Abstract
Hyperglycemia induced oxidative stress inside the cells. Myricitrin, as an antioxidant plant-derived component, may be useful in hyperglycemia. Hence, the aim of this study was conducted to evaluate the antioxidant effects of myricitrin on hyperglycemia-induced oxidative damage in myotubes (C2C12 cells). In this experimental study, mouse myoblast cell line (C2C12) was obtained and divided into five groups: control, hyperglycemia, hyperglycemia + myricitrin 1, 3, and 10 μM. After treatment period for 48 h, cells were collected, homogenized, and centrifuged at 2000 rpm for 10 min. All samples were kept at − 80 °C until experimental and real-time PCR assessments were performed. Hyperglycemia increased malondialdehyde (MDA) (p < 0.05), total antioxidant capacity (TAC) (p < 0.001), and cellular apoptosis, and decreased levels of superoxide dismutase (SOD), catalase (CAT) (p < 0.01), myotube glycogen content (p < 0.05), glucose transporter type 4 (Glut-4), and cellular viability (p < 0.001). Myricitrin administration improved SOD (p < 0.05), CAT (p < 0.01), muscle cell’s glycogen content (p < 0.01), Glut-4 gene expression (p < 0.001), Thiazolyl blue tetrazolium bromide (MTT) (p < 0.05), and Bax to Bcl-2 ratio (p < 0.001), and reduced MDA (p < 0.05) compared to hyperglycemia group. In conclusion, hyperglycemic condition induced oxidative stress along with cellular apoptosis, and myricitrin improved these disorders. Also, low and moderate doses of myricitrin are more efficient on skeletal muscle cells exposed to hyperglycemic statues than a high concentration of this antioxidant agent.
Keywords: Myricitrin, Hyperglycemia, Oxidative stress, Apoptosis, C2C12 cell line
Introduction
Hyperglycemia is an important statues to promote reactive oxygen species (ROS) accumulation through the several metabolic pathways such as decrease of antioxidant defenses, increased flux of glucose, formation of advanced glycation end products (AGEs), activation protein kinase C (PKC) isoforms- α, β, δ and hexosamine pathway. Glucose is converted to polyalcohol sorbitol and, reduced amount of antioxidant agents concomitant with induction of O2− overproduction during hyperglycemia (Fiorentino et al. 2013). The impairment of the oxidant and antioxidant balance is very important. Several studies have shown that elevated extra- and intracellular glucose concentrations result in the impairment of antioxidant defense in diabetic animals and patients (Matough et al. 2012). It was demonstrated that hyperglycemia decreases the function and viability of cells (Safi et al. 2014). Skeletal muscle cell is a primary site of glucose utilization which insulin stimulates uptake of glucose via the complex insulin signaling pathways. This cell’s function has been occurred by translocation of glucose transporters to the plasma membrane from intracellular medium (Park et al. 2014).
Recently, some studies evaluate the roles of antioxidants system against toxic ROS and their pathological processes or possible therapeutic implications (Matough et al. 2012). Antioxidant agents such as flavonoids reduced the formation of ROS and scavenge them (Zatalia and Sanusi 2013). Flavonoids are a large group of polyphenolic compounds which possess antioxidant, antidiabetic and, anti-inflammatory properties (Aloud et al. 2017).
Myricitrin is a major component of flavonol glycoside which distributed in the root bark of Myrica cerifera, Myrica esculenta, Ampelopsis grossedentata, Myrica rubra, Manilkara zapota, and Eugenia uniflora. This plan-derived flavonoid glycoside has anxiolytic, antinociceptive, anti-inflammatory, and antioxidant effects that lead to being an important supplement in medicines (Pereira et al. 2011). Myricitrin could decrease malondialdeid (MDA), H2O2-induced oxidative damage and, improved antioxidant enzyme activity in ROS-induced cells dysfunction more than rhamnosides and quercetin (Zhang et al. 2017). In addition, this antioxidant agent could afford protection from oxidative stress-induced apoptotic cell death and prevent endothelial cell apoptosis induced by high glucose (Zhang et al. 2017; Pyun et al. 2017). Therefore, since skeletal muscle is an important site of glucose utilization during hyperglycemia and hyperglycemic condition increase oxidative stress, and myricitrin has antioxidant effects, the aim of present study was conducted to evaluate the antioxidant effects of myricitrin on hyperglycemia-induced oxidative stress in myoblast cell line of the mouse (C2C12).
Methods
Materials
C2C12 cell line (Pasteur Institute, Iran), myricitrin (AvaChem Scientific, USA), potassium hydroxide (KOH), sodium sulfate (Na2SO4), D-glucose, saline 0.9%, phenol, sulfuric acid (Merck, Germaney), phosphate buffered saline (Pharmaceutical Technology Development Center of Ahvaz Jundishapur University of Medical Sciences, Iran; pH: 7.4), MDA, TAC, CAT (Zellbio, Germaney), SOD (Randox Laboratories Ltd. United Kingdom), RNeasy Mini Kit (Qiagen, Valencia, CA), cDNA Synthesis Kit (Takara, Japan), Sybergreen (Takara, Japan), Glut-4, Bax and Bcl-2 primers (Microsynth, Switzerland), fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM) (Solar bio, South Korea), Trypsin-EDTA (Gibco, Canada), Thiazolyl blue tetrazolium bromide (MTT), Penicillin-streptomycin (Sigma-Aldrich, Canada), dimethyl sulphoxide (DMSO) (Bio Idea, Iran).
Cell culture
The mouse skeletal muscle C2C12 cell line was obtained from the cellular bank of Pasteur Institute of Iran, Tehran, Iran. This cell was maintained in DMEM, supplemented with 10% FBS, 4 mM glutamine, streptomycin (100 μg/mL), and penicillin (100 U/mL), under a humidified atmosphere of 5% CO2/95% at 37 °C. The cells were cultured in 75-cm2 cell culture flasks and divided in 25-cm2 cell culture flasks for treatment (Botha et al. 2017; Son et al. 2015).
Experimental design
First, C2C12 cells were grown in 25-cm2 cell culture flasks. Next, control group received medium contained D-glucose 5 mM, and the hyperglycemic group maintained in medium contained D-glucose 100 mM without any treatment. Then, the concentrations of myricitrin (1, 3 and 10 μM) concomitant with D-glucose 100 mM were administered in hyperglycemic-treated groups. The period of treatment was 48 h, and all cell culture flasks were incubated at 37 °C, in a humidified incubator with atmosphere of 5% CO2/95% (Huang et al. 2014; Kang et al. 2015; Karbach et al. 2012). Therefore, all cultured cell flasks were divided into five groups (n = 3): control, hyperglycemia, hyperglycemia + myricitrin 1 μM, hyperglycemia + myricitrin 3 μM and, hyperglycemia + myricitrin 10 μM (Senesi et al. 2016). After the experimental period and treatment by myricitrin, cells were collected by trypsin-EDTA (0.05%) and, centrifuged at 1300 rpm for 15 min. Then, resuspended in 0.5 mL of PBS (pH 7.4) and, lysed using a Teflon homogenizer. All the samples were centrifuged at 2000 rpm for 10 min and, were kept at − 80 °C until experimental measurements were performed (Dimauro et al. 2012).
Lipid peroxidation and antioxidants enzyme measurements
The supernatant of homogenate cells was administrated to assess MDA, total antioxidant capacity (TAC), catalase (CAT) and, superoxide dismutase (SOD) levels by specific commercial kits and their manufacture instructions (Sharma and Singh 2014).
Cultured medium glucose assay
Samples of culture medium were aspirated at the end of experiment and, transferred immediately to microtubes on ice. Then, the glucose concentration of cultured medium was measured using biochemical assay kits (Pars Azmoon Inc., Iran) and autoanalyzer device (BT3000, Italy) (Elkalaf et al. 2013; Li et al. 2013).
Muscle glycogen content
The C2C12 myotube glycogen contents were measured using the phenol-sulfuric acid method. Differentiated C2C12 cells cultured in 25-cm2 flasks were removed, transferred into 500 μl of Na2SO4 -saturated 30% KOH solution and heated at boiling point for 30 min. Then, 600 μl ethanol (95%) was added to the samples, incubated on ice for 5 min and, centrifuge at 840 rpm for 30 min. Next, the sediment contains glycogen were resuspended in 50 μl distilled water and, 50 μl of phenol 5% solution was added to the aliquots of 50 μl glycogen solution. Finally, concentrated 250 μl of sulfuric acid 98% was rapidly added, and the absorbance of solutions was read at 490-nm wavelength with a plate reader (ELx808 Absorbance Microplate Reader, USA) (Uruno et al. 2016).
MTT assay experiment
MTT was dissolved in PBS at a final concentration of 5 mg/mL to determine cell survival and viability. The living cells convert the MTT compound to the formed formazan crystals. These crystals were solubilized by DMSO. So, cultured cells were removed and incubated in medium containing 0.5 mg/mL of 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide. After 4-h incubation at 5% CO2 and 37 °C, the purple formazan crystals were dissolved in 100 μL DMSO and shacked for 15 min. Finally, the absorbance of solutions was read at 540-nm wavelength using an ELISA reader (ELx808 Absorbance Microplate Reader, ELISA Technologies, Inc. USA) (Hosseinzadeh and Khorsandi 2017; Gomez Perez et al. 2017).
Quantitative real-time PCR analyses
Total RNA and cDNA were prepared from the myotube cells using the commercial instruction of Rneasy mini kit and Reverse Transcriptase kit respectively. Real-time PCR was performed in triplicate using SYBR Green Master Mix in ABI step one plus instrument (Thermofisher, USA). The relative Glut-4, Bax and, Bcl-2 gene expression levels to the expression level of GAPDH, as the endogenous reference gene, were calculated using a comparative CT method (2-ΔΔCT). Also, primer efficiency investigation was conducted to validate our experiment. The primer sequences of the forward and reverse regarding to Glut-4 and GAPDH gene were the following:
Glut-4 forward primer: 5′-CGCACTAGCTGAGCTGAAGG-3′;
Glut-4 reverse primer: 5′-GCAGCACCACTGCGATGATA-3′;
Bax forward primer, 5′- GCTGGACATTGGACTTCCTC-3′;
Bax reverse primer, 5′- ACCACTGTGACCTGCTCCA-3′;
Bcl-2 forward primer, 5′- GCTGGACATTGGACTTCCTC-3′;
Bcl-2 reverse primer, 5′- GCTGGACATTGGACTTCCTC-3′;
GAPDH forward primer: 5′-ACCCAGAAGACTGTGGATGG-3′;
GAPDH reverse primer: 5′-TTCTAGACGGCAGGTCAGGT-3′ (Son et al. 2015).
Statistical assessment
All results were statistically analyzed using SPSS v. XVI as mean ± standard error of mean (SEM) with one-way analysis of variance (ANOVA), followed by post hoc least significant difference tests (LSD). The differences between groups were considered statistically significant at p < 0.05.
Results
Effect of myricitrin on lipid peroxidation and antioxidant defense in C2C12 cells
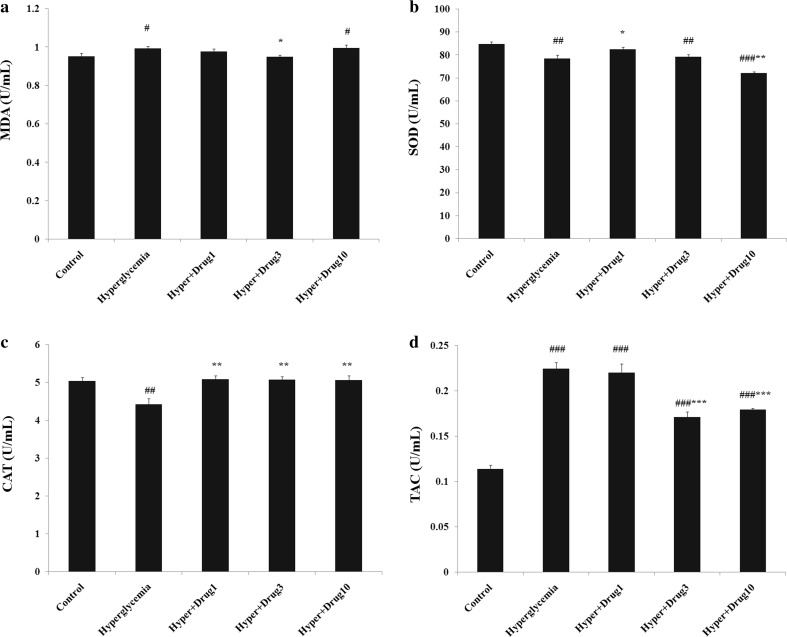
The level of MDA increased in hyperglycemia, hyperglycemia + myricitrin 10 μM compared with control (p < 0.05). This lipid peroxidation marker decreased in hyperglycemia + myricitrin 3 μM when compared to hyperglycemia (p < 0.05). SOD level assessment showed a significant decrease in hyperglycemia (p < 0.01), hyperglycemia + myricitrin 3 (p < 0.01) and 10 μM (p < 0.001) compared to control group. This antioxidant enzyme level increased in hyperglycemia + myricitrin 1 μM (p < 0.05) and, decreased in hyperglycemia + myricitrin 10 μM (p < 0.01) compared with hyperglycemia group. The cellular level of CAT demonstrates a significant decreased in hyperglycemia group compared to control (p < 0.01). Further, this variable increased in all treated groups when compared to hyperglycemia (p < 0.01). The results of TAC showed a remarkable increase in all groups versus to control (p < 0.001). In compared with untreated hyperglycemia exposed cells, this factor decreased in hyperglycemia + myricitrin 3 and 10 μM (p < 0.001; Fig. 1).
Fig. 1.
Effect of myricitrin on lipid peroxidation and antioxidant defense of C2C12 cell. Results are given as mean ± SEM; n = 3; #p < 0.05, ##p < 0.01, and ###p < 0.001 compared with the control group, *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the hyperglycemia group. a MDA; b SOD; c CAT; d TAC
Effect of myricitrin on glucose level of cultured medium
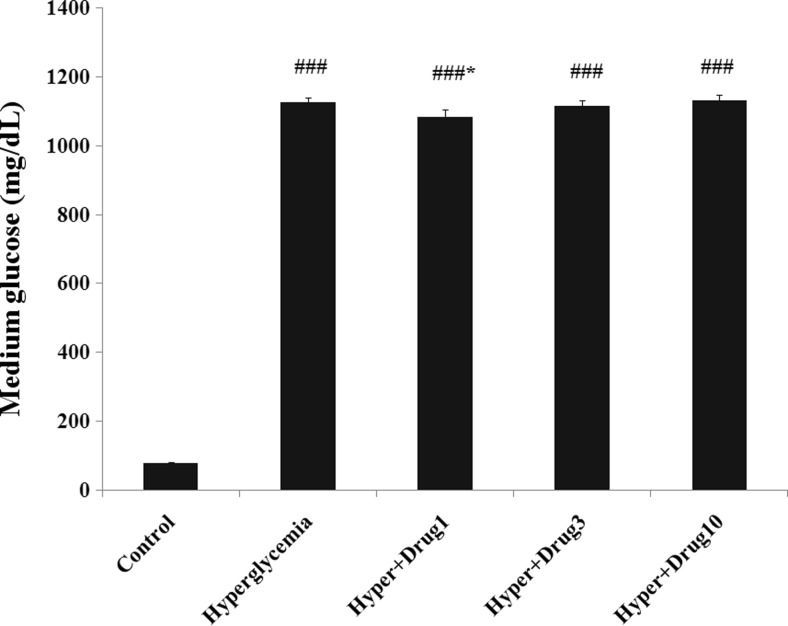
Since the high concentration of D-glucose has been used in treatment group, a significantly increased of glucose level was observed in all groups when compared to control (p < 0.001). The glucose level of cultured medium revealed a significant decrease in hyperglycemia + myricitrin 1 μM compared with hyperglycemia group (p < 0.05; Fig. 2).
Fig. 2.
Effect of myricitrin on glucose level of cultured medium. Results are given as mean ± SEM; n = 3; ###p < 0.001 compared with the control group, *p < 0.05 compared with the hyperglycemia group
Effect of myricitrin on C2C12 cell’s glycogen content
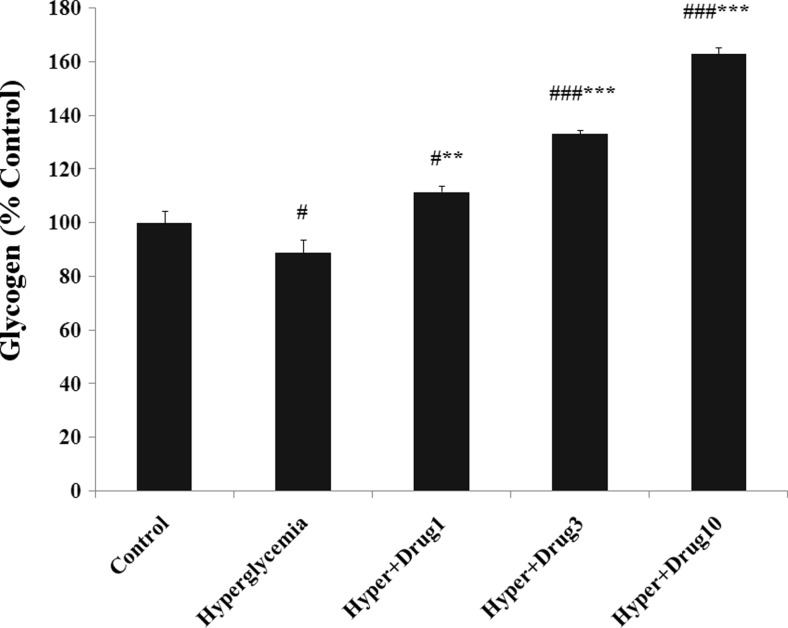
Glycogen content of myotubes deceased in hyperglycemia (p < 0.05), and increased in hyperglycemia + myricitrin 1 (p < 0.05), 3 and 10 μM (p < 0.001) compared with control. This variable showed a significant increase in hyperglycemia + myricitrin 1 (p < 0.01), 3 and 10 μM (p < 0.001) groups when compared with hyperglycemia (Fig. 3).
Fig. 3.
Effect of myricitrin on myotube glycogen content. Results are given as mean ± SEM; n = 3; #p < 0.05 and ###p < 0.001 compared with the control group, **p < 0.01 and ***p < 0.001 compared with the hyperglycemia group
Effect of myricitrin on C2C12 cell’s Glut-4 gene expression
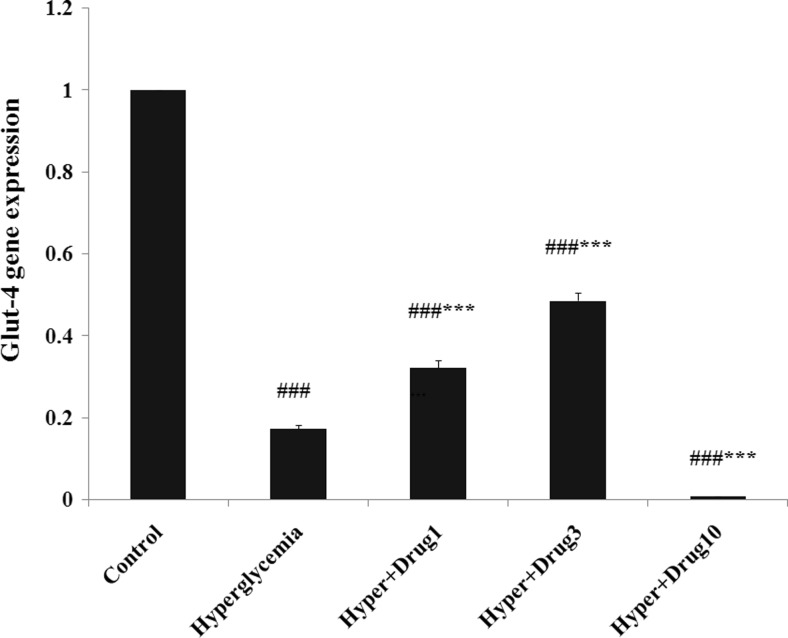
The results of Glut-4 gene expression revealed a significant decrease in all groups compared to control group (p < 0.001). Also, this variable increased in hyperglycemia + myricitrin 1 and 3 μM and, decreased in hyperglycemia + myricitrin 10 μM groups compared with hyperglycemia (p < 0.001; Fig. 4).
Fig. 4.
Effect of myricitrin on Glut-4 gene expression. Results are given as mean ± SEM; n = 3; ###p < 0.001 compared with the control group, ***p < 0.001 compared with the hyperglycemia group
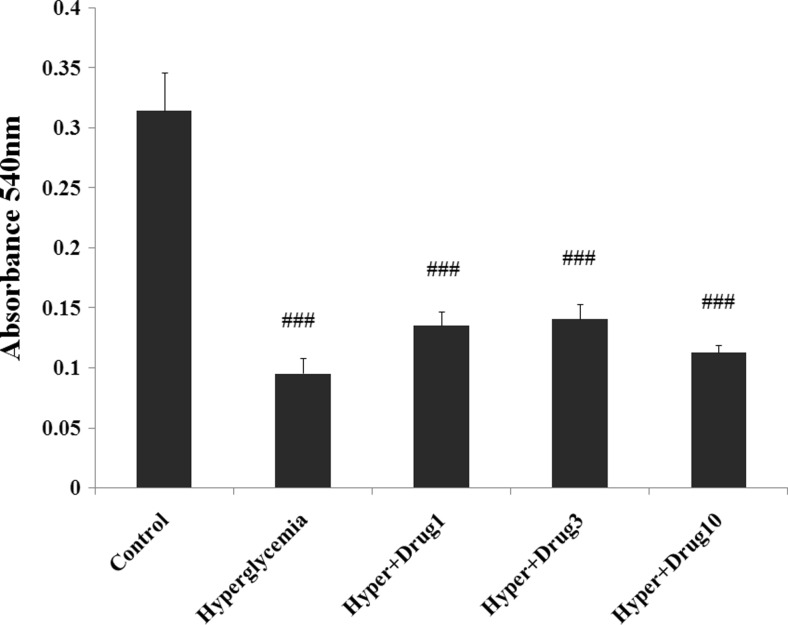
Effect of myricitrin on C2C12 cell’s viability
As shown in Fig. 5, MTT reduced in all groups compared to control (p < 0.001). However, this variable revealed a tendency to increase at doses 1 and 3 μM of myricitrin, but these changes are significant in hyperglycemia + myricitrin 3 μM (p < 0.05).
Fig. 5.
Effect of myricitrin MTT levels of C2C12 cells. Results are given as mean ± SEM; n = 3; ###p < 0.001 compared with the control group, *p < 0.05 compared with the hyperglycemia group
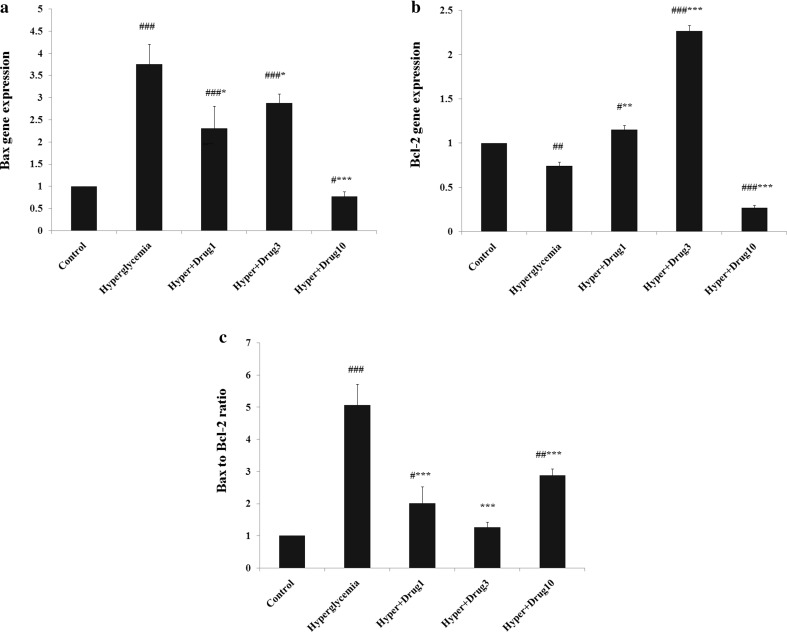
Effect of myricitrin on C2C12 cell’s apoptosis
Bax gene expression increased in hyperglycemia, hyperglycemia + myricitrin 1 and 3 μM (p < 0.001) compared with control. This gene expression decreased in hyperglycemia + myricitrin 1, 3 (p < 0.05) and 10 μM (p < 0.001) versus to hyperglycemia group. The expression of Bcl-2 gene decreased in hyperglycemia (p < 0.01) and hyperglycemia + myricitrin 10 μM (p < 0.001) and, increased in hyperglycemia + myricitrin 1 (p < 0.05) and 3 μM (p < 0.001) compared to control group. This gene expression assessment showed a significant increase in hyperglycemia + myricitrin 1 (p < 0.01) and 3 μM (p < 0.001) and, decreased in hyperglycemia + myricitrin 10 μM (p < 0.001) when compared to untreated hyperglycemic cell group. Bax to Bcl-2 ratio increased in hyperglycemia (p < 0.001), hyperglycemia + myricitrin 1 (p < 0.05) and 10 μM (p < 0.01) compared with control group. This ratio decreased in all treated groups compared to hyperglycemia (p < 0.001; Fig. 6).
Fig. 6.
Effect of myricitrin on mytube apoptosis gene expression. Results are given as mean ± SEM; n = 3; #p < 0.05, ###p < 0.01, and ###p < 0.001 compared with the control group, *p < 0.05, **p < 0.01, and ***p < 0.001 compared with the hyperglycemia group. a Bax; b Bcl-2; c Bax to Bcl-2 ratio
Discussion
Hyperglycemia generates free radicals such as a transient burst of H2O2 in various insulin-responsive tissues (Kabra et al. 2009).This condition can cause cellular damage through the several pathways including increased polyol and nonenzymatic glycation of intra and extracellular proteins, altered signal transduction pathways and increased oxidative stress. Previous in vitro and in vivo studies demonstrated that hyperglycemia leads to induced oxidative stress in endothelial cell (Jain et al. 2006). Further, the enhanced glucose concentration in cell culture medium elevates free fatty acid levels via the absence of an appropriate compensatory response such as antioxidant defense, which results in the increased expression of numerous cellular damage gene products (Evans et al. 2003). Chronic hyperglycemia down-regulate SOD and CAT levels in pancreas tissue, and administration of antioxidant components including aucubin, S-allylcysteine and Naringenin prevent MDA and improve the level of SOD and CAT (Annadurai et al. 2012). So, along with the previous researches, our results indicated that hyperglycemic medium induced oxidative stress in myotube cell through the elevated lipid peroxidation and reduced antioxidant enzyme level. Excessive production of antioxidant agents may eventually induce oxidative stress, and synergistically exacerbate the cellular component damage. A positive correlation of TAC and MDA levels has occurred in ischemic stroke assessment study which leads to increase oxidative stress and mortality (Lorente et al. 2016). Hence, according to the present results, it could be suggested that hyperglycemic medium induced more destruction in muscle cells through the increased TAC and MDA compared to normal condition in control group. It seems that myricitrin at dose of 1 μM improved hyperglycemia-induced oxidative stress through the increased SOD and CAT level in C2C12 cells. Further, the dose of 3 μM is more potent on lipid peroxidation than others, and it improved the TAC and CAT level which altered by hyperglycemic condition. However, myricitrin 10 μM increased CAT in myotubes, but this dose of myricitrin did not improve elevated level of MDA, and more reduced cellular level of TAC and SOD than untreated hyperglycemic cells. So, it may be suggested that this dose of myricitrin is not useful for this condition.
Normal cells are able to control the transport of glucose into the cell in hyperglycemic status. However, the cells injured by hyperglycemia cannot do this performance and increase the glucose levels inside the cell that leads to cellular oxidative stress (Naudi et al. 2012). Glycogen synthesis is the main pathway for glucose absorption into the skeletal muscle cells that regulate by hexokinase II and glucose-6-phosphate amidotransferase (GFAT) activity. Recently, it was demonstrated that hyperglycemia reduced glycogen content in myotube by disrupted the hexosamine biosynthetic pathway. The muscle’s activity of GFAT increase in this condition and results in the impairment of glucose uptake due to the decrease Glut-4 level in this tissue (Kato et al. 2004; Kim et al. 2005). Flavonol glycoside stimulate glucose uptake in muscle tissue and, improved the activity of the rate-limiting enzymes that involved in glucose metabolism such as glucokinase (Gupta et al. 2012). So, the present results suggested that hyperglycemia reduced glycogen content via the increased oxidative agents and reduced the glycogen synthesis enzyme. Also, myricitrin consumption increased C2C12 cell’s glycogen content in a dose-dependent manner. However, low and moderate doses of administered myricitrin improved myotube glycogen content through the increased Glut-4 gene expression and glucose uptake, but it seems that high dose of myricitrin was more efficient on glycogen synthesis enzymes than Glut-4 gene expression for glucose uptake. Hence, future studies are required to clarify the exact mechanism of this event.
The hyperglycemic condition increases the intracellular superoxide level and leads to mitochondrial dysfunction that eventually induced cellular apoptosis (Pyun et al. 2017). Mohammadi-Farani et al, showed that hyperglycemia has negative effects on adrenal medulla cell viability of rat through the reduced MTT (Mohammadi-Farani et al. 2014). Also, a similar effect in osteoblast cell was observed in Ogawa et al. research (Ogawa et al. 2007). Bax and Bcl-2 belong to a family of proteins which regulates apoptosis. An over expression of Bax promotes cell death, and the enhancement of Bcl-2 gene expression increases cells survival or viability, and protects them against apoptosis. It was demonstrated that Bcl-2 expression could significantly elevated during hydrogen peroxide-induced apoptosis in myotubes than other muscle cells. Further, overexpression of Bcl-2 results to decrease levels of pro-apoptotic Bax in the myotubes, without remarkable changes in the levels of other pro-apoptotic agent such as Bak and Bad. So, these genes are more important for apoptosis assessment than others (Schöneich et al. 2014). It was generally approved that Bax and Bcl-2 serve to control the release of mitochondria-housed apoptotic factors in C2C12 myotubes following oxidative stress treatment (Siu et al. 2009). Hyperglycemia significantly down-regulates Bcl-2 and up-regulates Bax in beta-cells (Hasnan et al. 2010). Exogenous antioxidants could improve hyperglycemia-induced apoptotic changes in tubular cells (Verzola et al. 2004). One study revealed that kaempferol, as an antioxidant agent, restored Bcl-2 protein expression which reduced by high glucose concentration in islets of langerhans (Zhang and Liu 2011). Apoptosis induction can increase the ratio of Bax to Bcl-2, and exacerbate the susceptibility of hematopoietic cell to apoptosis (Magistrelli et al. 2006). Therefore, consists of previous study present results indicated that hyperglycemic medium reduced C2C12 cell viability through the decrease MTT, Bcl-2, and increased Bax gene expression and Bax to Bcl-2 ratio compared to myricitrin treated muscle’s cell.
It was revealed that flavonoids possess both anti- and prooxidant action. Their antioxidant effect has been shown to account for biological effects of phenolic compounds and, some recent studies have demonstrated that anticancer activities of these agents may be mediated via pro-oxidant action. Anti- or pro-oxidant actions of flavonoid are depending on its dose. Flavonoids have been found to induce cytotoxicity only at a relatively high dose (Sak 2014). Also, one research indicated that some of the flavonoids appear to have several biological activities depending on the dose ingested (Kay et al. 2012). In previous studies, quercetin was implied as an apoptotic activator, free radical scavenger, and antiapoptotic agent. These effects are depending on the dose of administration. The results of one study showed that higher dose of quercetin than 200 μM act as a toxic dose and reduce K562 cell viability but low doses of quercetin (< 200 μM) could increase cell viability and considered as therapeutic dose (Akan and Garip 2013). Present study revealed that myricitrin treatment improved cell viability and apoptosis at the concentrations of 1 and 3 μM via the increase MTT and Bcl-2 levels, and decrease Bax gene expression and Bax to Bcl-2 ratio, but the dose of 3 μM are more potent on Bcl-2 and the ratio of Bax to Bcl-2 than 1 μM. Finally, it could be suggested myricitrin 10 μM improved cellular apoptosis by a direct effect on Bax gene expression, which this event leads to a remarkable reduction of Bcl-2. So, in line with previous research, this data demonstrated that the different roles of myricitrin on cellular viability and apoptosis is depending on the dose of ingestion and, it suggested that low and moderate doses of myricitrin may act as antioxidant agents and, high dose of this plant-derived antioxidant plays as a reducing cell viability agent which may be useful for cancer treatment. However, future studies are required to clarify the exact mechanism of these suggestions.
In conclusion, the hyperglycemic condition induced lipid peroxidation along with the cellular apoptosis, reduced antioxidant enzymes activity, myotube glycogen content, and cell’s viability. Myricitrin administration revealed antioxidant effects, and improved hyperglycemia-induced oxidative stress alterations due to the increase glucose uptake, C2C12 glycogen content, Glut-4 gene expression concomitant with decrease cellular apoptosis and increase the viability of myotubes. Ultimately, low and moderate doses of myricitrin are suggested as more efficient on skeletal muscle cells exposed to hyperglycemic statues than a high concentration of this antioxidant agent. Hence, myricitrin can be commercially viable, because it's more effective at a low dose of administration.
Acknowledgements
This study is a part of the Ph.D. thesis of Ali Akbar Oroojan was labeled Cell & Molecular Research Center project (CMRC-9509) and was supported financially by the vice-chancellor of research affairs of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Compliance with ethical standards
This research has been done in accordance with the principles and guidelines of Ahvaz Jundishapur University of Medical Sciences ethics committee with No. IR.AJUMS.REC.1395.136.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Akan Z, Garip AI. Antioxidants may protect cancer cells from apoptosis signals and enhance cell viability. Asian Pac J Cancer Prev. 2013;14(8):4611–4614. doi: 10.7314/APJCP.2013.14.8.4611. [DOI] [PubMed] [Google Scholar]
- Aloud AA, Veeramani C, Govindasamy C, Alsaif MA, El Newehy AS, Al-Numair KS. Galangin, a dietary flavonoid, improves antioxidant status and reduces hyperglycemia-mediated oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2017;22(6):290–300. doi: 10.1080/13510002.2016.1273437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annadurai T, Muralidharan AR, Joseph T, Hsu MJ, Thomas PA, Geraldine P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J Physiol Biochem. 2012;68(3):307–318. doi: 10.1007/s13105-011-0142-y. [DOI] [PubMed] [Google Scholar]
- Botha CJ, Clift SJ, Ferreira GCH, Masango MG. Geigerin-induced cytotoxicity in a murine myoblast cell line (C2C12) Onderstepoort J Vet Res. 2017;84(1):e1–e7. doi: 10.4102/ojvr.v84i1.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimauro I, Pearson T, Caporossi D, Jackson MJ. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Research Notes. 2012;5:513–513. doi: 10.1186/1756-0500-5-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkalaf M, Anděl M, Trnka J. Low glucose but not galactose enhances oxidative mitochondrial metabolism in C2C12 myoblasts and myotubes. PLoS One. 2013;8(8):e70772. doi: 10.1371/journal.pone.0070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress- activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52(1):1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- Gomez Perez M, Fourcade L, Mateescu MA, Paquin J. Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Anal Biochem. 2017;535(Supplement C):43–46. doi: 10.1016/j.ab.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4(2):164–171. doi: 10.1111/j.1753-0407.2011.00173.x. [DOI] [PubMed] [Google Scholar]
- Hasnan J, Yusoff M, Damitri T, Faridah A, Adenan A, Norbaini T. Relationship between apoptotic markers (Bax and Bcl-2) and biochemical markers in type 2 diabetes mellitus. Singap Med J. 2010;51(1):50–55. [PubMed] [Google Scholar]
- Hosseinzadeh R, Khorsandi K. Methylene blue, curcumin and ion pairing nanoparticles effects on photodynamic therapy of MDA-MB-231 breast cancer cell. Photodiagn Photodyn Ther. 2017;18(Supplement C):284–294. doi: 10.1016/j.pdpdt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- Huang Q, Gao B, Wang L, Hu YQ, Lu WG, Yang L, Luo ZJ, Liu J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol Appl Pharmacol. 2014;280(3):550–560. doi: 10.1016/j.taap.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Jain SK, McVie R, Bocchini JA. Hyperketonemia (ketosis), oxidative stress and type 1 diabetes. Pathophysiology. 2006;13(3):163–170. doi: 10.1016/j.pathophys.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Kabra DG, Gupta J, Tikoo K. Insulin induced alteration in post-translational modifications of histone H3 under a hyperglycemic condition in L6 skeletal muscle myoblasts. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2009;1792(6):574–583. doi: 10.1016/j.bbadis.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kang J, Boonanantanasarn K, Baek K, Woo KM, Ryoo H-M, Baek J-H, Kim G-S. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner. J Periodontal Implant Sci. 2015;45(3):101–110. doi: 10.5051/jpis.2015.45.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach S, Jansen T, Horke S, Heeren T, Scholz A, Coldewey M, Karpi A, Hausding M, Kröller-Schön S, Oelze M, Münzel T, Daiber A. Hyperglycemia and oxidative stress in cultured endothelial cells—a comparison of primary endothelial cells with an immortalized endothelial cell line. J Diabetes Complicat. 2012;26(3):155–162. doi: 10.1016/j.jdiacomp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Kato M, Suwa A, Shimokawa T. Glucose catabolic gene mRNA levels in skeletal muscle exhibit non-coordinate expression in hyperglycemic mice. Horm Metab Res. 2004;36(08):513–518. doi: 10.1055/s-2004-825752. [DOI] [PubMed] [Google Scholar]
- Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res. 2012;56(11):1605–1616. doi: 10.1002/mnfr.201200363. [DOI] [PubMed] [Google Scholar]
- Kim YB, Peroni OD, Aschenbach WG, Minokoshi Y, Kotani K, Zisman A, Kahn CR, Goodyear LJ, Kahn BB. Muscle-specific deletion of the Glut4 glucose transporter alters multiple regulatory steps in glycogen metabolism. Mol Cell Biol. 2005;25(21):9713–9723. doi: 10.1128/MCB.25.21.9713-9723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hu Z-F, Chen B, Ni G-X. Response of C2C12 myoblasts to hypoxia: the relative roles of glucose and oxygen in adaptive cellular metabolism. Biomed Res Int. 2013;2013:326346. doi: 10.1155/2013/326346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente L, María MM, Pérez-Cejas A, Abreu-González P, Ramos L, Argueso M, Cáceres JJ, Solé-Violán J, Jiménez A. Association between total antioxidant capacity and mortality in ischemic stroke patients. Ann Intensive Care. 2016;6:39. doi: 10.1186/s13613-016-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistrelli P, Coppola R, Tonini G, Vincenzi B, Santini D, Borzomati D, Vecchio F, Valeri S, Castri F, Antinori A. Apoptotic index or a combination of Bax/Bcl-2 expression correlate with survival after resection of pancreatic adenocarcinoma. J Cell Biochem. 2006;97(1):98–108. doi: 10.1002/jcb.20621. [DOI] [PubMed] [Google Scholar]
- Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12(1):5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi-Farani A, Ghazi-Khansari M, Sahebgharani M. Glucose concentration in culture medium affects mRNA expression of TRPV1 and CB1 receptors and changes capsaicin toxicity in PC12 cells. Iran J Basic Med Sci. 2014;17(9):673–378. [PMC free article] [PubMed] [Google Scholar]
- Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:696215. doi: 10.1155/2012/696215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res. 2007;39(12):871–875. doi: 10.1055/s-2007-991157. [DOI] [PubMed] [Google Scholar]
- Park SY, Kim MH, Ahn JH, Lee SJ, Lee JH, Eum WS, Choi SY, Kwon HY. The stimulatory effect of essential fatty acids on glucose uptake involves both Akt and AMPK activation in C2C12 skeletal muscle cells. Korean J Physiol Pharmacol. 2014;18(3):255–261. doi: 10.4196/kjpp.2014.18.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Siba IP, Chioca LR, Correia D, Vital MABF, Pizzolatti MG, Santos ARS, Andreatini R. Myricitrin, a nitric oxide and protein kinase C inhibitor, exerts antipsychotic-like effects in animal models. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1636–1644. doi: 10.1016/j.pnpbp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Pyun B-J, Kim YS, Lee I-S, Kim JS. Homonoia riparia and its major component, myricitrin, inhibit high glucose-induced apoptosis of human retinal pericytes. Int Med Res. 2017;6(3):300–309. doi: 10.1016/j.imr.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi SZ, Qvist R, Yan GOS, Ismail ISB. Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Med Genet. 2014;7:29–29. doi: 10.1186/1755-8794-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8(16):122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneich C, Dremina E, Galeva N, Sharov V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis. 2014;19(1):42–57. doi: 10.1007/s10495-013-0922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesi P, Montesano A, Luzi L, Codella R, Benedini S, Terruzzi I. Metformin treatment prevents sedentariness related damages in mice. J Diabetes Res. 2016;2016(8274689):30. doi: 10.1155/2016/8274689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Singh R. Effect of Momordica dioica fruit extract on antioxidant status in liver, kidney, pancreas, and serum of diabetic rats. Pharm Res. 2014;6(1):73–79. doi: 10.4103/0974-8490.122922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Wang Y, Alway SE. Apoptotic signaling induced by H(2)O(2)-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009;84(13–14):468–481. doi: 10.1016/j.lfs.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology. 2015;67(4):641–652. doi: 10.1007/s10616-014-9730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno A, Yagishita Y, Katsuoka F, Kitajima Y, Nunomiya A, Nagatomi R, Pi J, Biswal SS, Yamamoto M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol Cell Biol. 2016;36(11):1655–1672. doi: 10.1128/MCB.01095-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT, Garibotto G, Deferrari G. Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol. 2004;15(1 suppl):S85–S87. doi: 10.1097/01.ASN.0000093370.20008.BC. [DOI] [PubMed] [Google Scholar]
- Zatalia SR, Sanusi H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med Indones. 2013;45(2):141–147. [PubMed] [Google Scholar]
- Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670(1):325–332. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Zhang B, Shen Q, Chen Y, Pan R, Kuang S, Liu G, Sun G, Sun X. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Sci Rep. 2017;7:44239. doi: 10.1038/srep44239. [DOI] [PMC free article] [PubMed] [Google Scholar]