Abstract
Ubiquitin is a 76-amino acid protein that is highly conserved among higher and lower eukaryotes. The polyubiquitin gene UBI4 encodes a unique precursor protein that contains five ubiquitin repeats organized in a head-to-tail arrangement. Although the involvement of the yeast polyubiquitin gene UBI4 in the stress response was reported long ago, there are no reports regarding the underlying mechanism of this involvement. In this study, we used UBI4-deletion and UBI4-overexpressing yeast strains as models to explore the potential mechanism by which UBI4 protects yeast cells against paraquat-induced oxidative stress. Here, we show that ubi4Δ cells exhibit oxidative stress, an apoptotic phenotype, and a decreased replicative lifespan. Additionally, the reduced resistance of ubi4Δ cells to paraquat that was observed in this study was rescued by overexpression of either the catalase or the mitochondrial superoxide dismutase SOD2. We also demonstrated that only SOD2 overexpression restored the replicative lifespan of ubi4Δ cells. In contrast to the case of ubi4Δ cells, UBI4 overexpression in wild-type yeast increases the yeast’s resistance to paraquat, and this overexpression is associated with large pools of expressed ubiquitin and increased levels of ubiquitinated proteins. Collectively, these findings highlight the role of the polyubiquitin gene UBI4 in apoptosis and implicate UBI4 as a modulator of the replicative lifespan.
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0860-3) contains supplementary material, which is available to authorized users.
Keywords: Yeast, UBI4, Apoptosis, Replicative lifespan
Introduction
Ubiquitin (Ub) is a 76-amino acid protein that is highly conserved in higher and lower eukaryotes and exists in cells as either a free protein or covalently joined via its carboxyl-terminal Gly residue to a substrate protein (termed ubiquitination). Ubiquitination is well known for its role in protein degradation, protein quality control and cell division (Finley and Chau 1991).
Four different genes encode ubiquitin in the yeast Saccharomyces cerevisiae: UBI1, UBI2, UBI3 and UBI4. Interestingly, UBI1, UBI2 and UBI3 encode hybrid proteins in which ubiquitin is fused to unrelated amino acid sequences (UBI1 and UBI2 encode the same 52-residue tails, whereas UBI3 encodes a different, 76-residue tail), while UBI4 encodes a polyubiquitin precursor protein that contains five ubiquitin repeats that are arranged in a head-to-tail sequence. This polyubiquitin precursor protein is rapidly cleaved into ubiquitin monomers after its synthesis (Fraser et al. 1991; Ozkaynak et al. 1987). The unique precursor protein structure of UBI4 suggests that it plays a special role in biological processes.
UBI1, UBI2 and UBI3 are strongly expressed in exponentially growing yeast cells, whereas the UBI4 gene is relatively weakly expressed (Fraser et al. 1991). Previous studies suggested that UBI4-deleted mutants are hypersensitive to stress, such as high temperature, starvation, oxidative stress and the presence of amino acid analogues, and that the levels of the UBI4 transcript were markedly increased under the stressed conditions, but the underlying mechanism of these behaviours has not yet been reported (Chen and Piper 1995; Finley et al. 1987; Watt and Piper 1997).
We report here that deletion of the yeast polyubiquitin gene UBI4 results in reduced resistance to the oxidizing agent paraquat (PQ), and this decrease could be rescued by overexpression of catalase and mitochondrial superoxide dismutase SOD2. Ubi4Δ cells exhibit oxidative stress and apoptotic phenotypes as well as a decreased replicative lifespan (RLS). Moreover, overexpressing UBI4 in wild-type yeast increases yeast resistance to PQ, which may be due to large pools of expressed ubiquitin and increased levels of ubiquitinated proteins. These findings highlight the role of the polyubiquitin gene UBI4 in apoptosis and ageing.
Materials and methods
Yeast strains and culture conditions
All the yeast strains that were used in this paper were derived from haploid wild-type BY4742 cells (listed in Table 1).
Table 1.
The S. cerevisiae strains used in this work
| Strain name | Genotype | Comments | Source |
|---|---|---|---|
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | wild-type | Gift from Matt Kaeberlein |
| ubi4Δ | BY4742 ubi4:URA3 | Deletion of UBI4 in BY4742 | This study |
| UBI4OX | BY4742 UBI4OX:HIS3 | Overexpression UBI4 in BY4742 | This study |
| ubi4ΔpAUR123CTT1 | BY4742 ubi4:URA3CTT1OX | pAUR123CTT1 was transformed into ubi4Δ | This study |
| ubi4ΔpAUR123CTA1 | BY4742 ubi4:URA3CTA1OX | pAUR123CTA1 was transformed into ubi4Δ | This study |
| ubi4ΔpAUR123SOD1 | BY4742 ubi4:URA3SOD1OX | pAUR123SOD1 was transformed into ubi4Δ | This study |
| ubi4ΔpAUR123SOD2 | BY4742 ubi4:URA3SOD2OX | pAUR123SOD2 was transformed into ubi4Δ | This study |
| ubi4ΔpAUR123UBI4 | BY4742 ubi4:URA3UBI4OX | pAUR123UBI4 was transformed into ubi4Δ | This study |
| ubi4ΔpAUR123 | BY4742 ubi4:URA3pAUR123 | Empty vector pAUR123 was transformed into ubi4Δ | This study |
Polymerase chain reaction (PCR)-mediated gene disruption was used to generate ubi4Δ mutants (Baudin et al. 1993). First, a gene-specific disruption cassette (containing the selective marker URA3) was generated by PCR using the primers 5′-CTCGAACTCTCCCTCCCACTTTACTTTAACTAATAGATTAGATTGTACTGAGAGTGCAC-3′ and 5′-ATATATATATTGACATAATGAAAATATTGCGAGGACTGACTGTGCGGTATTTCACACCG-3′ and the template plasmid pRS306. Second, the PCR product was purified and transformed into the wild-type strain, which allowed the gene disruption cassette to replace the UBI4 ORF by recombination. Positive clones grew on selective plates (SD-URA), and gene disruption in these colonies was further confirmed by PCR (Suppl. Fig. 1).
To generate a UBI4 overexpression yeast strain (UBI4OX), a fragment that started 555 bp upstream from the UBI4 ORF and ended 318 bp downstream from the UBI4 ORF (thus containing the UBI4 ORF) was amplified from wild-type yeast genomic DNA using the primers UBI4-S (5′-TATACTAGTTCATCTTATTCGCGCAGGGC-3′) and UBI4-A (5′-GATGTCGACTTATGTGCGTTTACTGGAGA-3′). These primers included a SpeI site and a SalI site, respectively. The PCR product was then purified and cloned into the vector pRS303. Next, the recombinant plasmid pRS303-UBI4 was digested with Tth111I and transformed into the wild-type strain so that this linearized plasmid could recombine with genomic DNA. Positive clones grew on selective plates (SD-HIS), and these colonies were further confirmed by PCR (Suppl. Fig. 2). This method allowed an extra copy of UBI4 with its endogenous promoter to be integrated into strain BY4742 (Stearns et al. 1990).
Plasmid construction and yeast transformation
The constitutive expression vector pAUR123 (containing the aureobasidin A-resistance gene), which has the ADH1 promoter, was used to overexpress UBI4, CTT1, CTA1, SOD1 and SOD2. The entire ORF of each gene was amplified from wild-type yeast genomic DNA by PCR and then cloned individually into the empty pAUR123. The primers used to amplify these target genes are listed in Table S1.The recombinant plasmids were transformed into the Escherichia coli strain DH5α. DNA sequencing of these plasmids was performed by Life Technologies (Guangzhou, China).
The plasmids pAUR123UBI4, pAUR123CTT1, pAUR123CTA1, pAUR123SOD1 and pAUR123SOD2 were each transformed into ubi4Δ or wild-type cells. The transformants were screened by growing them at 30 °C for 2 days on YPD medium that had been supplemented with 0.2 g/ml aureobasidin A. The presence of the relevant overexpression vector in each of these strains was verified by using PCR.
Growth rate determination
The yeast growth rates were analysed by using a Bioscreen C machine (Growth Curves, USA) (Delaney et al. 2013). First, a single colony of each yeast strain was inoculated into YPD medium and was grown overnight at 30 °C with shaking. Next, overnight cultures were adjusted to an OD600 of 0.1 by diluting with fresh YPD medium or 2 mM PQ-supplemented YPD medium in culture plates, the plates were constantly shaken at 30 °C for more than 2 days, and the optical density (OD) values were recorded at 600 nm every 2 h. This experiment was repeated three times, and the averages were used to generate growth curves. Statistical significance was calculated by using the Friedman test. p < 0.05 was considered to indicate a significant difference.
Spot assay
Spot assays were used to analyse the resistance of yeast to PQ. Briefly, a single yeast colony was added to a volume of liquid YPD medium, and the culture was grown overnight at 30 °C. Next, 5 ml of fresh liquid YPD was inoculated with the overnight culture and grown to the exponential phase. Cells were collected and washed with phosphate-buffered saline (PBS), and the concentration was adjusted to an OD600 of 0.1. The cells were then diluted with sterile PBS in a 5-fold series, 5 μl of each dilution was spotted onto solid medium with or without PQ (2 mM), and the samples were allowed to grow for approximately 3 days at 30 °C.
RLS assay
The RLS assay was used to count the total number of daughter cells that were generated by individual mother cells. Briefly, an appropriate number of cells (grandmother cells) that were derived from a single colony were patched along a vertical line on a YPD plate, which was then kept at 30 °C for approximately 2 h. Next, virgin daughters (mother cells) were selected for RLS analysis. The daughter cells of these cells (granddaughter cells) were removed using a fibre-optic needle under a microscope. The total number of granddaughter cells that were generated by the mother cells was recorded, and growth curves were obtained by plotting the average number of granddaughter cells produced by a given strain as a function of the age point (Steffen et al. 2009). Statistical significance was calculated by using the Wilcoxon rank-sum test. p < 0.05 was considered to indicate a significant difference.
Real-time PCR
Exponential phase cells were pelleted and washed thrice with precooled PBS after they had been treated with or without 2 mM PQ for 1 h; total RNA was then extracted using a Yeast RNAiso Kit (Takara, Japan). This RNA was then reverse transcribed. Real-time quantitative PCR was performed in a LightCycler 480 instrument using the SYBR Green method. The expression of the target genes was quantified relative to that of the PRP8 housekeeping gene. The primers that were used for RT-PCR are listed in Table S2. Each experiment was repeated three times. Statistical significance was calculated by using the Student’s t test. p < 0.05 was considered to indicate a significant difference.
Analysis of ROS production and mitochondrial membrane potential
Intracellular reactive oxygen species (ROS) production was quantified by using the dichlorodihydrofluorescein diacetate (DCFH)-DA method (Chen et al. 2003). Briefly, DCFH-DA was de-esterified to DCFH and then further oxidized to green fluorescent DCF by ROS. To analyse intracellular ROS production, exponential phase cells were pelleted and washed thrice with precooled PBS after they had been treated with or without 2 mM PQ for 12 h; the cells were then harvested and incubated with 5 M DCFH-DA at 30 °C in the dark for 1 h. Finally, the pellet was washed three times with precooled PBS, and the mean green fluorescence intensity was determined by using flow cytometry (BD FACS Canto II). The mitochondrial membrane potential was determined by using the fluorescent dye rhodamine 123, which is incorporated specifically into the mitochondria in a manner that depends on the mitochondrial membrane potential (Ludovico et al. 2001). To analyse the mitochondrial membrane potential, exponential phase cells were pelleted and washed thrice with precooled PBS after they had been treated with or without PQ for 12 h. The samples were then incubated for 30 min with 10 mM rhodamine 123 at 30 °C in the dark, after which the mitochondrial membrane potential of the yeast was analysed by using flow cytometry (BD FACS Canto II). Statistical significance was determined using the Student’s t test. p < 0.05 was considered to indicate a significant difference.
Enzyme activity assay
Exponential phase cells were pelleted and washed three times with precooled PBS after being treated with or without 2 mM PQ. Cell extracts were generated by using glass beads to disrupting cells in buffer (50 mM Tris–HCl, pH 8.0, 50 mM KCl, 2 M citrate, 10% glycerol and 1 mM PMSF); after centrifugation, the supernatants were used for enzymatic assays. Protein concentrations were determined by using a Bradford Protein Assay Kit (Sangon Biotech, China). Catalase activity, superoxide dismutase activity, glutathione peroxidase activity, and hydrogen peroxide content were quantified using commercial assay kits (Beyotime Biotechnology, China).
Apoptotic marker assay
Apoptosis was analysed by using Annexin V-fluorescein isothiocyanate (FITC) staining and quantifying caspase-3 activity. Annexin V labelling (Dojindo Molecular Technologies, Japan) was performed as described previously (Herker et al. 2004; Ligr et al. 2001). Briefly, exponential phase cells were pelleted and washed three times with precooled PBS after they had been treated with or without 2 mM PQ; the cell wall was then digested with 120 U of zymolyase in a solution that was composed of 1.2 M sorbitol, 0.5 mM MgCl2, and 35 mM PO43 at pH 6.8 and 30 °C. After the cell walls had been digested, the cells were washed with PBS. The protoplasts were then resuspended in Annexin V binding solution, incubated with FITC-conjugated Annexin V for 15 min at room temperature, and analysed with a flow cytometer.
The caspase-3 activity assay is based on spectrophotometric detection of the chromophore p-nitroaniline (pNA) after it has been cleaved from the labelled substrate DEVD-pNA. The pNA light emission was quantified at 400 nm using a spectrophotometer or a microtitre-plate reader. Briefly, exponential phase cells were pelleted and washed three times with precooled PBS after they had been treated with or without 2 mM PQ. Cell extracts were generated using glass beads to disrupt the cells in buffer (50 mM Tris–HCl, pH 8.0, 50 mM KCl, 2 M citrate, 10% glycerol and 1 mM PMSF). After centrifugation, the supernatants were used to determine the caspase-3 activity with the Caspase-3 Assay Kit according to the manufacturer’s instructions (Abcam, USA).
Western blot analysis
To analyse the ubiquitination profile of the yeast strains (except for the detection of free ubiquitin proteins), whole extracts of yeast were analysed by SDS-PAGE using 15% Tris–glycine gels. The separated proteins were then transferred to a PVDF membrane (0.45 μM, Millipore, USA). To better separate low-molecular mass-free ubiquitin proteins (8.5 kDa), total extracts from yeast were separated by Tricine–SDS-PAGE (16.5% tricine–SDS-polyacrylamide gel, with a 10% spacer gel) (Schagger 2006) and transferred to a PVDF membrane (0.22 μM, Millipore, USA). The membrane was blocked with 3% BSA powder in TBST buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, and 0.1% Tween 20) and incubated with the first antibody (1:1000 dilution) at 4 °C overnight; after this step, the membrane was incubated with an AP-labelled secondary antibody (1:2000 dilution). The immunoreactive signal was revealed by NBT/BCIP regents. Anti-GAPDH and anti-ubiquitin monoclonal antibodies were purchased from Cell Signaling Technology (CA, USA).
Results
ubi4Δ cells are sensitive to PQ
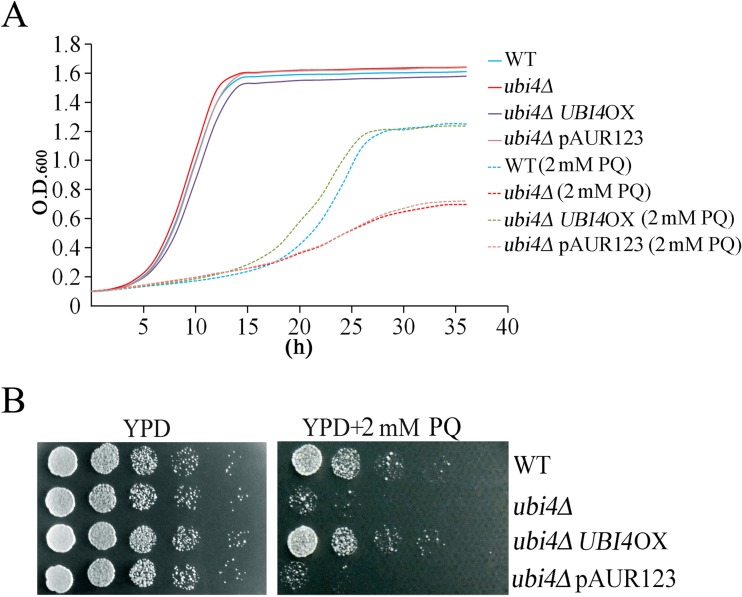
PQ is commonly used to generate ROS and oxidative stress in cells (Suntres 2002). To explore the potential mechanism of UBI4 action on the oxidative stress response, a UBI4-deletion strain of yeast was generated using a homologous recombination-mediated one-step gene disruption method. The growth rate of the ubi4Δ cells and their sensitivity to oxidative stress caused by PQ were then examined. No obvious differences were observed between the ubi4Δ and the wild-type cells when they were under unstressed conditions; when under PQ-stressed conditions, the ubi4Δ cells exhibited hypersensitivity to PQ (Fig. 1a, b).
Fig. 1.
UBI4-deleted cells are sensitive to PQ. Cell proliferation assays with wild-type and ubi4Δ cells in YPD medium with or without PQ were performed by the Bioscreen C MBR machine (a). *p < 0.05 indicated a significant difference. Wild-type and ubi4Δ cells were 5-fold serially diluted and spotted onto YPD plates or PQ-supplemented YPD plates. These plates were kept at 30 °C until colonies formed (b). pAUR123, empty plasmid; pAUR123UBI4, UBI4 overexpression plasmid
Increased ROS production and mitochondrial hyperpolarization in ubi4Δ cells
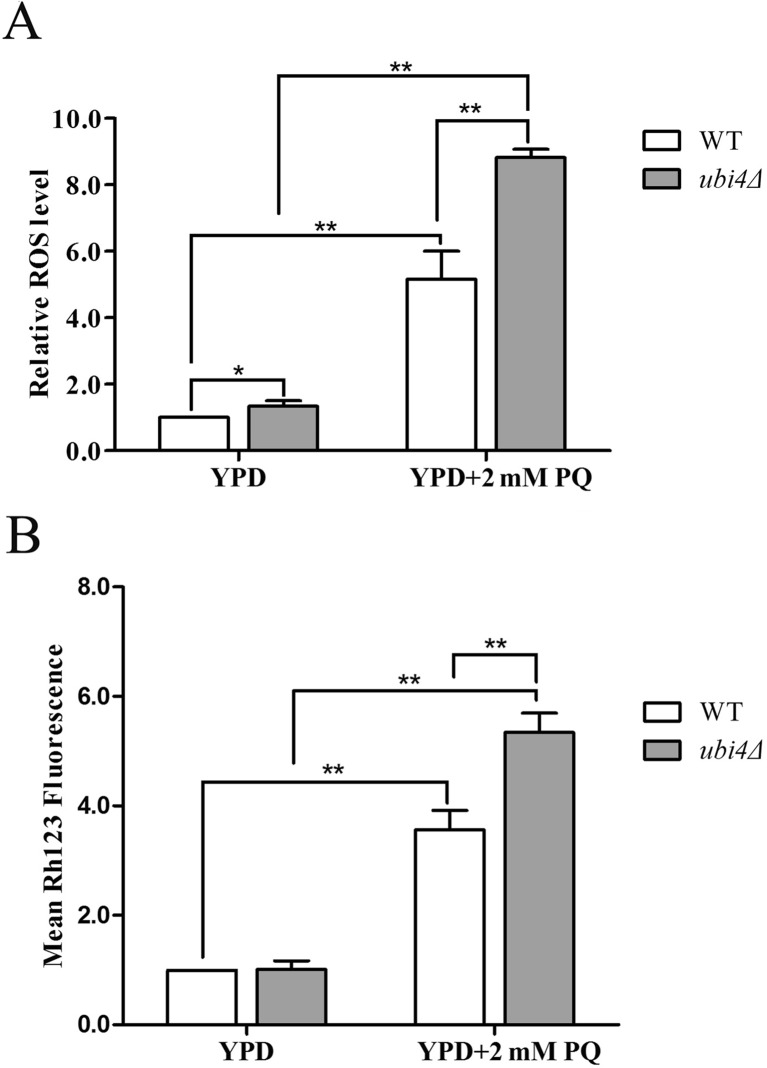
Complex I is the main site where PQ produces mitochondrial superoxide, and PQ can induce cell death by impairing mitochondrial membrane permeability (Cocheme and Murphy 2008; Huang et al. 2016). UBI4 deficiency increases the sensitivity of yeast cells to PQ. An investigation of the ROS level by flow cytometry showed that the intracellular ROS level was higher in ubi4Δ cells than in wild-type control cells under unstressed conditions, suggesting the oxidative stress in ubi4Δ cells. After stressing the cells with 2 mM PQ for 12 h, the difference became more evident (Fig. 2a). Accordingly, ubi4Δ cells that were stressed with PQ also exhibited augmented induction of mitochondrial hyperpolarization (Fig. 2b).
Fig. 2.
Increased ROS production and mitochondrial hyperpolarization in ubi4Δ cells. Wild-type and ubi4Δ cells were grown with or without 2 mM PQ stress in liquid YPD medium for 12 h, then stained with DCFH-DA and subjected to flow cytometry to detect intracellular ROS (a) or stained with rhodamine123 and subjected to flow cytometry to determine mitochondrial membrane potentials (b). The results are displayed as the mean fluorescence value. *p < 0.05 was considered to indicate a significant difference, and **p < 0.01 was considered to indicate a highly significant difference
ubi4Δ cells exhibit decreased catalase expression
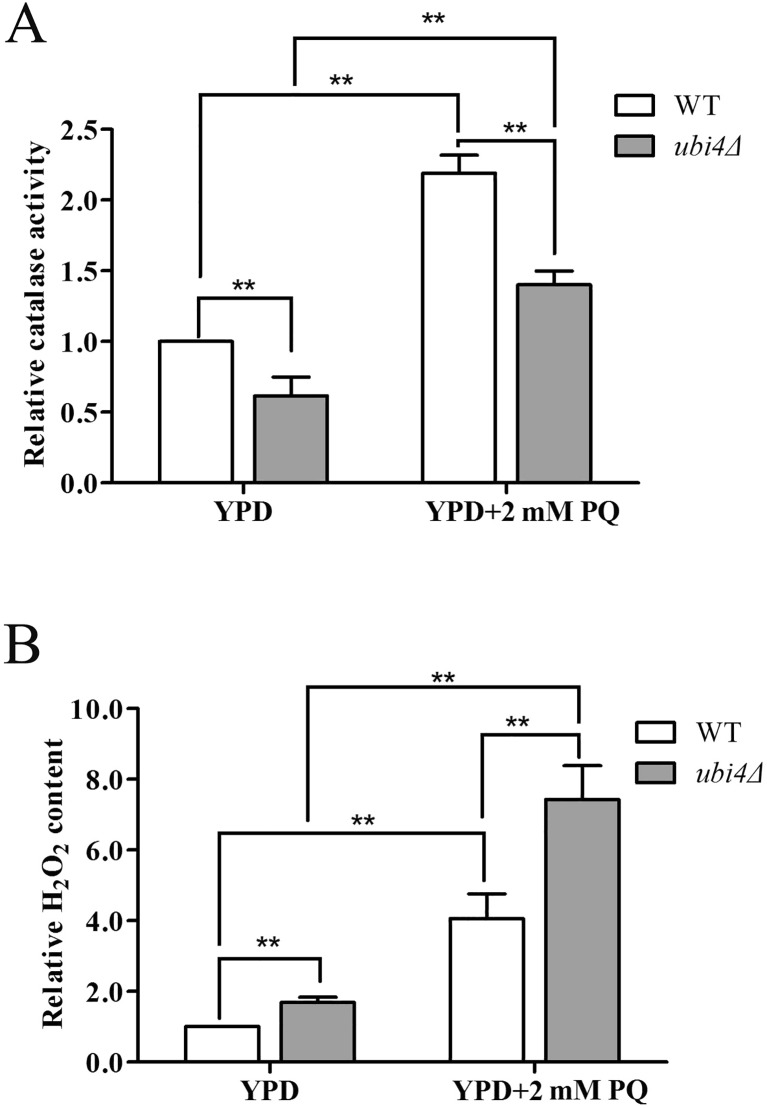
Increased intracellular ROS concentrations are always due to an imbalance of pro-oxidants and anti-oxidants (Ayer et al. 2014); thus, we determined the activity of several canonical anti-oxidative enzymes, such as superoxide dismutase, catalase and glutathione peroxidase under both unstressed and stressed conditions. Catalase activity was obviously lower in ubi4Δ cells than in wild-type cells under both unstressed and stressed conditions (Fig. 3a), but superoxide dismutase and glutathione peroxidase activities were identical in the two strains (data not shown). Catalase is the enzyme that primarily decomposes H2O2; thus, we next determined the level of intracellular H2O2 under both unstressed and stressed conditions. As expected, the H2O2 level in ubi4Δ cells was greater than that in wild-type cells (Fig. 3b). The elevated intracellular H2O2 levels also indicated oxidative stress in ubi4Δ cells.
Fig. 3.
Ubi4Δ cells have decreased catalase activity. Catalase activity assay of the wild-type and ubi4Δ cells (a) under the unstressed and PQ-stressed conditions. Intracellular H2O2 levels of the wild-type and ubi4Δ cells (b) under the unstressed and PQ-stressed conditions. *p < 0.05 was considered to indicate a significant difference, and **p < 0.01 was considered to indicate a highly significant difference
UBI4 deficiency induces early apoptosis in yeast
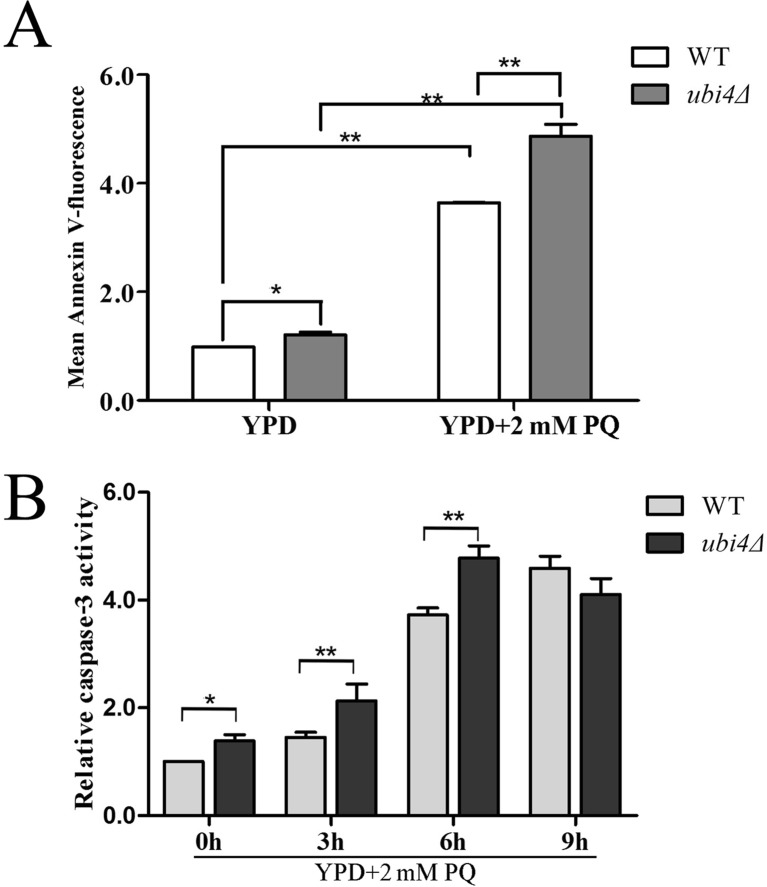
ROS production and hyperpolarization of the mitochondrial membrane are always associated with increased apoptosis (Frohlich and Madeo 2001; Kim et al. 2003). The yeast cell wall was digested, and the samples were subjected to an Annexin V-FITC-labelled apoptosis assay. The mean FITC fluorescence intensity under unstressed conditions was stronger in ubi4Δ cells than in wild-type control cells. After stressing the cells with 2 mM PQ for 12 h, the difference became more evident (Fig. 4a). Caspase-3 activity, an early marker of cells that are undergoing apoptosis, was also greater in ubi4Δ cells than in wild-type cells under both unstressed and PQ-stressed conditions. Moreover, the caspase-3 activity in the PQ-stressed ubi4Δ cells gradually increased during the first 6 h of incubation and slightly decreased after incubation for 9 h (Fig. 4b).
Fig. 4.
UBI4 deficiency induces apoptosis in yeast. Wild-type cells and ubi4Δ cells were grown with or without PQ stress in liquid YPD medium; the cell wall was then digested, and the cells were subjected to an Annexin V-FITC-labelled apoptosis assay (a). Total protein extracts were collected and subjected to a caspase-3 activity assay (b). Caspase-3 activity was detected by the production of pNA from the cleavage of Ac-DEVD-pNA substrate. The pNA light emission at 400 nm was quantified by a microtitre plate reader. *p < 0.05 was considered to indicate a significant difference, ** p < 0.01 was considered to indicate a highly significant difference
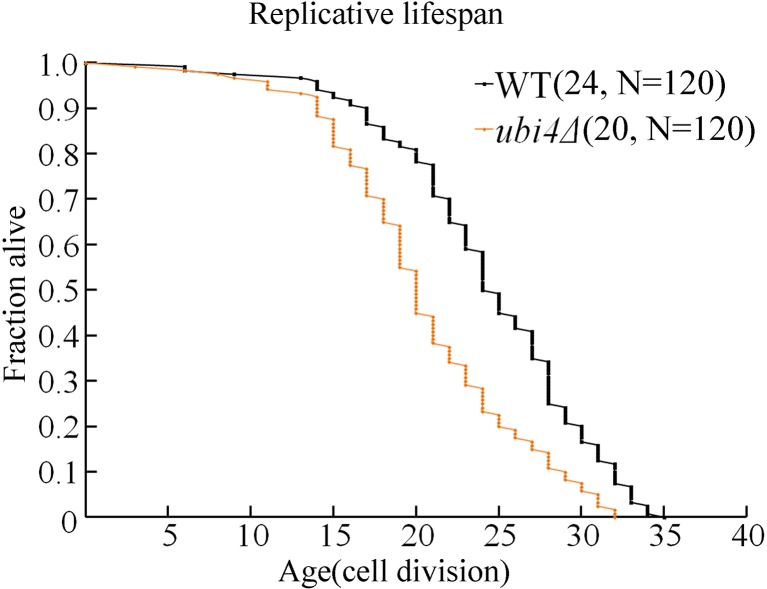
UBI4 deficiency decreases the RLS
Much evidence indicates that dysregulation of the apoptotic process may be involved in some ageing processes. Therefore, we further investigated the RLS of ubi4Δ cells on standard YPD plates and determined that fewer daughter cells were generated by ubi4Δ cells (mean RLS = 20, decreased by 20%, p < 0.05) than by wild-type cells (mean RLS = 24) (Fig. 5).
Fig. 5.
RLS lifespan is shorter in ubi4Δ cells. Yeast RLSs were analysed on a YPD plate. Mean lifespans and cell counts are shown in parentheses, and N is the total number of mother cells that were used for the RLS assay. Statistical significance was calculated by using the Wilcoxon rank-sum test, and *p < 0.05 indicated a significant difference
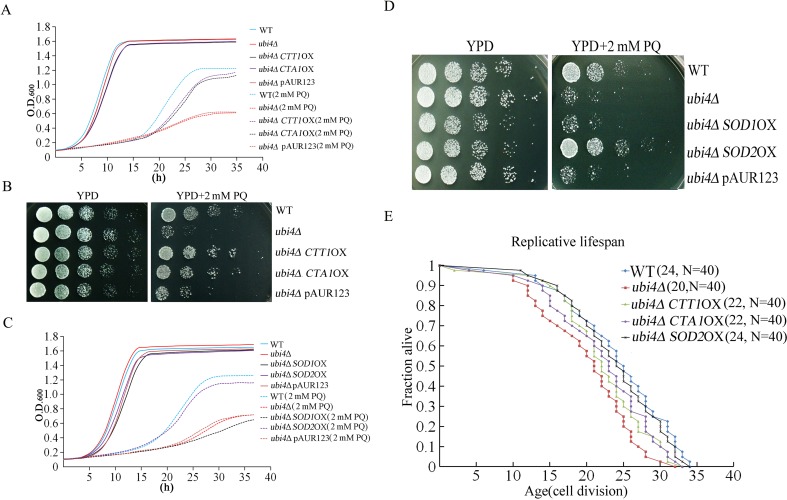
Overexpression of catalase and superoxide dismutase can rescue the phenotype that is associated with UBI4 deficiency
Since ubi4Δ cells exhibit reduced catalase expression, we overexpressed the catalase-coding genes CTT1 and CTA1 in cells and found that overexpression of either CTT1 or CTA1 could restore the resistance of ubi4Δ to 2 mM PQ (Fig. 6a, b). We also overexpressed the superoxide dismutase-coding genes SOD1 and SOD2 in ubi4Δ cells; interestingly, only SOD2 overexpression restored the resistance of ubi4Δ to 2 mM PQ (Fig. 6c, d). Moreover, we demonstrated that only SOD2 overexpression restored the RLS (increased by 20%) of ubi4Δ cells to the level of that exhibited by wild-type cells (Fig. 6e).
Fig. 6.
Overexpression of catalase and superoxide dismutase can rescue the phenotype that is associated with UBI4 deficiency. Cell proliferation (a) and spot assays (b) of CTT1- or CTA1-overexpressing ubi4Δ cells. Cell proliferation (c) and spot assays (d) of SOD1- or SOD2-overexpressing ubi4Δ cells. RLS lifespan assay of the CTT1-, CTA1-, and SOD2-overexpressing ubi4Δ cells (e), Mean lifespans and cell counts are shown in parentheses, and N is the total number of mother cells used for the RLS assay. Statistical significance was calculated by using the Wilcoxon rank-sum test, and *p < 0.05 indicated a significant difference
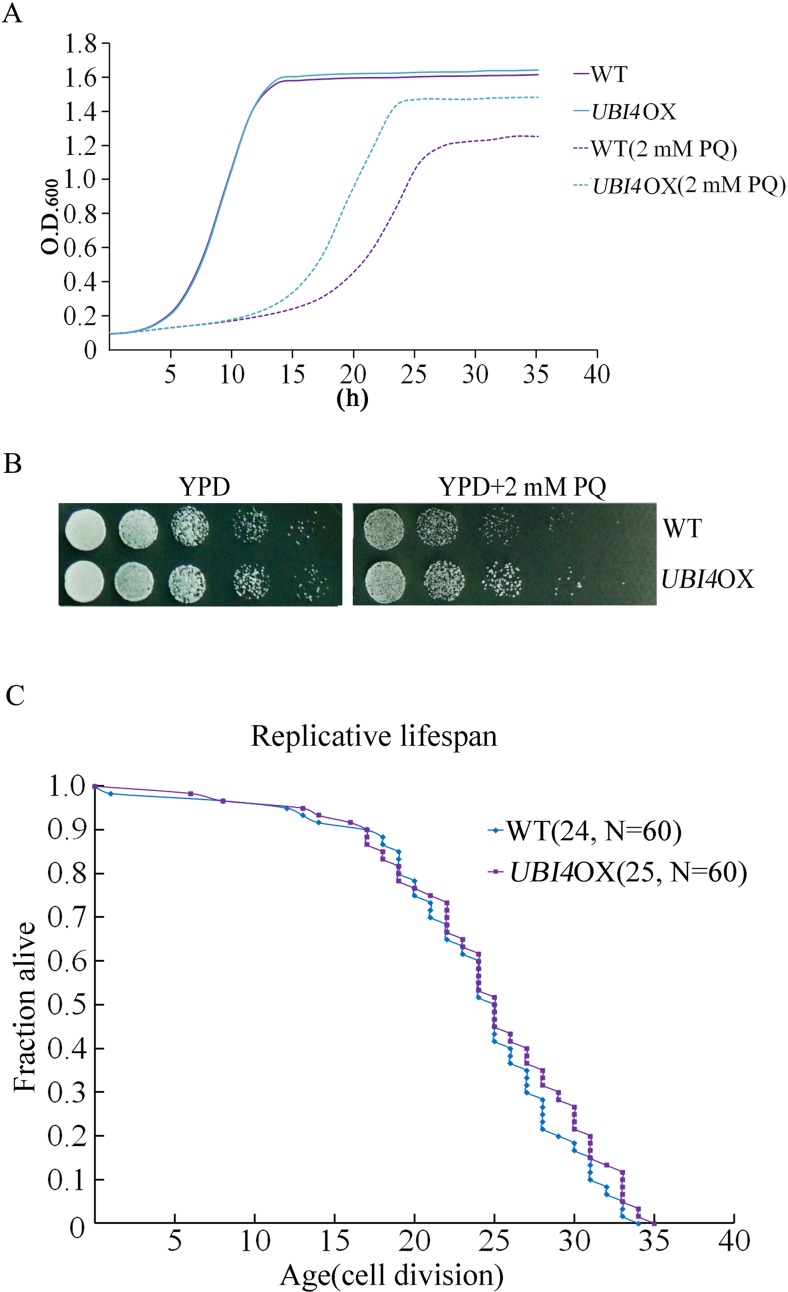
Overexpression of UBI4 increases resistance to PQ
We next investigated the effect of UBI4 overexpression on yeast resistance to PQ. We made a UBI4 overexpression (UBI4OX) strain that expresses UBI4 protein under its natural promoter. We used qPCR to evaluate the expression level of UBI4 in the UBI4OX strain and found that the UBI4 messenger RNA (mRNA) expression level in the UBI4OX strain was about 3-fold higher than that of the wild-type strain (data not shown). A growth curve analysis showed that the UBI4OX strain was more tolerant to PQ stress (Fig. 7a), which was consistent with the spot assay results (Fig. 7b).
Fig. 7.
Overexpression of UBI4 increases yeast resistance to PQ. Cell proliferation assays for wild-type and UBI4OX cells in YPD medium with or without 2 mM PQ were performed by a Bioscreen C MBR machine (a). *p < 0.05 indicated a significant difference. Wild-type and UBI4OX cells were 5-fold serially diluted, spotted onto YPD or PQ-supplemented YPD plates. These plates were kept at 30 °C until colonies formed (b). RLS of UBI4OX cells (c). Mean lifespans and cell counts are shown in parentheses, and N is the total number of mother cells that were used for the RLS assay. Statistical significance was calculated by using the Wilcoxon rank-sum test, and *p < 0.05 indicated a significant difference
Although overexpression of UBI4 increases the resistance of yeast to PQ, the RLS assay showed that no obvious difference existed between UBI4OX and wild-type cells (Fig. 7c). This result indicated that enhanced resistance of the UBI4OX strain to PQ was irrelevant to RLS.
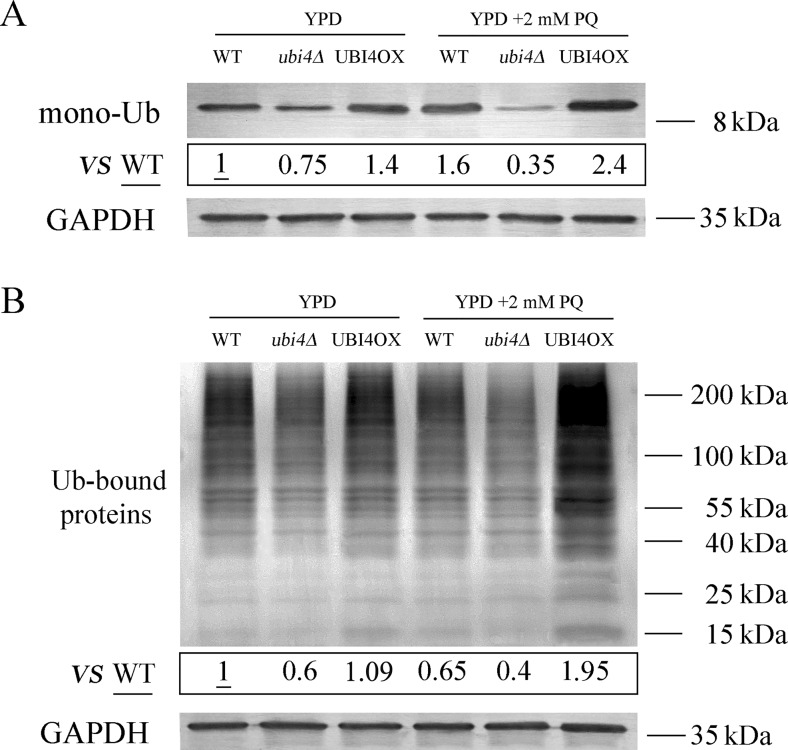
UBI4 overexpression leads to significant increases in ubiquitination under PQ-stressed conditions
Since UBI4 deficiency decreases resistance to PQ and overexpression of UBI4 increases resistance to PQ, we examined the patterns of ubiquitination in wild-type, ubi4Δ and UBI4OX cells. Total protein extracts from untreated and PQ-treated cells were examined by using Western blots. Tricine–SDS-PAGE was used to separate free ubiquitin proteins (8.5 kDa). As expected, mono-ubiquitin levels were lower in ubi4Δ cells and higher in UBI4OX cells than in wild-type cells under unstressed conditions (Fig. 8a). After the cells were stressed with PQ, mono-ubiquitin levels in wild-type and UBI4OX cells both increased; in contrast, the mono-ubiquitin levels in stressed ubi4Δ cells were much lower than those in unstressed ubi4Δ cells.
Fig. 8.
UBI4 overexpression increases ubiquitination under PQ-treated conditions. Changes in the levels of Ub monomers were evaluated by Western blotting (Tricine–SDS-PAGE) in lysates from yeast cells that had not been treated with PQ and had been treated with 2 mM PQ for 12 h (a). Changes in the levels of ubiquitinated proteins were evaluated by Western blotting (15% SDS-PAGE) using lysates from yeast cells that had not been treated with PQ and had been treated with 2 mM PQ for 12 h (b). Quantification of ubiquitin monomers and ubiquitinated proteins by densitometry
We next examined the patterns of ubiquitinated proteins in wild-type, ubi4Δ and UBI4OX cells. Under unstressed conditions, ubiquitinated protein levels were lower in ubi4Δ cells than in UBI4OX and wild-type cells, which had similar levels (Fig. 8b). Ubiquitinated protein levels were significantly higher in PQ-treated UBI4OX cells than in unstressed UBI4OX cells. Ubiquitinated protein levels in PQ-treated wild-type and ubi4Δ cells were decreased compared to their unstressed counterparts.
Discussion
ubi4Δ cells exhibit oxidative stress
Although the involvement of the yeast polyubiquitin gene UBI4 in the stress response was reported long ago, there are no reports regarding the underlying mechanism (Chen and Piper 1995; Finley et al. 1987; Fraser et al. 1991). Deleting (or overexpressing) specific genes from yeast and studying the responses of the resulting strains to stress agents constitutes an ideal methodology for the characterization of gene functions. In this study, we used UBI4-deleted and UBI4-overexpressing yeast strains as models to explore the potential mechanism of UBI4 action on the oxidative stress response.
First, we have shown that ubi4Δ cells exhibited obvious growth defects under PQ-stressed conditions. This behaviour is consistent with the previous finding that inactivation of UBI4 results in reduced resistance to oxidative stress.
PQ inducing the formation of intracellular ROS is one mechanism of PQ-induced cell toxicity. Therefore, we further investigated the level of intracellular ROS by using flow cytometry. The results showed that the intracellular ROS level was higher in ubi4Δ cells than in wild-type control cells under both unstressed and stressed conditions, and this difference became more pronounced when cells were treated with PQ, indicating intracellular oxidative stress in ubi4Δ cells.
Intracellular excess ROS production is always associated with the imbalance between pro-oxidant and anti-oxidant systems. Budding yeast, as a higher eukaryote, has two defence systems to eliminate intracellular ROS, enzymatic (such as catalase and superoxide dismutase) and non-enzymatic systems (such as glutathione and thioredoxin) (Jamieson 1998). We monitored the activity of several canonical anti-oxidative enzymes, such as superoxide dismutases, catalase and glutathione peroxidases. Catalase activity was obviously lower in ubi4Δ cells under both unstressed and PQ-stressed conditions, but superoxide dismutase and glutathione peroxidase activities were identical in the two strains (data not shown). Catalase is the principal H2O2-scavenging enzyme in yeast; thus, we further investigated intracellular H2O2 levels in ubi4Δ cells. Accordingly, the H2O2 levels were higher in ubi4Δ cells under both unstressed and PQ-stressed conditions, providing additional evidence for oxidative stress in ubi4Δ cells.
Unlike humans, budding yeast cells possess two different types of catalase enzymes to remove H2O2: peroxisome-localized catalase A (encoded by CTA1) and cytosol-localized catalase T (encoded by CTT1) (Cohen et al. 1988; Hartig and Ruis 1986). The regulation of catalase under oxidative stress is complex and unpredictable (Choi et al. 2009). To determine the mechanisms by which UBI4 deficiency inhibits catalase activity, we examined whether catalase activity was regulated at the mRNA level in ubi4Δ cells, and the data showed that there was no significant difference in the mRNA expression levels of CTA1 and CTT1 between the ubi4Δ cells and wild-type cells (data not shown), suggesting that UBI4-deficiency-induced inhibition of catalase activity was not mediated by a decrease of catalase at the mRNA level. Previous research has suggested that catalase could be inactivated by increased H2O2 (Altomare et al. 1974). Moreover, activated caspase-3 has also been suggested to inhibit catalase activity because of its proteolysis, not because of mRNA expression inhibition (Iwai et al. 2003).
UBI4 deficiency induces early apoptosis in yeast
Excessive intracellular amounts of ROS can cause oxidative damage to mtDNA and mitochondrial membrane proteins, resulting in structural changes and dysfunction of mitochondria and subsequently triggering the initiation of apoptosis (Breitenbach 2005; Lin and Beal 2006; Pyatrikas et al. 2015; Zhang et al. 2015). Furthermore, mitochondrial hyperpolarization is an event that is associated with the early stages of apoptosis (Fedoseeva et al. 2017; Gao et al. 2008). Interestingly, ubi4Δ cells exhibited augmented mitochondrial hyperpolarization under the PQ-stressed conditions.
Similar apoptotic pathways have been identified in organisms from yeast to mammalian cells. An early, sensitive cellular marker of apoptosis is the exposure of phosphatidylserines at the outer leaflet of the cytoplasmic membrane (Martin et al. 1995). In yeast, exposed phosphatidylserines can be detected by using FITC-labelled Annexin V upon cell wall digestion. The mean fluorescence strength is higher in ubi4Δ cells than in the wild-type strain under the unstressed conditions, and the difference became larger after the cells were stressed with PQ, indicating that UBI4 deficiency induces early apoptosis in yeast and that the UBI4-deleted cells were more susceptive to PQ-induced apoptosis.
Another hallmark of early apoptosis is the presence of caspase activity, which was also detected in this study. Caspase-3 is one of the most important caspases and plays a central role in mediating nuclear apoptosis (Rona et al. 2015). As shown in Fig. 4b, caspase-3 activity was greater in ubi4Δ cells than in wild-type cells under both unstressed and PQ-stressed conditions. Moreover, the caspase-3 activity in the PQ-stressed ubi4Δ cells gradually increased during the first 6 h of incubation and slightly decreased after incubation for 9 h. These data suggest that UBI4 could be a potential anti-apoptotic gene in yeast.
Approximately 17 pro-apoptotic genes and 4 anti-apoptotic genes are related to the yeast ageing process and in nearly all cases; the deletion of one of these apoptosis-related genes impacts either the chronological or replicative lifespans (Laun et al. 2012). No study has been carried out to determine the correlation between apoptosis-related genes and lifespan (Laun et al. 2008). We also demonstrated that the mean replicative lifespan was lower (decreased by approximately 20%, p < 0.05) in ubi4Δ cells than in wild-type mother cells.
Upregulation of anti-oxidative enzymes can rescue the phenotype that is associated with UBI4 deficiency
As discussed above, ubi4Δ cells exhibit markedly low catalase activity. Therefore, we overexpressed the catalase-coding genes CTT1 or CTA1 in ubi4Δ cells, resulting in increased resistance of ubi4Δ cells to PQ; thus, the low resistance might due to decreased catalase activity in ubi4Δ cells.
In contrast, ubi4Δ cells that were stressed with PQ exhibited augmented induction of mitochondrial hyperpolarization, suggesting impaired mitochondrial function. Therefore, we determined whether overexpression of the mitochondrial superoxide dismutase SOD2 reduces oxidative stress defects that are associated with UBI4 deficiency; SOD2 overexpression does indeed restore the resistance of ubi4Δ to PQ, although not quite as well as the wild-type cells. We also overexpressed the cytoplasm-localized SOD1 in ubi4Δ cells and found that the rescue effect that was observed for SOD2 overexpression was absent under these conditions.
Moreover, we investigated whether overexpression of catalase and superoxide dismutase could rescue the decreased RLS that is associated with UBI4 deficiency, but only SOD2 overexpression could restore the RLS of ubi4Δ cells. By contrast, we previously reported that overexpression of SOD2 cannot extend the RLS of wild-type budding yeast (Zhao et al. 2014). Hence, these results are indirect evidence for mitochondrial dysfunction in ubi4Δ cells.
Overexpression of UBI4 increases both mono-ubiquitin levels and ubiquitinated proteins levels under the PQ-stressed condition
Ubiquitin is a small protein that is highly conserved in organisms from yeast to high eukaryotes and can be covalently linked to itself or other proteins (termed ubiquitination). Ubiquitination plays a significant role in the targeting of specific proteins (such as abnormal, short-lived and denatured proteins) for cytoplasmic degradation (Goldberg 2003). Ubiquitin is also involved in the regulation of gene expression, membrane protein delivery and receptor internalization (Aguilar and Wendland 2003; Jackson and Durocher 2013).
Growth curve analysis and spot assays clearly showed that the UBI4OX strain is resistant to PQ. Therefore, we examined the ubiquitination profile of ubi4Δ cells and UBI4OX cells. On the one hand, the mono-ubiquitin levels were lower in ubi4Δ cells and higher in UBI4OX cells than in wild-type cells under the unstressed conditions. After the cells were stressed with PQ, the mono-ubiquitin levels in wild-type (increased 60%) and UBI4OX cells (increased 71%) had both increased, while the mono-ubiquitin levels in ubi4Δ cells were much lower (decreased 53%) than those found under unstressed conditions.
Since the mono-ubiquitin levels significantly increased in UBI4OX cells and ubiquitin is also involved in the regulation of gene expression and DNA damage responses (Buckley et al. 2012; Rajendra et al. 2014), one possible explanation of the increased resistance of UBI4OX cells to PQ is that a set of specific genes that are involved in DNA damage responses was upregulated in UBI4OX cells, making it able to withstand PQ-induced oxidative stress. Additionally, why were mono-ubiquitin levels in ubi4Δ cells decreased under PQ stress? This effect might due to UBI1, UBI2 and UBI3 genes, which according to a previous report, were weakly expressed under the stress condition (Fraser et al. 1991).
We also showed that total ubiquitinated protein levels decreased in both wild-type (by 35%) and ubi4Δ cells (by 33%), but increased significantly in UBI4OX cells (by 79%) when PQ stress was applied. Increased ubiquitinated protein levels have been suggested to be concomitant with increased proteolysis, which could reduce the toxic effects of the oxidized proteins on yeast cells (Myeku and Figueiredo-Pereira 2011; Shang and Taylor 2011; Wing et al. 1995). Therefore, we speculated that the harmful oxidized protein that had been generated by PQ was easily degraded by the ubiquitin-mediated degradation pathway. This explanation might account for the increased resistance of UBI4OX cells to PQ.
It should be noted that total ubiquitinated protein levels were decreased in wild-type cells under the PQ-stressed condition, although mono-ubiquitin levels were obviously increased in wild-type cells under the PQ-stressed condition; in contrast, both the mono-ubiquitin levels and total ubiquitinated protein levels were increased in UBI4OX cells under the PQ-stressed condition. We speculated that PQ-induced high levels of mono-ubiquitin expression on wild-type cells may be an adaptive response of the yeast to oxidative stress induced by PQ but may not increase the ubiquitination of target proteins. Another possible explanation is that PQ may, at least to some extent, impair the protein ubiquitination pathway. Previous studies also reported that PQ can induce ubiquitin/proteasome system dysfunction (Navarro-Yepes et al. 2016; Yang et al. 2007).
In summary, the present study demonstrates that the deletion of yeast polyubiquitin gene UBI4 results in reduced resistance to the oxidizing agent PQ and that this phenotype is rescued by overexpression of either catalase or mitochondrial superoxide dismutase SOD2. Additionally, ubi4Δ cells exhibit greater oxidative stress and an apoptotic phenotype, and these factors may cause the decreased RLS that is observed in this study. In addition, we demonstrated that only SOD2 overexpression restores the RLS of ubi4Δ cells. Moreover, overexpression of UBI4 in wild-type yeast increases the yeast’s resistance to PQ; this result is associated with large pools of expressed ubiquitin and increased levels of ubiquitinated proteins. These findings highlight the role of polyubiquitin gene UBI4 in apoptosis and ageing, although no exact molecular mechanism linking UBI4 and these processes has been elucidated. Investigations of the molecular mechanisms that form the basis of these observations will be informative.
Electronic supplementary material
(DOCX 119 kb).
(DOCX 87 kb).
(XLS 27 kb).
(XLS 25 kb).
Acknowledgements
We are grateful to Brian K. Kennedy (Buck Institute), Matt Kaeberlein, and Brian M. Wasko (University of Washington) for technical assistance.
Funding information
This work was supported by the China National Natural Science Foundation (31101051, 81671399), the Ordinary University Innovation Team Construction Project of Guangdong Province (2015KCXTD022), the Unique Innovative Projects in Ordinary University of Guangdong Province (2015KTSCX049), and the Guangdong Medical Research Foundation (A2016257).
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12192-017-0860-3) contains supplementary material, which is available to authorized users.
References
- Aguilar RC, Wendland B. Ubiquitin: not just for proteasomes anymore. Curr Opin Cell Biol. 2003;15(2):184–190. doi: 10.1016/S0955-0674(03)00010-3. [DOI] [PubMed] [Google Scholar]
- Altomare RE, Greenfield PF, Kittrell JR. Letter: inactivation of immobilized fungal catalase by hydrogen peroxide. Biotechnol Bioeng. 1974;16(12):1675–1680. doi: 10.1002/bit.260161209. [DOI] [PubMed] [Google Scholar]
- Ayer A, Gourlay CW, Dawes IW. Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2014;14(1):60–72. doi: 10.1111/1567-1364.12114. [DOI] [PubMed] [Google Scholar]
- Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21(14):3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach M. Apoptosis, ageing and redox homeostasis in yeasts. FEMS Yeast Res. 2005;5(12):1191–1192. doi: 10.1016/S1567-1356(05)00147-9. [DOI] [PubMed] [Google Scholar]
- Buckley SM, Aranda-Orgilles B, Strikoudis A, Apostolou E, Loizou E, Moran-Crusio K, Farnsworth CL, Koller AA, Dasgupta R, Silva JC, Stadtfeld M, Hochedlinger K, Chen EI, Aifantis I. Regulation of pluripotency and cellular reprogramming by the ubiquitin-proteasome system. Cell Stem Cell. 2012;11(6):783–798. doi: 10.1016/j.stem.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Dunigan DD, Dickman MB. Bcl-2 family members inhibit oxidative stress-induced programmed cell death in Saccharomyces cerevisiae. Free Radic Biol Med. 2003;34(10):1315–1325. doi: 10.1016/S0891-5849(03)00146-1. [DOI] [PubMed] [Google Scholar]
- Chen Y, Piper PW. Consequences of the overexpression of ubiquitin in yeast: elevated tolerances of osmostress, ethanol and canavanine, yet reduced tolerances of cadmium, arsenite and paromomycin. Biochim Biophys Acta. 1995;1268(1):59–64. doi: 10.1016/0167-4889(95)00044-S. [DOI] [PubMed] [Google Scholar]
- Choi SI, Kim TI, Kim KS, Kim BY, Ahn SY, Cho HJ, Lee HK, Cho HS, Kim EK. Decreased catalase expression and increased susceptibility to oxidative stress in primary cultured corneal fibroblasts from patients with granular corneal dystrophy type II. Am J Pathol. 2009;175(1):248–261. doi: 10.2353/ajpath.2009.081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283(4):1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Cohen G, Rapatz W, Ruis H. Sequence of the Saccharomyces cerevisiae CTA1 gene and amino acid sequence of catalase A derived from it. Eur J Biochem. 1988;176(1):159–163. doi: 10.1111/j.1432-1033.1988.tb14263.x. [DOI] [PubMed] [Google Scholar]
- Delaney JR, Ahmed U, Chou A, Sim S, Carr D, Murakami CJ, Schleit J, Sutphin GL, An EH, Castanza A, Fletcher M, Higgins S, Jelic M, Klum S, Muller B, Peng ZJ, Rai D, Ros V, Singh M, Wende HV, Kennedy BK, Kaeberlein M. Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell. 2013;12(1):156–166. doi: 10.1111/acel.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoseeva IV, Pyatrikas DV, Stepanov AV, Fedyaeva AV, Varakina NN, Rusaleva TM, Borovskii GB, Rikhvanov EG. The role of flavin-containing enzymes in mitochondrial membrane hyperpolarization and ROS production in respiring Saccharomyces cerevisiae cells under heat-shock conditions. Sci Rep. 2017;7(1):2586. doi: 10.1038/s41598-017-02736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987;48(6):1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Fraser J, Luu HA, Neculcea J, Thomas DY, Storms RK. Ubiquitin gene expression: response to environmental changes. Curr Genet. 1991;20(1–2):17–23. doi: 10.1007/BF00312760. [DOI] [PubMed] [Google Scholar]
- Frohlich KU, Madeo F. Apoptosis in yeast: a new model for aging research. Exp Gerontol. 2001;37(1):27–31. doi: 10.1016/S0531-5565(01)00177-2. [DOI] [PubMed] [Google Scholar]
- Gao C, Xing D, Li L, Zhang L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta. 2008;227(4):755–767. doi: 10.1007/s00425-007-0654-4. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- Hartig A, Ruis H. Nucleotide sequence of the Saccharomyces cerevisiae CTT1 gene and deduced amino-acid sequence of yeast catalase T. Eur J Biochem. 1986;160(3):487–490. doi: 10.1111/j.1432-1033.1986.tb10065.x. [DOI] [PubMed] [Google Scholar]
- Herker E, Jungwirth H, Lehmann KA, Maldener C, Frohlich KU, Wissing S, Buttner S, Fehr M, Sigrist S, Madeo F. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164(4):501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Chao CC, Lee YC, MK L, Cheng JJ, Yang YC, Wang VC, Chang WC, Huang NK. Paraquat induces cell death through impairing mitochondrial membrane permeability. Mol Neurobiol. 2016;53(4):2169–2188. doi: 10.1007/s12035-015-9198-y. [DOI] [PubMed] [Google Scholar]
- Iwai K, Kondo T, Watanabe M, Yabu T, Kitano T, Taguchi Y, Umehara H, Takahashi A, Uchiyama T, Okazaki T. Ceramide increases oxidative damage due to inhibition of catalase by caspase-3-dependent proteolysis in HL-60 cell apoptosis. J Biol Chem. 2003;278(11):9813–9822. doi: 10.1074/jbc.M201867200. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49(5):795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast. 1998;14(16):1511–1527. doi: 10.1002/(SICI)1097-0061(199812)14:16<1511::AID-YEA356>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kim JM, Bae HR, Park BS, Lee JM, Ahn HB, Rho JH, Yoo KW, Park WC, Rho SH, Yoon HS, Yoo YH. Early mitochondrial hyperpolarization and intracellular alkalinization in lactacystin-induced apoptosis of retinal pigment epithelial cells. J Pharmacol Exp Ther. 2003;305(2):474–481. doi: 10.1124/jpet.102.047811. [DOI] [PubMed] [Google Scholar]
- Laun P, Buttner S, Rinnerthaler M, Burhans WC, Breitenbach M. Yeast aging and apoptosis. Subcell Biochem. 2012;57:207–232. doi: 10.1007/978-94-007-2561-4_10. [DOI] [PubMed] [Google Scholar]
- Laun P, Heeren G, Rinnerthaler M, Rid R, Kossler S, Koller L, Breitenbach M. Senescence and apoptosis in yeast mother cell-specific aging and in higher cells: a short review. Biochim Biophys Acta. 2008;1783(7):1328–1334. doi: 10.1016/j.bbamcr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Ligr M, Velten I, Frohlich E, Madeo F, Ledig M, Frohlich KU, Wolf DH, Hilt W. The proteasomal substrate Stm1 participates in apoptosis-like cell death in yeast. Mol Biol Cell. 2001;12(8):2422–2432. doi: 10.1091/mbc.12.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Ludovico P, Sansonetty F, Corte-Real M. Assessment of mitochondrial membrane potential in yeast cell populations by flow cytometry. Microbiology. 2001;147(Pt 12):3335–3343. doi: 10.1099/00221287-147-12-3335. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myeku N, Figueiredo-Pereira ME. Dynamics of the degradation of ubiquitinated proteins by proteasomes and autophagy: association with sequestosome 1/p62. J Biol Chem. 2011;286(25):22426–22440. doi: 10.1074/jbc.M110.149252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Yepes J, Anandhan A, Bradley E, Bohovych I, Yarabe B, de Jong A, Ovaa H, Zhou Y, Khalimonchuk O, Quintanilla-Vega B, Franco R. Inhibition of protein ubiquitination by paraquat and 1-methyl-4-phenylpyridinium impairs ubiquitin-dependent protein degradation pathways. Mol Neurobiol. 2016;53(8):5229–5251. doi: 10.1007/s12035-015-9414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Finley D, Solomon MJ, Varshavsky A. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987;6(5):1429–1439. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyatrikas DV, Fedoseeva IV, Varakina NN, Rusaleva TM, Stepanov AV, Fedyaeva AV, Borovskii GB, Rikhvanov EG. Relation between cell death progression, reactive oxygen species production and mitochondrial membrane potential in fermenting Saccharomyces cerevisiae cells under heat-shock conditions. FEMS Microbiol Lett. 2015;362(12):82. doi: 10.1093/femsle/fnv082. [DOI] [PubMed] [Google Scholar]
- Rajendra E, Oestergaard VH, Langevin F, Wang M, Dornan GL, Patel KJ, Passmore LA. The genetic and biochemical basis of FANCD2 monoubiquitination. Mol Cell. 2014;54(5):858–869. doi: 10.1016/j.molcel.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rona G, Herdeiro R, Mathias CJ, Torres FA, Pereira MD, Eleutherio E. CTT1 overexpression increases life span of calorie-restricted Saccharomyces cerevisiae deficient in Sod1. Biogerontology. 2015;16(3):343–351. doi: 10.1007/s10522-015-9550-7. [DOI] [PubMed] [Google Scholar]
- Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1(1):16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51(1):5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Ma H, Botstein D. Manipulating yeast genome using plasmid vectors. Methods Enzymol. 1990;185:280–297. doi: 10.1016/0076-6879(90)85025-J. [DOI] [PubMed] [Google Scholar]
- Steffen KK, Kennedy BK, Kaeberlein M (2009) Measuring replicative life span in the budding yeast. J Vis Exp 28:1209 [DOI] [PMC free article] [PubMed]
- Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180(1):65–77. doi: 10.1016/S0300-483X(02)00382-7. [DOI] [PubMed] [Google Scholar]
- Watt R, Piper PW. UBI4, the polyubiquitin gene of Saccharomyces cerevisiae, is a heat shock gene that is also subject to catabolite derepression control. Mol Gen Genet. 1997;253(4):439–447. doi: 10.1007/s004380050341. [DOI] [PubMed] [Google Scholar]
- Wing SS, Haas AL, Goldberg AL. Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem J. 1995;307(Pt 3):639–645. doi: 10.1042/bj3070639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen L, Ding Y, Zhuang X, Kang UJ. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16(23):2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ren L, Chen GQ, Zhang J, Reed BM, Shen XH. ROS-induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep. 2015;34(9):1499–1513. doi: 10.1007/s00299-015-1802-0. [DOI] [PubMed] [Google Scholar]
- Zhao W, Fang BX, Niu YJ, Liu YN, Liu B, Peng Q, Li JB, Wasko BM, Delaney JR, Kennedy BK, Suh Y, Zhou ZJ, Kaeberlein M, Liu XG. Nar1 deficiency results in shortened lifespan and sensitivity to paraquat that is rescued by increased expression of mitochondrial superoxide dismutase. Mech Ageing Dev. 2014;138:53–58. doi: 10.1016/j.mad.2014.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 119 kb).
(DOCX 87 kb).
(XLS 27 kb).
(XLS 25 kb).