Abstract
Heat stress is exacerbated by global warming and affects human and animal health, leading to heart damage caused by imbalances in reactive oxygen species (ROS) and the antioxidant system, acid-base chemistry, electrolytes and respiratory alkalosis. Vitamin C scavenges excess ROS, and sodium bicarbonate maintains acid-base and electrolyte balance, and alleviates respiratory alkalosis. Herein, we explored the ability of vitamin C alone and in combination with equimolar sodium bicarbonate (Vitamin C-Na) to stimulate endogenous antioxidants and heat shock proteins (HSPs) to relieve heat stress in H9C2 cells. Control, vitamin C (20 μg/ml vitamin C for 16 h) and vitamin C-Na (20 μg/ml vitamin C-Na for 16 h) groups were heat-stressed for 1, 3 or 5 h. Granular and vacuolar degeneration, karyopyknosis and damage to nuclei and mitochondria were clearly reduced in treatment groups, as were apoptosis, lactate dehydrogenase activity and ROS and malondialdehyde levels, while superoxide dismutase activity was increased. Additionally, CRYAB, Hsp27, Hsp60 and Hsp70 mRNA levels were upregulated at 3 h (p < 0.01), and protein levels were increased for CRYAB at 0 h (p < 0.05) and 1 h (p < 0.01), and for Hsp70 at 3 and 5 h (p < 0.01). Thus, pre-treatment with vitamin C or vitamin C-Na might protect H9C2 cells against heat damage by enhancing the antioxidant ability and upregulating CRYAB and Hsp70.
Keywords: Vitamin C, Sodium bicarbonate, Heat stress, Antioxidant, H9C2 cells, ROS
Introduction
Stress is defined as a nonspecific general reaction when an organism is forced to respond to environmental challenges such as exposure to cold, surgical injury or hyperthermia (Zulkifli et al. 2009). Heat stress is a major environmental issue exacerbated by global warming that influences human health, affects animal growth and development, decreasing livestock production and causing sudden death in extreme cases (Crandall and Wilson 2014; Niu et al. 2009). Heat stress can also damage important organs like the heart (Cui and Sinoway 2014; Tang et al. 2016b) and induce tissue damage associated with sudden death. Heat stress can also destroy the integrity of the tight junctions of myocardial cells, and injure the structure and function of cell membranes (Aitken-Buck and Lamberts 2017; Crandall and Wilson 2014; Cui and Sinoway 2014). The heat damage is involved in excessive generation of reactive oxygen species (ROS) and the destruction of the antioxidant system (Ahmad et al. 2017; Lin et al. 2006; Panda et al. 2008). Effective antioxidants that can be used to decrease heat stress damage are therefore of much interest in human and veterinary medicine.
Vitamin C (ascorbic acid) is an effective antioxidant that acts as a cofactor for at least 15 mammalian enzymes (Aditi and Graham 2012). Vitamin C can be synthesised by all plants and most animals, but not by humans and other primates (Aditi and Graham 2012; Padayatty and Levine 2016). Dietary supplementation with vitamin C is beneficial, especially in stress and injury conditions that induce the production of ROS. Many studies have indicated that vitamin C, as dietary supplement, is beneficial for humans (Ahmad et al. 2017; Khassaf et al. 2003; Morrison et al. 2015) and for animals to reduce stress (Imik et al. 2012; Mahmoud et al. 2003; Mahmoud et al. 2004; Niu et al. 2009; Sahin et al. 2004; Sahin et al. 2009; Szczubial 2015). Evidence suggests that supplementation with vitamin C can alleviate heat-related disruption of metabolic processes, and enhance the immune competence of animals (Jang et al. 2014; Niu et al. 2009; Panda et al. 2008; Rafiee et al. 2016; Redmond et al. 2010). Feed supplemented with 200–400 mg/kg vitamin C can enhance feed intake, survivability and laying performance in hens reared under heat stress conditions (Attia et al. 2016). Similar effects of vitamin C have been observed in broiler chicken, sows, cows and other livestock (Chand et al.,2014; Liu et al. 2016; Mahmoud et al. 2003; Mahmoud et al. 2004; Sahin et al. 2004; Sharma et al. 2013; Sinkalu and Ayo 2016; Szczubial 2015). However, due to the strong acidity of vitamin C, people with gastrointestinal conditions might suffer side effects of its administration.
As a dietary supplement, sodium bicarbonate can help to maintain acid-base and electrolyte balance, and alleviate respiratory alkalosis following exposure to high temperatures (Mujahid 2011). Sodium bicarbonate supplementation in hens could improve shell quality, and in chickens to enhance body weight (Ahmad et al. 2009; Grizzle et al. 1992; Yörük et al. 2004). Additionally, as a weak base, sodium bicarbonate can neutralise the strong acidity of vitamin C without destroying its electron donor and antioxidant activities (Padayatty and Levine 2016). However, the exact mechanisms of the protective effects of vitamin C combined with sodium bicarbonate against heat stress remain unknown.
The heat shock response (HSR) is an evolutionarily conserved endogenous protective mechanism characterised by transcriptional activation and accumulation of heat shock proteins (HSPs) (Collier et al. 2008; Kishore et al. 2014). The HSR can be activated by many stresses including heat stress (Ruell et al. 2009; Tower 2011). HSPs are highly conserved and are expressed in almost all living organisms from bacteria to humans (Tang et al. 2016a). Based on molecular weight, HSPs can be divided into Hsp110, Hsp90, Hsp70, Hsp60, Hsp47 and small HSPs such as Hsp27 and CRYAB (Garrido et al. 2001). HSPs act as molecular chaperones to stabilise newly synthesised proteins and promote the correct folding and refolding of damaged proteins (Sottile and Nadin 2017; Tower 2011). Under heat stress, denaturation or misfolding of proteins can lead to cell dysfunction and even death. HSPs play an important role in relieving stress-induced damage (Garrido et al. 2001; Ruell et al. 2009; Sottile and Nadin 2017). Vitamin C has the potential function as HSP inducer. Camins indicated that vitamin C could increase dog neutrophils cell-surface Hsp27, Hsp72 and Hsp90 expression after oxidative stress (Camins et al. 1999). Vitamin C supplement induced Hsp60 and Hsp70 expression in human lymphocyte and skeletal muscle (Khassaf et al. 2003); however, the mechanism of induction was unclear.
In our previous studies, pre-treatment with aspirin or co-enzyme Q10 can upregulate HSPs to protect heart tissue against heat stress (Tang et al. 2016a; Tang et al. 2016b; Xu et al. 2017b; Xu et al. 2017c). However, the protective effect of vitamin C against heat stress on heart tissue and its relationship with HSPs were less known; moreover, whether the effect of combination with equimolar sodium bicarbonate (vitamin C-Na) is better than vitamin C alone is not known. In the present study, we explored the ability of pre-treatment with vitamin C (VC) alone or in combination with sodium bicarbonate (VC-Na) to protect heart cells against heat stress, and demonstrated stimulation of natural antioxidant functions and upregulation of HSPs.
Materials and methods
Cell culture
H9C2 cells, derived from rat myocardium, were purchased from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco), 100 U penicillin and 100 μg/ml streptomycin (Gibco) at 37 °C in a 5% CO2 humidified atmosphere (Thermo, USA). When cells formed a complete monolayer, they were passaged into dishes or 96-well plates for subsequent experiments.
Cell viability analysis
Cell viability analysis was performed using a cell counting kit-8 (CCK-8, Beyotime, China) according to the manufacturer’s instructions. Firstly, we measured the maximum safe concentration of vitamin C alone and in combination with an equimolar amount of sodium bicarbonate (vitamin C-Na) for H9C2 cells. Vitamin C and vitamin C-Na were provided by Ningxia Zhihong Biotechnology Company. H9C2 cells were seeded in 96-well plates (1 × 104 cells/well). After ~ 16−18 h, the cell confluence reached 70−80%, and the medium was replaced with fresh medium containing vitamin C or the vitamin C-Na mixture at 0, 10, 20, 50, 100, 200, 400, 600, 800 or 1000 μg/ml. Plates were incubated at 37 °C for 16 h, and 10 μl CCK-8 solution was added to each well and incubation continued for 1 h at 37 °C. The absorbance at 450 nm was then measured with a microplate reader (NanoQuant, USA). In order to discriminate between the protective effects of vitamin C and the vitamin C-Na mixture on H9C2 cells under heat stress using the CKK-8 method, we transferred the plates into a 5% CO2-humidified atmosphere at 42 °°C for 5 h. At the end of the treatment, 10 μl of CCK-8 solution was added and incubation continued for 1 h at 37 °C. GraphPad Prism 5 software was used for data analysis.
The H9C2 cell heat stress model
To evaluate the protective effects of vitamin C or the vitamin C-Na mixture, H9C2 cells were seeded in a 60-mm dish and randomly divided into control, vitamin C and vitamin C-Na groups. After 16–18 h, the medium was changed to fresh medium for the control group, fresh medium containing 20 μg/ml vitamin C for the VC group and fresh medium containing 20 μg/ml vitamin C-Na for VC-Na group. Culturing was continued at 37 °C for 16 h, cells were transferred into a 5% CO2-humidified atmosphere at 42 °C for 0, 1, 3 or 5 h and cells or supernatants were immediately harvested for analysis.
Histological analysis of H9C2 cells
Histological analysis was performed to detect cell damage. H9C2 cells seeded on coverslips were collected after heat stress treatment, washed three times with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 30 min, washed three more times with PBS, stained with haematoxylin for 5 min, eosin for 3 min and passed immediately through an ethanol gradient consisting of 75% ethanol for 2 min, 85% ethanol for 2 min, 95% ethanol for 2 min, 95% ethanol for 2 min, 100% ethanol for 2 min, 100% ethanol for 2 min, xylene for 2 min and xylene for 2 min. Coverslips were mounted using neutral resin for light microscopic analysis using an Axio Imager.A2 instrument (Zeiss, German).
Transmission electron microscopy analysis of H9C2 cells
Transmission electron microscopy (TEM) analysis was performed to detect damage to subcellular structures, especially mitochondria. H9C2 cells were seeded in a 10 mm dish collected after heat treatment for 0 or 3 h. After heat stress treatment, the medium was discarded and cells were washed three times with PBS, then gently harvested using a cell scraper. After centrifugation at 800 g for 5 min, the supernatant was discarded and cells were fixed in 2.5% glutaraldehyde for TEM analysis using Libra 120 instrument (Zeiss).
Flow cytometry analysis of apoptosis
Flow cytometry analysis was performed to detect apoptosis using an Annexin V-FITC/PI Apoptosis Detection kit (Vazyme, China). Cells after heat stress were treated with EDTA-free trypsin (Gibco), collected, washed with cold PBS three times, suspended in 100 μl binding buffer and 5 μl annexin V-FITC and 5 μl PI solution were added. All samples were analysed by flow cytometry (BD FACSAria, USA) within 1 h, and data were analysed using FlowJo 7.6.
Measurement of lactate dehydrogenase, malondialdehyde and superoxide dismutase
H9C2 cells were seeded in 30 mm dishes, subjected to heat stress and the supernatant was collected for lactate dehydrogenase (LDH) analysis using a commercial kit (Nanjing Jiancheng Biochemical Reagent, China), while cells were treated with 100 μl RIPA lysis buffer for malondialdehyde (MDA) and superoxide dismutase (SOD) analysis. MDA was detected using an ELISA kit (Mlbio, China) according to the manufacturer’s instruction. SOD activity was measured using a commercial kit (Nanjing Jiancheng Biochemical Reagent), and protein concentration was measured using a BCA assay kit (Life Technologies, USA) with protein standards to normalise SOD activity to protein content.
Measurement of reactive oxygen species
Intracellular free radical production was measured using a reactive oxygen species (ROS) assay kit (Beyotime, China) following manufacturer’s instructions, followed by flow cytometry (BD FACSAria, USA) and Axio Imager.A2 fluorescence microscopy (Zeiss). For flow cytometry, H9C2 cells were seeded in 30 mm dishes, subjected to heat stress, treated with trypsin (Gibco), harvested, washed once with cold PBS, then suspended in 1 ml serum-free DMEM with 10 μM DCFH-DA. Cells were then incubated at 37 °C for 20 min, mixed every 5 min, washed with serum-free DMEM three times to remove free DCFH-DA and finally resuspended in 100 μl PBS. All samples were immediately analysed using flow cytometry. For fluorescence microscopy, H9C2 cells were seeded on coverslips in 24-well plates, subjected to heat stress, the supernatant was discarded, cells were washed with PBS three times, 500 μl serum-free DMEM and 10 μM DCFH-DA were added and cells were incubated at 37 °C for 20 min. After washing with PBS three more times, coverslips were placed on slides for fluorescence microscopy analysis.
Real-time quantitative PCR
H9C2 cells were seeded in 24-well plates, and total RNA was extracted from heat-stressed cells using TRIzol reagent (TaKaRa, Japan) and quantified with a Nanodrop 2000 (Thermo, USA) by measuring the absorbance at 260 nm and A260/A280 ratio. Reverse transcription was then carried out with a real-time quantitative PCR (RT-PCR kit) (Vazyme, China). Synthesised cDNA was used for RT-PCR with Power SYBR Green master mix (Vazyme) according to the manufacturer’s instructions. The relative expression level of genes was normalised against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and quantified using the comparative Ct (2-ΔΔCt) method. Primer sequences are shown in Table 1.
Table 1.
Sequences of primers used for real-time PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Hsp27 | 5′-CGTGGTGGAGATCACTGGCAAGC-3′ | 5′-CGGGCCTCGAAAGTGACCGG-3′ |
| CRYAB | 5′-CACGAAGAGCGCCAGGACGA-3′ | 5′-CGTCGGCTGGGATCCGGTACT-3′ |
| Hsp47 | 5′-TCTCCTTCTGGGCACCTTA-3′ | 5′-CTCCACCGCCTGATCTTT-3′ |
| Hsp60 | 5′-CCGCCCCGCAGAAATGCTTCGAA-3′ | 5′-AGGCTCGAGCATCCGCACCAA-3′ |
| Hsp70 | 5′-GCTGACCAAGATGAAGGAGAT-3′ | 5′-GCTGCGAGTCGTTGAAGTAG-3′ |
| Hsp90 | 5′-CCCGGTGCGGTTAGTCACGT-3′ | 5′-TCCAGAGCGTCTGAGGAGTTGGA-3′ |
| Hsp110 | 5′-GCGTGGAGCAGATAACA-3′ | 5′-AAGCAACAGCCGTCAT-3′ |
| GAPDH | 5′-GCAAGTTCAACGGCACAG-3′ | 5′-GCCAGTAGACTCCACGACAT-3′ |
Western blotting analysis
H9C2 cells were washed with cold PBS three times and lysed on ice using RIPA lysis buffer (Dingguo Changsheng Biotechnology, China) containing 1% protease inhibitor (Nanjing Jiancheng Biochemical Reagent) for 10 min. The supernatant was collected after centrifugation at 12,000 g for 10 min, and the protein concentration was measured with a BCA Protein Assay Kit (Life Technologies, USA). Samples were combined with sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled for 15 min, stored at – 20 °C until needed, and 15 μg of each protein sample was separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Bio-Rad, California). After blocking with 5% non-fat dry milk in Tris-buffered saline and Tween 20 (TBST) buffer for 2 h, the membrane was incubated overnight at 4 °C with primary antibodies including anti-CRYAB, anti-Hsp27, anti-Hsp47, anti-Hsp70, anti-Hsp90, anti-Hsp110 (ENZO, USA), anti-Hsp60 (CST, USA) and GAPDH (Abcam, USA). Membranes were washed six times with TBST for 5 min each time, then incubated for 2 h with the corresponding peroxidase-conjugated goat IgG antibody (Boster, China). Membranes were washed six times with TBST for 5 min each time, enhanced chemiluminescence detection regents (Thermo, USA) were added and detection was performed using an ImageQuant LAS4000 digital imaging system (GE Healthcare, Japan). The intensity of scanned bands was determined using Quantity One.
Statistical analysis
All experiments were performed in triplicate (n = 3), and data are presented as mean ± standard deviation (SD). Differences between experimental groups were analysed by one-way analysis of variance (ANOVA) with the least significant difference (LSD) multiple comparison test using SPSS software v.20.0 (IBM, Armonk, NY). Statistical significance was assumed at p < 0.05 (* or #) or p < 0.01(** or ##).
Results
Cell viability
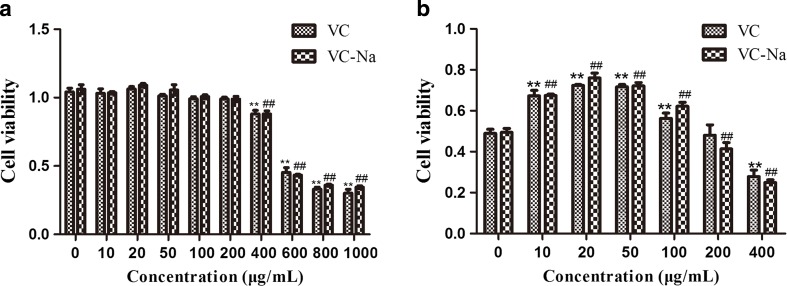
H9C2 cell viability following treatment with vitamin C and vitamin C-Na is shown in Fig. 1a. Under normal culture conditions, 200 μg/ml vitamin C or vitamin C-Na had no effect on H9C2 cells, but a dose of 400 μg/ml lowered cell viability significantly (p < 0.01), and addition of 600 μg/ml VC or VC-Na reduced cell viability by ~ 50%. The effects of VC and VC-Na on H9C2 cells suffering heat stress for 5 h are shown in Fig. 1b. A 10–100 μg/ml concentration increased cell viability (p < 0.01), and a dose of 20 μg/ml enhanced viability ~ 1.5-fold under heat stress conditions. However, when the concentration was increased to 200 μg/ml, the protective effects disappeared and a dose of 400 μg/ml was injurious to cells. In summary, 20 μg/ml of vitamin C and vitamin C-Na was optimal and used in subsequent protection experiments.
Fig. 1.
Viability of H9C2 cells. a Cell viability upon addition of various concentrations of vitamin C (VC) and vitamin C-Na (VC-Na) without heat stress. Addition of 200 μg/ml vitamin C and vitamin C-Na had no effect on H9C2 cells, but 400 μg/ml reduced cell viability. b Cell viability upon addition of various concentrations of VC and VC-Na following heat stress for 5 h. Addition of between 10 and 100 μg/ml increased cell viability, but 20 μg/ml resulted in the largest increase (~ 1.5-fold). *p < 0.05, **p < 0.01compared with 0 h in the control group; #p < 0.05, ##p < 0.01 means supplements group compared with the control group or compared between the two supplements at the same timepoint
Histological analysis of H9C2 cells
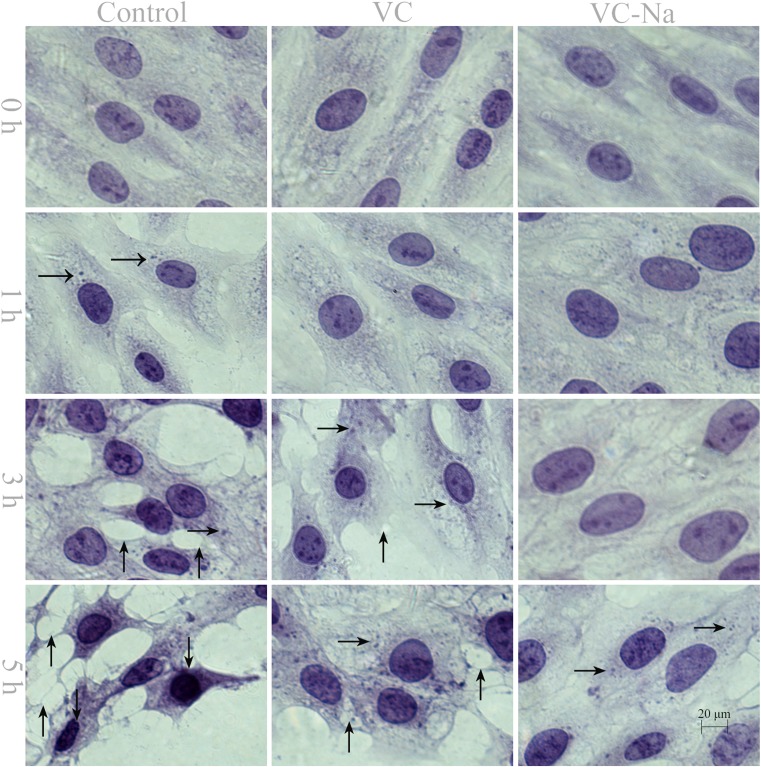
Pathological cell lesions occurred when H9C2 cells were exposed to heat stress (Fig. 2). Normal H9C2 cells are spindle-shaped, but heat stress causes them to adopt a polygon configuration, and the intercellular space becomes wider than normal. In the control group, H9C2 cells exhibited granular degeneration when suffering from heat stress for 1 h, vacuolar degeneration after 3 h and karyopyknosis after 5 h. By contrast, in the VC and VC-Na groups, only slight granular degeneration appeared after heat stress for 1 h, less vacuolar degeneration was observed after 3 h and karyopyknosis was evident after 5 h.
Fig. 2.
Pathological lesions in H9C2 cells following heat stress for 0, 1, 3 and 5 h (bar = 20 μm) shown by haematoxylin and eosin staining. Heat exposure used granular degeneration (rightwards arrow) after 1 h, vacuolar degeneration (upwards arrow) after 3 h and karyopyknosis (downwards arrow) after 5 h in the control group. Supplementation with vitamin C or vitamin C-Na resulted in less granular degeneration and vacuolar degeneration after 1 and 3 h, and no karyopyknosis after 5 h
TEM analysis of H9C2 cells
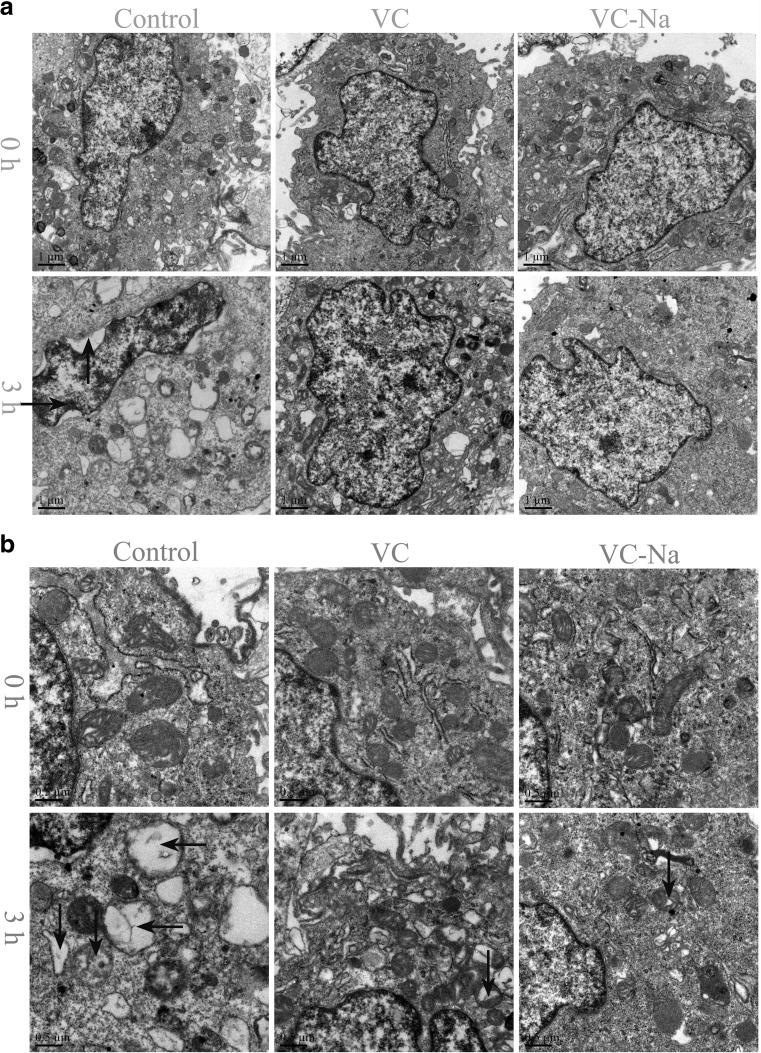
The results of TEM analysis are shown in Fig. 3a, and mitochondrial changes are shown in Fig. 3b. Without heat stress, the VC and VC-Na groups displayed no visible differences compared with the control group; chromatin was uniformly distributed in the nucleus, no gaps were present between the cytoplasm and nucleus and mitochondrial cristae were intact and clearly visible. However, when H9C2 cells were subjected to heat stress for 3 h, the control group presented condensed and marginalised metachromatin, nucleocytoplasmic separation with many gaps between the cytoplasm and nucleus and most mitochondrial cristae were partly or fully disintegrated to form vacuoles. By contrast, VC and VC-Na groups presented no metachromatin marginalisation, no nucleocytoplasmic separation and less mitochondrial cristae disintegration.
Fig. 3.
Transmission electron microscopy (TEM) of H9C2 cells following heat stress for 0 and 3 h. a Changes in nuclei (bar = 1 μm) and b mitochondria (bar = 0.5 μm). Exposure to heat stress for 3 h caused metachromatin condensation and marginalisation (rightwards arrow), nucleocytoplasmic separation (upwards arrow), mitochondrial conversion to vacuoles (leftwards arrow) and mitochondrial cristae disintegration (downwards arrow) in control cells. Supplementation with vitamin C or vitamin C-Na prevented metachromatin marginalisation and nucleocytoplasmic separation, and decreased mitochondrial cristae disintegration
Measurement of apoptosis, LDH, MDA and SOD
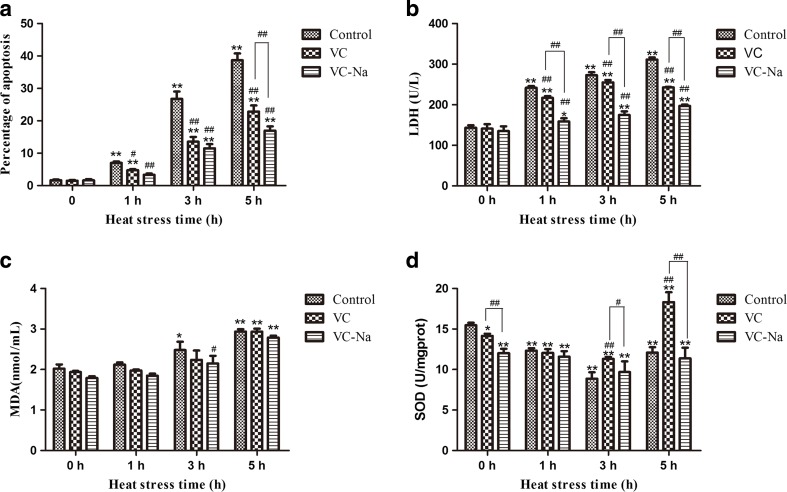
Figure 4 shows the results of measurement of LDH, MDA and SOD. Supplementation with vitamin C and vitamin C-Na for 16 h had no significant influence on apoptosis, LDH or MDA, but SOD activity was significantly reduced about 8.6% for VC (p < 0.05) and about 22.3% lower for VC-Na (p < 0.01). In the control group, when cells suffered increasing heat stress, apoptosis, LDH and MDA all increased compared with the 0 h timepoint. However, thermal injury was significantly reduced in the VC group (p < 0.01 at 1, 3 and 5 h for apoptosis and LDH; slight reduction at 1 and 3 h for MDA) and the VC-Na group (p < 0.01 at 1, 3 and 5 h for apoptosis and LDH; p < 0.05 at 3 h and slight reduction at 1 and 5 h for MDA). In addition, compared with the VC group, the VC-Na group displayed a significant reduction at 5 h for apoptosis, and at 1, 3 and 5 h for LDH. Meanwhile, SOD activity in the control group was decreased at 1 and 3 h following heat stress (p < 0.01), and was still lower than the 0 h timepoint at 5 h (p < 0.01). Compared with the control group, SOD activity in the VC group was significantly higher (p < 0.01) at 3 h, and even higher at 5 h (p < 0.01).
Fig. 4.
Cell damage associated with heat stress (HS). a Apoptosis, b LDH and c MDA are increased following heat stress, but this increase is reversed by pre-treatment with vitamin C (VC) and vitamin C-Na (VC-Na). d SOD activity decreases following heat stress, and supplementation with vitamin C and vitamin C-Na decreases basal level SOD activity at 0 h compared to controls. SOD activity is strongly increased following exposure to heat stress for 3 and 5 h, respectively. *p < 0.05, **p < 0.01compared with 0 h in the control group; #p < 0.05, ##p < 0.01 means supplements group compared with the control group or compared between the two supplements at the same timepoint
Measurement of ROS
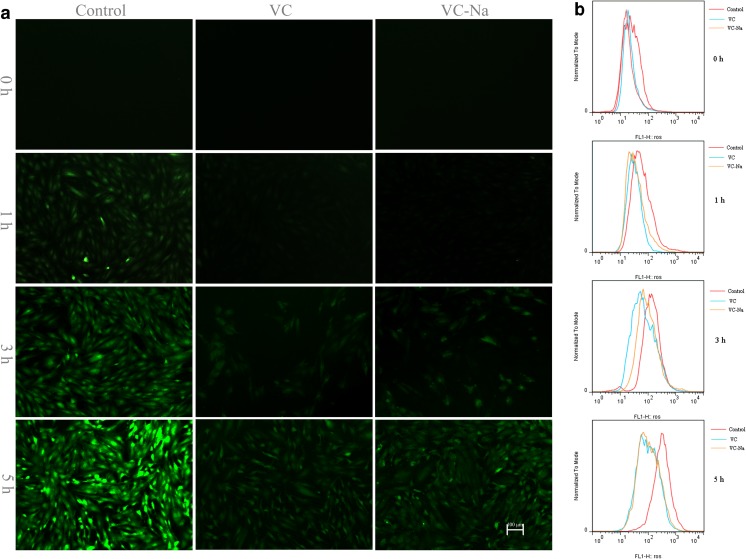
The results of fluorescence microscopy analysis of ROS are shown in Fig. 5a. The intensity of the green fluorescence is proportional to intracellular free radical production, and it was clearly enhanced with continued heat stress, but much weaker in the VC and VC-Na groups than the control group, indicating ROS scavenging. The results of flow cytometry analysis of ROS are shown in Fig. 5b. When suffering heat stress, the peak shifted to the right, indicating increased ROS production, and ROS scavenging in the VC and VC-Na groups was indicated by a peak shift to the left compared with the control group at the same time point.
Fig. 5.
Levels of ROS in H9C2 cells following heat stress for 1, 3 and 5 h after pre-treatment with vitamin C or vitamin C-Na, or without pre-treatment (controls). a Fluorescence microscopy (bar = 100 μm) showing an enhancement in green fluorescence following continued heat stress in controls, and much weaker fluorescence in the vitamin C group and vitamin C-Na groups. b Flow cytometry showing a peak shift to the right following heat stress, and a peak shift to the left in cells supplemented with vitamin C or vitamin C-Na
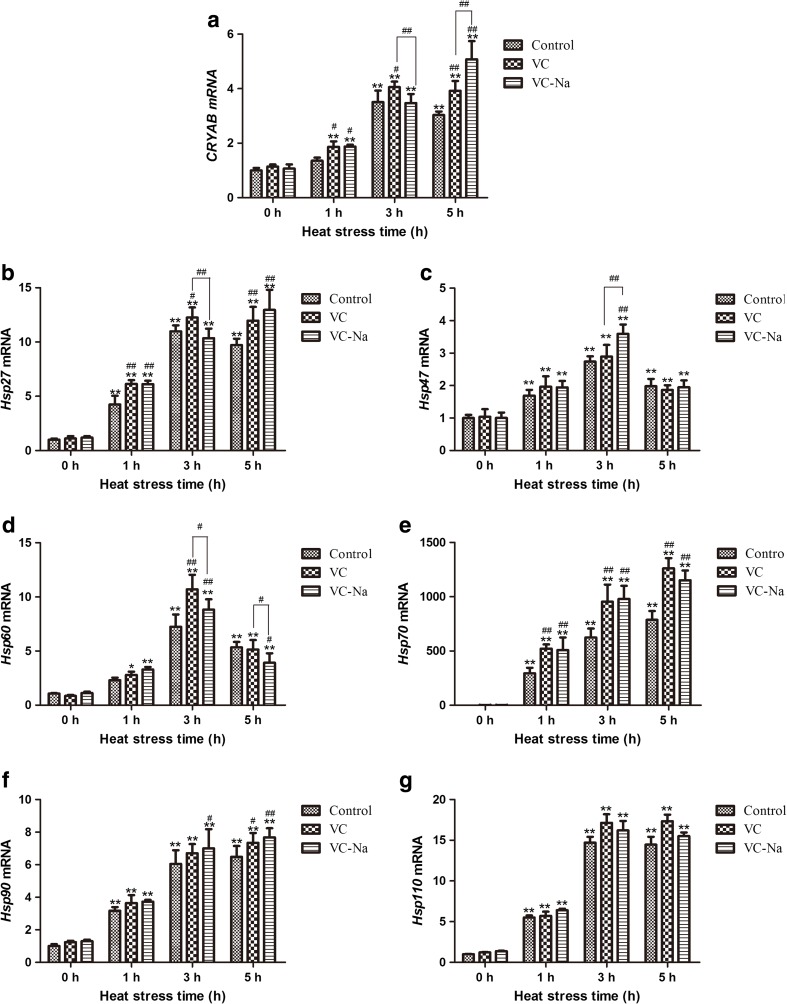
Real-time quantitative PCR
Transcription of HSPs was measured relative to the GAPDH housekeeping gene, which did not change in response to heat stress and the data were analysis using the formula as follow: HSP transcriptional level = 2− △ △ Ct . The results are shown in Fig. 6. Transcription of CRYAB, Hsp27, Hsp47, Hsp60, Hsp70, Hsp90 and Hsp110 was not obviously changed by pre-treatment with vitamin C or vitamin C-Na for 16 h in the absence of heat stress. For the control group, heat stress significantly increased the transcription of all HSPs at 1 h (p < 0.01) except CRYAB and Hsp60 compared with levels at 0 h. Upon continued heat stress, transcription of HSPs was further increased at 3 h (p < 0.01), and Hsp70 and Hsp90 were still upregulated at 5 h, although transcription of other HSPs had a lesser degree compared to 3 h by this timepoint. Pre-treatment with vitamin C and vitamin C-Na led to similar HSP transcriptional changes to those observed in the control group except for CRYAB and Hsp27, which were still induced at 5 h for the VC-Na group. However, compared with the control group, only CRYAB, Hsp27, Hsp60 and Hsp70 mRNA levels were comparable, and all were induced, especially at 3 h.
Fig. 6.
Transcription of CRYAB, Hsp27, Hsp47, Hsp60, Hsp70, Hsp90, Hsp110 detected using the RT-PCR method relative to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Heat stress induces transcription of HSPs at 1 and 3 h. Upon continued heat stress, transcription of Hsp70 and Hsp90 were still upregulated at 5 h, although transcription of other HSPs had a lesser degree compared to 3 h by this timepoint. Following supplementation with vitamin C or vitamin C-Na, only transcription of CRYAB, Hsp27, Hsp60 and Hsp70 is comparable with controls, and all were induced at 3 h. *p < 0.05, **p < 0.01compared with 0 h in the control group; #p < 0.05, ##p < 0.01 means supplements group compared with the control group or compared between the two supplements at the same timepoint
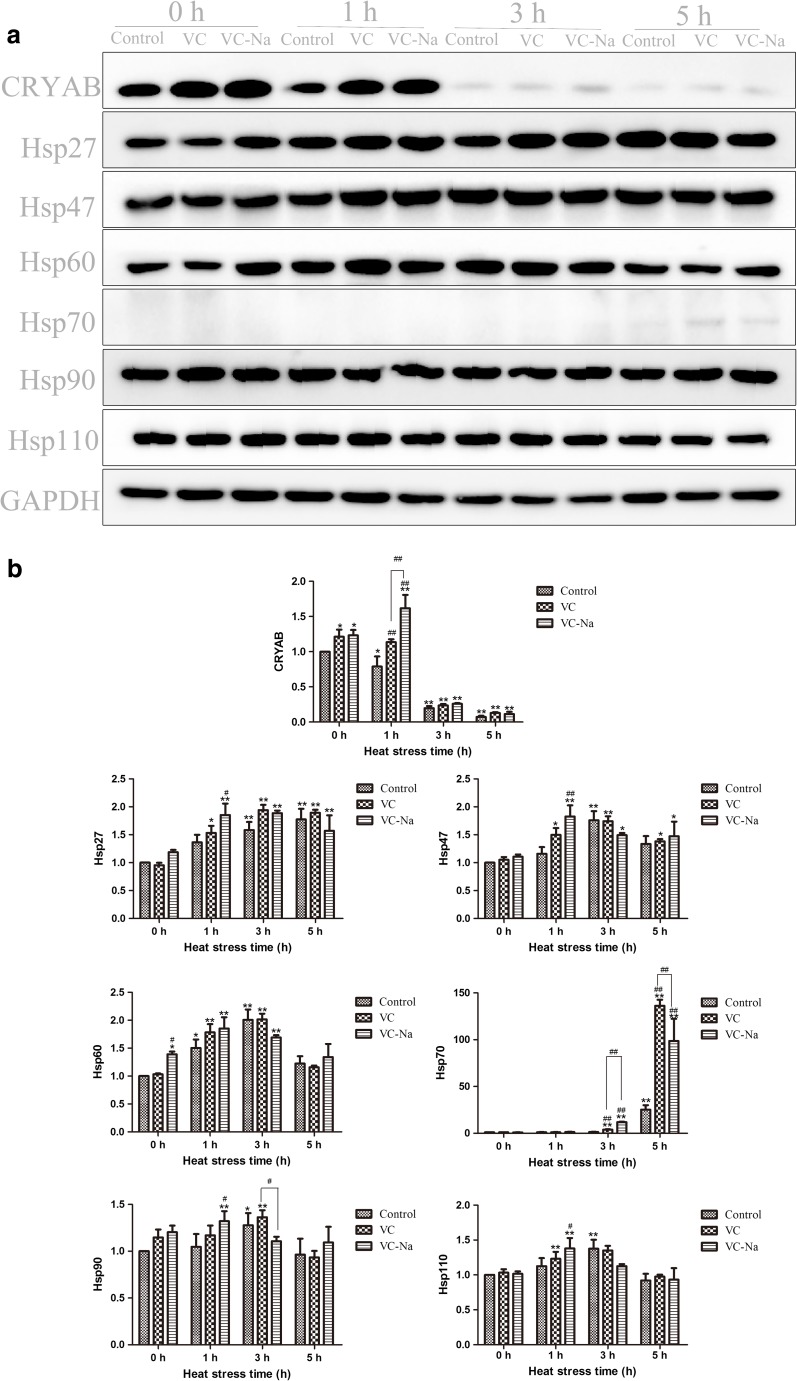
Western blotting analysis
Protein bands were scanned using Quantity One software and the HSP protein levels were measured by normalising against the GAPDH housekeeping protein with the following formula: . The results were shown in Fig. 7. Supplementation with vitamin C or vitamin C-Na did not induce any obvious changes in HSP protein levels except for CRYAB (p < 0.05 in both VC and VC-Na groups) and Hsp60 (p < 0.05 in the VC-Na group). In the control group, when cells suffered heat stress, expression of CRYAB was decreased at 1 h (p < 0.05) and was even lower at 3 and 5 h (p < 0.01) compared with the 0 h timepoint. However, other HSPs were increased. Hsp27 and Hsp70 were increased from 1 to 5 h, and levels were significantly elevated between 3 and 5 h (p < 0.01). Hsp47, Hsp60, Hsp90 and Hsp110 were induced at 1 and 3 h, but levels had begun to decrease at 5 h. Compared with the control group, the VC and VC-Na groups displayed a similar trend in HSP protein levels upon suffering heat stress, but only for CRYAB or Hsp70 was compared; vitamin C and vitamin C-Na significantly induced CRYAB expression at 1 h (p < 0.01), and upregulated Hsp70 at 3 and 5 h (p < 0.01).
Fig. 7.
CRYAB, Hsp27, Hsp47, Hsp60, Hsp70, Hsp90 and Hsp110 protein levels detected using Western blotting relative to the housekeeping protein GAPDH. Heat stress decreased expression of CRYAB, but induced other HSPs. Hsp27 and Hsp70 were increased from 1 to 5 h, Hsp47, Hsp60, Hsp90 and Hsp110 were induced at 1 and 3 h, but levels had begun to decrease at 5 h. Following supplementation with vitamin C or vitamin C-Na, only for CRYAB or Hsp70 was compared with controls; vitamin C and vitamin C-Na significantly induced CRYAB expression at 0 and 1 h, and upregulated Hsp70 at 3 and 5 h. *p < 0.05, **p < 0.01compared with 0 h in the control group; #p < 0.05, ##p < 0.01 means supplements group compared with the control group or compared between the two supplements at the same timepoint
Discussion
Heat stress can lead to cell membrane lipid peroxidation, DNA destruction and protein oxidative damage, all of which can result from enhanced ROS generation and disturb the oxidant/antioxidant balance (Lin et al. 2006). A crucial factor in thermal injury is the increase in free radicals produced by metabolic disorders (Lin et al. 2006; Malcolm et al. 2002; Matsumura et al. 2017; Tan et al. 2010). Vitamin C is a natural antioxidant that can help to eliminate free radicals (Imik et al. 2012; Khassaf et al. 2003). For human, vitamin C is widely used for antioxidation, anti-ageing, antioxidative stress toxicity and by athletes to scavenge exercise-induced free radicals (Jaiswal et al. 2017; Khassaf et al. 2003; Morrison et al. 2015). In livestock, vitamin C also is widely used to relieve heat stress-induced losses in productivity and improve meat quality (Ferreira et al. 2015; Imik et al. 2012; Torki et al. 2014). However, the heart protection potential of vitamin C has not been thoroughly investigated using appropriate pathological studies. In the present work, we pre-treated H9C2 cells with vitamin C alone or in combination with sodium bicarbonate to assess protection from heat stress damage and explore the molecular mechanisms.
The protective functions of drugs are generally dose-dependent and time-dependent, and this holds true for vitamin C (Jaiswal et al. 2017; Padayatty and Levine 2016). An important difference between drugs and toxicants is this dose-dependence (Bae et al. 2008; Zhou et al. 2015). The physiology of vitamin C is closely related to its dose (Levine et al. 2011). Under normal conditions, 200 μg/ml vitamin C or vitamin C-Na proved safe for H9C2 cells. However, following exposure to heat stress, 200 μg/ml vitamin C or vitamin C-Na damaged H9C2 cells, as demonstrated by lower cell viability compared with the control group. Within the safe concentration of 100 μg/ml, the protective functions of vitamin C and vitamin C-Na did not increase linearly between 10 and 100 μg/ml, but rather increased initially then decreased with increasing concentration. The reason for this might be related to cell membrane permeability. Cell membranes usually regulate the balance of materials entering or leaving the cell, but heat stress can damage them and increase cell permeability, allowing larger materials to enter the cell (Tan et al. 2010). Thus, under heat stress conditions, the concentration of vitamin C entering the cell could be much higher than in normal conditions. In our experiments, 20 μg/ml vitamin C or vitamin C-Na was found to be the optimal concentration for protecting H9C2 cells against heat stress.
Pathological sectioning and TEM analysis are commonly used to investigate cell lesions (Xu et al. 2017b). Our previous study revealed necrosis in rat myocardium following heat stress for 40 min (Tang et al. 2016b). Pathological lesions in cells can include granular degeneration, vacuolar degeneration and karyopyknosis (Xu et al. 2017b). In the present study, heat stress caused granular degeneration after 1 h in H9C2 cells, vacuolar degeneration after 3 h and karyopyknosis after 5 h. However, pre-treatment with vitamin C or vitamin C-Na decreased granular degeneration and vacuolar degeneration after heat stress for 1 and 3 h, and karyopyknosis was not observed after 5 h. TEM analysis also revealed minimal damage to nuclei and mitochondria following treatment. Indices of apoptosis, LDH and MDA are widely used to investigate oxidative damage (Szczubial 2015; Tan et al. 2010; Yuan et al. 2017). LDH is released from the cytoplasm into the supernatant when cell membrane permeability increases, and MDA levels increase following biofilm lipid peroxidation induced by ROS (Tan et al. 2010). Our current results indicated a continuous increase in apoptosis, LDH and MDA upon increasing duration of heat stress from 1 to 5 h. However, pre-treatment with vitamin C significantly reduced apoptosis, LDH and MDA, and the vitamin C-Na group displayed a significant reduction at 5 h for apoptosis, and at 1, 3 and 5 h for LDH. These results suggest that vitamin C protects H9C2 cells against heat stress damage, and the effects of vitamin C are enhanced by the inclusion of sodium bicarbonate in the pre-treatment.
To explore the underlying mechanisms of the protection to H9C2 cells by vitamin C against heat stress-induced damage, we measured the antioxidation ability of cells, including SOD activity and ROS levels, and investigated the expression of HSPs. ROS and SOD levels reflect the prooxidant and antioxidant balance; ROS are induced by heat stress, which enhances the ability of oxidants and decreases the ability of antioxidants (Lin et al. 2006). In our experiments, the intensity of green fluorescence increased with increasing duration of heat stress, and the peak shifted to the right with increasing heat stress. However, compared with the control group at the same timepoint, green fluorescence was weaker in the vitamin C group and vitamin C-Na groups, and the peak was shifted to the left, indicating less ROS generation under the same heat stress conditions. These results suggest that vitamin C and vitamin C-Na efficiently scavenged heat-induced ROS, consistent with previous studies (Kazim et al. 2002; Morrison et al. 2015; Tan et al. 2010).
SOD is an important endogenous antioxidant enzyme that scavenges ROS, and SOD activity reflects the endogenous antioxidant ability of cells (Tan et al. 2010). Cell damage can decrease SOD activity (Ahmad et al. 2017), as demonstrated following heat stress for 1 and 3 h. However, after further heat stress at 5 h, SOD activity recovered slightly but was still lower than that at 1 h. Surprisingly, compared with the 0 h timepoint in the control group, the SOD activity at 0 h in the vitamin C and vitamin C-Na groups was significantly reduced. This could be because supplementation with vitamin C or vitamin C-Na greatly enhanced exogenous antioxidants, which decreased the need for endogenous antioxidants. Similarly, supplementation with vitamins C and E prevented SOD2 protein expression and lowered total SOD activity in a previous study (Cumming et al. 2014).
Heat shock proteins (HSPs) mediate important endogenous protective mechanisms to assist acclimatisation to changing environments and protect against various stressors such as heat, cold, bacteria, viruses and UV (Garrido et al. 2001; Ruell et al. 2009). Under stress condition, HSPs can be considered an important indicator of the ability of cells to resist damage and adapt to environmental stress (Xu et al. 2017b). Heat stress can induce expression of HSPs to protect cells against thermal injury (Dangi et al. 2015; Sottile and Nadin 2017; Tang et al. 2016b). CRYAB, expressed in most organisms, stabilises the cell cytoskeleton, and has anti-apoptotic activities associated with the cell cycle (Garrido et al. 2001; Tang et al. 2016a; Tang et al. 2016b). Hsp27 is a small HSP that functions as a molecular chaperone and has anti-apoptotic activities that are closely related to the p38 pathway (Shashidharamurthy et al. 2005). Hsp60 is mostly distributed in mitochondria and plays an important role in refolding misfolded proteins under various stress conditions (Song et al. 2016). Importantly, inducing Hsp70 expression can effectively improve cell survival and attenuate stress damage (Sahin et al. 2009; Xu et al. 2017a; Xu et al. 2017b). Heat stress also induces Hsp90, which can help its client proteins mostly associated with cellular pro-survival/anti-apoptotic signal transduction pathway to maintain their correct molecular structure (Chrisostomos et al. 2000; Wang et al. 2017). Hsp110 also performs an important protective function by suppressing protein aggregation induced by stress (Yamagishi et al. 2003). In the present work, six of the seven HSPs (Hsp27, Hsp47, Hsp60, Hsp70, Hsp90 and Hsp110) were upregulated at both the mRNA and protein levels following heat stress while CRYAB was downregulated. The production of HSP induced by heat stress has close relationship with generation of ROS. Several studies have shown that some level of ROS is needed for proper HSF1/HSP activation, and the use of antioxidants (Quercetin or Gingko biloba) can inhibit heat shock response (Dokladny et al. 2006; Westman et al. 2000; Zanini et al. 2007). However, induction was not increased linearly with increasing heat stress in all cases; when heat stress was continued for 5 h, transcription of Hsp70 and Hsp90 began to decrease, and the same was true for Hsp27 and Hsp90 protein levels. The reason for this may be associated with a decrease in the ability of cells to the thermal damage accrued. Surprisingly, CRYAB expression decreased following heat stress, although this is consistent with the observed decrease in CRYAB in rat heart following heat exposure for 20, 60, 80 and 100 min reported previously (Tang et al. 2016a). The underlying reasons clearly require further study. CRYAB acts as a molecular chaperone to suppress cellular apoptosis, and it combines with F-actin to maintain cytoskeletal structure and regulate the cell cycle (Bakthisaran et al. 2015). Thus, we hypothesise that the observed decrease in CRYAB expression may reflect overutilisation/consumption.
Pre-treatment with 20 μg/ml vitamin C or vitamin C-Na for 16 h induced basal expression of CRYAB and Hsp60 at 0 h, and increased expression of CRYAB and Hsp70 further upon heat stress, which may contribute to the protective functions, consistent with previous reports (Camins et al. 1999; Khassaf et al. 2003). However, supplementation with vitamin C could decrease the expression of Hsp70, possibly because vitamin C scavenges ROS, thereby lowering ROS levels and weakening stress-induced Hsp70 expression (Mahmoud et al. 2004). We believe these differing responses to vitamin C are closely related to the concentration of vitamin C and the incubation time. When the vitamin C concentration and incubation time are below the threshold to initiate stress, vitamin C may only perform an antioxidant function without induction of HSPs. However, when the vitamin C concentration and incubation time are sufficient to initiate slight stress without excessive damage, vitamin C may act as an antioxidant and upregulate HSPs. A 20 μg/ml pre-treatment of vitamin C for 16 h appeared to induce slight stress in H9C2 cells in the present study.
Conclusion
Supplementation with 20 μg/ml vitamin C or vitamin C-Na protected H9C2 cells from heat damage by increasing the antioxidant ability and inducing expression of CRYAB and Hsp70.
Acknowledgements
The current study was supported by grants from the National Natural Science Foundation of China (grant no. 31672520), the Fundamental Research Funds for the Central Universities (grant no. KJQN201709), the National Natural Science Foundation of China (grant no. 31602027), the National Natural Science Foundation of China (grant no. 31372403), Jiangsu Natural Science Foundation of China (grant no. BK20160732), China Postdoctoral Science Foundation (2016M591860) and the Priority Academic Program Development of Jiangsu Higher Education Institutions, Graduate Research and Innovation Projects in Jiangsu Province.
References
- Aditi A, Graham DY. Vitamin C, gastritis, and gastric disease: a historical review and update. Dig Dis Sci. 2012;57(10):2504–2515. doi: 10.1007/s10620-012-2203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad G, Agarwal A, Esteves SC, Sharma R, Almasry M, Al-Gonaim A, Alhayaza G, Singh N, Al Kattan L, Sannaa WM, Sabanegh E (2017) Ascorbic acid reduces redox potential in human spermatozoa subjected to heat-induced oxidative stress. Andrologia e12773:1–8. 10.1111/and.12773 [DOI] [PubMed]
- Ahmad T, Mushtaq T, Khan MA, Babar ME, Yousaf M, Hasan ZU, Kamran Z. Influence of varying dietary electrolyte balance on broiler performance under tropical summer conditions. J Anim Physiol Anim Nutr. 2009;93(5):613–621. doi: 10.1111/j.1439-0396.2008.00840.x. [DOI] [PubMed] [Google Scholar]
- Aitken-Buck HM, Lamberts RR. To the heart of activation heat. J Physiol. 2017;595(14):4577–4578. doi: 10.1113/JP274582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia YA, Abd El-Hamid Ael H, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, Sahin K, Abou-Shehema BM. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus. 2016;5(1):1619–1631. doi: 10.1186/s40064-016-3304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae ON, Lim KM, Han JY, Jung BI, Lee JY, Noh JY, Chung SM, Lee MY, Lee JY, Chung JH. U-shaped dose response in vasomotor tone: a mixed result of heterogenic response of multiple cells to xenobiotics. Toxicological sciences : an Official Journal of the Society of Toxicology. 2008;103(1):181–190. doi: 10.1093/toxsci/kfn023. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao Ch M. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Camins C, Diezfernandez C, Prieo P. Cell-surface expression of heat shock proteins in dog neutrophils after oxidative stress. Toxicilogy in Vitro. 1999;13(3):437–443. doi: 10.1016/S0887-2333(99)00012-0. [DOI] [PubMed] [Google Scholar]
- Chand N, Naz S, Khan A, Khan S, Khan RU. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int J Biometeorol. 2014;58(10):2153–2157. doi: 10.1007/s00484-014-0815-7. [DOI] [PubMed] [Google Scholar]
- Chrisostomos P, Barry P, Shahzad C, Giuliano S, Ronan O, John EL, Smark R, Peter WP, Laurence HP. The ATPase cycle of Hsp90 drives a molecular 'clamp' via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier RJ, Collier JL, Rhoads RP, Baumgard LH. Invited review: genes involved in the bovine heat stress response. J Dairy Sci. 2008;91(2):445–454. doi: 10.3168/jds.2007-0540. [DOI] [PubMed] [Google Scholar]
- Crandall, C.G., Wilson, T.E., 2014. Human cardiovascular responses to passive heat stress. 17-43 [DOI] [PMC free article] [PubMed]
- Cui J, Sinoway LI. Cardiovascular responses to heat stress in chronic heart failure. Current Heart Failure Reports. 2014;11(2):139–145. doi: 10.1007/s11897-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming KT, Raastad T, Holden G, Bastani NE, Schneeberger D, Paronetto MP, Mercatelli N, Ostgaard HN, Ugelstad I, Caporossi D, Blomhoff R, Paulsen G. Effects of vitamin C and E supplementation on endogenous antioxidant systems and heat shock proteins in response to endurance training. Physiological Reports. 2014;2:1–14. doi: 10.14814/phy2.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi SS, Gupta M, Dangi SK, Chouhan VS, Maurya VP, Kumar P, Singh G, Sarkar M. Expression of HSPs: an adaptive mechanism during long-term heat stress in goats (Capra Hircus) Int J Biometeorol. 2015;59(8):1095–1106. doi: 10.1007/s00484-014-0922-5. [DOI] [PubMed] [Google Scholar]
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G204–G212. doi: 10.1152/ajpgi.00401.2005. [DOI] [PubMed] [Google Scholar]
- Ferreira IB, Matos Junior JB, Sgavioli S, Vicentini TI, Morita VS, Boleli IC. Vitamin C prevents the effects of high rearing temperatures on the quality of broiler thigh meat1. Poult Sci. 2015;94(5):841–851. doi: 10.3382/ps/pev058. [DOI] [PubMed] [Google Scholar]
- Garrido C, Gurbuxani S, Ravagnan L, Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286(3):433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- Grizzle J, Iheanacho M, Saxton A, Broaden J. Nutritional and environmental factors involved in egg shell quality of laying hens. Br Poult Sci. 1992;33(4):781–794. doi: 10.1080/00071669208417520. [DOI] [PubMed] [Google Scholar]
- Imik H, Ozlu H, Gumus R, Atasever MA, Urcar S, Atasever M. Effects of ascorbic acid and alpha-lipoic acid on performance and meat quality of broilers subjected to heat stress. Br Poult Sci. 2012;53(6):800–808. doi: 10.1080/00071668.2012.740615. [DOI] [PubMed] [Google Scholar]
- Jaiswal SK, Gupta VK, Ansari MD, Siddiqi NJ, Sharma B. Vitamin C acts as a hepatoprotectant in carbofuran treated rat liver slices in vitro. Toxicol Rep. 2017;4:265–273. doi: 10.1016/j.toxrep.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Ko YH, Moon YS, Sohn SH. Effects of vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian Australas J Anim Sci. 2014;27(5):749–756. doi: 10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim S, Osman K, Nurhan S, Mustafa S. Effects of vitamin C and vitamin E on lipid peroxidation status. Serum Hormone, Metabolite, and Mineral Concentrations of Japanese Quails Reared under Heat Stress Int J Vitam. 2002;72:91–100. doi: 10.1024/0300-9831.72.2.91. [DOI] [PubMed] [Google Scholar]
- Khassaf M, Mcardle A, Esanu C, Vasilaki A, Mcardle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol. 2003;549(2):645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore A, Sodhi M, Kumari P, Mohanty AK, Sadana DK, Kapila N, Khate K, Shandilya U, Kataria RS, Mukesh M. Peripheral blood mononuclear cells: a potential cellular system to understand differential heat shock response across native cattle (Bos indicus), exotic cattle (Bos taurus), and riverine buffaloes (Bubalus bubalis) of India. Cell Stress Chaperones. 2014;19(5):613–621. doi: 10.1007/s12192-013-0486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Decuypere E, Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp Biochem Physiol A Mol Integr Physiol. 2006;144(1):11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Liu F, Cottrell JJ, Furness JB, Rivera LR, Kelly FW, Wijesiriwardana U, Pustovit RV, Fothergill LJ, Bravo DM, Celi P, Leury BJ, Gabler NK, Dunshea FR. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp Physiol. 2016;101(7):801–810. doi: 10.1113/EP085746. [DOI] [PubMed] [Google Scholar]
- Mujahid A. Nutritional strategies to maintain efficiency and production of chickens under high environmental temperature. J Poult Sci. 2011;48(3):145–154. doi: 10.2141/jpsa.010115. [DOI] [Google Scholar]
- Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. Effect of ascorbic acid and acute heat exposure on heat shock protein 70 expression by young white leghorn chickens. Comparative biochemistry and physiology Toxicology & pharmacology : CBP. 2003;136(4):329–335. doi: 10.1016/j.cca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Mahmoud KZ, Edens FW, Eisen EJ, Havenstein GB. Ascorbic acid decreases heat shock protein 70 and plasma corticosterone response in broilers (Gallus gallus domesticus) subjected to cyclic heat stress. Comp Biochem Physiol B: Biochem Mol Biol. 2004;137(1):35–42. doi: 10.1016/j.cbpc.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Malcolm JJ, Sergio P, Juan B, Richard B, Harald C, Ruan ME, Jacoba F, Helen RG. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Asp Med. 2002;23:209–285. doi: 10.1016/S0098-2997(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Matsumoto H, Hayakawa Y. Heat stress hardening of oriental armyworms is induced by a transient elevation of reactive oxygen species during sublethal stress. Arch Insect Biochem Physiol. 2017;96:1–10. doi: 10.1002/arch.21421. [DOI] [PubMed] [Google Scholar]
- Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, Wadley GD. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009;88(10):2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Vitamin C: the known and the unknown and goldilocks. Oral Dis. 2016;22(6):463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda AK, Ramarao SV, Raju MV, Chatterjee RN. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of white leghorn layers under tropical summer conditions. Br Poult Sci. 2008;49(5):592–599. doi: 10.1080/00071660802337233. [DOI] [PubMed] [Google Scholar]
- Rafiee F, Mazhari M, Ghoreishi M, Esmaeilipour O. Effect of lemon verbena powder and vitamin C on performance and immunity of heat-stressed broilers. J Anim Physiol Anim Nutr. 2016;100(5):807–812. doi: 10.1111/jpn.12457. [DOI] [PubMed] [Google Scholar]
- Redmond SB, Tell RM, Coble D, Mueller C, Palic D, Andreasen CB, Lamont SJ. Differential splenic cytokine responses to dietary immune modulation by diverse chicken lines. Poult Sci. 2010;89(8):1635–1641. doi: 10.3382/ps.2010-00846. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Thompson MW, Hoffman KM. Heat shock proteins as an aid in the treatment and diagnosis of heat stroke. J Therm Biol. 2009;34(1):1–7. doi: 10.1016/j.jtherbio.2008.09.004. [DOI] [Google Scholar]
- Sahin N, Onderci M, Sahin K, Gursu MF, Smith MO. Ascorbic acid and melatonin reduce heat-induced performance inhibition and oxidative stress in Japanese quails. Br Poult Sci. 2004;45(1):116–122. doi: 10.1080/00071660410001668941. [DOI] [PubMed] [Google Scholar]
- Sahin N, Tuzcu M, Orhan C, Onderci M, Eroksuz Y, Sahin K. The effects of vitamin C and E supplementation on heat shock protein 70 response of ovary and brain in heat-stressed quail. Br Poult Sci. 2009;50(2):259–265. doi: 10.1080/00071660902758981. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ramesh K, Hyder I, Uniyal S, Yadav VP, Panda RP, Maurya VP, Singh G, Kumar P, Mitra A, Sarkar M. Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Rumin Res. 2013;112(1-3):216–223. doi: 10.1016/j.smallrumres.2012.12.008. [DOI] [Google Scholar]
- Shashidharamurthy R, Koteiche HA, Dong J, Mchaourab HS. Mechanism of chaperone function in small heat shock proteins: dissociation of the HSP27 oligomer is required for recognition and binding of destabilized T4 lysozyme. J Biol Chem. 2005;280(7):5281–5289. doi: 10.1074/jbc.M407236200. [DOI] [PubMed] [Google Scholar]
- Sinkalu VO, Ayo JO (2016) Combined effects of retinol, ascorbic acid and alpha-tocopherol on diurnal variations in rectal temperature of Black Harco pullets subjected to heat stress. Int J Biometeorol. 10.1007/s00484-00016-01157-00484 [DOI] [PubMed]
- Song E, Tang S, Xu J, Yin B, Bao E, Hartung J. Lenti-siRNA Hsp60 promote bax in mitochondria and induces apoptosis during heat stress. Biochem Biophys Res Commun. 2016;481(1-2):125–131. doi: 10.1016/j.bbrc.2016.10.153. [DOI] [PubMed] [Google Scholar]
- Sottile ML, Nadin SB (2017) Heat shock proteins and DNA repair mechanisms: an updated overview. Cell Stress Chaperones. 10.1007/s00484-00016-01157-00484 [DOI] [PMC free article] [PubMed]
- Szczubial M. Effect of supplementation with vitamins E, C and beta-carotene on antioxidative/oxidative status parameters in sows during the postpartum period. Pol J Vet Sci. 2015;18(2):299–305. doi: 10.1515/pjvs-2015-0039. [DOI] [PubMed] [Google Scholar]
- Tan GY, Yang L, Fu YQ, Feng JH, Zhang MH. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult Sci. 2010;89(1):115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Tang S, Chen H, Cheng Y, Nasir MA, Kemper N, Bao E. Expression profiles of heat shock protein 27 and alphaB-crystallin and their effects on heat-stressed rat myocardial cells in vitro and in vivo. Mol Med Rep. 2016;13(2):1633–1638. doi: 10.3892/mmr.2015.4693. [DOI] [PubMed] [Google Scholar]
- Tang S, Yin B, Song E, Chen H, Cheng Y, Zhang X, Bao E, Hartung J. Aspirin upregulates alphaB-crystallin to protect the myocardium against heat stress in broiler chickens. Sci Rep. 2016;6(1):37273. doi: 10.1038/srep37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torki M, Zangeneh S, Habibian M. Performance, egg quality traits, and serum metabolite concentrations of laying hens affected by dietary supplemental chromium picolinate and vitamin C under a heat-stress condition. Biol Trace Elem Res. 2014;157(2):120–129. doi: 10.1007/s12011-013-9872-8. [DOI] [PubMed] [Google Scholar]
- Tower J. Heat shock proteins and Drosophila aging. Exp Gerontol. 2011;46(5):355–362. doi: 10.1016/j.exger.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Sun GB, Du YY, Tian Y, Liao P, Liu XS, Ye JX, Sun XB. Myricitrin protects cardiomyocytes from hypoxia/reoxygenation injury: involvement of heat shock protein 90. Front Pharmacol. 2017;8:353. doi: 10.3389/fphar.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman J, Drieu K, Sharma H. Antioxidant compounds EGB-761 and BN-52021 attenuate heat shock protein (HSP72 kD) response, edema and cell changes following hyperthermic brain injury. Amino Acids. 2000;19(1):339–350. doi: 10.1007/s007260070065. [DOI] [PubMed] [Google Scholar]
- Xu J, Tang S, Song E, Yin B, Bao E. Inhibition of heat shock protein 70 intensifies heat-stressed damage and apoptosis of chicken primary myocardial cells in vitro. Mol Med Rep. 2017;15(5):2881–2889. doi: 10.3892/mmr.2017.6337. [DOI] [PubMed] [Google Scholar]
- Xu J, Tang S, Song E, Yin B, Wu D, Bao E. Hsp70 expression induced by co-enzyme Q10 protected chicken myocardial cells from damage and apoptosis under in vitro heat stress. Poult Sci. 2017;96(5):1426–1437. doi: 10.3382/ps/pew496. [DOI] [PubMed] [Google Scholar]
- Xu J, Tang S, Yin B, Sun J, Song E, Bao E. Co-enzyme Q10 and acetyl salicylic acid enhance Hsp70 expression in primary chicken myocardial cells to protect the cells during heat stress. Mol Cell Biochem. 2017;435(1-2):73–86. doi: 10.1007/s11010-017-3058-1. [DOI] [PubMed] [Google Scholar]
- Yörük MA, Gül M, Hayirli A, Laçin E. Laying performance and egg quality of hens supplemented with Humate and sodium bicarbonate during the late laying period. J Appl Anim Res. 2004;26(1):17–21. doi: 10.1080/09712119.2004.9706498. [DOI] [Google Scholar]
- Yamagishi N, Ishihara K, Saito Y, Hatayama T. Hsp105 but not Hsp70 family proteins suppress the aggregation of heat-denatured protein in the presence of ADP. FEBS Lett. 2003;555(2):390–396. doi: 10.1016/S0014-5793(03)01292-4. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Pan S, Yang S-L, Liu Y-L, Xu Q-M. Antioxidant and cardioprotective effects of Ilex cornuta on myocardial ischemia injury. Chin J Nat Med. 2017;15(2):94–104. doi: 10.1016/S1875-5364(17)30025-0. [DOI] [PubMed] [Google Scholar]
- Zanini C, Giribaldi G, Mandili G, Carta F, Crescenzio N, Bisaro B, Doria A, Foglia L, Di Montezemolo LC, Timeus F, Turrini F. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and Ewing's sarcoma cell lines. J Neurochem. 2007;103(4):1344–1354. doi: 10.1111/j.1471-4159.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- Zhou H, Malik MA, Arab A, Hill MT, Shikanov A. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLoS One. 2015;10(10):e0140205. doi: 10.1371/journal.pone.0140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I, Al-Aqil A, Omar AR, Sazili AQ, Rajion MA. Crating and heat stress influence blood parameters and heat shock protein 70 expression in broiler chickens showing short or long tonic immobility reactions. Poult Sci. 2009;88(3):471–476. doi: 10.3382/ps.2008-00287. [DOI] [PubMed] [Google Scholar]