Abstract
Bovine milk is rich in exosomes, which contain abundant miRNAs and play important roles in the regulation of neonatal growth and development of adaptive immunity. Here, we analyzed miRNA expression profiles of bovine milk exosomes from three healthy and three mastitic cows, and then six miRNA libraries were constructed. Interestingly, we detected no scRNAs and few snRNAs in milk exosomes; this result indicated a potential preference for RNA packaging in milk exosomes. A total of 492 known and 980 novel exosomal miRNAs were detected, and the 10 most expressed miRNAs in the six samples accounted for 80–90% of total miRNA-associated reads. Expression analyses identified 18 miRNAs with significantly different expression between healthy and infected animals; the predicted target genes of differentially expressed miRNAs were significantly enriched in immune system process, response to stimulus, growth, etc. Moreover, target genes were significantly enriched in several Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways including inflammatory, immune, and cancer pathways. Our survey provided comprehensive information about milk exosomes and exosomal miRNAs involved in mastitis. Moreover, the differentially expressed miRNAs, especially miR-223 and miR-142-5p, could be considered as potential candidates for mastitis.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0876-3) contains supplementary material, which is available to authorized users.
Keywords: Bovine, Milk, Exosome, Mastitis, miRNA-sequencing
Introduction
Bovine mastitis is an inflammatory reaction of the mammary gland and is usually a consequence of microbial infection, caused by pathogens such as staphylococci, streptococci and coliform bacteria (Zadoks et al. 2011). Mastitis has long been considered a highly prevalent and costly disease with clinical or subclinical manifestations in the dairy industry (DeGraves and Fetrow 1993). To date, numerous studies have focused on bovine mastitis, including the nosogenesis, regulation mechanisms, and therapeutic approaches. For example, cytokines and other inflammatory mediators showed lower expression in bovine milk infected by Escherichia coli than in milk infected by Staphylococcus aureus (Bannerman et al. 2004). Moreover, Toll-like receptor-4 has been suggested as an important contributor to robust mammary gland immune defense against E. coli mastitis (De Schepper et al. 2008). Nonetheless, few studies have focused on bovine mastitis milk, its vesicles, and gene expression.
Exosomes are a type of extracellular vesicle, such as microvesicles, apoptotic bodies, or ectosomes with a diameter of 50~150 nm, which are derived from MVBs fusing with the plasma membrane (Chivet et al. 2013; Kowal et al. 2016). Therefore, exosomal membrane molecules such as CD9, CD63, and CD81, as well as the proteins involved in exosomal biogenesis such as tumor susceptibility gene 101 (Tsg101), are considered to be exosomal markers (Kumar et al. 2015). Exosomes can be released from many cell types, including epithelial cells, endothelial cells, immunocytes, platelets, blood cells, and smooth muscle cells (Liao et al. 2014; Saunderson et al. 2014; Wieckowski and Whiteside 2006). Secreted exosomes can regulate the activity of recipient cells by delivering lipids, proteins, and nucleic acids, thus representing a novel intercellular communication pathway (Février and Raposo 2004). Furthermore, exosomes have been found to be involved in both immune stimulation and tolerance, depending on the cell origin (Raposo et al. 1996), and several studies have proposed the potential use of exosomes in immunotherapy (Aline et al. 2004; Pêche et al. 2003). Exosomes are present in body fluids including urine (Pisitkun et al. 2004), plasma (Wahlgren et al. 2012), saliva (Michael et al. 2010), malignant effusions (Andre et al. 2002), breast milk (Admyre et al. 2007), bronchoalveolar lavage fluid (Levänen et al. 2013), synovial fluid (Kolhe et al. 2017), and epididymal fluid (Gatti et al. 2005). The phenotype of exosomes derived from human breast milk varies with maternal sensitization and lifestyle, which might influence allergy development in the child (Torregrosa Paredes et al. 2014). Bovine milk exosomes exhibit cross-species tolerance with no adverse immune and inflammatory response, which can be considered as a carrier to enhance oral bioavailability and improve efficacy and safety of drugs (Munagala et al. 2016).
MicroRNAs (miRNAs) are stable small non-coding RNAs, typically 22 nucleotides in length, with high evolutionary sequence conservation. Numerous studies have found that miRNAs play crucial roles in the process of cellular proliferation and differentiation, tissue development, and immune response. Furthermore, only a few studies investigated the regulatory roles of miRNA in bovine immunity and infection. For example, a recent study revealed that miR-9, miR-125b, miR-146a, miR-233, and miR-155 were closely related with inflammation after stimulation of bovine monocytes with lipopolysaccharide and S. aureus enterotoxin B (Dilda et al. 2012). In another study, miRNAs were detected in exosomes and shown to be delivered to another cell as functional molecules (Valadi et al. 2007). Subsequently, numerous studies focused on the function of exosomal miRNAs. For example, miR-29c derived from urinary exosomes was discovered as a novel non-invasive marker for renal fibrosis (Lv et al. 2013). In addition, miR-21 was found to be enriched in serum exosomes and might serve as a potential biomarker for hepatocellular carcinoma (Wang et al. 2014). However, few studies focused on the function of bovine milk derived miRNAs.
The objective of this study was to investigate features of small RNAs, the expression pattern of miRNAs in bovine milk exosomes, and to comprehensively characterize differential expression of milk-derived exosomal miRNAs between normal and infected cows.
Materials and methods
Milk collection
Six 3- to 4-year-old Chinese female Holstein cattle were obtained from a dairy farm and divided into two groups randomly. For the three healthy cows, the milk was inspected to have SCC < 100,000 cells/mL (Supplementary Table S1), and the udders were observed to show no mastitic symptoms, such as redness, swelling, or pain; bacterial isolation and identification from the milk showed the absence of pathogens. In the mastitic group, Holstein cows were infused with approximately 105 colony forming units (CFU) of S. aureus (ATCC29213, purchased from BNCC) suspended in 5 mL PBS. After 48 h, the milk SCC was > 1 million/mL (Supplementary Table S1), and the presence of mastitis was confirmed. Then, 400 mL of milk was aseptically collected under sterile conditions and stored at 4 °C until use.
Isolation of exosomes
Milk exosomes were isolated by differential centrifugation as described previously (Munagala et al. 2016). Briefly, milk was centrifuged at 10,000×g in 50-mL centrifuge bottles (Thermofisher Scientific) at 4 °C for 30 min to remove fat globules, casein aggregates, and other debris. The whey was collected and transferred into 50-mL polycarbonate tubes and centrifuged at 100,000×g at 4 °C for 60 min to remove large particles and microvesicles. The supernatant was carefully removed and filtered through 0.75 μm. This supernatant was centrifuged at 150,000×g for 90 min at 4 °C. The exosome pellet was re-suspended in PBS and filtered through 0.22 μm and then collected and washed twice with PBS. Finally, exosomes were re-suspended in 200 μL PBS and stored at − 80 °C until use.
NTA
Size distribution and particle concentration in bovine milk exosomes were analyzed by NanoSight and Zetasizer (Malvern Instruments Ltd., Malvern, UK). The particle Brownian movement was analyzed by NTA software (version 2.3, NanoSight). Ten microliters of exosomes was diluted in 1 mL with PBS, and size distribution and parameters were analyzed at 37 °C according to the manufacturer’s instructions.
TEM
A drop of the re-suspended exosomes was placed on a sheet and then floated a carbon-coated copper grid on the drop for 10~30 s. The grid was removed, and excess liquid was drained by a piece of sterile filter paper. The same side of the grid was covered by a drop of 2% phosphotungstic acid, pH 7.0, for 5 s, and the excess liquid was removed. After several minutes, when the grid was dry, it was examined with Tecnai G2 F20 S-TWIN (FEI, Hillsboro, USA).
Western blot
For total protein, samples were incubated with RIPA (Beyotime, Shanghai, China) reagent for 10 min on a shaker, centrifuged at 10,000×g for 5 min, and then the supernatant was collected. The protein content of the supernatant was measured with a BCA kit (Beyotime, Shanghai, China). Twenty micrograms of protein was loaded per well onto a 12% acrylamide gel. The protein was transferred onto a PVDF membrane and blocked with non-fat milk at room temperature for 2 h. Then, the membrane was incubated with either anti-calnexin (1:2000) (Abcam, USA), anti-Tsg-101 (1:300) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-CD81 (1:300) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in primary antibody diluent overnight at 4 °C. The membrane was washed 5 × 10 min with TBST. The secondary antibodies (1:5000) (Servicebio, Wuhan, China) diluted in TBST were incubated with the membrane at room temperature for 2 h. The membrane was washed 5 × 10 min and then incubated with ECL kit (Servicebio, Wuhan, China) following the manufacturer’s recommendations.
RNA sequencing
Total RNA was extracted from bovine milk exosomes using the Trizol reagent (TAKARA, Japan) according to the manufacturer’s protocol and dissolved in RNase free water. RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA), and the RIN was 7.0–8.0. A total amount of 2.5 ng RNA per sample was used as input material for the RNA sample preparations. Sequencing libraries were generated using NEBNext Ultra small RNA Sample Library Prep Kit for Illumina (NEB, USA) following manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System TruSeq PE Cluster Kit v4-cBot-HS (Illumina, NEB, USA) according to the manufacturer’s instructions. After cluster generation, the librares were sequenced on an Illumina Hiseq 2500 platform and paired-end reads were generated. The raw FASTq files (raw reads) were first processed through in-house perl scripts. In this step, clean data (clean reads) were obtained by removing reads containing adapter, reads containing ploy-N, and low-quality reads from raw data. Next, reads were trimmed and cleaned by removing the sequences smaller than 18 nt or longer than 30 nt. At the same time, Q20, Q30, GC-content, and sequence duplication level of the clean data were calculated.
cDNA synthesis and qRT-PCR
Fourteen differentially expressed miRNAs were reverse transcribed into cDNA by using mir-X™ miRNA First-Strand Synthesis Kit (Takara, Dalian, China) according to the manufacturer’s protocol. Then, the SYBR™ Green II qRT-PCR kit (Takara, Dalian, China) was used to detect and quantify miRNA expression on a Bio-Rad CFX 96 (Bio-Rad, CA, USA) following manufacturer’s recommendations. The cycle conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, and 60 °C 30 s. U6 small nuclear RNA was used as internal control to normalize the copy number of the miRNAs by the 2−ΔΔCt method. After normalization, the relative miRNA levels of the infected group were expressed as fold change relative to those of the healthy group. The miRNA-specific primers were synthesized by Sangon Biotech Co. (Shanghai) (Supplementary Table 2). A miRNA was considered to be present only if its mean threshold cycle (Ct) was < 34 (Thomou et al. 2017).
Results
Isolation and characterization of bovine milk exosomes
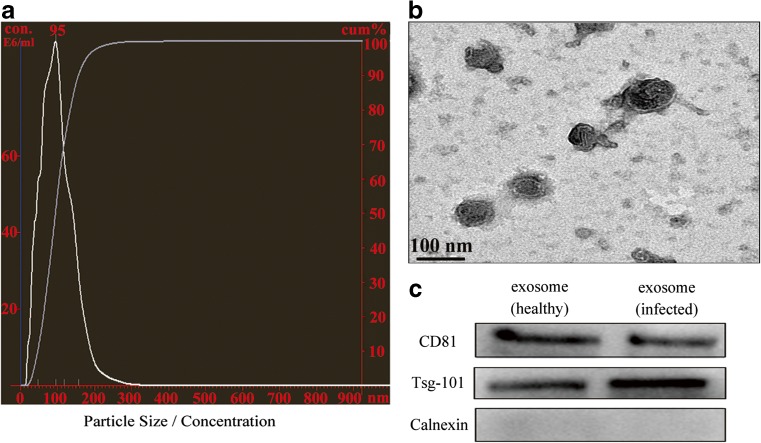
Bovine milk exosomes were isolated by a serial centrifugations. Particle size ranged between 40 and 120 nm in diameter (Fig. 1a). Furthermore, exosomal size and morphology were confirmed to be around 100 nm and presented distinct membrane structure by TEM (Fig. 1b). Exosomal protein lysates were prepared and verified with western blot analysis for special exosomal membrane markers Tsg101 and CD81. The absence of the endoplasmic reticulum marker calnexin indicated that milk-derived vesicles were not contaminated with other multivesicular bodies (Fig. 1c). These results are consistent with previous exosome observations.
Fig. 1.
Isolation and characterization of bovine milk-derived exosomes. a The exosome suspension was analyzed by NanoSight. X-axis represented the size of particles, left Y-axis represented the concentration of particles (E6 particles/mL), and right Y-axis represented the cumulative percentage of particles. b A drop of exosomal suspension was imaged in TEM. c Exosomes isolated in two groups were analyzed for marker proteins with western blot
miRNA sequencing
Approximately 200 μL resuspended exosomes of each sample were used for RNA extraction and next-generation sequencing; six small RNA libraries were constructed and sequenced simultaneously. A total of 11.87 million high-quality reads were generated after removing low-quality reads and adaptor sequences (Table 1). Between the two groups (HH for Holstein healthy group and HM for Holstein mastitic group), nearly 96.72% of the total reads and 9.56% of unique reads were common (Fig. S1). After alignment with small RNA databases, Silva, GtRNAdb, Rfam, and Repbase databases, nearly 90.01% of the clean reads in the HH group and 83.26% in the HM group were identified as alignment reads (Fig. S1). Particularly, no small cytoplasmic RNA (scRNA) and few small nuclear RNA (snRNA) (389 reads) were detected in either group. The remaining reads were used to detect known miRNA, and novel miRNAs predicted based on the miRBase database.
Table 1.
Total number of sequence reads from six small RNA sequencing libraries with number of alignments
| HH1 | HH2 | HH3 | HM1 | HM2 | HM3 | |
|---|---|---|---|---|---|---|
| Total raw reads | 21,050,342 | 23,613,507 | 20,946,610 | 28,659,439 | 20,224,894 | 28,260,353 |
| Clean reads | 17,897,078 | 22,034,379 | 16,171,810 | 26,511,440 | 16,088,610 | 20,025,618 |
| Q30 (%) | 98.27 | 98.78 | 98.79 | 98.8 | 98.4 | 97.08 |
| Number of alignments | 161,14,983 | 20,570,860 | 13,810,757 | 24,904,303 | 14,142,023 | 13,097,810 |
| Mapped reads | 10,204,349 | 16,070,343 | 9,262,399 | 18,930,800 | 9,440,008 | 8,406,855 |
Characteristics of known and novel miRNAs
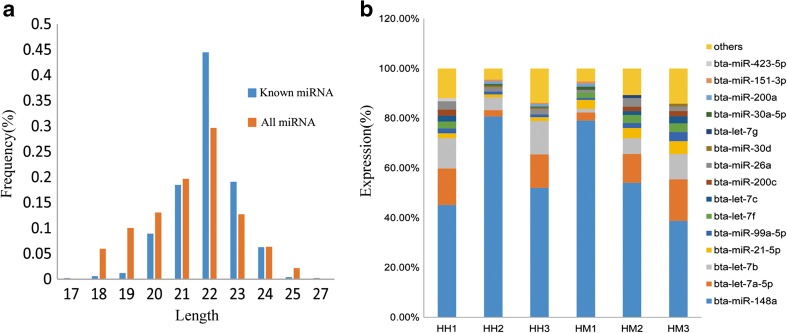
In total, miDeep2 generated 1472 miRNAs in the high-throughput sequencing data from both groups; from these, 492 miRNAs were identified as known bovine miRNAs. The majority of total and known miRNAs were 18–24 nucleotides in length (Fig. 2a; Supplementary Table 3), consistent with the characteristics of miRNA. Among the known miRNAs in the six libraries, ten highly expressed miRNAs accounted for approximately 80–90% of the total reads (Fig. 2b). In addition, 980 unique reads were considered as novel miRNAs (Supplementary Table 4). The novel miRNAs were named as unconservative miRNAs, followed by chromosome number and the serial number generated when the sequence was detected. On average, the total read counts of novel miRNAs were much lower than of known miRNAs. A total of 613 unconservative miRNAs had read counts < 10, and only 66 unique sequences had a total read count > 100 (Supplementary Table 4). Furthermore, removing the 613 unique sequences, 179 sequences belonged to 40 miRNA families, and 115 sequences had significant homology with mir-2284.
Fig. 2.
The length and expression pattern of miRNAs in all libraries. a The distribution by length (nt) of all and known miRNA from bovine milk exosomes. b Top ten miRNAs with the highest expression levels in six bovine milk exosomal miRNA libraries. And 15 miRNAs that are present in the top 10 miRNAs
Differential expression of miRNAs
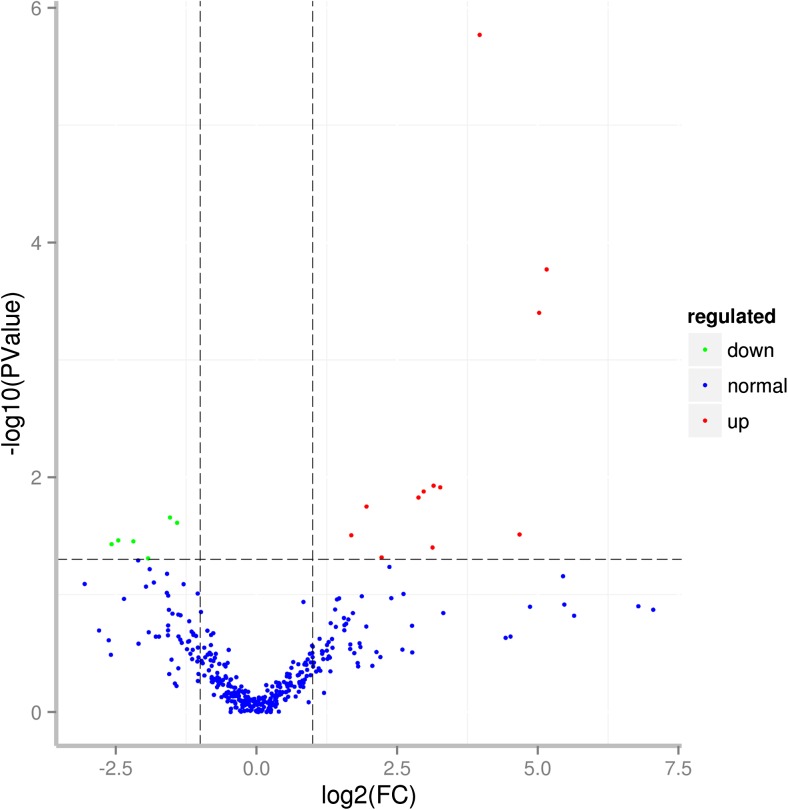
Differential expression (DE) analysis of the two groups was performed using the DESeq R package (1.10.1), and miRNAs with an adjusted p < 0.05 were assigned as differentially expressed. Finally, a total of 18 differentially expressed miRNAs were detected, of which 12 miRNAs were upregulated and six were downregulated. Four of these DE miRNAs were novel (Table 2; Supplementary Table 5). Among these miRNAs, bta-miR-142-5p and bta-miR-223 were also identified to be significantly upregulated in a previous study (Sun et al. 2015). The other differentially expressed miRNAs showed different expression patterns in our studies. Differences in miRNA expression between the two groups are illustrated by Volcano Plot (Fig. 3).
Table 2.
Differential expression of miRNAs in bovine milk-derived exosomes between HH and HM. (*p < 0.05, **p < 0.01)
| miRNA name | p value | log2FC | Regulated | Significance level |
|---|---|---|---|---|
| bta-let-7b | 2.20E−02 | − 1.54 | Down | * |
| bta-miR-103 | 3.12E−02 | 1.69 | Up | * |
| bta-miR-142-3p | 1.22E−02 | 3.27 | Up | * |
| bta-miR-142-5p | 3.07E−02 | 4.68 | Up | * |
| bta-miR-1468 | 4.92E−02 | − 1.92 | Down | * |
| bta-miR-146a | 1.18E−02 | 3.15 | Up | * |
| bta-miR-146b | 1.70E−06 | 3.97 | Up | ** |
| bta-miR-147 | 3.97E−02 | 3.13 | Up | * |
| bta-miR-221 | 1.32E−02 | 2.97 | Up | * |
| bta-miR-223 | 1.70E−04 | 5.16 | Up | ** |
| bta-miR-2284w | 3.97E−04 | 5.02 | Up | ** |
| bta-miR-2285b | 4.82E−02 | 2.22 | Up | * |
| bta-miR-23a | 1.78E−02 | 1.96 | Up | * |
| bta-miR-423-5p | 2.44E−02 | − 1.41 | Down | * |
| unconservative_25_329551a | 3.52E−02 | − 2.19 | Down | * |
| unconservative_25_333271a | 3.72E−02 | − 2.58 | Down | * |
| unconservative_3_387931a | 1.49E−02 | 2.88 | Up | * |
| unconservative_8_520328a | 3.45E−02 | − 2.46 | Down | * |
aNovel miRNAs were named as unconservative miRNAs, which were named as “unconservative_ the number of chromosome_ the serial-number generated when the sequence was detected”
Fig. 3.
Volcano plot for differential expression of miRNAs (red points represent miRNAs significantly upregulated, and green points represent miRNAs significantly downregulated) (color figure online)
Target gene prediction and enriched GO functional categories and KEGG pathways
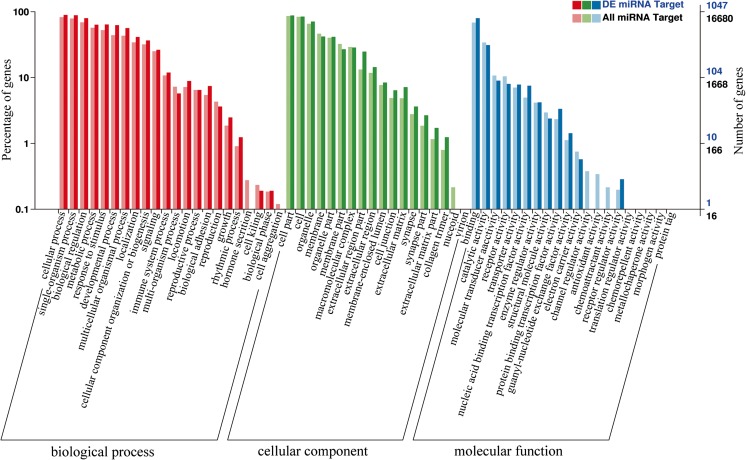
Two software, miRanda and TargetFinder, were employed to predict target genes. In total, 18,028 target genes were identified for all miRNAs, and 1112 target genes might be regulated by the 18 DE miRNAs. Gene ontology (GO) enrichment analysis of all miRNAs and differentially expressed genes was performed by the GOseq R packages based on Wallenius non-central hyper-geometric distribution. The results revealed enrichment of 22 biological processes, such as immune system process, response to stimulus, and growth (Fig. 4). KOBAS software was used to statistically test the enrichment of differential expression genes in KEGG pathways. Interestingly, the target genes of the 18 DE miRNAs were enriched in 11 pathways (Supplementary Table 6).
Fig. 4.
Gene ontology functional annotation of the target genes of differentially expressed and all miRNAs in HH and HM
Verification of miRNA expression by RT-PCR
Fourteen known differentially expressed miRNAs were verified by real time PCR. Among them, three miRNAs (miR-423-5p, miR-1468, let-7b) were downregulated, while nine (miR-142-5p, miR-142-3p, miR-103, miR-147, miR-23a, miR-223, miR-146a, miR-146b, and miR-221) were upregulaed, in agreement with the high-throughput sequencing results (Fig. S2). However, miR-2284w expression levels were below the threshold cycle (Ct) 34, and the expression pattern of miR-2285b detected in qRT-PCR was different from miRNA-seq.
Discussion
Effective ways to cure mastitis include stimulation of the immune responses related to mastitis at the molecular level and effective prophylactic treatments in the early stage. So far, several pathways and cytokines have been identified as inflammatory responses and tissue injuries, such as JAK-STAT, NF-κB, TNF-α, iNOS, and COX-2 (Rossol et al. 2011). A genome-wide association study revealed TRAPPC9 and ARHGAP39 as indicators for bovine mastitis susceptibility (Wang et al. 2015); miRNA profiling of bovine mammary epithelial cells (Jin et al. 2014; Li et al. 2015) and mammary gland (Kirsty et al. 2013) identified miRNAs and mRNAs related to mastitis. Recent studies have hypothesized that exosomal miRNA may be a potential biomarker for disease (Lv et al. 2013). The present study confirmed the presence of exosomes derived from bovine milk, identified the characteristics of exosomal small RNAs, and measured the expression of miRNA in exosomes.
In this study, we performed miRNA sequencing of milk exosomes to provide insight on differential expression of miRNAs and the molecular mechanisms of mastitis. We identified 11.87 million high-quality reads. Interestingly, no scRNAs and few snRNAs were identified based on alignments with small RNA databases. It has been proposed that exosomal cargo can change in response to different cellular conditions (Skog et al. 2008). The mechanisms for selective packaging and release of exosomal RNAs are not well known; however, it has been reported that RNA profiles from exosomes are different from those of host cells. RNA packaging may occur by specific interactions with RNA binding proteins; these interactions are necessary for membrane invagination into the interior of an MVB (Janas et al. 2015). Previous studies have reported that exosomes and parental cells have different types of RNA, with the apparent lack of ribosomal RNA in exosomes (Hessvik et al. 2012; Mittelbrunn et al. 2011).
Only a few miRNAs were highly expressed, ranking in the top ten within each of the six samples and accounting for approximately 80–90% of all known bovine miRNAs. This finding was consistent with those of studies on human breast milk exosomes (Zhou et al. 2012); bovine mammary epithelial cells infected with Streptococcus uberis (Lawless et al. 2013), S. aureus, and E. coli (Jin et al. 2014); and bovine milk exosomes (Sun et al. 2015). Among these miRNAs, bta-miR-148a and bta-30a-3p were also identified by Sun. miR-148a was reported to be abundant in human breast milk exosomes, porcine breast milk exosomes (Gu et al. 2012), and in human and murine plasma cells (Porstner et al. 2015). miR-148a was also highly expressed in bovine milk and was predicted to be a potential biomarker for identifying the quality of raw milk or milk-related commercial products (Chen et al. 2010). miR-30a-5p might ameliorate inflammatory response and oxidative stress by targeting Neurod 1 (Fu et al. 2017). Moreover, the other four miRNAs (miR-200a, miR-21-5p, let-7g, and let-7c) identified in our study have been reported to play roles in immunity. Using miRDeep 2 for novel miRNA prediction, we identified 980 novel miRNAs in bovine milk exosomes. Only 367 novel miRNAs had read counts > 10, which is consistent with previous reports (Sun et al. 2015). A previous study identified seven members of the bta-miR-2284/2285 family among the eight more novel candidate miRNAs (Guduricfuchs et al. 2012). We also detected 115 unique sequences showing significant homology with mir-2284. It is possible that these highly expressed miRNAs in bovine milk exosomes may be ingested and play post-transcriptional regulation in calves.
A total of 492 known miRNAs were identified, and 14 known differentially expressed miRNAs were found in the two groups. Among these DE miRNAs, miR-223 (log2FC = 5.16) and miR-142-5p (log2FC = 4.68) were highly regulated post-infection, which is consistent with previous reports on bovine milk exosomes (Sun et al. 2015) and bovine mammary gland infected with S. aureus or S. uberis (Li et al. 2015; Naeem et al. 2012). In bovine colostrum milk, a higher level of miR-223 was reported to be involved in immune response and immune system development (Fazi et al. 2005). Interestingly, miR-223 was also identified to inhibit classic pro-inflammatory pathways and was therefore considered as a novel regulator of diet-induced adipose tissue inflammatory response (Chen et al. 2004). Furthermore, a novel miR-223–CEBP-β–LMO2–miR-142 regulatory pathway has been proposed to have pivotal functions in hematopoiesis (Sun et al. 2010). Thus, a deeper analysis of the biological regulation of miR-223 and miR-142-5p in mammary epithelial cells is needed, as these miRNAs may play crucial roles in bovine mastitis. Further, miR-146a/b was also significantly upregulated in milk exosomes post-infection. Similar upregulation of miR-146a and miR-146b was detected in bovine mammary gland after injection with S. aureus (Wang et al. 2016) and in human monocytes reacting to lipopolysaccharides (Taganov et al. 2006). Furthermore, miR-146b was proposed to be involved in luminal alveolar progenitor cell maintenance by regulating STAT3 (Elsarraj et al. 2013). Regarding the downregulation of miRNAs, miR-1468 participates in the regulation of cell cycle progression by targeting RRM1 (Jiang et al. 2017). Let-7b (Teng et al. 2013) and miR-423 (Wang et al. 2017) have been shown to regulate the activation of NF-κB signaling by targeting TLR4 and TNIP2, respectively. Our results provide important information about miRNAs differentially expressed in bovine milk exosomes and specific miRNAs that could be potential candidate for early diagnostic detection of mastitis. Furthermore, differentially expressed miRNAs packaged by exosomes post-infection might be delivered to recipient cells, such as macrophages, for cell-to-cell communication, and may be functional in the new location.
It was notable that predicted gene targets of DE miRNAs were enriched in GO terms, such as cellular process, developmental process, metabolic process, immune system process, and growth. This result indicated the involvement of miRNAs in numerous developmental and physiological processes, including disease and immune development. The results of the KEGG pathway analysis indicated that target genes were associated with cancer, inflammatory, and immune pathway. This result further illuminated the function of bovine milk-derived exosomes in growth and productive capacities as well as defense capabilities in infants.
Conclusion
Using deep sequencing, we characterized the miRNA profiling of bovine milk-derived exosomes in healthy and S. aureus-infected mastitic groups. We found that no scRNA and few snRNA existed in bovine milk exosomes. In addition, 492 known and 980 novel miRNAs were detected, and ten highly expressed miRNAs accounted for 80–90% of the total reads. Differential expression analysis showed that 14 known miRNAs and four novel miRNAs had significant differential expression. Among these miRNAs, miR-223 and miR-142-5p were considered as potential targets for early detection of mastitis. GO and KEGG pathway analysis showed that predicted target genes were significantly enriched in immunity.
Electronic supplementary material
Distribution and classification of small RNA reads in the HH and HM. (PPT 442 kb)
Comparison of the results of miRNA-sequencing and qRT-PCR. (PPT 159 kb)
The SCC of six Holstein cows. (XLSX 9 kb)
Primer sequences for qRT-PCR. (XLSX 10 kb)
The length and expression of miRNAs. (XLSX 10 kb)
The read counts and miRNA family analysis of novel miRNAs (XLSX 69 kb)
The expression of differentially expressed miRNAs. (XLSX 12 kb)
KEGG pathways of the target genes. (XLSX 10 kb)
Author contributions
SJL and MCC contributed to concept design. HBH and WDX performed the animal experiments. MCC, BWL, and YS performed the RNA and protein isolation and western blot. MCC, HBH, and XBJ performed data analysis. MCC, SYC, and JW prepared the manuscript. All authors read and approved the final manuscript.
Funding information
This research was supported by the Science and Technology Support Program in Sichuan (No. 2014NZ0032-A).
Compliance with ethical standards
All experiments were carried out according to the Regulations for the Administration of Affairs Concerning Experimental Animals published by the Ministry of Science and Technology, China in 2004 and approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University, China.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Mingcheng Cai and Hongbing He contributed equally to this work
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0876-3) contains supplementary material, which is available to authorized users.
References
- Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EPA, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72(7):4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F, Schartz NEC, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Paape MJ, Lee J-W, Zhao X, Hope JC, Rainard P. Escherichia coli and Staphylococcus aureus elicit differential innate immune responses following intramammary infection. Clin Diagn Lab Immunol. 2004;11(3):463–472. doi: 10.1128/CDLI.11.3.463-472.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Z, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science (80- ) 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen X, Gao C, Li H, Huang L, Sun Q, Dong Y, Tian C, Gao S, Dong H, Guan D, Hu X, Zhao S, Li L, Zhu L, Yan Q, Zhang J, Zen K, Zhang CY. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010;20(10):1128–1137. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- Chivet M, Javalet C, Hemming F, Pernet-Gallay K, Laulagnier K, Fraboulet S, Sadoul R. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241–244. doi: 10.1042/BST20120266. [DOI] [PubMed] [Google Scholar]
- De Schepper S, De Ketelaere A, Bannerman DD, Paape MJ, Peelman L, Burvenich C. The toll-like receptor-4 (TLR-4) pathway and its possible role in the pathogenesis of Escherichia coli mastitis in dairy cattle. Vet Res. 2008;39(1):1–23. doi: 10.1051/vetres:2007044. [DOI] [PubMed] [Google Scholar]
- DeGraves FJ, Fetrow J. Economics of mastitis and mastitis control. Vet Clin North Am Food Anim Pract. 1993;9(3):421–434. doi: 10.1016/S0749-0720(15)30611-3. [DOI] [PubMed] [Google Scholar]
- Dilda F, Gioia G, Pisani L, Restelli L, Lecchi C, Albonico F, Bronzo V, Mortarino M, Ceciliani F. Escherichia coli lipopolysaccharides and Staphylococcus aureus enterotoxin B differentially modulate inflammatory microRNAs in bovine monocytes. Vet J. 2012;192(3):514–516. doi: 10.1016/j.tvjl.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Elsarraj HS, YH KV, Carletti M, Salah SM, Raimo M, Taverna D, Prochasson P, Bharadwaj U, Tweardy DJ, Christenson LK, Behbod F. A novel role of microRNA146b in promoting mammary alveolar progenitor cell maintenance. J Cell Sci. 2013;126(11):2446–2458. doi: 10.1242/jcs.119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPα regulates human granulopoiesis. Cell. 2005;123(5):819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Février B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fu X, Shen Y, Wang W, Li X. MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting Neurod 1 through MAPK/ERK signaling. Clin Exp Pharmacol Physiol. 2017;45(1):68–74. doi: 10.1111/1440-1681.12856. [DOI] [PubMed] [Google Scholar]
- Gatti J-L, Métayer S, Belghazi M, Dacheux F, Dacheux J-L. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles 1. Biol Reprod. 2005;72(6):1452–1465. doi: 10.1095/biolreprod.104.036426. [DOI] [PubMed] [Google Scholar]
- Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang X, Zhou Q, Chen L, Lang Q, He Z, Chen X, Gong J, Gao X, Li X, Lv X. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PLoS One. 2012;7(8):e43691. doi: 10.1371/journal.pone.0043691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guduricfuchs J, et al. Deep sequencing reveals predominant expression of miR-21 amongst the small non-coding RNAs in retinal microvascular endothelial cells. J Cell Biochem. 2012;113(6):2098–2111. doi: 10.1002/jcb.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta. 2012;1819(11-12):1154–1163. doi: 10.1016/j.bbagrm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Janas T, Janas MM, Sapoń K, Janas T. Mechanisms of RNA loading into exosomes. FEBS Lett. 2015;589(13):1391–1398. doi: 10.1016/j.febslet.2015.04.036. [DOI] [PubMed] [Google Scholar]
- Jiang K, et al. MicroRNA-1468-5p inhibits glioma cell proliferation and induces cell cycle arrest by targeting RRM1. Am J Cancer Res. 2017;7:784. [PMC free article] [PubMed] [Google Scholar]
- Jin W, Ibeagha-Awemu EM, Liang G, Beaudoin F, Zhao X. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genomics. 2014;15(1):181. doi: 10.1186/1471-2164-15-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsty J, et al. Escherichia coli- and Staphylococcus aureus-induced mastitis differentially modulate transcriptional responses in neighbouring uninfected bovine mammary gland quarters. BMC Genomics. 2013;14(1):36. doi: 10.1186/1471-2164-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, Isales CM, Guldberg RE, Hamrick MW, Fulzele S. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Gupta D, Shankar S, Srivastava RK. Biomolecular characterization of exosomes released from cancer stem cells: possible implications for biomarker and treatment of cancer. Oncotarget. 2015;6(5):3280–3291. doi: 10.18632/oncotarget.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless N, Foroushani AB, McCabe MS, O’Farrelly C, Lynn DJ. Next generation sequencing reveals the expression of a unique miRNA profile in response to a gram-positive bacterial infection. PLoS One. 2013;8(3):e57543. doi: 10.1371/journal.pone.0057543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levänen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Sköld CM, Svartengren M, Grunewald J, Gabrielsson S, Eklund A, Larsson BM, Woodruff PG, Erle DJ, Wheelock ÅM. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131(3):894–903.e8. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang CL, Liao XX, Chen D, Wang WQ, Zhu YH, Geng XH, Ji DJ, Mao YJ, Gong YC, Yang ZP. Transcriptome microRNA profiling of bovine mammary glands infected with Staphylococcus aureus. Int J Mol Sci. 2015;16(3):4997–5013. doi: 10.3390/ijms16034997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Liu R, Yin L, Pu Y. Expression profiling of exosomal miRNAs derived from human esophageal cancer cells by Solexa high-throughput sequencing. Int J Mol Sci. 2014;15(9):15530–15551. doi: 10.3390/ijms150915530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv L-L, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- Michael A, Bajracharya S, Yuen P, Zhou H, Star R, Illei G, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16(1):34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371(1):48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem A, Zhong K, Moisá S, Drackley J, Moyes K, Loor J. Bioinformatics analysis of microRNA and putative target genes in bovine mammary tissue infected with Streptococcus uberis. J Dairy Sci. 2012;95(11):6397–6408. doi: 10.3168/jds.2011-5173. [DOI] [PubMed] [Google Scholar]
- Pêche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection1. Transplantation. 2003;76(10):1503–1510. doi: 10.1097/01.TP.0000092494.75313.38. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porstner M, Winkelmann R, Daum P, Schmid J, Pracht K, Côrte-Real J, Schreiber S, Haftmann C, Brandl A, Mashreghi MF, Gelse K, Hauke M, Wirries I, Zwick M, Roth E, Radbruch A, Wittmann J, Jäck HM. miR-148a promotes plasma cell differentiation and targets the germinal center transcription factors Mitf and Bach2. Eur J Immunol. 2015;45(4):1206–1215. doi: 10.1002/eji.201444637. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31(5):379–446. doi: 10.1615/CritRevImmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Shen W, Yang S, Hu F, Li H, Zhu T-H. miR-223 and miR-142 attenuate hematopoietic cell proliferation, and miR-223 positively regulates miR-142 through LMO2 isoforms and CEBP-β. Cell Res. 2010;20(10):1158–1169. doi: 10.1038/cr.2010.134. [DOI] [PubMed] [Google Scholar]
- Sun J, Aswath K, Schroeder SG, Lippolis JD, Reinhardt TA, Sonstegard TS. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genomics. 2015;16(1):806. doi: 10.1186/s12864-015-2044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng GG, Wang WH, Dai Y, Wang SJ, Chu YX, Li J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS One. 2013;8(2):e56709. doi: 10.1371/journal.pone.0056709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosa Paredes P, Gutzeit C, Johansson S, Admyre C, Stenius F, Alm J, Scheynius A, Gabrielsson S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69(4):463–471. doi: 10.1111/all.12357. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wahlgren J, Karlson TDL, Brisslert M, Sani FV, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:1–5. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma P, Liu J, Zhang Q, Zhang Y, Ding X, Jiang L, Wang Y, Zhang Y, Sun D, Zhang S, Su G, Yu Y. Genome-wide association study in Chinese Holstein cows reveal two candidate genes for somatic cell score as an indicator for mastitis susceptibility. BMC Genet. 2015;16(1):1–9. doi: 10.1186/s12863-015-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Zhuo ML, Zan LS, Raza SHA, Li F, Li N, Liu S. Expression patterns of miR-146a and miR-146b in mastitis infected dairy cattle. Mol Cell Probes. 2016;30(5):342–344. doi: 10.1016/j.mcp.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Wang W, Gao J, Wang F. MiR-663a/MiR-423-5p are involved in the pathogenesis of lupus nephritis via modulating the activation of NF-κB by targeting TNIP2. Am J Transl Res. 2017;9(8):3796–3803. [PMC free article] [PubMed] [Google Scholar]
- Wieckowski E, Whiteside TL. Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res. 2006;36(1-3):247–254. doi: 10.1385/IR:36:1:247. [DOI] [PubMed] [Google Scholar]
- Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J Mammary Gland Biol Neoplasia. 2011;16(4):357–372. doi: 10.1007/s10911-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8(1):118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution and classification of small RNA reads in the HH and HM. (PPT 442 kb)
Comparison of the results of miRNA-sequencing and qRT-PCR. (PPT 159 kb)
The SCC of six Holstein cows. (XLSX 9 kb)
Primer sequences for qRT-PCR. (XLSX 10 kb)
The length and expression of miRNAs. (XLSX 10 kb)
The read counts and miRNA family analysis of novel miRNAs (XLSX 69 kb)
The expression of differentially expressed miRNAs. (XLSX 12 kb)
KEGG pathways of the target genes. (XLSX 10 kb)