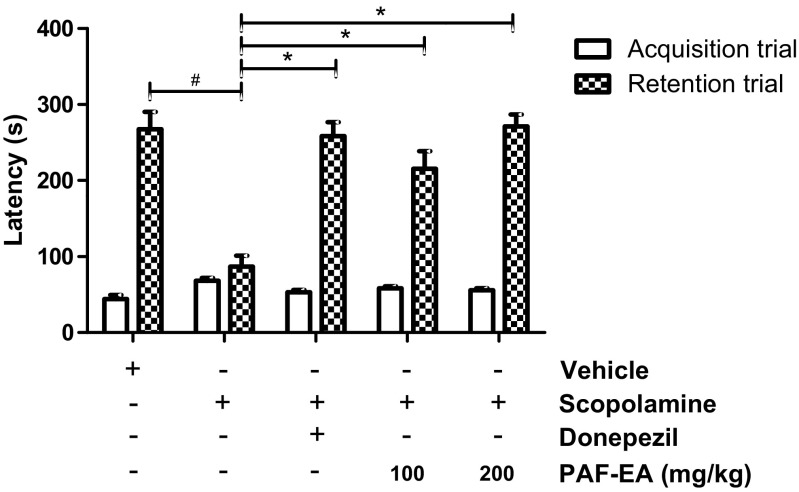
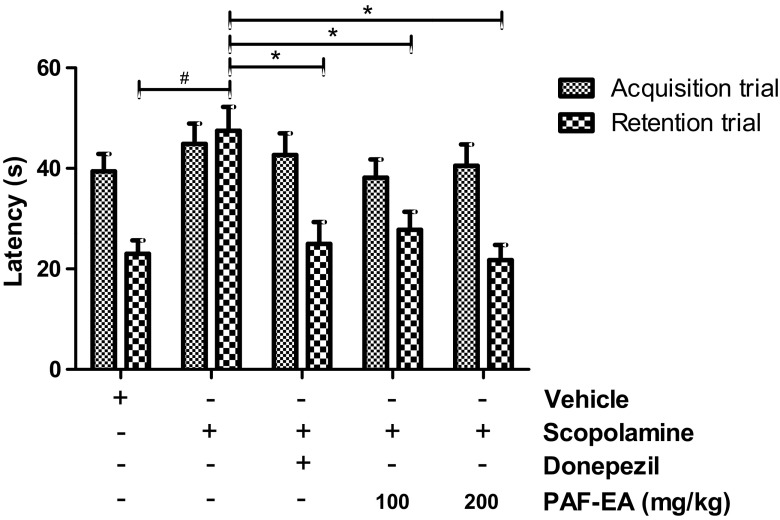
Learning and memory are complex processes and thought not to be regulated by only one muscarinic receptor subtype (Herrera-Morales et al. 2007). Scopolamine, a non-selective muscarinic receptor antagonist, has been reported to cause oxidative stress in the hippocampus and impair both acquisition and long-term memory formation (Herrera-Morales et al. 2007; Fan et al. 2005). In the passive avoidance task, there were no significant differences observed in the latency times during the acquisition trial across all groups. However, there were significant group effects in terms of step-through latency. A reduction in step-through latency was observed in the scopolamine-injected group, and the decreased latency induced by scopolamine was significantly reversed following the administration of PAF-EA (100 and 200 mg/kg, p < 0.05, Fig. 4). In addition, PAF-EA produced similar effects in the elevated plus maze test. None of the treatments produced any significant differences in the acquisition trial; however, significantly (p < 0.05) ameliorated scopolamine-induced amnesia in the retention trial (Fig. 5). Therefore, these results suggest that the PAF-EA ameliorates scopolamine-induced memory deficits possibly through the restoration of redox imbalance induced by scopolamine.
Fig. 4.
PAF-EA ameliorates scopolamine-induced memory deficits in passive avoidance test. Following 30 min of desired treatments, mice were injected with scopolamine (3 mg/kg). After 30 min, the animals were scored for acquisition trial and exposed to electric shock on their foot (0.5 mA for 5 s). Twenty-four hours following the shock, animals were again scored for step through latency. Data represent the mean ± SEM (n = 8; #p < 0.05 vs. control group; *p < 0.05 vs. scopolamine group)
Fig. 5.
PAF-EA ameliorates scopolamine-induced memory deficits in elevated plus maze test. Following 30 min of desired treatments, mice were injected with scopolamine (3 mg/kg). After 30 min, the animals were scored for acquisition trial from the open arm of the maze. Twenty-four hours following the acquisition trial, animals were scored for retention trial (b). Data represent the mean ± SEM (n = 8; #p < 0.05 vs. control group; *p < 0.05 vs. scopolamine group)
From HPLC analysis of EA fraction, luteolin-7-O-β-D-glucoside emerged as a major constituent (1.78%) at 48.4 min (Fig. 6, Suppl Fig. 1) (Moniruzzaman et al. 2016). It has been found that luteolin-7-O-β-D-glucoside possesses antioxidant properties through scavenging free radicals as well as inhibiting lipid peroxidation (Gamal-Eldeen et al. 2004). It has also been reported to show strong antioxidant activity through modulation of Akt and MAPK pathways as well as induction of HO-1 in RAW264.7 cells (Song and Park 2014). In PC12 cell line, a widely used model of dopaminergic neuron, luteolin-7-O-β-D-glucoside, shows a protective role against 6-hydroxydopamine toxicity (Hanrott et al. 2006). Therefore, we tried to understand whether luteolin-7-O-β-D-glucoside is responsible behind the HO-1-dependent antioxidant effects of PAF-EA in BV2 microglial cells. The results revealed that the compound at 10 μM concentration significantly (p < 0.05) suppressed LPS-induced production of NO and intracellular ROS which have been reversed by the treatment with ZnPP (the HO-1 inhibitor) (Fig. 7). Based on these evidences, it is clear that being one of the active components in PAF-EA, luteolin-7-O-β-D-glucoside plays critical role in HO-1-dependent antioxidant effects which also could be implicated in the observed anti-amnestic activities of PAF-EA.
Fig. 6.
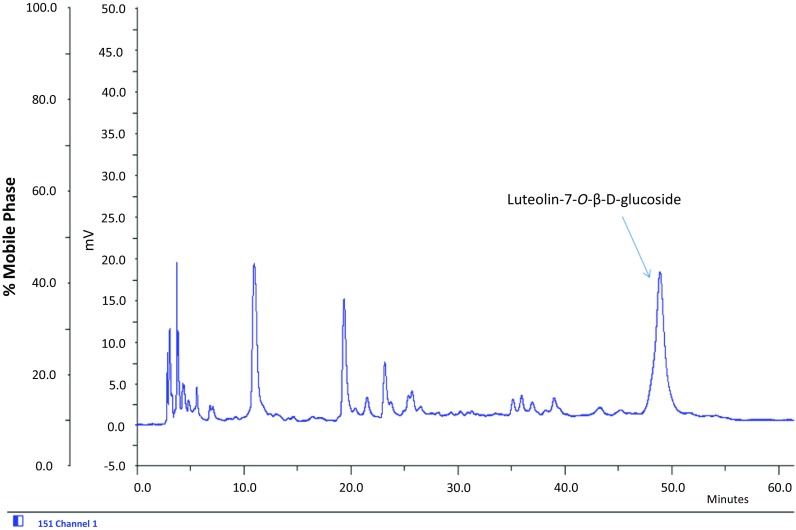
HPLC chromatogram of EA fraction of PAF
Fig. 7.
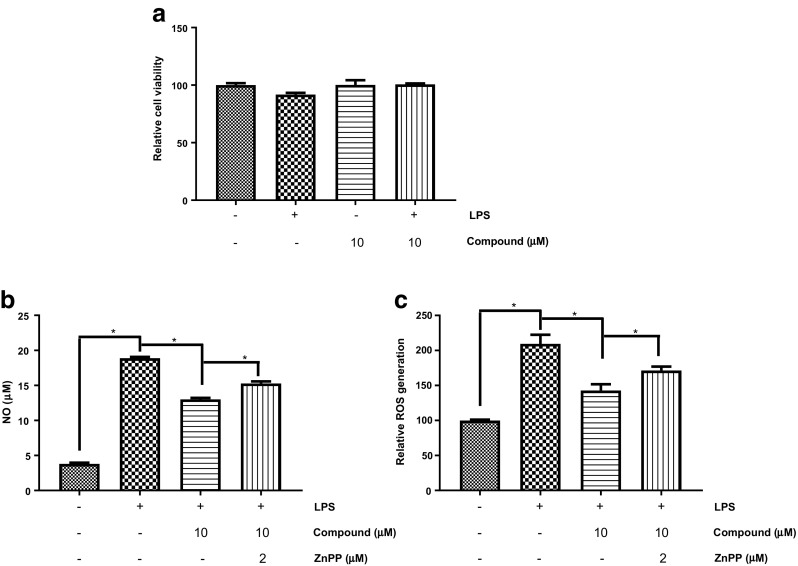
Luteolim-7-O-β-D-glucoside (compound) exhibited antioxidant activities potentially through HO-1 pathway. Cells were incubated with Luteolim-7-O-β-D-glucoside (10 μM) in absence or presence of LPS (1 μg/ml) for 24 h and then viability was checked using MTT assay (a). Cells were pretreated with 10 μM of Luteolim-7-O-β-D-glucoside in the presence or absence of 2 μM ZnPP for 1 h and then co-treated with 1 μg/ml LPS for 24 h. The amount of NO production in the culture medium was measured using the Griess reaction (b) and ROS was determined using DCFH-DA as a fluorescent probe (c). Data represent as the mean ± SEM of at least three independent experiments (*p < 0.05)
Conclusion
In conclusion, the present study strongly demonstrated HO-1-dependent antioxidant effects of the ethyl acetate fraction of PAF which may confer neuroprotection in scopolamine-induced oxidative damage and supported its traditional uses. Therefore, these results suggest that PAF-EA could be used as a potential therapy in treatment of neurological disorders such as dementia.
Electronic supplementary material
(DOCX 152 kb)
Acknowledgements
Md. Moniruzzaman was supported by SRD-II scholarship from Dongguk University. Jungsook Cho was funded by the GRRC Program of Gyeonggi province [GRRC DONGGUK2016-B01], Republic of Korea.
Abbreviation
- HO-1
Heme oxygenase 1
- AD
Alzheimer’s disease
- CNS
Central nervous system
- LPS
Lipopolysaccharide
- ROS
Reactive oxygen species
- H2O2
Hydrogen per oxide
- PAF
Physalis alkekengi fruit
- MAPKs
Mitogen-activated protein kinases
- DCF-HAD
2′,7′-dichloro dihydro fluorescein diacetate
- MTT
3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- DMEM
Dulbecco’s modified eagle’s medium
- FBS
Fetal bovine serum
- DMSO
Dimethyl sulfoxide
- NO
Nitric oxide
- EPM
Elevated plus maze
- ZnPP
Zinc protoporphyrine
Compliance with ethical standards
All experimental protocols used in this study were performed following “Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, USA” (National Academy Press: Washington D.C., 1996) and approved by the Institutional Animal Ethical Committee of Dongguk University (Approval Number: IACUC-2013-0005).
Competing interests
Author does not have any conflict of interests associated with this publication.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0887-0) contains supplementary material, which is available to authorized users.
Contributor Information
Md. Moniruzzaman, Phone: +61469714887, Email: moniruzzaman.babu@yahoo.com
Jungsook Cho, Phone: +821050612419, Email: neuroph@dongguk.edu.
References
- Asilbekova DT, Ul’chenko NT, Glushenkova AI. Lipids from Physalis alkekengi. Chem Nat Comp. 2016;52(1):96–97. doi: 10.1007/s10600-016-1556-0. [DOI] [Google Scholar]
- Balmus IM, Ciobica A, Antioch I, Dobrin R, Timofte D. Oxidative stress implications in the affective disorders: main biomarkers, animal models relevance, genetic perspectives, and antioxidant approaches. Oxidative Med Cell Longev. 2016;2016:1–25. doi: 10.1155/2016/3975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron KD. The microglial cell. A historical review. J Neurol Sci. 1995;134(Suppl):57–68. doi: 10.1016/0022-510X(95)00209-K. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chien CC, Shen SC, Yang LY, Chen YC. Prostaglandins as negative regulators against lipopolysaccharide, lipoteichoic acid, and peptidoglycan-induced inducible nitric oxide synthase/nitric oxide production through reactive oxygen species-dependent heme oxygenase 1 expression in macrophages. Shock (Augusta, Ga) 2012;38(5):549–558. doi: 10.1097/SHK.0b013e31826b2826. [DOI] [PubMed] [Google Scholar]
- Deb D, Bairy KL, Nayak V, Rao M. Comparative effect of Lisinopril and Fosinopril in mitigating learning and memory deficit in scopolamine-induced amnesic rats. Adv Pharmacol Sci. 2015;2015:521718–521711. doi: 10.1155/2015/521718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374(3):222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- Gamal-Eldeen AM, Kawashty SA, Ibrahim LF, Shabana MM, El-Negoumy SI. Evaluation of antioxidant, anti-inflammatory, and antinociceptive properties of aerial parts of Vicia Sativa and its flavonoids. J Nat Rem. 2004;4(1):81–96. [Google Scholar]
- Hanrott K, Gudmunsen L, O'Neill MJ, Wonnacott S. 6-hydroxydopamine-induced apoptosis is mediated via extracellular auto-oxidation and caspase 3-dependent activation of protein kinase Cdelta. J Biol Chem. 2006;281(9):5373–5382. doi: 10.1074/jbc.M511560200. [DOI] [PubMed] [Google Scholar]
- Herrera-Morales W, Mar I, Serrano B, Bermudez-Rattoni F. Activation of hippocampal postsynaptic muscarinic receptors is involved in long-term spatial memory formation. Eur J Neurosci. 2007;25(5):1581–1588. doi: 10.1111/j.1460-9568.2007.05391.x. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275(21):16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Jung WY, Kim H, Park HJ, Jeon SJ, Park HJ, Choi HJ, Kim NJ, Jang DS, Kim DH, Ryu JH. The ethanolic extract of the Eclipta Prostrata L. ameliorates the cognitive impairment in mice induced by scopolamine. J Ethnopharmacol. 2016;190:165–173. doi: 10.1016/j.jep.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee Y, Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37(6):938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- Kim S, Chin YW, Cho J. Protection of cultured cortical neurons by Luteolin against oxidative damage through inhibition of apoptosis and induction of Heme Oxygenase-1. Biol Pharm Bull. 2017;40(3):256–265. doi: 10.1248/bpb.b16-00579. [DOI] [PubMed] [Google Scholar]
- Kranjc E, Albreht A, Vovk I, Makuc D, Plavec J. Non-targeted chromatographic analyses of cuticular wax flavonoids from Physalis Alkekengi L. J Chromatogr A. 2016;1437:95–106. doi: 10.1016/j.chroma.2016.01.061. [DOI] [PubMed] [Google Scholar]
- Li A-L, Chen B-J, Li G-H, Zhou M-X, Li Y-R, Ren D-M, Lou H-X, Wang X-N, Shen T. Physalis Alkekengi L. Var. Franchetii (mast.) Makino: an ethnomedical, phytochemical and pharmacological review. J Ethnopharmacol. 2018;210(Supplement C):260–274. doi: 10.1016/j.jep.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76(8):967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniruzzaman M, Bose S, Kim YM, Chin YW, Cho J. The ethyl acetate fraction from Physalis Alkekengi inhibits LPS-induced pro-inflammatory mediators in BV2 cells and inflammatory pain in mice. J Ethnopharmacol. 2016;181:26–36. doi: 10.1016/j.jep.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science (New York, NY) 1997;278(5337):412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nie K, Yu JC, Fu Y, Cheng HY, Chen FY, Qu Y, Han JX. Age-related decrease in constructive activation of Akt/PKB in SAMP10 hippocampus. Biochem Biophys Res Commun. 2009;378(1):103–107. doi: 10.1016/j.bbrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Qiu L, Zhao F, Jiang ZH, Chen LX, Zhao Q, Liu HX, Yao XS, Qiu F. Steroids and flavonoids from Physalis Alkekengi Var. Franchetii and their inhibitory effects on nitric oxide production. J Nat Prod. 2008;71(4):642–646. doi: 10.1021/np700713r. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995;9(7):526–533. doi: 10.1096/fasebj.9.7.7737461. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AMK. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234(1):249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Sharma AC, Kulkarni SK. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 1992;16(1):117–125. doi: 10.1016/0278-5846(92)90014-6. [DOI] [PubMed] [Google Scholar]
- Song YS, Park CM. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem Toxicol. 2014;65:70–75. doi: 10.1016/j.fct.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Syapin PJ. Regulation of haeme oxygenase-1 for treatment of neuroinflammation and brain disorders. Br J Pharmacol. 2008;155(5):623–640. doi: 10.1038/bjp.2008.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Naito Y, Uchiyama K, Yoshikawa T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox Rep. 2010;15(5):193–201. doi: 10.1179/174329210X12650506623889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Callahan PM, Hall B, Webster SJ. Alzheimer's disease and age-related memory decline (preclinical) Pharmacol Biochem Behav. 2011;99(2):190–210. doi: 10.1016/j.pbb.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ML, Ho YC, Lin CY, Yet SF. Heme oxygenase-1 in inflammation and cardiovascular disease. Am J Cardiovasc Dis. 2011;1(2):150–158. [PMC free article] [PubMed] [Google Scholar]
- Yang H, Han S, Zhao D, Wang G. Adjuvant effect of polysaccharide from fruits of Physalis Alkekengi L. in DNA vaccine against systemic candidiasis. Carbohydr Polym. 2014;109:77–84. doi: 10.1016/j.carbpol.2014.03.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 152 kb)