Abstract
In this study, we studied the effect of 2.0 GHz radio frequency electromagnetic field (RF-EMF) and 50 Hz extremely low frequency electromagnetic field (ELF-EMF) exposure on prion generation and propagation using two budding yeast strains, NT64C and SB34, as model organisms. Under exposure to RF-EMF or ELF-EMF, the de novo generation and propagation of yeast prions [URE3] were elevated in both strains. The elevation increased over time, and the effects of ELF-EMF occurred in a dose-dependent manner. The transcription and expression levels of the molecular chaperones Hsp104, Hsp70-Ssa1/2, and Hsp40-Ydj1 were not statistically significantly changed after exposure. Furthermore, the levels of ROS, as well as the activities of superoxide dismutase (SOD) and catalase (CAT), were significantly elevated after short-term, but not long-term exposure. This work demonstrated for the first time that EMF exposure could elevate the de novo generation and propagation of yeast prions and supports the hypothesis that ROS may play a role in the effects of EMF on protein misfolding. The effects of EMF on protein folding and ROS levels may mediate the broad effects of EMF on cell function.
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0867-9) contains supplementary material, which is available to authorized users.
Keywords: Prion, RF-EMF, URE3, ELF-EMF, ROS
Introduction
Along with the notable growth of electric power and wireless communication equipment utilization, the strength, complexity and range of extremely low frequency electromagnetic field (ELF-EMF) and radio frequency electromagnetic field (RF-EMF) are increasing rapidly. Consequently, concern about the health effects of ELF-EMF and RF-EMF has attracted significant attention.
Proteins must fold into correct conformations in order to execute their proper functions, while incorrect folding could impair function and lead to disease. The amyloid form is a self-propagating, highly ordered, β-sheet rich fibrous aggregate with a strong resistance to proteinase K and detergent (Moore et al. 2014). In the human nervous system, some proteins that fold to the amyloid structure are associated with a number of amyloid diseases, such as Alzheimer’s (AD), Parkinson’s, and prion diseases. It was reported that the protein folding could be affected by exposure to EMF (Mancinelli et al. 2004; Solomentsev et al. 2012). Exposure to ELF-EMF could elevate the risk of AD (Davanipour and Sobel 2009; Davanipour et al. 2007; Sobel et al. 1995, 1996), and increase production of amyloid beta (Davanipour and Sobel 2009; Sobel and Davanipour 1996). Interestingly, it was found that RF-EMF exposure has some beneficial effects on AD pathology in a transgenic model (Arendash et al. 2010, 2012; Banaceur et al. 2013; Dragicevic et al. 2011; Jeong et al. 2015), which indicates that RF-EMF may affect AD pathology. A similar beneficial effect of RF-EMF on AD was also observed in an epidemiological survey. A 30–40% decrease in standardized hospitalization ratios (SHRs) was observed for dementia (AD, vascular and other dementia), Parkinson’s disease, and epilepsy among men who had used mobile phones for more than 10 years (Schuz et al. 2009).
A prion is an alternative conformational form of a specific protein that can be transmitted between cells of a given species or between species in some cases. Once established, the prion can replicate by recruiting the soluble form of the protein and converting it into the same misfolded form (Castellani et al. 2004). As in mammals, several proteins in the yeast Saccharomyces cerevisiae can misfold and propagate as prions that possess amyloid properties (Wickner et al. 1995). The first identified yeast prion [URE3], the prion form of the Ure2 protein, has attracted much attention and been studied in depth (Lian et al. 2006). The Ure2 protein (Ure2p) is responsible for the catabolism of nitrogen sources. In the presence of a rich nitrogen source, Ure2p blocks the uptake of poor nitrogen sources by sequestering the transcription factor Gln3p in the cytoplasm (Cox et al. 2000). When converting into the prion form, the aggregated Ure2p loses its normal function (Masison and Wickner 1995) and makes it possible to take up ureidosuccinate (USA) (Hong et al. 2011). Yeast prions provide an ideal model system for studying the amyloid formation and propagation that are applicable to mammalian and human diseases.
Studies have confirmed that molecular chaperones play an important role in helping proteins fold correctly to their native functional conformations by preventing proteins from misfolding and aggregating, particularly under conditions of stress, such as heat shock or disease (Chen and Inouye 2008). Heat-shock proteins, Hsp104, Hsp70 (e.g. Ssa1 and Ssa2) and the co-chaperone Hsp40 (e.g., Ydj1 and Sis1), are involved in the propagation of yeast prions (Moriyama et al. 2000). Hsp104 is a crucial factor in the propagation of [URE3] and another yeast prion, [PSI+] (Moriyama et al. 2000). Hsp70, including the cytoplasmic Ssa1 to Ssa4, Ssb1 and Ssb2, and its co-chaperone Hsp40, including Ydj1 and Sis1, are also critical in the propagation of yeast prions (Kryndushkin and Wickner 2007). Overproduction of Ssa1p, but not the nearly identical Ssa2p, cures [URE3], while not affecting [PSI+] (Schwimmer and Masison 2002). Overproduction of Hsp40-Ydj1p could cure [URE3] in vivo (Moriyama et al. 2000), and purified Ydj1 could inhibit amyloid formation of Ure2 in vitro (Lian et al. 2007).
It is important to maintain the balance between the biochemical processes leading to production and the removal of reactive oxygen species (ROS). When production of ROS exceeds the antioxidant capacity of a cell, the ROS level will increase and cause molecular damage, leading to a critical failure of biological function (Yu 1994). Oxidative stress will also affect protein folding and lead to protein misfolding diseases. Oxidative stress plays a significant role in the formation of AD pathology (Su et al. 2008). The frequency of [PSI+] prion formation could be elevated under conditions of oxidative stress and in mutants lacking key antioxidants (Doronina et al. 2015). Interestingly, it was also reported that exposure to RF-EMF could increase free radical production in yeast (Crouzier et al. 2009) and ROS levels in the cell line V79 (Marjanovic et al. 2015), while exposure to ELF-EMF could increase cell ROS levels in various organisms and cells (Goraca et al. 2010; Simko and Mattsson 2004).
Here, we studied the effects of RF-EMF and ELF-EMF exposure on de novo generation and propagation of prions, using the budding yeast prion [URE3] as a model organism. We also investigated the expression levels of prion-related chaperones, as well as ROS levels and activities of related enzymes.
Materials and methods
Yeast strains and prion assay
The strain NT64C (MAT a, ade2-1, his3-11,15, leu2, 3-112, trp1-1, ura3,1, pDAL5::ADE2, [URE3]) and SB34 (MATa; erg6::TRP1; pDAL5::ADE2; ade2-1; trp1-1; leu2-3112; his3-11,15; ura2::HIS3, [URE3]) were used for the assessment of in vivo [URE3] prion status. NT64C contains the ADE2 coding region under control of the DAL5 promoter, which is inhibited by active Ure2 protein. The lack of Ade2p will make cells appear red, and allows rapid identification of the [URE3] and [ure-o] cells by color: white and red, respectively. SB34 is in a different strain background as NT64C and was used as another strain to exclude the possibility of strain specificity. Similar to NT64C, the SB34 again made use of the red/white methods based on the ADE2 gene and DAL5 promoter.
Exposure conditions
An RF electromagnetic field was generated using a vector signal generator (Agilent E8267D PSG, USA) and signal amplifier (AV38701E, the 41st Institute of CETC, China). The signal amplifier was used to amplify the RF/MW (Microwaves) signal induced by the signal generator. The signal in the sample position was measured by an electromagnetic radiation analyzer (PMM 8053B, Narta-STS, Italy) and a signal analyzer (Agilent N9030A). In this study, yeast cells were exposed to 2000 MHz RF/MW.
At the position of yeast cells, the RF electromagnetic field strength was 20 V/m, and the temperature was 30 °C. The average specific absorption rate (SAR) for a single cell was 0.12 W/kg. The SAR was calculated using finite difference time-domain (FDTD) analysis methods (Schuderer et al. 2004).
The ELF-EMF exposure system consists of five parts: a power regulator, two Helmholtz coils, a biochemical incubator, some temperature recorders, and a temperature control system. Two vertical coils (150 turns of 1 mm copper wire) were placed into a biochemical incubator (Jinghong SHP-250, China). The two coils were connected in parallel, and a 50 Hz sinusoidal magnetic field controlled by a power regulator (PS-7005, China) was generated by feeding a line current. When energized, a uniform magnetic field (0 to 7.0 mT) was generated in the center of the coils where the culture dishes were placed. The magnetic flux densities were then measured using a portable field meter (PM 8053B, Italy). A silicone tube connected to a condenser was wound around the coils to counteract the generated heat.
In order to ensure temperature accuracy and stability, a series of operations were applied. Firstly, we put the temperature probe of the incubator itself in the position of the sample area so that the incubator maintained its temperature according to the temperature at the position of our samples. Secondly, we calibrated the temperature of the two incubators for the control and exposure samples using the same thermometer routinely. Thirdly, during incubation and exposure, we continuously monitored the sample area temperature using temperature probes around the control and exposure samples. Fourthly, we checked the surface temperature of samples during incubation using a thermal imager (Testo 890). All the monitoring data showed that the temperature of the control samples and exposure samples was maintained accurately and stably.
Prion status assay
To obtain the [ure-o] strains, the yeast cells were put on YPD plates containing 3 mM guanidine hydrochloride, which inactivates Hsp104 and causes a loss of prions as cells divide (Ferreira et al. 2001; Grimminger et al. 2004; Jung and Masison 2001). Red colonies from the plates containing guanidine hydrochloride were then streaked onto YPD plates containing guanidine hydrochloride again. Then, the red colonies were streaked onto YPD plates and red [ure-o] colonies were isolated. To obtain the [URE3] strains, the yeast cells were put on SC-Ade plates, which do not allow [ure-o] cells to grow due to the lack of Ade2 protein. White colonies from –Ade plates were then streaked onto –Ade plates again, and white colonies were streaked onto YPD plates. Finally, the white colonies were isolated and used as the [URE3] strain.
The selected yeast cells were grown on YPD liquid medium at 30 °C until the logarithmic phase. The yeast incubates were diluted according to the concentration measured by OD absorbance, and then spread onto YPD plates. About 300 colonies were expected to grow on each plate. At least three parallel plates were exposed to EMF, while at least another 3 parallel plates were incubated in the control conditions as well. After a 96-h incubation, the number of red and white colonies on each plate was counted. Each experiment was repeated at least three times independently.
ROS level assay
Intracellular ROS levels were detected using the fluorescent probe DCFH-DA (2′7’-dichlorodihydrofluorescein diacetate). After yeast cells in liquid medium containing 10 μm DCFH-DA were incubated for 20 min at 30 °C in the dark, the cells were split by every 4 ml and incubated in the EMF and control incubator respectively. Then, yeast cells were collected at different time points by centrifugation and washed three times by phosphate buffer. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm by flow cytometry (Beckman, USA).
Real-time reverse transcription-polymerase chain reaction (RT-PCR)
After exposure, the yeast cells on the plates were washed in 10 ml water and were then quickly frozen by liquid nitrogen. The frozen cells were then smashed with acid-washed glass beads, and total RNA was isolated from the lysate using an RNA purification kit (Omega). First-stand complementary DNA synthesis was performed with hexamers as primers using a cDNA synthesis kit (TaKaRa) following the manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using the SYBR Premix Ex Taq II kit (TaKaRa) and the Roche LightCycle 480 II sequence detection system (Roche, Switzerland). Gene expression studies were performed in triplicate, and the formation of a single PCR product was confirmed using dissociation curves. Negative controls comprised all components of the PCR mix, except cDNA. The program used was the following: 1 cycle at 95 °C for 2 min; 40 cycles of 20 s at 95 °C, 20 s at 55 °C, and 30 s at 68 °C; 1 cycle at 68 °C for 1 min. After this, the melting curve data were collected. Gene expression levels are shown as the concentration of the studied gene, normalized to the concentration of the housekeeping ACT1 gene. Each experiment was repeated at least three times independently. Table S1 shows the primer sequences used in this study.
Western blotting assay
After exposure, the yeast cells on the plates were washed in 10 ml water and were then quickly harvested by spinning and suspended in 150 μl cold cell lysis buffer (Beyotime, China) and 100 μl acid-washed glass beads (0.5 mm, Sigma) followed by three cycles of vortexing for 30 s and alternating cooling for 30 s on ice. Proteins in lysate were separated by electrophoresis and then transferred to a PVDF membrane. Membranes were blocked, probed, washed, and developed according to the manufacturer’s instructions using the SuperSignal West Pico Chemiluminescent Substrate kit (cat. no. 34080, Thermo Scientific). Blots were imaged by chemiluminescence System (Image Station 4000 mm, Kodak, USA) and densitometric analysis was performed using image-pro plus 6.0 image-processing software.
Primary antibodies were rabbit anti-Hsp104 antibody (ab69549, Abcam), goat anti-Hsp70-Ssa1/2(yT-14) antibody (sc-23,752, Santa Cruz), mouse anti-Hsp40-Ydj1 antibody (ab74442, Abcam) and mouse anti-beta actin antibody (66009-1-Ig, proteintech). Secondary antibodies were donkey anti-mouse IgG antibody (ab97030, Abcam), goat anti-rabbit IgG antibody (ab136817, Abcam), and donkey anti-goat IgG antibody (sc-2020, Santa Cruz).
SOD and CAT activity assay
After exposure, the yeast cells on the plates were washed in 10 ml water and were then quickly harvested by spinning and suspended in cold cell lysis buffer (Beyotime, China) and 100 μl acid-washed glass beads (0.5 mm, Sigma) followed by three cycles of vortexing for 30 s and alternating cooling for 30 s on ice. After centrifuged at 10000 rpm for 5 min, the supernatant was applied for protein concentration measurements using the BCA (Thermo Scientific, USA) method, and enzyme activity of SOD and CAT was measured using SOD (Nanjing Jiancheng, China) and CAT (Nanjing Jiancheng, China) enzymatic activity detection kits.
The activity of SOD was measured using the xanthine-xanthine oxidase system to produce superoxide ions, which then reacted with 2-(4-iodophenyl)-3-(4-nitrophenol-5-phenlyltetrazolium chloride) to form a red formazan dye, and the absorbance at 550 nm was determined. One unit of SOD was defined as the amount of SOD inhibiting the rate of reaction by 50% at 25 °C. CAT activity was measured by analyzing the rate of H2O2 decomposition, which was monitored by absorbance at 240 nm. The activity of SOD is expressed as units per milligrams of protein (U/mg protein) and the activity of CAT is expressed as (U/g protein).
Results
Prion status assay
We used the yeast prion [URE3] as a model to analyze whether the exposure to EMF affects protein folding or misfolding. [URE3] is a yeast prion phenotype, in which its prion determinant protein, Ure2, is misfolded and aggregated. Firstly, we used a [URE3] strain, NT64C, to assay the effects of EMF on prion status. NT64C contains an ADE2 coding region under the control of the DAL5 promoter. In [ure-o] (in non-prion state) cells, soluble Ure2p will interact with Gln3p and prevent transcription of ADE2 from the DAL5 promoter. In [URE3] (in prion state) cells, the Ure2p is aggregated and cannot prevent transcription of ADE2 expression. Lack of Ade2p expression will make cells appear red due to the accumulation of a pigmented substrate of Ade2p. Hence, we can use this strain to identify the [URE3] and [ure-o] cells initially by color: white and red, respectively. During cell propagation, the prion phenotype [URE3] will be spontaneously lost at some rate, which can be seen as an indicator of the robustness of prion phenotype propagation. When we use the [URE3] strain as the starting strain, the spontaneous loss of [URE3] will be observed by the appearance of red, [ure-o] colonies.
Effects of RF-EMF on [URE3] de novo generation
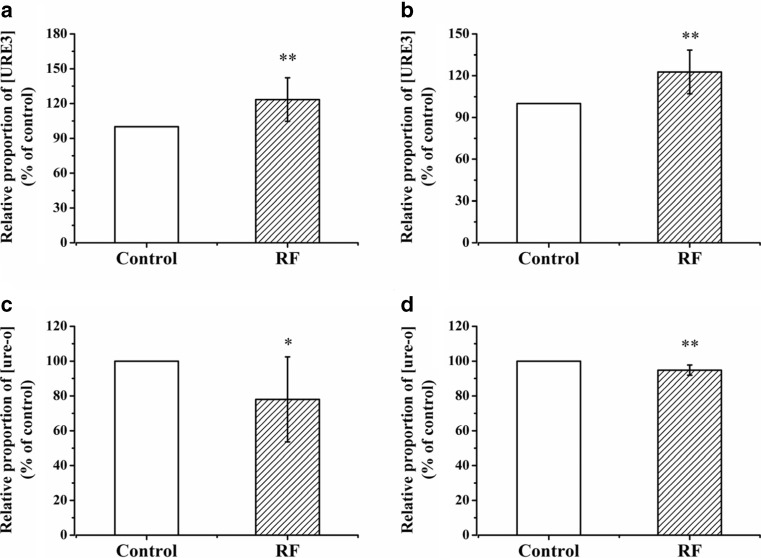
After being selected by YPD plates containing 3 mM GuHCl, the red, [ure-o] colony cells were incubated in liquid YPD medium until the lag-phase. The cells in the lag-phase were then spread in parallel lines onto several YPD plates. At least three plates were exposed to 2.0 GHz RF-EMF at 30 °C, while at least another three plates were incubated in the control incubator at 30 °C. To obtain large enough colonies to facilitate the counting work, the plates were allowed to grow for 96 h. In each plate, the ratio of the white colony number to the red colony number was calculated. The differences in the rate of appearance of white colonies will reflect the effects of exposure to RF-EMF on prion de novo generation. As the effects that we found were relatively weak, this experiment was repeated eight times independently, with at least three parallel samples each time, to ensure the reliability and credibility of the results. The starting strain of each batch (repetition) of experiment was picked from different single colonies, so the ratio of white/red colony numbers in the control sample plate varied among different batches of experiments. In order to get comparable results, the calculated ratio of the control and exposure samples in each batch of experiments was normalized according to the ratio of the control sample in that batch. Compared with the control group, the exposure group had a higher ratio of white/red colony numbers, which meant that the NT64C cells exposed to RF-EMF contained more white colonies (Fig. 1a). Under exposure to RF-EMF, the de novo generation of [URE3] in the NT64C strain was promoted.
Fig. 1.
Short-term (96 h) effects of 2.0 GHz, 40 dBm RF-EMF exposure on the relative proportion of [URE3] or [ure-o] in yeast cells. a Relative proportion of [URE3] in NT64C [ure-o] after RF exposure versus control. b Relative proportion of [URE3] in SB34 [ure-o] after RF exposure versus control. c Relative proportion of [ure-o] in NT64C [URE3] after RF exposure versus control. d Relative proportion of [ure-o] in SB34 [URE3] after RF exposure versus control. All data represent more than seven independent experiments. Bars represent S.D. of the mean. “*” means P < 0.05, compared with the control, while “**” represents P < 0.01, with respect to the control
In order to exclude the interference of yeast strain specificity, we used another yeast strain SB34 to repeat the experiments above, which also contained the [URE3] prion form but the genetic background was quite different from NT64C. Similar to NT64C, the SB34 made use of the red/white methods based on the ADE2 gene and the DAL5 promoter. In the SB34 strain, we obtained results similar to those in NT64C (Fig. 1b).
Effects of RF-EMF on [URE3] propagation
By using the non-prion phenotype [ure-o] as the starting strain, we found exposure to RF-EMF could promote spontaneous transformation from the non-prion form to the prion form. We also used the NT64C prion phenotype [URE3] as the starting strain to study the effects of RF-EMF exposure on prion propagation. After selection by SC-Ade plates, the white, [URE3] colony cells were incubated in liquid YPD medium until the lag-phase. The cells were then spread in parallel onto several YPD plates. At least three plates were incubated in 2.0 GHz RF-EMF and control conditions for 96 h, respectively. In each plate, the ratio of red colony numbers to white colony numbers was calculated. To obtain reliable results, this experiment was repeated nine times independently, with at least three parallel samples each time. Compared with the control group, the exposure group had a lower ratio of red/white colony numbers, which meant that the NT64C cells exposed to RF-EMF obtained fewer red colonies (Fig. 1c). Under exposure to RF-EMF, the spontaneous loss of [URE3] in the NT64C strain was inhibited, while on the other hand, the prion propagation of [URE3] was promoted.
In another yeast strain SB34, we performed similar experiments as above, and obtained similar results as in NT64C (Fig. 1d).
Long-term effects of RF-EMF on [URE3] generation and propagation
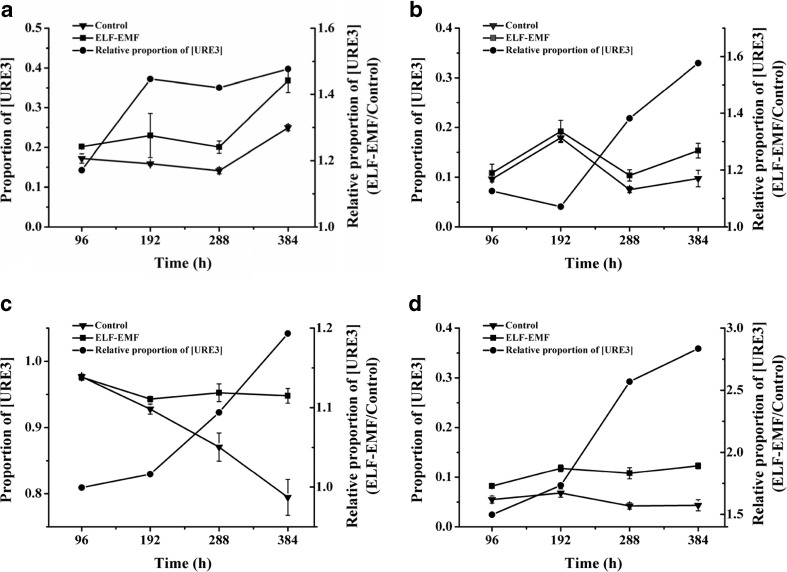
After we found the elevation of prion generation and propagation after 96-h RF-EMF exposure, we wondered whether a longer time exposure would lead to stronger effects. Therefore, we carried out long-term exposure experiments. After the yeast plate was exposed to RF-EMF for 96 h as the short-term exposure introduced above, the red/white colony numbers were counted and the cells were washed, suspended and spread onto a new YPD plate. The new plate was exposed to RF-EMF for another 96 h again, and the cells were then counted and spread onto another new plate. By this method, we exposed the cells to RF-EMF continuously for 384 h. While the NT64C cells in the non-prion state were exposed to RF-EMF for 384 h (Fig. 2a), the exposure group always contained more prion state colonies than the control group (i.e., the relative proportion was above 1.0, and the value of the exposure group was higher than the control group.), and the difference between the exposure and control groups tended to increase with time. In another [ure-o] strain, SB34, we obtained similar results (Fig. 2b).
Fig. 2.
Long-term effects of 2.0 GHz, 40 dBm RF-EMF exposure on the relative proportion of [URE3] in yeast cells. a Relative proportion of [URE3] in NT64C [ure-o] after RF exposure versus control. b Relative proportion of [URE3] in SB34 [ure-o] after RF exposure versus control. c Relative proportion of [URE3] in NT64C [URE3] after RF exposure versus control. d Relative proportion of [URE3] in SB34 [URE3] after RF exposure versus control. Bars represent S.D. of the mean
When we exposed the prion state strains to RF-EMF for long periods of time, we also found a time-dependent effect. While NT64C [URE3] (Fig. 2c) and SB34 [URE3] (Fig. 2d) cells were exposed to RF-EMF for 384 h, the exposure group maintained more prion state colonies than the control group (i.e., the relative proportion was above 1.0), and the difference between the exposure and control groups tended to increase with time.
When either starting from the prion state or from non-prion state cells, the white/red ratios were higher in the exposure group, and the differences tended to increase with time (Fig. 2). Similar to what we found in short-term experiments, long-term exposure to RF-EMF also led to the promotion of de novo generation and propagation of [URE3] in both strains.
Effects of ELF-EMF on [URE3] de novo generation and propagation
Using similar methods as RF-EMF, we also assayed the effects of ELF-EMF exposure on [URE3] de novo generation and propagation. After the yeast strains in the YPD plates were exposed to 6.0 mT ELF-EMF at 30 °C for 96 h, at least another three plates were incubated in the control incubator at 30 °C, and the ratio of white colony numbers to red colony numbers in each plate was calculated. This experiment was repeated eight times independently, with at least three parallel samples each time, to ensure the reliability and credibility of the result.
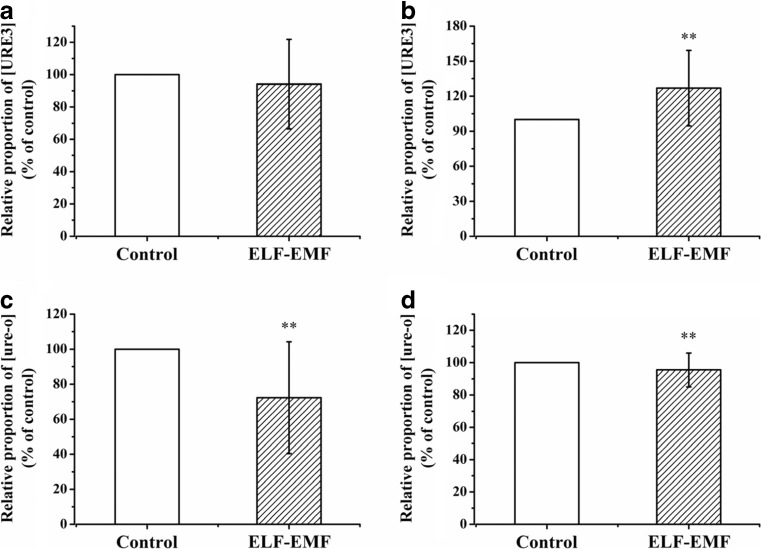
When using the non-prion phenotype NT64C [ure-o] as the starting strain, we did not find significant differences in the ratio of white/red colony numbers between the exposure group and control group (Fig. 3a). However, after SB34 [ure-o] cells were exposed to 6.0 mT ELF-EMF, the ratio of white/red colony numbers was significantly higher than the control group (Fig. 3b), which meant the SB34 cells exposed to ELF-EMF contained more white colonies. Under exposure to ELF-EMF, the de novo generation of [URE3] in the SB34, but not in the NT64C, strain was promoted.
Fig. 3.
Short-term (96 h) effects of 6.0 mT ELF-EMF exposure on the relative proportion of [URE3] or [ure-o] in yeast cells. a Relative proportion of [URE3] in NT64C [ure-o] after ELF-EMF exposure versus control. b Relative proportion of [URE3] in SB34 [ure-o] after ELF-EMF exposure versus control. c Relative proportion of [ure-o] in NT64C [URE3] after ELF-EMF exposure versus control. d Relative proportion of [ure-o] in SB34 [URE3] after ELF-EMF exposure versus control. All data represent more than eight independent experiments. Bars represent S.D. of the mean. “*” means P < 0.05, compared with the control, while “**” represents P < 0.01, with respect to the control
In the analysis of ELF-EMF effects on prion propagation, the NT64C [URE3] (Fig. 3c) and the SB34 [URE3] (Fig. 3d) cells were exposed to ELF-EMF for 96 h. In both strains, the exposure groups had a significantly (p < 0.01) lower ratio of red/white colony numbers compared with the control groups, which meant the cells exposed to ELF-EMF contained fewer red colonies (Fig. 3c, d). Under exposure to ELF-EMF, the spontaneous loss of [URE3] in the NT64C and SB34 strains was inhibited, and the prion propagation of [URE3] was promoted.
Long-term effects of ELF-EMF on [URE3] generation and propagation
In the analysis of ELF-EMF effects, we also performed long-term exposure experiments to measure whether longer time exposure could cause stronger effects. Using similar methods as in the RF-EMF assay, the yeast cells were exposed to ELF-EMF for 384 h, with counting, washing, resuspending and spreading every 96 h. When the NT64C cells in the non-prion state were exposed to ELF-EMF (Fig. 4a), the ratios of the white/red colony numbers in the exposure group were always higher than the control group (i.e. the relative proportion was above 1.0), and the difference between the exposure and control groups increased with time. In another [ure-o] strain, SB34, we obtained similar results (Fig. 4b).
Fig. 4.
Long-term effects of 6.0 mT ELF-EMF exposure on the relative proportion of [URE3] or [ure-o] in yeast cells. a Relative proportion of [URE3] in NT64C [ure-o] after ELF-EMF exposure versus control. b Relative proportion of [URE3] in SB34 [ure-o] after ELF-EMF exposure versus control. c Relative proportion of [URE3] in NT64C [URE3] after ELF-EMF exposure versus control. d Relative proportion of [URE3] in SB34 [URE3] after ELF-EMF exposure versus control. Bars represent S.D. of the mean
When we used the prion state strains as the starting strain to study the effects of long-term exposure, we also found a time-dependent effect. After the NT64C [URE3] (Fig. 4c) and SB34 [URE3] (Fig. 4d) cells were exposed to ELF-EMF for 384 h, the ratios of the white/red colony numbers in the exposure group were always higher than the control group (i.e., the relative proportion was above 1.0), and the difference between the exposure and control groups tended to increase with time.
Effects of ELF-EMF in different strengths on [URE3] de novo generation and propagation
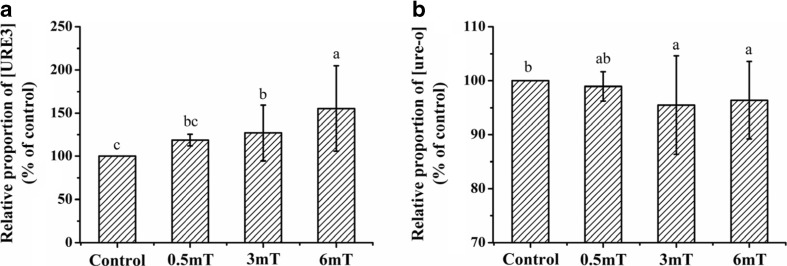
After we found the ELF-EMF exposure could elevate the generation and propagation of [URE3] in both short-term and long-term conditions, we analyzed the effects of ELF-EMF at different strengths. We constructed three ELF-EMF exposure systems with similar power supplies, Helmholtz coils, biochemical incubators, temperature recorders, and temperature control systems. The ELF-EMF strength of the three exposure systems was set to 0.5, 3.0, and 6.0 mT. After the cells starting from one single colony were incubated and spread onto YPD plates, the plates were exposed to 0, 0.5, 3.0, and 6.0 mT at 30 °C for 96 h.
When using the non-prion phenotype SB34 [ure-o] as the starting strain, the relative ratio of white/red colony numbers between the exposure group and the control group increased along with the increase of ELF-EMF exposure strength (Fig. 5a). Exposure to 3.0 and 6.0 mT, but not 0.5 mT, ELF-EMF significantly increased the [URE3] generation. The effect of 6.0 mT was significantly stronger than 3.0 and 0.5 mT, while the effect of 3.0 mT was significantly stronger than 0.5 mT.
Fig. 5.
Short-term (96 h) effects of different strengths of ELF-EMF exposure on the relative proportion of [URE3] or [ure-o] in SB34 yeast cells. a Relative proportion of [URE3] in SB34 [ure-o] after exposure versus control. b Relative proportion of [ure-o] in SB34 [URE3] after exposure versus control. All data represent more than three independent experiments. Bars represent S.D. of the mean. Bars marked with different letter means P < 0.05, compared with each other
Similarly, when using the prion phenotype SB34 [URE3] as the starting strain, the relative ratio of red/white colony numbers between the exposure group and control group also decreased along with the increase of ELF-EMF exposure strength (Fig. 5b). 3.0 and 6.0 mT, but not 0.5 mT, ELF-EMF exposure significantly inhibited the appearance of non-prion colonies, and increased the [URE3] proportion.
Effects of RF-EMF and ELF-EMF on expression levels of chaperones
Since Hsp chaperones play very important roles in prion generation and propagation in yeast, quantitative real-time PCR was applied to detect whether RF-EMF and ELF-EMF exposure could affect the cellular gene transcription levels of HSP104, HSP70-SSA1/2, and HSP40-YDJ1 (Fig. 6a–d and Fig. S1). After two prion strains NT64C and SB34 were grown on YPD plates under the exposure/control conditions for 4 days, the cells were collected and subjected to RT-PCR analysis. Quantitative RT-PCR analysis showed that cellular gene transcription levels of HSP104, HSP70-SSA1/2, and HSP40-YDJ1 were not significantly affected by the RF-EMF and ELF-EMF exposure in both strains, whether starting from the non-prion state or the prion state.
Fig. 6.
Effects of RF-EMF and ELF-EMF exposure on the expression of molecular chaperones including HSP104, SSA1/2 and YDJ1 in SB34 strain. The transcription levels of HSP104, SSA1/2 and YDJ1 in, SB34 [ure-o] (a), SB34 [URE3] (b) after 2.0 GHz, 40 dBm RF-EMF exposure versus control as well as SB34 [ure-o] (c), SB34 [URE3] (d) after 6.0 mT ELF-EMF exposure versus control were detected by quantitative RT-PCR. e Western blot analysis showing the molecular chaperones expression in SB34 [ure-o] cells exposed to 2.0 GHz, 40 dBm RF-EMF, compared with the control. f Quantitative analysis of (e). g Western blot analysis showing the molecular chaperones expression in SB34 [ure-o] cells exposed to 6.0 mT ELF-EMF, compared with the control. h Quantitative analysis of (g). The β-actin protein was used as internal control. Values indicate integrated optical density (IOD) ratio of target proteins/β-actin in immunoblots. All data represent three independent experiments. Bars represent S.D. of the mean
We also analyzed the protein expression levels of the three Hsp proteins by western blotting. After non-prion form SB34 strains were grown on YPD plates under exposure/control conditions for 96 h, the cells were collected and subjected to western blotting analysis. Consistent with the transcription level results, the protein expression levels of HSP104, HSP70-SSA1/2, and HSP40-YDJ1 were also not significantly changed (Fig. 6e–h).
Effects of RF-EMF on ROS level and enzyme activity of SOD and CAT
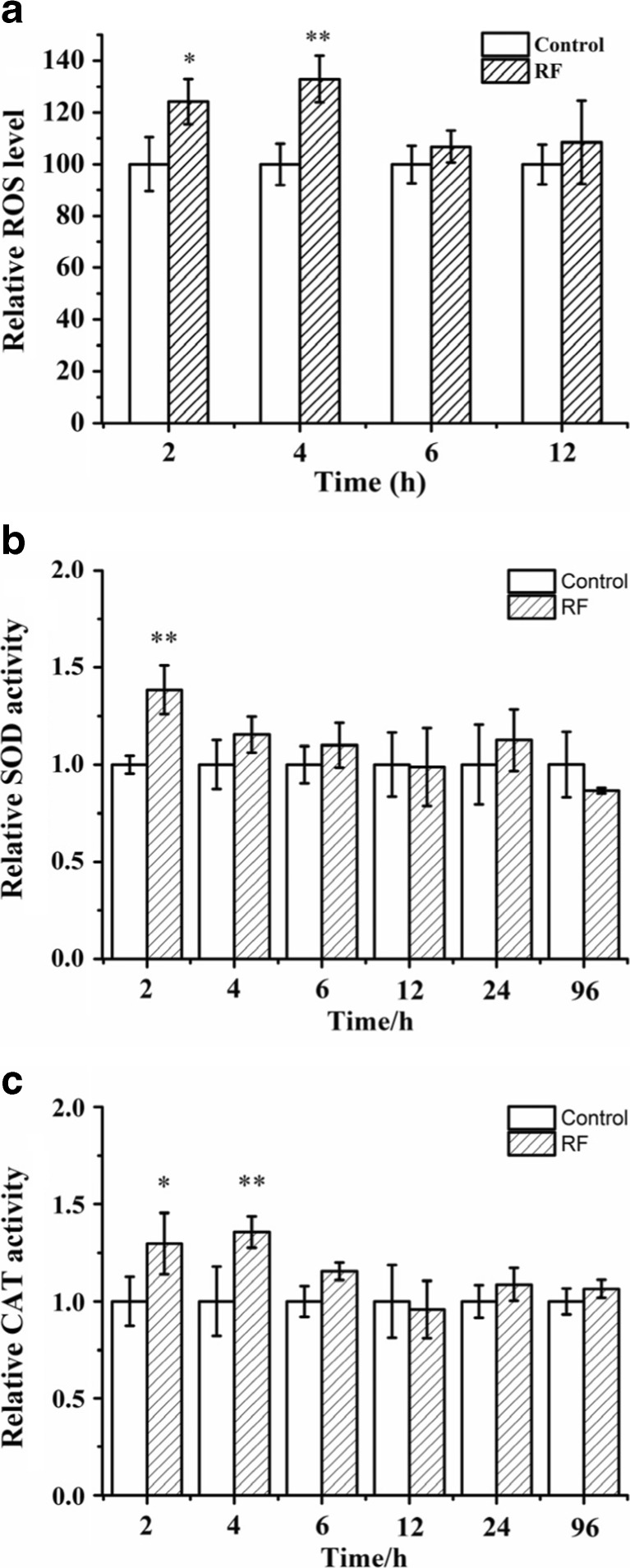
Since the RF-EMF exposure could increase the formation and propagation of yeast prions, and improved ROS level could also increase the formation and propagation of amyloid structures, we wondered whether ROS levels played some role in the effects of RF-EMF on yeast prions. Therefore, ROS levels were measured after RF-EMF exposure. SB34 cells in liquid medium were exposed to RF-EMF and were sampled at 2.0-, 4.0-, 6.0-, and 12.0-h time points. Under exposure, ROS levels increased significantly in SB34 [ure-o] cells at 2.0 and 4.0 h; however, at the 6.0- and 12.0-h time points, we did not observe a significant increase (Fig. 7a).
Fig. 7.
Effects of 2.0 GHz, 40 dBm RF-EMF exposure on ROS levels and related enzymatic activity in SB34 [ure-o] cells. a Relative ROS level in SB34 [ure-o] cells after RF-EMF exposure versus control. b Enzymatic activity of SOD after RF-EMF exposure versus control. c Enzymatic activity for CAT after RF-EMF exposure versus control. All data represent three independent experiments. Bars represent S.D. of the mean. “*” means P < 0.05, compared with the control, while “**” represents P < 0.01, with respect to the control
Since ROS levels were affected by exposure, the activities of two ROS level related enzymes, superoxide dismutase (SOD) and catalase (CAT), were measured after exposure. SB34 [ure-o] cells were exposed to RF-EMF and were sampled at different time points. Similar to the ROS level, the enzyme activities also increased at the beginning time points under exposure. Activities of both SOD and CAT significantly increased at the 2- and 4-h time points (Fig. 7b, c). At other time points, enzyme activities were still generally higher under exposure; however, the difference was not statistically significant.
Effects of ELF-EMF on ROS level and enzyme activity of SOD and CAT
SB34 cells in liquid medium were exposed to 6.0 mT ELF-EMF and were sampled at the 0.5-, 1.0-, 2.0-, 6.0-, 12.0-, and 24.0-h time points. Under exposure, ROS levels increased significantly in the SB34 [ure-o] cells at 0.5, 1.0, and 2.0 h; however, at the 6.0-, 12.0- and 24.0-h time points, we did not observe a significant increase (Fig. 8a).
Fig. 8.
Effects of 6.0 mT ELF-EMF exposure on ROS levels and related enzymatic activity in SB34 [ure-o] cells. a Relative ROS level in SB34 [ure-o] cells after ELF-EMF exposure versus control. b Enzymatic activity of SOD after ELF-EMF exposure versus control. c Enzymatic activity for CAT after ELF-EMF exposure versus control. All data represent three independent experiments. Bars represent S.D. of the mean. “*” means P < 0.05, compared with the control, while “**” represents P < 0.01, with respect to the control
SB34 [ure-o] cells were exposed to ELF-EMF and were sampled at 0.5-, 1.0-, 2.0-, 6.0-, 12.0-, and 24.0-h time points. Activities of SOD significantly increased at the 1.0-h time point (Fig. 8b). Activities of CAT significantly increased at the 0.5- and 2.0-h time points (Fig. 8c). At other time points, the enzyme activities of SOD and CAT in the exposure group were not significantly different from the control group. Under ELF-EMF exposure, ROS levels and enzyme activities were increased at the beginning time points.
Discussion
Up to now, studies on the health effects of RF-EMF and ELF-EMF have focused on an epidemiological survey on the health effects on human and laboratory study for biological effects on animals. Due to the complexity of animals, as well as the lack of reproducibility and the difficulty of analysis, the biological effects of EMF still remain unclear. In this study, we studied the effects of RF-EMF and ELF-EMF on prions using budding yeast as a model organism. After short-term exposure to RF-EMF, we found de novo generation and propagation of prion were elevated in both strains (Fig. 1). Interestingly, it has been found that the exposure to RF-EMF usually had beneficial effects on AD in epidemiological survey (Schuz et al. 2009) and AD pathology transgenic model study (Arendash et al. 2012; Dragicevic et al. 2011). To ensure the reliability and credibility of our results, these experiments were repeated eight or nine times independently, with at least three parallel samples each time. Furthermore, we observed increasing tendency with time in long-term exposure (Fig. 2), which strongly supported the elevation effects of RF-EMF on generation and propagation of prions. To exclude the possibility that other mutations resulted in a conversion from red to white cells, we treated newly formed white colonies with guanidine hydrochloride, and the colonies were cured. The oxidizing environment blocked the development of red color in ade mutant cells and make the ade mutant cells appear white (Bharathi et al. 2016). The white colonies formed during EMF exposure maintained their white color under non-EMF conditions, and turned back to red on guanidine hydrochloride plates; therefore, the white colonies were not caused by an oxidizing environment in cells.
In the analysis of ELF-EMF effects, we found the de novo generation of prions was elevated in the SB34, but not in the NT64C strain, while the propagation of prions was elevated in both strains after short-term exposure to ELF-EMF (Fig. 3). The lack of difference in the effects of short-term exposure on prion generation in NT64C somewhat disappointed us. We believe this result is true, however, as these experiments were repeated at least eight times independently, with three parallel samples each time, and we obtained a similar result in 3.0 mT exposure experiments (data not shown). The possible reason for this may be due to the difference in strain backgrounds which give the stains different sensitivity to EMF exposure. In general, we still believe exposure to ELF-EMF could elevate the de novo generation of prions, because we observed a significant difference after the effects was accumulated in the long-term exposure experiment (Fig. 4). In the ELF-EMF effects analysis, we studied the effects of ELF-EMF in different strengths on yeast prion, and found the elevation effects of ELF-EMF on yeast prion generation and formation was in a dose-dependent manner (Fig. 5). This result also supports that the effects we found were indeed caused by ELF-EMF exposure.
Hsp104 and Hsp70 (e.g., Ssa1 and Ssa2), together with the co-chaperone Hsp40 (e.g., Ydj1 and Sis1), have been reported to be involved in the propagation of yeast prions in vivo and fibril formation in vitro (Wickner et al. 2015). Therefore, we tried to measure the expression levels of molecular chaperones under RF-EMF and ELF-EMF stress conditions in order to explain how EMF exposure affected [URE3] generation and propagation. However, in our conditions, we did not find a significant difference in the transcription levels and protein expression levels (Fig. 6 and S1). As the effects of EMF are generally weak, the change in the chaperones amounts might be too small to be found in this study. Since the ROS levels and activities of SOD and CAT exhibited some elevation in the early hours of exposure, but not in the later time points, it may be that the chaperones have a similar time course. It would be interesting to measure the time course, especially in the beginning time points, for expression levels of chaperones in a further study.
In the ROS level analysis, we found that the RF-EMF and ELF-EMF could increase cell ROS levels at the beginning time points, but not at the later time points (Figs. 7a and 8a). Probably, a self-adaptive mechanism, in particular the compensatory upregulation of antioxidant enzymes, of the cells was induced after a few hours under EMF stress. Under physiological conditions, ROS levels are tightly controlled by antioxidants and antioxidant enzymes such as SOD and CAT (Yu 1994). A strong oxidant factor, superoxide, could be converted by SOD to a weaker oxidant factor, hydrogen peroxide, which could then be converted to water and oxygen by CAT (Johns and Platts 2014; Wu et al. 2013). To study how the yeast cells recovered their high ROS levels to normal levels after a few hours, we measured the enzyme activities of SOD and CAT (Figs. 7 and 8). Consistent with ROS levels, the activities of both SOD and CAT also increased at the beginning time points under EMF stress. Probably, the accumulation of ROS activated the antioxidant system in the cell, and activities of SOD and CAT were elevated to defend against oxidative stress under EMF stress. After a few hours, the ROS levels dropped, and the activities of SOD and CAT recovered to normal levels accordingly.
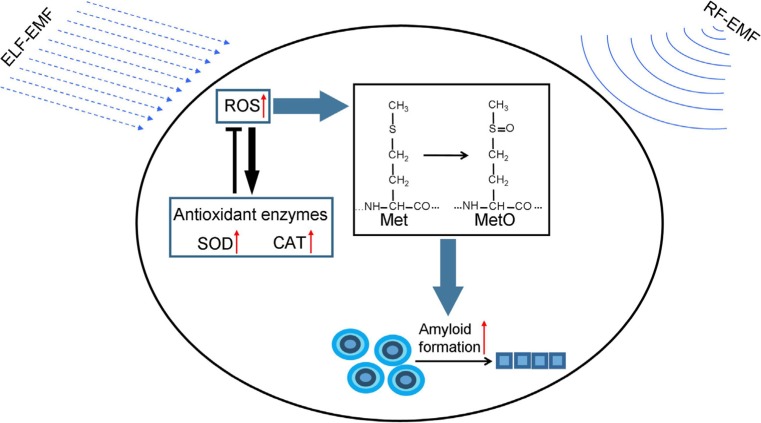
Under oxidative stress, the moderately hydrophobic thioester side chain of methionine could be converted into the hydrophilic sulphoxide form (MetO), which can significantly influence protein folding and conformation. Oxidation of methionine can induce amyloid fibril formation of Apolipoprotein A-I in vitro (Wong et al. 2010) and MetO levels were found to be elevated in persons carrying familial Alzheimer’s disease mutations that also correlated with other indices of oxidative stress (Ringman et al. 2012). MetO could be detected from the PrPSc brain, but could not be detected in the PrPC brain (Canello et al. 2008). In a [PIN+][psi-] yeast strain, the MetO levels and the frequency of another yeast prion, [PSI+], formation were significantly increased under oxidative stress (Doronina et al. 2015). In addition, similar increases in MetO levels and [PSI+] prion formation were detected in antioxidant mutants, including mutants deficient in superoxide dismutases, catalases, and peroxiredoxins, while the Sup35 aggregation and methionine oxidation in these antioxidant mutants could be prevented by overexpression of methionine sulphoxide reductase (MSRA) (Doronina et al. 2015). These data confirmed that methionine oxidation of Sup35 plays a critical role in the increase of de novo formation of [PSI+] under oxidative stress. Similar to Sup35, Ure2 contains 10 methionine residues (Fig. S2), so it is possible that Ure2 share some similar mechanism in the effects of oxidative stress on protein folding (Fig. 9).
Fig. 9.
Model of the biological effect of EMF. ELF-EMF exposure and RF-EMF exposure both can increase cell ROS level, which subsequently elevates the generation and propagation of amyloid, probably via the oxidation of methionine. Increase of ROS levels activated the antioxidant system in the cell, and activities of SOD and CAT were elevated to defend the oxidative stress and prevent ROS levels from increasing. Changes in ROS levels and protein folding will widely interfere with cell function, and are therefore hypothesized to mediate various biological effect of EMF. Red, black and green arrow represents “increase,” “process,” and “lead to,” respectively
It is still debatable whether the EMF has biological impact, due to the lack of repeatability in the experimental region and to the lack of convincing mechanisms in the theoretical region. Theoretically, the energy brought by radio frequency electromagnetic wave and extremely low frequency electromagnetic field is not sufficient to break molecular bonds (Adair 1998); therefore, it is difficult to explain the effects of EMF on cells. The effects of EMF on free radicals, which was thought to be mediated by an imbalance in iron homeostasis and the following formation of hydroxyl radicals via Fenton reaction (Lai and Singh 2004), might be an explanation. Free radicals are very reactive species. Elevation in the levels of free radicals, especially oxygen radicals, will dramatically damage biomolecules, such as lipids, proteins, and nucleic acids, and interfere with cell processes (Falletti et al. 2007; Itoh et al. 2007; Stadtman 1990). Excessive reactive species have been implicated in the pathogenesis of many neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), AD, and Huntington’s disease (HD) (Niedzielska et al. 2016). Elevation of free radical levels could also promote the formation of amyloid aggregates in vitro; on the other hand, the use of antioxidant agents could decrease the occurrence rate of amyloid diseases (Chakrabarti et al. 2013; Hensley et al. 1994; Zhao and Zhao 2013). In yeast, the [PSI+] prion formation could be induced under conditions of oxidative stress (Doronina et al. 2015; Tyedmers et al. 2008) and in mutants lacking key antioxidants (Doronina et al. 2015). It was found that exposure to RF-EMF and ELF-EMF could lead to the elevation of ROS levels in this study (Fig. 6) as well as in various organisms and cells in other studies (Doronina et al. 2015; Goraca et al. 2010; Simko and Mattsson 2004). Besides ROS levels, the levels of some compounds and proteins involved in energy metabolism were also found to be enhanced under EMF exposure in various model organisms in our previous work ((Li et al. 2013; Shi et al. 2015), Sun et al., Unpublished data). Therefore, it is possible that free radicals play some roles in the effects of EMF on amyloid formation and propagation (Fig. 9 and should therefore receive more attention in further research.
Proteins are involved in almost all cell processes, so defects in protein structure and function will lead to abnormality in all the related processes. We found both exposure to RF-EMF and ELF-EMF could cause the elevation of prion generation and propagation, which suggests that the folding of proteins could be affected by EMF exposure. In spite of some evidence supporting the hypothesis that ROS levels mediate the effects of EMF on protein folding, the actual mechanism is still unclear and we cannot exclude the possibility that EMF could affect the process of protein folding directly or via some unknown pathway. Although various biological effects of EMF exposure have been reported, the mechanism remains unknown. EMF exposure could affect ROS levels and protein folding, both of which are widely involved in biological processes. Therefore, the effects of EMF exposure on ROS levels or protein folding may initiate a cascade of effects on many biological processes to some extent under EMF stress (Fig. 9).
Electronic supplementary material
(DOC 553 kb)
(DOC 219 kb)
(DOC 26.5 kb)
Acknowledgements
We express our gratitude to Dr. Gary Jones (NUI Maynooth, Ireland) for kindly providing the yeast strains. The valuable assistance of Mr. Shen Tian on painting of Fig. 9 is gratefully acknowledged.
Author contribution
KWL and HYL performed all experiments. CJY, HYL, and KWL constructed and maintained the exposure systems. HYL conceived the study. HYL, KWL, and PC wrote the manuscript. PC led the project and reviewed the manuscript.
Funding information
This work was supported by the Scientific Equipment Development Project of the Chinese Academy of Sciences (CAS) (YZ201104&YZ201205), the Xiamen Science and Technology Plans Project (3502Z20126012), the National Natural Science Foundation of China (31270888), and the Natural Science Foundation of Fujian Province (2012J01157).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0867-9) contains supplementary material, which is available to authorized users.
Contributor Information
Hui-Yong Lian, Phone: +865926190569, Email: hylian@qzu.edu.cn.
Peng Cai, Phone: +865926190996, Email: pcai@iue.ac.cn.
References
- Adair RK. Extremely low frequency electromagnetic fields do not interact directly with DNA. Bioelectromagnetics. 1998;19(2):136–138. doi: 10.1002/(SICI)1521-186X(1998)19:2<136::AID-BEM14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M, Wang L, Zhang G, Sava V, Tan J, Cao C. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer's disease mice. J Alzheimers Dis. 2010;19(1):191–210. doi: 10.3233/JAD-2010-1228. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Dorsey M, Gonzalez R, Tajiri N, Borlongan C. Electromagnetic treatment to old Alzheimer's mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One. 2012;7(4):e35751. doi: 10.1371/journal.pone.0035751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaceur S, Banasr S, Sakly M, Abdelmelek H. Whole body exposure to 2.4 GHz WIFI signals: effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer's disease (3xTg-AD) Behav Brain Res. 2013;240:197–201. doi: 10.1016/j.bbr.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Bharathi V, Girdhar A, Prasad A, Verma M, Taneja V, Patel BK. Use of ade1 and ade2 mutations for development of aversatile red/white colour assay of amyloid-induced oxidative stress in saccharomyces cerevisiae. Yeast. 2016;33(12):607–620. doi: 10.1002/yea.3209. [DOI] [PubMed] [Google Scholar]
- Canello T, Engelstein R, Moshel O, Xanthopoulos K, Juanes ME, Langeveld J, Sklaviadis T, Gasset M, Gabizon R. Methionine sulfoxides on PrPSc: a prion-specific covalent signature. Biochemistry. 2008;47(34):8866–8873. doi: 10.1021/bi800801f. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Perry G, Smith MA. Prion disease and Alzheimer's disease: pathogenic overlap. Acta Neurobiol Exp. 2004;64:11–17. doi: 10.55782/ane-2004-1487. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sinha M, Thakurta IG, Banerjee P, Chattopadhyay M. Oxidative stress and amyloid beta toxicity in Alzheimer's disease: intervention in a complex relationship by antioxidants. Curr Med Chem. 2013;20(37):4648–4664. doi: 10.2174/09298673113209990152. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Inouye M. The intramolecular chaperone-mediated protein folding. Curr Opin Struct Biol. 2008;18(6):765–770. doi: 10.1016/j.sbi.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Cox KH, Rai R, Distler M, Daugherty JR, Coffman JA, Cooper TG. Saccharomyces cerevisiae GATA sequences function as TATA elements during nitrogen catabolite repression and when Gln3p is excluded from the nucleus by overproduction of Ure2p. J Biol Chem. 2000;275(23):17611–17618. doi: 10.1074/jbc.M001648200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzier D, Perrin A, Torres G, Dabouis V, Debouzy JC. Pulsed electromagnetic field at 9.71 GHz increase free radical production in yeast (Saccharomyces cerevisiae) Pathol Biol (Paris) 2009;57(3):245–251. doi: 10.1016/j.patbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Davanipour Z, Sobel E. Long-term exposure to magnetic fields and the risks of Alzheimer's disease and breast cancer: further biological research. Pathophysiology. 2009;16(2-3):149–156. doi: 10.1016/j.pathophys.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Davanipour Z, Tseng CC, Lee PJ, Sobel E. A case-control study of occupational magnetic field exposure and Alzheimer's disease: results from the California Alzheimer's disease diagnosis and treatment centers. BMC Neurol. 2007;7(1):13. doi: 10.1186/1471-2377-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina VA, Staniforth GL, Speldewinde SH, Tuite MF, Grant CM. Oxidative stress conditions increase the frequency of de novo formation of the yeast [PSI+] prion. Mol Microbiol. 2015;96(1):163–174. doi: 10.1111/mmi.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragicevic N, Bradshaw PC, Mamcarz M, Lin X, Wang L, Cao C, Arendash GW. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer's transgenic mice and normal mice: a mechanism for electromagnetic field-induced cognitive benefit? Neuroscience. 2011;185:135–149. doi: 10.1016/j.neuroscience.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Falletti O, Cadet J, Favier A, Douki T. Trapping of 4-hydroxynonenal by glutathione efficiently prevents formation of DNA adducts in human cells. Free Radic Biol Med. 2007;42(8):1258–1269. doi: 10.1016/j.freeradbiomed.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol Microbiol. 2001;40(6):1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- Goraca A, Ciejka E, Piechota A. Effects of extremely low frequency magnetic field on the parameters of oxidative stress in heart. J Physiol Pharmacol. 2010;61(3):333–338. [PubMed] [Google Scholar]
- Grimminger V, Richter K, Imhof A, Buchner J, Walter S. The prion curing agent guanidinium chloride specifically inhibits ATP hydrolysis by Hsp104. J Biol Chem. 2004;279(9):7378–7383. doi: 10.1074/jbc.M312403200. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney JM, Mattson MP, Aksenova M, Harris M, Wu JF, Floyd RA, Butterfield DA. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91(8):3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JY, Mathur V, Liebman SW. A new colour assay for [URE3] prion in a genetic background used to score for the [PSI(+)] prion. Yeast. 2011;28(7):555–560. doi: 10.1002/yea.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Cao J, Chen ZH, Yoshida Y, Niki E. Advantages and limitation of BODIPY as a probe for the evaluation of lipid peroxidation and its inhibition by antioxidants in plasma. Bioorg Med Chem Lett. 2007;17(7):2059–2063. doi: 10.1016/j.bmcl.2007.01.080. [DOI] [PubMed] [Google Scholar]
- Jeong YJ, et al. 1950 MHz electromagnetic fields ameliorate Abeta pathology in Alzheimer's disease mice. Curr Alzheimer Res. 2015;12(5):481–492. doi: 10.2174/156720501205150526114448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JR, Platts JA. Theoretical insight into the antioxidant properties of melatonin and derivatives. Org Biomol Chem. 2014;12(39):7820–7827. doi: 10.1039/C4OB01396D. [DOI] [PubMed] [Google Scholar]
- Jung G, Masison DC. Guanidine hydrochloride inhibits Hsp104 activity in vivo: a possible explanation for its effect in curing yeast prions. Curr Microbiol. 2001;43(1):7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- Kryndushkin D, Wickner RB. Nucleotide exchange factors for Hsp70s are required for [URE3] prion propagation in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18(6):2149–2154. doi: 10.1091/mbc.e07-02-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, Singh NP. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. 2004;112(6):687–694. doi: 10.1289/ehp.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS, Zhang ZY, Yang CJ, Lian HY, Cai P. Gene expression and reproductive abilities of male Drosophila melanogaster subjected to ELF-EMF exposure. Mutat Res. 2013;758(1-2):95–103. doi: 10.1016/j.mrgentox.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Lian HY, Jiang Y, Zhang H, Jones GW, Perrett S. The yeast prion protein Ure2: structure, function and folding. Biochim Biophys Acta. 2006;1764(3):535–545. doi: 10.1016/j.bbapap.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Lian HY, Zhang H, Zhang ZR, Loovers HM, Jones GW, PJE R, Itzhaki LS, Zhou JM, Perrett S. Hsp40 interacts directly with the native state of the yeast prion protein Ure2 and inhibits formation of amyloid-like fibrils. J Biol Chem. 2007;282(16):11931–11940. doi: 10.1074/jbc.M606856200. [DOI] [PubMed] [Google Scholar]
- Mancinelli F, Caraglia M, Abbruzzese A, Ambrosio G, Massa R, Bismuto E. Non-thermal effects of electromagnetic fields at mobile phone frequency on the refolding of an intracellular protein: myoglobin. J Cell Biochem. 2004;93(1):188–196. doi: 10.1002/jcb.20164. [DOI] [PubMed] [Google Scholar]
- Marjanovic AM, Pavicic I, Trosic I (2015) Cell oxidation-reduction imbalance after modulated radiofrequency radiation. Electromagn Biol Med 34:381–386 [DOI] [PubMed]
- Masison DC, Wickner RB. Prion-inducing domain of yeast Ure2p and protease resistance of Ure2p in prion-containing cells. Science. 1995;270(5233):93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- Moore RA, Sturdevant DE, Chesebro B, Priola SA. Proteomics analysis of amyloid and nonamyloid prion disease phenotypes reveals both common and divergent mechanisms of neuropathogenesis. J Proteome Res. 2014;13(11):4620–4634. doi: 10.1021/pr500329w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama H, Edskes HK, Wickner RB. [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol Cell Biol. 2000;20(23):8916–8922. doi: 10.1128/MCB.20.23.8916-8922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M. Oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2016;53(6):4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Fithian AT, Gylys K, Cummings JL, Coppola G, Elashoff D, Pratico D, Moskovitz J, Bitan G. Plasma methionine sulfoxide in persons with familial Alzheimer's disease mutations. Dement Geriatr Cogn Disord. 2012;33(4):219–225. doi: 10.1159/000338546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuderer J, Samaras T, Oesch W, Spat D, Kuster N. High peak SAR exposure unit with tight exposure and environmental control for in vitro experiments at 1800 MHz. IEEE Trans Microw Theory. 2004;52:2057–2066. doi: 10.1109/TMTT.2004.832009. [DOI] [Google Scholar]
- Schuz J, Waldemar G, Olsen JH, Johansen C. Risks for central nervous system diseases among mobile phone subscribers: a Danish retrospective cohort study. PLoS One. 2009;4(2):e4389. doi: 10.1371/journal.pone.0004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer C, Masison DC. Antagonistic interactions between yeast [PSI(+)] and [URE3] prions and curing of [URE3] by Hsp70 protein chaperone Ssa1p but not by Ssa2p. Mol Cell Biol. 2002;22(11):3590–3598. doi: 10.1128/MCB.22.11.3590-3598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Yu H, Sun Y, Yang C, Lian H, Cai P. The energy metabolism in Caenorhabditis elegans under the extremely low-frequency electromagnetic field exposure. Sci Rep. 2015;5(1):8471. doi: 10.1038/srep08471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simko M, Mattsson MO. Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: possible immune cell activation. J Cell Biochem. 2004;93(1):83–92. doi: 10.1002/jcb.20198. [DOI] [PubMed] [Google Scholar]
- Sobel E, Davanipour Z. Electromagnetic field exposure may cause increased production of amyloid beta and eventually lead to Alzheimer's disease. Neurology. 1996;47(6):1594–1600. doi: 10.1212/WNL.47.6.1594. [DOI] [PubMed] [Google Scholar]
- Sobel E, Davanipour Z, Sulkava R, Erkinjuntti T, Wikstrom J, Henderson VW, Buckwalter G, Bowman JD, Lee PJ. Occupations with exposure to electromagnetic fields: a possible risk factor for Alzheimer's disease. Am J Epidemiol. 1995;142(5):515–524. doi: 10.1093/oxfordjournals.aje.a117669. [DOI] [PubMed] [Google Scholar]
- Sobel E, Dunn M, Davanipour Z, Qian Z, Chui HC. Elevated risk of Alzheimer's disease among workers with likely electromagnetic field exposure. Neurology. 1996;47(6):1477–1481. doi: 10.1212/WNL.47.6.1477. [DOI] [PubMed] [Google Scholar]
- Solomentsev GY, English NJ, Mooney DA. Effects of external electromagnetic fields on the conformational sampling of a short alanine peptide. J Comput Chem. 2012;33(9):917–912. doi: 10.1002/jcc.22912. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990;29(27):6323–6331. doi: 10.1021/bi00479a001. [DOI] [PubMed] [Google Scholar]
- Su B, Wang X, Nunomura A, Moreira P, Hg L, Perry G, Smith M, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr Alzheimer Res. 2008;5(6):525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Madariaga ML, Lindquist S. Prion switching in response to environmental stress. PLoS Biol. 2008;6:e294. doi: 10.1371/journal.pbio.0060294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB, Masison DC, Edskes HK. [PSI] and [URE3] as yeast prions. Yeast. 1995;11(16):1671–1685. doi: 10.1002/yea.320111609. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Shewmaker FP, Bateman DA, Edskes HK, Gorkovskiy A, Dayani Y, Bezsonov EE. Yeast prions: structure, biology, and prion-handling systems. Microbiol Mol Biol Rev. 2015;79(1):1–17. doi: 10.1128/MMBR.00041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YQ, Binger KJ, Howlett GJ, Griffin MD. Methionine oxidation induces amyloid fibril formation by full-length apolipoprotein A-I. Proc Natl Acad Sci U S A. 2010;107(5):1977–1982. doi: 10.1073/pnas.0910136107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Kosten TR, Zhang XY. Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;46:200–206. doi: 10.1016/j.pnpbp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 553 kb)
(DOC 219 kb)
(DOC 26.5 kb)