Abstract
Spinal cord injury (SCI) is generally divided into primary and secondary injuries, and apoptosis is an important event of the secondary injury. As an endogenous bile acid and recognized endoplasmic reticulum (ER) stress inhibitor, tauroursodeoxycholic acid (TUDCA) administration has been reported to have a potentially therapeutic effect on neurodegenerative diseases, but its real mechanism is still unclear. In this study, we evaluated whether TUDCA could alleviate traumatic damage of the spinal cord and improve locomotion function in a mouse model of SCI. Traumatic SCI mice were intraperitoneally injected with TUDCA, and the effects were evaluated based on motor function assessment, histopathology, apoptosis detection, qRT-PCR, and western blot at different time periods. TUDCA administration can improve motor function and reduce secondary injury and lesion area after SCI. Furthermore, the apoptotic ratios were significantly reduced; Grp78, Erdj4, and CHOP were attenuated by the treatment. Unexpectedly, the levels of CIBZ, a novel therapeutic target for SCI, were specifically up-regulated. Taken together, it is suggested that TUDCA effectively suppressed ER stress through targeted up-regulation of CIBZ. This study also provides a new strategy for relieving secondary damage by inhibiting apoptosis in the early treatment of spinal cord injury.
Keywords: Tauroursodeoxycholic acid, Spinal cord injury, ER stress, Apoptosis, CIBZ
Introduction
Spinal cord injury (SCI) is one of the central nervous system (CNS) diseases with a high disablement rate, and which still lacks effective clinical treatment (Crowe et al. 1997; McDonald and Sadowsky 2002). SCI results in many complications, such as pressure sores, chronic limb pain, urinary and respiratory system infections, and a decline in muscle function (Furlan and Fehlings 2008), despite efforts to relieve its complications. SCI comprises primary and secondary injury; the pathological and physiological reactions induce nerve cell damage, eventually causing tissue and organ dysfunction in the secondary injury (Lu et al. 2000; Noreau et al. 2000). Apoptosis dominates the secondary injury, but the real mechanisms are extremely complicated; various stimulus signal pathways are involved in apoptosis in SCI (Ahn et al. 2006). At this time, the physiological and pathological processes are still unclear.
Endoplasmic reticulum (ER) stress plays an important protective role in keeping physiological balance and homeostasis, when cells are exposed to various cellular stresses including infection, trauma, and oxidative damage (Boyce and Yuan 2006). However, persistent or excessive ER stress will induce cell death via activations of mitochondrial apoptotic and other cell death pathways (Xu et al. 2005). In many diseases, like cardiovascular, endocrine, metabolic, and nervous system diseases, ER stress is the pathological and physiological basis and participates in the process and development of the disease (Keene et al. 2002; Ramalho et al. 2008; Rodrigues et al. 2003). Previous studies have demonstrated the important role of ER stress in traumatic SCI. Different cell types have different sensitivities to ER stress induced by SCI. Oligodendrocytes are the most sensitive and are a key cytotoxic target of the ERSR in response to SCI (Ohri et al. 2011; Penas et al. 2007). CIBZ, a BTB domain-containing protein, participates in the negative regulation of apoptosis in murine cells (Oikawa et al. 2008). We have reported that CIBZ is a novel target of spinal cord injury, because its expression is significantly reduced in the early stage of SCI, and it induces apoptosis via the p53-independent caspase-3 pathway (Cai et al. 2012). Additionally, we have further demonstrated that knockdown of CIBZ results in the elevation of ER stress; interestingly, CIBZ overexpression can inhibit ER stress-associated apoptosis and improve spinal cord function (Cai et al. 2017). Therefore, our previous study provides a promising therapeutic way to solve this problem.
TUDCA is the conjugate form of ursodeoxycholic acid (UDCA), widely present in animal bile, and at low levels in humans. It is usually used for the treatment of liver diseases (Podda et al. 1995). Recent studies have found that TUDCA acts as a potent inhibitor on apoptosis, decreasing apoptosis in cardiovascular and neurodegenerative diseases and in retinal degenerative disorders and stroke (Keene et al. 2002; Ramalho et al. 2008; Rodrigues et al. 2003). TUDCA can inhibit apoptosis, negatively modulate the mitochondrial pathway, interfere with the death receptor pathway, block caspase-3 activation, and suppress ER stress through inhibiting calpain and caspase-12 activation (Vang et al. 2014). TUDCA also modulates Aβ-induced apoptosis through an E2F-1/p53/Bax pathway by interfering with crucial events of the mitochondrial pathway (Ramalho et al. 2004). However, little is known about whether and how the inhibition of ER stress can effectively reduce traumatic spinal cord injury.
In this study, we successfully built a mouse model of traumatic SCI and confirmed the therapeutic effect of TUDCA on motor functional recovery. Our results indicate that TUDCA effectively decreases the secondary lesion and preserves spinal cord functions. Finally, TUDCA protects nerve cells from ER stress-associated apoptosis by elevating levels of CIBZ. Therefore, our study provides a new strategy for the treatment of SCI patients.
Materials and methods
Animal preparation and group
Eight-week-old KM male mice weighing 30–35 g were purchased from the Qinglongshan Animal Farm (Nanjing, China). The mice were raised separately in clean animal cages on an unrestricted diet at a room temperature of 22–25 °C with 12–12-h light–dark cycles. All animal experiments complied with the ARRIVE guidelines and were carried out according to the National Institutes of Health guide for the care and use of laboratory animals. All animal experiments were approved by the Anhui Normal University Academic Ethics Committee. Mice were randomly divided into four groups, including a sham group, a sham/TUDCA group, an SCI group, and an SCI/TUDCA group. Sham and sham/TUDCA group mice were treated with normal saline (NS). The dose of TUDCA (100 mg/kg, ip; Aladdin, Shanghai, China) has been reported to offer the maximum cytoprotection (Rodrigues et al. 2003). This study, to determine the optimal therapeutic concentration of TUDCA in a mouse model of SCI, was carried out using a range of dosages of TUDCA (50, 100, 150, and 200 mg/kg of bw). Mice were immediately injected once with TUDCA post SCI at the same time each day.
Experimental design
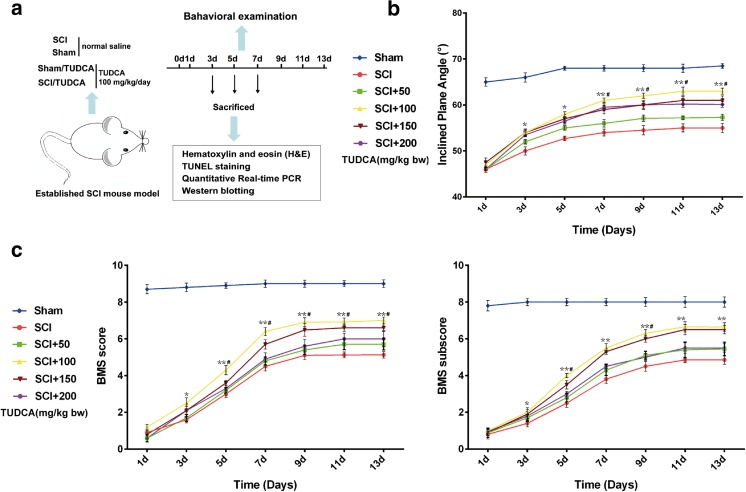
The motor function score tests were performed and recorded for 1–13 days post injury. We collected spinal segments Th7–Th9 respectively, and observed traumatic changes of spinal cord at 1, 3, and 7 days under a digital microscope (KEYENCE, VHX-5000), and then tissues of spinal cord were picked up and embedded with paraffin for histology staining. Additionally, the tissue samples were used to extract the RNA and protein for qPCR and western blot analysis after SCI (Fig. 1a).
Fig. 1.
TUCA improves the recovery of motor function after SCI. a Implementation of group and the experimental process. b, c Time course of functional recovery of hind limbs was evaluated by BMS scores and inclined plane test in sham, SCI, and SCI/TUDCA mice. Data are presented as mean ± SEM for each group (n = 5). *p < 0.05, **p < 0.01 (significantly different from the SCI group); # p < 0.05 (significantly different at the same times in different dosages)
Mouse model of SCI
Spinal cord injury was performed as described previously (Cai et al. 2011; Cai et al. 2012). We used sterilized instruments, and all the surgical operations were completed in clean conditions. First, mice were anesthetized with chloral hydrate (4 mg/kg, ip). Then, all groups were treated with surgery to expose Th7–Th9 to the spinal cord, but the SCI and SCI/TUDCA groups were subjected to compression of the spinal cord with a 30-g weight drop. In the compressed injury mice, the tail cocked up and the lower limbs experienced spasticity at the moment of injury; subsequently, mice suffered paralysis of the lower limbs after continuous compression for 3 min. Finally, we used an alcohol sponge to stanch the bleeding and clean the wound, which was stitched immediately. All groups were raised in clean cages. To prevent from urinary retention, we squeezed the animals’ bladders two or three times a day to assist urination, until the mice regained the automatic micturition reflex.
Behavioral analysis
The Basso mouse scale (BMS) was used for the comprehensive rate of motor function after SCI as previously described. Hind limb function could be evaluated according to the BMS motor function rating gauge. The scale ranges from 0 (complete paralysis) to 9 (normal movement of the hind limbs). For the BMS score, the mice were placed in an open field (50 × 50 × 30 cm) divided into 25 sections (10 × 10 cm each). The mice were allowed to walk about in the open field for several days before SCI surgery to acclimatize themselves to the apparatus. The open field test was recorded throughout by video camera, and was assessed by repeated observation (Basso et al. 2006). The mice were observed for 4 min by three independent observers who were unaware of the groupings and who all received the same score training. Scores were obtained for each hind limb and averaged for the three observers on each day. The strength of hind limb recovery was evaluated with inclined plane angles. The inclined plane test was placed on a rubber plate, and then gradually raised on one side of the plane, and the maximum angle at which mice could remain stable for at least 5 s was noted (Wells et al. 2003). Each mouse was assessed three times, and the average was taken. The mean of all mice in each group was the final result. We followed a previously mentioned procedure to analyze the data.
Tissue preparation and histology staining
Mice were anesthetized, and exposed to heart perfusion after the chest was opened. Spinal cord (Th7–Th9) tissues at the injury site were harvested, fixed in PFA overnight, and embedded in paraffin wax. Tissues were transversely and longitudinally sectioned at 7-μm thickness. HE staining followed a standard procedure, and these slides were stained with hematoxylin for 3 min, differentiated into 1% hydrochloric acid alcohol for a few seconds, rinsed with running water for 5 min, and then stained with Eosin Y for 3 min. Finally, the slides were mounted in neutral balsam, and the images were observed and captured with a microscope (Olympus, BX61).
The area of lesion was measured with Photoshop software according to images from longitudinal sections of each spinal cord. The five digital HE images of longitudinal sections were captured with the microscope, the contours of lesion area were selected with Photoshop, and the corresponding pixel values were obtained. Then the corresponding pixel value per unit area was calculated according to the scale of the image, and finally the lesion area was calculated.
Tissue paraffin sections were deparaffinized according to standard procedures. Antigen retrieval was performed in citrate buffer with 750-W microwave irradiation for 1 min, and sections were blocked with Tris-HCl (0.1 M, pH 7.5) containing 3% BSA and 20% normal bovine serum for 30 min at 37 °C. After washing with PBS, sections were incubated with 50 μl TUNEL reaction mixture (In Situ Cell Death Detection Kit, Roche, Indianapolis, IN, USA) for 1 h at 37 °C in a humidified atmosphere in the dark. Finally, the slides were mounted with the DAPI mounting medium (Solarbio, Beijing, China) to mount slides. Slides were observed with the fluorescence microscope (Olympus, BX61), and positive cells were counted from digitally captured images.
Quantitative real-time PCR
Total RNA was extracted from injured spinal cord tissues by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA); isolated RNA was reversely transcribed based on the FastQuant RT Kit (Tiangen, Beijing, China). Amplification and real-time detection were performed on an IQ5 instrument (Bio-Rad, Hercules, CA, USA) by using the SuperReal PreMix Plus (Tiangen). The improved four-step reaction was used: 95 °C 15 min; 95 °C 10s, 60 °C 32 s, 72 °C 32 s, 85 °C 6 s, for 40 cycles; the melting curve analysis ranging from 60 to 95 °C, gradually increasing at a speed of 0.5 °C every 10 s. Relative quantitative analysis of the final results was normalized to GAPDH by using the 2−ΔΔCt method. The primers for SCI mouse were as follows:
GAPDHF 5′-AACTTTGGCATTGTGGAAGG-3′, R 5′-CACATTGGGGGTAGGAACA-3′; Grp78 F 5′-TAAAAGCCCTGATGCTGAAGC-3′, R 5′-TCCGACTATTGGCATCCGA-3′; ERdj4 F 5′-GCATGAAGGAGAAGGAGCAG-3′, R 5′-GCATGAAGGAGAAGGAGCAG-3′; CHOP F 5′-GCATGAAGGAGAAGGAGCAG-3′, R 5′-TGGTGCTGGGTACACTTCC-3′; CIBZ F 5′-CCAGAAAATAGGATTGGCGA-3′, R 5′-GTTGCAATAATGGCAAGGGT-3′.
Western blot
The spinal cord tissues were homogenized and lysed in RIPA buffer (Boster, Wuhan, China) with protease inhibitors, and the protein concentration was measured with a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The proteins were denatured and diluted to 1 μg/μl. Then equal amounts of the proteins were separated by SDS-PAGE, and transferred on Immobilon-P Transfer Membrane (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% BSA in TBS containing 0.1% Tween-20 for 1 h at room temperature; primary antibodies were incubated overnight at 4 °C and the secondary antibody for 1 h at room temperature. The blots were developed with ECL reagents (TransGen Biotech, Beijing, China). The gray intensities of blots were measured using ImageJ software (Bethesda, MD, USA) and were normalized for GAPDH. Anti-GAPDH (TA-08; 1:1000) and the secondary antibody (anti-mouse-HRP ZB-5305 and anti-rabbit-HRP ZB-5301; 1:5000) were from ZSGB-Bio. CHOP (2895; 1:1000) and Grp78 (3177; 1:1000) were from Cell Signaling Technology. CIBZ (NBP1-80263; 1:1000) and ERdj4 (NBP2-17246; 1:500) were from Novus Biologicals.
Statistical analysis
The experiment data were performed to statistical analysis with SPSS version 20.0 (IBM, Armonk, NY, USA). We set up the significant level as α = 0.05; when p < 0.05, there is statistical significance. Measurement data were shown as the mean ± SEM. The compared data were applied to perform a one-way analysis of variance. Comparisons among groups were executed with the LSD method, when comparing differences between the groups that were statistically significant.
Results
TUDCA improves motor functional recovery after SCI
To investigate the effect of TUDCA on SCI, a variety of behavioral examinations were performed after SCI. Compared with the sham group, mice in the SCI and SCI/TUDCA groups showed different degrees of bilateral hind limb paralysis. The growth of inclined plane angle began to accelerate in the SCI/TUDCA group after 1 day, but the two groups showed a similar repair tendency (Fig. 1b). Compared with the sham group, BMS scores declined significantly in the SCI and SCI/TUDCA groups. The BMS score began to increase on the 1st day post SCI in the SCI and SCI/TUDCA groups (Fig. 1c). The mice had the highest scores at 100 mg/kg of bw TUDCA, but there were no differences between the scores at the 150-mg/kg dose. Therefore, 100 mg/kg was performed in the next study. There was no significant difference between the scores of SCI/TUDCA groups before 3 days, but the SCI/TUDCA group scores were significantly higher than the SCI group after 3 days. Mice treated with TUDCA recovered better as early as 3 days following SCI, and exhibited the best locomotor recovery on the 9th day. Moreover, there was a similar trend towards change between the BMS score and the sub-score (Fig. 1c).
TUDCA decreases the secondary injury and lesion area after SCI
Drug therapy can relieve compression of the spinal cord, alleviate cell edema and secondary damage, and improve microcirculation in the early stages of injury (Lu et al. 2000). The center of the lesions expanded with the increase of days after SCI. The size of lesions reached 3.9 ± 0.22 mm2 on the 7th day, but the expansion was dramatically inhibited in the SCI/TUDCA group, in which the size was 2.3 ± 0.13 mm2 on the 7th day (Fig. 2a). Compared with the sham operation group, there were a few hemorrhagic foci and a large number of vacuolar structures, which severely damaged the gray matter, and neuronal loss in the SCI and SCI/TUDCA groups (Fig. 2c). The number of survival neurons was significantly decreased in both the SCI and SCI/TUDCA groups; in contrast, the SCI group (29 ± 2.10) was more severely affected than that of the SCI/TUDCA (41 ± 2.99) on the 7th day (Fig. 2b).
Fig. 2.
TUDCA decreases the secondary injury and lesion size after SCI. a The lesion area of spinal cords was measured under a digital microscope. Five longitudinal HE staining images from each spinal cord were captured with the microscope, and the damaged area was selected with Photoshop. The damage area was calculated by the pixel and scale of the image. All data are presented as means ± SEM for each group (n = 5). *p < 0.05 vs. SCI groups. b Quantification of the number of neurons from sham, sham/TUDCA, SCI, and SCI/TUDCA mice normalized to the total area imaged. The number of survival neurons was calculated under a bright-field microscope. All data are presented as mean ± SEM for each group (n = 5). *p < 0.05 vs. SCI groups. c Representative H&E staining of spinal cord obtained from sham, sham/TUDCA, SCI, and SCI/TUDCA mice. Scale bar 100 μm. Abbreviations are as follows: DH, dorsal horn; VH, ventral horn; CC, central canal; LF, lateral funiculus; DF, dorsal funiculus; VF, ventral funiculus
TUDCA reduces apoptosis of lesion spinal cord after SCI
TUDCA acts as an inhibitor of apoptosis in several models of neurodegenerative diseases, and possesses cytoprotective properties on rat cortical neurons treated with glutamate in vitro (Vang et al. 2014). Almost no TUNEL-positive cells (in red) were found in the sham group, but a large number of positive cells were scattered around the lesion center, and in the gray matter and white matter in the SCI and SCI/TUDCA groups (Fig. 3a). The average number of TUNEL positive cells was 99.8 ± 6.5 in the SCI group and 72.2 ± 2.8 in the SCI/TUDCA group on the 1st day after SCI; however, it decreased after 3 and 7 days. The cell death ratio of the SCI/TUDCA group was noticeably less than that of the SCI group (Fig. 3b).
Fig. 3.
TUDCA attenuates neuronal death after SCI. a TUNEEL staining of spinal cord from sham, sham/TUDCA, SCI, and SCI/TUDCA mice. TUNEL-positive cells (red) distributed around the lesion epicenter. Nuclei were stained by DAPI (blue). Scale bar 200 μm. b Quantification of the number of apoptotic cells from SCI and SCI/TUDCA mice normalized to total area imaged. Mean apoptotic cell counts for each group for 1–7 days. All data are presented as mean ± SEM for each group (n = 5). *p < 0.05 vs. SCI groups
TUDCA inhibits ER stress by increasing the expression of CIBZ
Recently, several studies have shown that TUDCA can ameliorate ER stress by preventing UPR dysfunction (Xie et al. 2002). To clarify the role of TUDCA in the inhibition of ER stress-induced apoptosis in SCI, we identified the expression of the gene associated with ER stress. The mRNA and protein expressions of CHOP, Erdj4, and Grp78 were remarkably up-regulated in mice that were subjected to SCI (Fig. 4a, b). A considerable expansion of Grp78 and ERdj4 helps to re-fold the deformed proteins, restore the correct conformation of the protein, and ease the ER stress (Shuda et al. 2003). The level of Grp78 and ERdj4 decreased in the TUDCA treatment mice, which indicated that ER stress was blocked (Fig. 4a, b). CHOP is a specific pro-apoptotic factor, which is expressed in various cells in normal circumstances, but the expression is greatly increased in ER stress (Wang et al. 2013). But deletion of CHOP attenuates UPR and cell death, and enhances functional recovery after SCI (Ohri et al. 2011). Our results show that the level of CHOP is decreased after the administration of TUDCA (Fig. 4a, b). In addition, the degree of apoptosis was reduced and motor function was also improved (Figs. 1b, c and 3). Interestingly, we found that mRNA and protein expressions of CIBZ were reduced after the injury, but the expressions were recovery after the injection of TUDCA (Fig. 4a, b). The protein level of CIBZ is also significantly increased in a certain concentration range, with the increasing concentration of TUDCA (Fig. 5).
Fig. 4.
TUDCA inhibits ER stress by increasing the expression of CIBZ. a Relative expression of genes associated with ER stress in mice treated with sham, SCI, and SCI/TUUDCA. Total RNA from spinal cord injured for 1–7 days either in SCI or SCI/TUDCA mice. b Immunoblot analysis of ERdj4, CIBZ, CHOP, and Grp78 in lysates from spinal cord of sham, sham/TUDCA, SCI, and SCI/TUDCA mice for 3 days. c Quantification of protein expression was performed by densitometric analysis. Bar represent mean ± SEM for each group (n = 3). *p < 0.05 vs. SCI groups
Fig. 5.
The protein level of CIBZ is constantly up-regulated with the increased concentrations of TUDCA. a Immunoblot analysis of CIBZ from mice treated with different concentrations of TUDCA at 3 days post injury. b Quantification of CIBZ protein expression was analyzed. Bar represent mean ± SEM for each group (n = 3)
Discussion
In this study, the administration of TUDCA dramatically promoted motor function and strength recovery of the hind limbs, and decreased the secondary injury. The expression of Grp78, Erdj4, and CHOP was blocked, but the levels of CIBZ were elevated. Our previous studies also confirmed that the expression of CIBZ plays an important role in the repair of SCI. Our results reveal that TUDCA effectively attenuates ER stress by up-regulating the expression of CIBZ, which adds to our previously reported results.
The clinical therapy of SCI generally includes three approaches: limiting the death of living cells, promoting the growth of living cells, and replacing the damaged cells (Schroeder et al. 2016). In the early stages of SCI, inhibition of apoptosis can reduce secondary injury and prevent the expansion of lesions (Lu et al. 2000). Anti-apoptosis drug administration is one of the most indispensable treatments in the early treatment stage. Nimodipine, methylprednisolone, and ganglioside are potent clinical medicines for an anti-apoptosis effect on SCI (Cai et al. 2011; Wells et al. 2003). Additionally, lanthionine ketimine ester and metformin have been recently reported to treat SCI as potential anti-apoptotic drugs in an SCI animal model (Kotaka et al. 2017; Wang et al. 2016; Yin et al. 2012).
TUDCA is a kind of conjunction-type natural bile acid, commonly used to treat liver disease (Lee et al. 2010). Several studies have demonstrated that TUDCA is a potent inhibitor of apoptosis; it can regulate the apoptosis pathway to reduce cell death in many diseases. Obesity, stroke, and neurodegenerative diseases associated with apoptosis are potential therapeutic targets for TUDCA (Vang et al. 2014). TUDCA may have a therapeutic effect on SCI. The behavioral results showed that TUDCA-treated mice had better motor function and strength recovery of the hind limbs. The damaged spinal cord experienced obvious morphological changes of apoptosis, expansion of lesion area, a large number of apoptotic cells, and the appearance of vacuoles in the SCI group. However, these unfavorable pathological changes were significantly reversed in the SCI/TUDCA group. These results suggest that TUDCA blocks secondary damage and ameliorates motor function in a mouse model of SCI. In addition to the direct neuroprotective effect, TUDCA exhibits a direct anti-inflammatory effect on both astrocytes and microglial cells in vitro and in a mouse model of acute neuroinflammation (Yanguas-Casas et al. 2014). TUDCA treatment inhibits the NF-κB pathway and increases further the TGF-β pathway leading to an increase in neuronal survival and a faster restoration of neural function in the CNS (Romero-Ramirez et al. 2017; Yanguas-Casas et al. 2016). In this study, we also found that TUDCA treatment decreased inflammation after SCI. Further studies are needed to understand whether it has an anti-inflammatory effect in a mouse model of traumatic SCI, and whether it plays an anti-inflammatory role through an ER-stress signaling pathway.
Previous studies have demonstrated two main pathways that induce apoptosis including the mitochondrial-dependent pathway (extrinsic pathway) and the death receptor pathway (intrinsic pathway) after SCI (Szegezdi et al. 2006). Recently, ER stress has been confirmed as a new apoptosis pathway. Although ER stress protects cells in the early stage of the stress response through the activation of the UPR which temporarily inhibits the synthesis of proteins and stabilizes ER homeostasis, persistent ER stress will result in neuronal apoptosis at the injury site. As a result, removing the excessive ER stress response can improve functional recovery after SCI (Ohri et al. 2011). Grp78 and CHOP serve as the markers of ER stress, with mutually facilitated structures formed by unique self-regulatory loops. These loops build a construction to ensure the robust and uniform regulation of Grp78, CHOP, and XBP1 genes during an ER stress response (Takayanagi et al. 2013; Wang et al. 2009). Our research indicated that the expressions of Grp78 and CHOP in the SCI/TUDCA group decreased significantly compared with the SCI group. ERdj4, an ER-resident chaperone (Kurisu et al. 2003), also decreased. CHOP null mice exhibited significant attenuation of the UPR, fewer apoptosis cells, and an enhancement of functional recovery after SCI (Ohri et al. 2011). Overexpression of Grp78 coupled with ERdj4 attenuates the induction of CHOP in ER stress and decreases ER stress-induced apoptosis. Meanwhile, motor function was significantly enhanced; lesion areas and apoptotic ratios were reduced in our study. Our results suggest that ER stress was effectively inhibited by the administration of TUDCA. CIBZ, a BTB domain zinc finger transcriptional factor, regulates ESC proliferation through the regulation of the expression of Nanog (Nishii et al. 2012). Unexpectedly, as a novel target of SCI (Cai et al. 2017), CIBZ was regulated by TUDCA in our study. Our previous studies found that CIBZ would be decreased after SCI (Cai et al. 2012); in vitro knockdown CIBZ induced ER stress and apoptosis in SH-SY5Y cells (Cai et al. 2017). The levels of CIBZ were constantly up-regulated with increased concentrations of TUDCA, indicating that TUDCA plays an important role in the expression of CIBZ. In combination with our previous reports, we suggest that TUDCA increases CIBZ expression after SCI, eventually suppressing the ER stress response. The experimental model of TUDCA in SCI is shown in Fig. 6.
Fig. 6.
Model of the repair role of TUDCA in mouse of SCI. ER stress is activated after SCI, up-regulating the expression of associated genes including CHOP, GRP78, and ERdj4, but blocks the expression of CIBZ. The expression of CIBZ was restored after administrating TUDCA; ER stress and secondary injury were also alleviated. TUDCA might alleviate secondary injury in the spinal cord through up-regulation of CIBZ
In summary, TUDCA is one of the bile acid drugs, naturally producing bile acid, which has been used to treat numerous health problems for more than 3000 years in China. The protective effects of TUDCA have been studied in animal models of human disease, including stroke, neurodegenerative diseases, diabetes, and cardiovascular disease (Vang et al. 2014). In this study, our results showed a neuroprotective effect for TUDCA in a mouse model of SCI. TUDCA reduced apoptosis through the inhibition of ER stress-associated apoptosis and restored motor function at the early injury stage. Furthermore, our results suggest that TUDCA may be a potent drug for inhibiting cell death after SCI.
Funding information
This study was supported by the National Natural Science Foundation of China (NSFC, 31372207 and 81570094), the Innovation Team of Scientific Research Platform in Anhui Province, a start-up grant from Nanjing Agricultural University (804090), and the “Sanxin” Research Program of Jiangsu Province (SXGC[2016]312).
Compliance with ethical standards
All animal experiments complied with the ARRIVE guidelines and were carried out according to the National Institutes of Health guide for the care and use of laboratory animals. All animal experiments were approved by the Anhui Normal University Academic Ethics Committee.
Footnotes
Zongmeng Zhang, Jie Chen, and Fanghui Chen are co-first authors.
Contributor Information
Jun Li, Phone: +86-18855356196, Email: lijunplant@163.com.
Yafei Cai, Phone: +86-13151568780, Email: ycai@njau.edu.cn.
References
- Ahn YH, Lee G, Kang SK. Molecular insights of the injured lesions of rat spinal cords: inflammation, apoptosis, and cell survival. Biochem Biophys Res Commun. 2006;348:560–570. doi: 10.1016/j.bbrc.2006.07.105. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- Cai Y, Fan R, Hua T, Liu H, Li J. Nimodipine alleviates apoptosis-mediated impairments through the mitochondrial pathway after spinal cord injury. Curr Zool. 2011;57:340–349. doi: 10.1093/czoolo/57.3.340. [DOI] [Google Scholar]
- Cai Y, Li J, Yang S, Li P, Zhang X, Liu H. CIBZ, a novel BTB domain-containing protein, is involved in mouse spinal cord injury via mitochondrial pathway independent of p53 gene. PLoS One. 2012;7:e33156. doi: 10.1371/journal.pone.0033156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Li J, Zhang Z, Chen J, Zhu Y, Li R, Chen J, Gao L, Liu R, Teng Y. Zbtb38 is a novel target for spinal cord injury. Oncotarget. 2017;8:45356–45366. doi: 10.18632/oncotarget.17487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus. 2008;25:E13. doi: 10.3171/FOC.2008.25.11.E13. [DOI] [PubMed] [Google Scholar]
- Keene CD, Rodrigues CM, Eich T, Chhabra MS, Steer CJ, Low WC. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc Natl Acad Sci U S A. 2002;99:10671–10676. doi: 10.1073/pnas.162362299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaka K, Nagai J, Hensley K, Ohshima T. Lanthionine ketimine ester promotes locomotor recovery after spinal cord injury by reducing neuroinflammation and promoting axon growth. Biochem Biophys Res Commun. 2017;483:759–764. doi: 10.1016/j.bbrc.2016.12.069. [DOI] [PubMed] [Google Scholar]
- Kurisu J, Honma A, Miyajima H, Kondo S, Okumura M, Imaizumi K. MDG1/ERdj4, an ER-resident DnaJ family member, suppresses cell death induced by ER stress. Genes Cells. 2003;8:189–202. doi: 10.1046/j.1365-2443.2003.00625.x. [DOI] [PubMed] [Google Scholar]
- Lee YY, Hong SH, Lee YJ, Chung SS, Jung HS, Park SG, Park KS. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem Biophys Res Commun. 2010;397:735–739. doi: 10.1016/j.bbrc.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P. Advances in secondary spinal cord injury: role of apoptosis. Spine. 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- Nishii T, Oikawa Y, Ishida Y, Kawaichi M, Matsuda E. CtBP-interacting BTB zinc finger protein (CIBZ) promotes proliferation and G1/S transition in embryonic stem cells via Nanog. J Biol Chem. 2012;287:12417–12424. doi: 10.1074/jbc.M111.333856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreau L, Proulx P, Gagnon L, Drolet M, Laramee MT. Secondary impairments after spinal cord injury: a population-based study. Am J Phys Med Rehabil. 2000;79:526–535. doi: 10.1097/00002060-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59:1489–1502. doi: 10.1002/glia.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa Y, Matsuda E, Nishii T, Ishida Y, Kawaichi M. Down-regulation of CIBZ, a novel substrate of caspase-3, induces apoptosis. J Biol Chem. 2008;283:14242–14247. doi: 10.1074/jbc.M802257200. [DOI] [PubMed] [Google Scholar]
- Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- Podda M, Crosignani A, Battezzati P, Buscarini L, Conte D, Notarbartolo A, Floreani A, Stabilini R, Manghisi OG. Tauroursodeoxycholic acid for the treatment of chronic hepatitis: a dose-response study. Hepatology. 1995;22:A120. doi: 10.1016/0270-9139(95)94204-1. [DOI] [Google Scholar]
- Ramalho RM, Ribeiro PS, Sola S, Castro RE, Steer CJ, Rodrigues CM. Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J Neurochem. 2004;90:567–575. doi: 10.1111/j.1471-4159.2004.02517.x. [DOI] [PubMed] [Google Scholar]
- Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM. Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med. 2008;14:54–62. doi: 10.1016/j.molmed.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Nan Z, Castro RE, Ribeiro PS, Low WC, Steer CJ. Tauroursodeoxycholic acid reduces apoptosis and protects against neurological injury after acute hemorrhagic stroke in rats. Proc Natl Acad Sci U S A. 2003;100:6087–6092. doi: 10.1073/pnas.1031632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramirez L, Nieto-Sampedro M, Yanguas-Casas N. Tauroursodeoxycholic acid: more than just a neuroprotective bile conjugate. Neural Regen Res. 2017;12:62–63. doi: 10.4103/1673-5374.198979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder GD, Kepler CK, Vaccaro AR. The use of cell transplantation in spinal cord injuries. J Am Acad Orthop Surg. 2016;24:266–275. doi: 10.5435/JAAOS-D-14-00375. [DOI] [PubMed] [Google Scholar]
- Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, Yamamoto N, Yamamoto M. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38:605–614. doi: 10.1016/S0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi S, Fukuda R, Takeuchi Y, Tsukada S, Yoshida K. Gene regulatory network of unfolded protein response genes in endoplasmic reticulum stress. Cell Stress Chaperones. 2013;18:11–23. doi: 10.1007/s12192-012-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang S, Longley K, Steer CJ, Low WC. The unexpected uses of urso- and tauroursodeoxycholic acid in the treatment of non-liver diseases. Glob Adv Health Med. 2014;3:58–69. doi: 10.7453/gahmj.2014.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang P, Peng JL, Wu S, Zhao XP, Li L, Shen GX. The altered expression of glucose-regulated proteins 78 in different phase of streptozotocin-affected pancreatic beta-cells. Cell Stress Chaperones. 2009;14:43–48. doi: 10.1007/s12192-008-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhang C, Hong Z, Chen H, Chen W, Chen G. C/EBP homologous protein (CHOP) mediates neuronal apoptosis in rats with spinal cord injury. Exp Ther Med. 2013;5:107–111. doi: 10.3892/etm.2012.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu C, Gao K, Zhao H, Zhou Z, Shen Z, Guo Y, Li Z, Yao T, Mei X. Metformin preconditioning provide neuroprotection through enhancement of autophagy and suppression of inflammation and apoptosis after spinal cord injury. Biochem Biophys Res Commun. 2016;477:534–540. doi: 10.1016/j.bbrc.2016.05.148. [DOI] [PubMed] [Google Scholar]
- Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanguas-Casas N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramirez L. Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. J Neuroinflammation. 2014;11:50. doi: 10.1186/1742-2094-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanguas-Casas N, Barreda-Manso MA, Perez-Rial S, Nieto-Sampedro M, Romero-Ramirez L (2016) TGFbeta contributes to the anti-inflammatory effects of tauroursodeoxycholic acid on an animal model of acute neuroinflammation. Mol Neurobiol. 10.1007/s12035-016-0142-6 [DOI] [PubMed]
- Yin X, Yin Y, Cao FL, Chen YF, Peng Y, Hou WG, Sun SK, Luo ZJ. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One. 2012;7:e38381. doi: 10.1371/journal.pone.0038381. [DOI] [PMC free article] [PubMed] [Google Scholar]