Abstract
Hsp70-1A—the major stress-inducible member of the HSP70 chaperone family—is being implicated in cancer diseases with the development of resistances to standard therapies. In normal cells, the protein is purely cytosolic, but in a growing number of tumor cells, a significant fraction can be identified on to the cell surface. The anchoring mechanism is still under debate, as Hsp70-1A lacks conventional signaling sequences for translocation from the cytosol to exoplasmic leaflet of the plasma membrane and common membrane binding domains. Recent reports propose a lipid-mediated anchoring mechanism based on a specific interaction with charged, saturated lipids such as dipalmitoyl phosphatidylserine (DPPS). Here, we prepared planar supported lipid bilayers (SLBs) to visualize the association of Hsp70-1A directly and on the single molecule level by atomic force microscopy (AFM). The single molecule sensitivity of our approach allowed us to explore the low concentration range of 0.05 to 1.0 μg/ml of Hsp70-1A which was not studied before. We compared the binding of the protein to bilayers with 20% DPPS lipid content both in the absence and presence of cholesterol. Hsp70-1A inserted exclusively into DPPS domains and assembled in clusters with increasing protein density. A critical density was reached for incubation with 0.5 μg/ml (7 nM); at higher concentrations, membrane defects were observed that originated from cluster centers. In the presence of cholesterol, this critical concentration leads to the formation of membrane blebs, which burst at higher concentrations supporting a previously proposed non-classical pathway for the export of Hsp70-1A by tumor cells. In the discussion of our data, we attempt to link the lipid-mediated plasma membrane localization of Hsp70-1A to its potential involvement in the development of resistances to radiation and chemotherapy based on our own findings and the current literature.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0879-0) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein, Cancer, Hsp70, Supported lipid bilayer, DPPS, Membranes, Stress
Introduction
Heat shock protein 70-1A (Hsp70-1A) is the major stress-inducible member of the HSP70 chaperone family. Under normal conditions, it resides in low concentrations in the cytosol. Under stressful conditions, it is highly expressed to support folding, refolding, and assembly of nascent polypeptides, prevent protein aggregation, and assist transport of other proteins across membranes. In a majority of human tumors, Hsp70-1A is permanently overexpressed, mediating tumor progression and conferring improved survival chances to the tumor cells such as increased resistance to apoptosis (Juhasz et al. 2013; Radons 2016). In a growing number of tumor cells, a significant fraction of Hsp70-1A has been detected on the cell surface where it is integrated into the extracellular leaflet of the plasma membrane (Hantschel et al. 2000; Multhoff 2007; Multhoff et al. 1995; Schilling et al. 2009). The cancer cell specific plasma membrane expression of Hsp70-1A (mHsp70) has been implicated with the development of tumor resistance to radiation therapy, increased invasiveness, the development of distant metastasis, and a poor prognosis for the patient (Farkas et al. 2003; Gehrmann et al. 2005; Murakami et al. 2015; Pfister et al. 2007; Schilling et al. 2009). However, the exact mechanisms of transport and anchorage of Hsp70 to the plasma membrane remain still unresolved, and it is hypothesized that these may hold a key to deciphering the plasma membrane associated functions.
Hsp70-1A lacks a consensus signaling sequence for translocation from the cytosol (Multhoff 2007); thus, the possibility of specific lipid-binding domains is currently debated (Armijo et al. 2014; Mahalka et al. 2014; McCallister et al. 2016). The lipids of the plasma membrane are asymmetrically distributed across the bilayer (Kiessling et al. 2009). In the exoplasmic leaflet, mainly phosphatidylcholine (PC) and sphingomyelin (SM) with neutral head groups are found. The cytoplasmic leaflet is rich in PC and negatively charged phosphatidylserine (PS), phosphatidylethanolamine, and phosphatidylinositols. The groups of De Maio and Multhoff were the first to discover specific interactions of Hsp70-1A with anionic lipids and PS in particular in artificial membranes (Arispe et al. 2002; Arispe et al. 2004; Armijo et al. 2014), whereas interaction with neutral lipids such as PC was only weak and unspecific. These observations were confirmed independently by other researchers (Mahalka et al. 2014; McCallister et al. 2016). Emphasizing the relevance of PS for membrane anchorage and in elucidating the role of mHsp70 in cancer, it was demonstrated that certain stressful conditions such as hypoxia initiate the synthesis and export of Hsp70-1A in vitro in conjunction with translocation of PS to the cell surface (Schilling et al. 2009).
Recently, the interaction of Hsp70-1A with different PS lipids was characterized in greater detail employing lipid vesicle sedimentation assays (LVS) (Armijo et al. 2014; McCallister et al. 2016). Liposomes were incubated with the protein, centrifuged, and analyzed by gel electrophoresis. It was found that insertion into liposomes was enhanced by increasing the saturation level of the fatty acid chains of the lipid. Association with dioleoyl phosphatidylserine (DOPS; two mono-unsaturated fatty acid chains) was significantly reduced compared to 1-palmitoyl-2-oleoyl phosphatidylserine (POPS; one mono-unsaturated and one saturated fatty acid chain), whereas incorporation into dipalmitoyl phosphatidylserine (DPPS; two saturated lipid tails) was clearly elevated. This finding may be of high biomedical relevance, as the plasma membrane of tumor cells is often enriched in saturated phospholipids, which protect the cell from oxidative damage, inhibit uptake of chemotherapeutics, and alter signal transduction (van Meer et al. 2008).
In this study, we employed atomic force microscopy (AFM) imaging to investigate the interaction of Hsp70-1A with PS lipids in planar supported lipid bilayers (SLBs). AFM provides the lateral and vertical resolution to visualize individual proteins on the surface of flat membrane preparations under physiological conditions, directly and without labeling. Apart from the saturation level of the fatty acid chains in PS, we compared the influence of presence of cholesterol in the membrane. The results are discussed in light of the previously suggested link between a PS-mediated mechanism for export and anchorage of Hsp70-1A to the cell surface and a potential role of mHsp70 in the development of resistances to radiation therapy.
Experimental section
Chemicals
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC) (transition temperature − 17 °C), sphingomyelin from chicken egg yolk (eSM) (transition temperature ~ 41 °C), and cholesterol (chol) were purchased as lyophilized powders from Sigma-Aldrich (Vienna, Austria). 1,2-Dioleoyl-sn-glycero-3-phospho-L-serine (sodium salt) (DOPS) (transition temperature − 11 °C) and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (transition temperature 41 °C) were obtained dissolved in chloroform and 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DPPS) (transition temperature 54 °C) as powder from Avanti Polar Lipids (Alabaster, AL, United States). Human Hsp70-1A was purchased from Enzo Life Science (ADI-ESP-555-D, Lausen, Switzerland). Buffer A containing 10 mM HEPES, 150 mM NaCl, 2 mM CaCl2 at pH 7.4 was used for sample preparation and buffer B with 10 mM HEPES and 150 mM NaCl at pH 7.4 for imaging. Buffers were prepared with MilliQ water and filtered through 0.22-μm membrane filters (Rotilabo®-syringe filters; Carl Roth, Karlsruhe, Germany).
Lipid mixtures
Mixtures with 5 mg total lipid with the following compositions and molar ratios were prepared: DPPC/DPPS 80:20, DPPC/DPPS/chol 64:16:20, DOPC/DOPS 80:20, DOPC/DOPS/chol 64:16:20, and DOPC/eSM/chol 40:40:20. For each mixture, the corresponding amounts of lipids were co-dissolved in a pear-shaped flask in 3 ml chloroform/methanol (2:1 v/v), and the solvents were evaporated on a rotary evaporator for 30 min. The lipid film was dissolved in 3 ml chloroform followed again by evaporation on a rotary evaporator. Residual solvent was removed under high vacuum for 2 h. The dry lipid films were re-hydrated in 500 μl MilliQ water for 15 min at 70 °C for DPPS containing mixtures, at 50 °C for eSM mixtures, and at ambient temperature for DOPS containing mixtures, and then vigorously vortexed resulting in milky lipid suspensions (10 mg/ml). All solutions were subjected to 8 freeze-thaw cycles to break down large multilamellar vesicles, aliquoted, and stored at − 20 °C until further processing.
Preparation of supported lipid bilayers
SLBs were prepared by the vesicle fusion method. For this procedure, aliquoted lipid solutions were first diluted in buffer A to a concentration of 1 mg/ml and extruded (Avanti Mini Extruder, Avanti Polar Lipids, Alabaster, AL, USA) through polycarbonate membranes with 50 nm pore size (Merck Millipore, Vienna, Austria) to obtain small unilamellar vesicles (SUVs). After extrusion, the solutions were further diluted in buffer A to a concentration of 0.5 mg/ml and then incubated on freshly cleaved mica for 30 min.
Depending on the lipid composition and under consideration of the respective lipid transition temperatures, both extrusion and incubation were done at following temperatures: 70 °C for DPPC/DPPS and DPPC/DPPS/chol, 50 °C for DOPC/eSM/chol, ambient temperature for DOPC/DOPS, and DOPC/DOPS/chol. For incubation on mica at elevated temperatures, samples were placed in a pre-heated humidified chamber to prevent evaporation of liquid during SLB formation. The heat-treated samples were slowly cooled down to 21 °C at a rate of approximately 0.5 °C/min. For AFM imaging, samples were mounted into a sample holder equipped with a fluid cell (fluid volume 600 μl) and rinsed thoroughly with buffer B 50 times. During all steps of preparation, great care was taken to keep the mica substrates under liquid (droplet) to prevent disruption of the SLBs.
Incubation of SLBs with Hsp70-1A
Hsp70-1A was diluted in 300 μl of buffer B to different concentrations (0.1, 0.15, 0.2, 0.5, 1.0, or 2.0 μg/ml) and well dispersed. The 300-μl protein solution was then added to 300 μl of buffer B that were already in the fluid cell with the SLBs and carefully mixed with the pipette, yielding final protein concentrations of 0.05, 0.075, 0.1, 0.25, 0.5, and 1.0 μg/ml, respectively. The SLBs were incubated with Hsp70-1A for 30 min at either 41 °C for DPPS containing SLBs, at 37 °C for eSM containing SLBs, or at ambient temperature for DOPS containing SLBs. After incubation, the buffer in the fluid cell was exchanged 5 times with buffer of 21 °C to remove unbound Hsp70-1A. The samples were left to settle and equilibrate to ambient temperature for another 10 min.
AFM imaging
Topographical imaging of SLBs was performed in liquid under ambient conditions on a PicoPlus 5500 AFM setup (Keysight, USA) equipped with a fluid chamber (volume 600 μl). Images were recorded with 512 scan lines at scan rates of 1–2.5 Hz in acoustic oscillation (intermittent contact) mode using MSCT-F cantilevers (Bruker AFM Probes, Camarillo, CA, USA) with a nominal spring constant of 0.6 N/m and a resonance frequency in buffer of ~ 30 kHz. For large-scale images (50 × 50 to 20 × 20 μm2), the vertical range of the AFM scanner was reduced to 3 μm. For small-scale images (< 20 × 20 μm2), the vertical range was reduced to 2 μm in order to improve vertical resolution on the flat SLBs. AFM nanolithography was done in selected small areas (500 × 500 nm2) to remove surface-bound molecules by performing a sequence of 10 rapid scans at a scan rate of 20 lines/s using high forces of typically > 20 nN.
Data processing and analysis
Image processing and extraction of cross sections were done in Gwyddion FreeSPM 2.44 (Nečas and Klapetek 2012). Image processing involved multiple steps. After an initial simple tilt fit, followed by coarse line correction (median difference method), a mask was applied to exclude the topographically higher liquid ordered and/or gel phase in the next step of background leveling. Then, a polynomial background with a third-order polynomial fit in horizontal direction and a second-order polynomial fit in vertical direction was subtracted. Finally, a second step of line correction (median method) and removal of scars were applied. Cross sections were taken from processed images and exported as text files for plotting in OriginPro8. Area fractions of ordered domains in SLBs were discriminated by the z-scale of the processed AFM images using ImageJ (Schindelin et al. 2015; Schneider et al. 2012). Figures were assembled in CorelDrawX7.
Results
Characterization of the pristine DPPC/DPPS (80:20 mol%) bilayer and the height of Hsp70-1A proteins
Following previous studies (Armijo et al. 2014; McCallister et al. 2016), we prepared a lipid mixture of DPPC (16:0 PC) and DPPS (16:0 PS) with a molar ratio of 80:20 and used the vesicle fusion method to obtain planar SLBs on freshly cleaved ultra-flat mica sheets. Extrusion of the lipid mixture through 50-nm filter membranes and incubation of the resulting small unilamellar vesicles (SUV) to mica were performed at 70 °C, well above the transition temperatures of DPPC (41 °C) and DPPS (54 °C). The samples were kept at 70 °C for 30 min followed by cooling to 21 °C ambient temperature at a slow rate of 0.5 °C/min. After vigorous rinsing, the resulting bilayers were examined by AFM imaging in buffer solution. Imaging was done in acoustic oscillation (AAC mode), where the AFM cantilever touches the sample only intermittently during downward movement of the cantilever oscillation, thus providing for gentle imaging conditions with reduced physical perturbation of the sample.
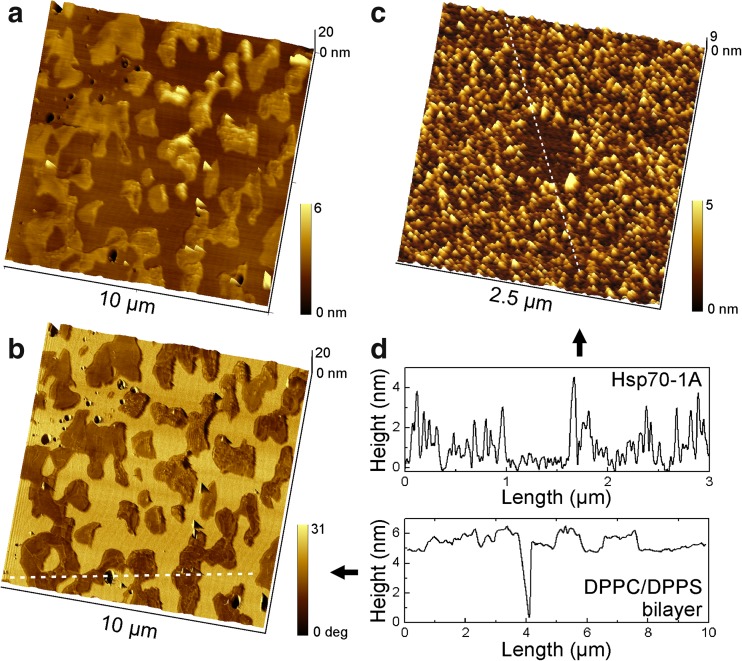
Figure 1a shows a representative topographical image of a cohesive DPPC/DPPS bilayer. The image reveals segregation of DPPS lipid domains from a homogenous phase of DPPC lipid. This segregation emerges during cooling from 70 °C to ambient temperature when DPPS starts to transit from the liquid phase into gel phase below 54 °C while DPPC continues to remain in the liquid phase until the temperature drops below 41 °C. The segregation was also well distinguishable in the phase contrast image (Fig. 1b), which results from the shift of the cantilever oscillation in response to the different physical properties of DPPS domains and the surrounding DPPC lipid phase. In Fig. 1b, the DPPS domains appear as a dark contrast (brown) in the surrounding DPPC phase (yellow). A cross section through the different phases and a small bilayer defect (Fig. 1d, bottom graph) indicates a layer height of ~ 5 nm for the DPPC ‘background’. The protruding DPPS domains exhibited a corrugated surface with rather irregular height making it difficult to estimate the layer thickness. A deviation from the height of the DPPC background between − 0.5 and + 1.5 nm within a single domain was commonly observed.
Fig. 1.
a AFM topography image of a phase separated DPPC/DPPS (80:20) bilayer on mica, captured in intermittent contact mode in buffer solution at ambient temperature. The DPPS phase emerged as islands with a corrugated surface from the surrounding smooth DPPC phase. b The overlay of the topography with the phase shift image shows DPPS domains as dark contrast. c Human Hsp70-1A adsorbed on ultra-flat mica. In the center of the image, proteins were removed by AFM lithography (square). d Cross sections along the indicated lines in b and c illustrate typical height profiles
Next, a solution of 2 μg/ml Hsp70-1A in buffer (10 mM HEPES, 150 mM NaCl, pH 7.4) was incubated to freshly cleaved mica for 20 min to determine the height of the protein molecules and compare with the topography of the bilayer. Freshly cleaved mica has a negative surface charge at neutral pH, which facilitates immobilization of Hsp70-1A via electrostatic adsorption. The sample was carefully rinsed to remove unattached molecules and imaged in AAC mode (Fig. 1c). In order to facilitate height determination, a small square (500 by 500 nm2) was cleared of protein by AFM lithography. Section analysis (Fig. 1d, top graph) across this area displayed a height of ∼ 2.5 nm. Bare mica exhibited a typical surface roughness of less than 0.5 nm.
Selective insertion of Hsp70-1A into DPPS domains in DPPC/DPPS bilayers
Having established the characteristic topography of the DPPC/DPPS bilayer and the height of Hsp70-1A molecules, we incubated bilayers with different concentrations of protein ranging from 0.05 to 1.0 μg/ml for 30 min. Unbound protein was removed through several steps of buffer exchange. In a first set of experiments, incubation was done at ambient temperature (23 °C), but we found that Hsp70-1A was absent on all bilayers irrespective of the protein concentration. As DPPC and DPPS assume a gel phase at ambient temperature, the rigidity of the bilayer might have prevented insertion of the protein.
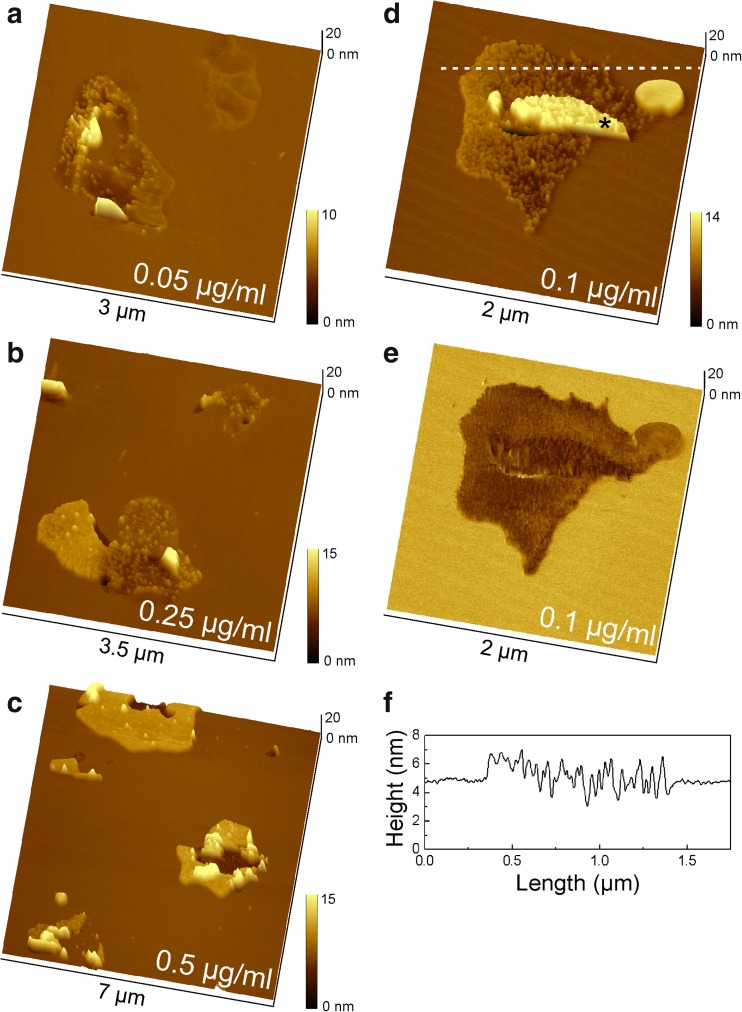
Figure 2a–d shows DPPC/DPPS bilayers that were incubated at 41 °C with Hsp70-1A concentrations of 0.05 to 0.5 μg/ml. The samples were equilibrated to ambient temperature after washing. Hsp70-1A molecules became visible at small scan ranges and were found to associate only with DPPS domains and not with the surrounding DPPC lipids. The overlay of topography image and phase contrast image (Fig. 2e) clearly shows localization of the molecules in areas with dark phase contrast, which was indicative of DPPS domains in the pristine bilayer (Fig. 1b). The height of SLB-associated Hsp70-1A was around 1.5 nm (Fig. 2f), though an accurate height estimation was difficult, because of the corrugated and irregular height profile of DPPS domains. With the estimate of 1.5 nm, the height of SLB-associated Hsp70-1A was lower than the height of the free protein on bare mica support, indicating either insertion or a structural rearrangement of the protein on the membrane.
Fig. 2.
DPPC/DPPS (80:20) bilayer after incubation with Hsp70-1A for 30 min. Molecules selectively associated with DPPS domains of the bilayer and formed clusters in a concentration-dependent manner. a Protein concentration of 0.05 μg/ml, b 0.25 μg/ml, and c 0.5 μg/ml. At a maximum concentration of 0.5 μg/ml, Hsp70-1A was densely packed and large membrane defects appeared in the center of each protein cluster. d Topography and corresponding phase shift image (e) at a protein concentration of 0.1 μg/ml clearly show that the protein associated only with DPPS domains. Note that part of the protein layer (marked with an asterisk *) is covered by an additional bilayer patch. f Representative height profile across a section marked in subfigure (d) for approximation of the height of inserted proteins
Interestingly, the associated molecules appeared to progress towards centralized clusters within DPPS domains. This tendency was visible even at the lowest concentration of 0.05 μg/ml (Fig. 2a). The packing density reached a maximum at a concentration of ~ 0.5 μg/ml, where all DPPS islands were covered with a dense monolayer of protein (Fig. 2c). Further increase of the Hsp70-1A concentration did not change that picture. At close packing, the lipid domains became prone to disruption and defects, which were enlarged probably due to shear during rinsing and scanning. However, proteins did not dissociate during continuous scanning hinting at firm insertion into the DPPS domains.
Hsp70-1A localizes in the liquid ordered phase of DPPC/DPPS/chol bilayers
Next, we included 20 mol% of cholesterol into the 80:20 mixture of DPPC/DPPS, resulting in a ratio of 64:16:20 mol% of DPPC/DPPS/chol. Cholesterol is an essential component of the eukaryotic plasma membrane with up to 45 mol% of the total lipid content, depending on the cell type (Marquardt et al. 2016). Cholesterol has an ordering effect on the bilayer (Marquardt et al. 2016; van Duyl et al. 2003), i.e., the hydroxyl group of cholesterol interacts with the polar head groups of the lipids and its acyl chain aligns with the lipid fatty acids, resulting in increased lipid chain order and bilayer thickening. We were thus interested, whether the presence of cholesterol would markedly influence the insertion of Hsp70-1A into the lipid bilayer.
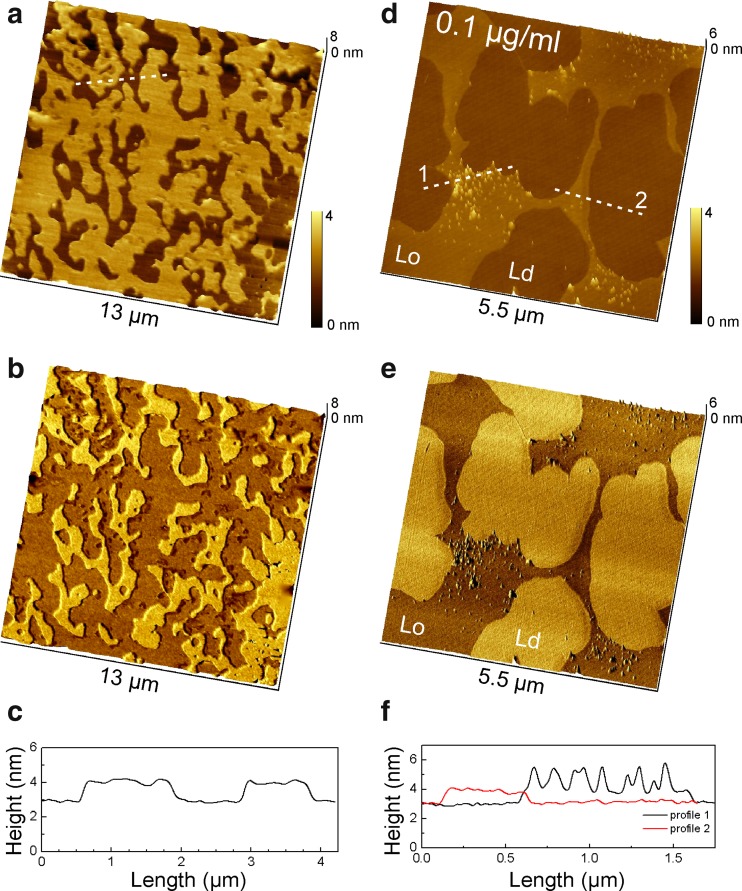
Similarly to the cholesterol-free condition, the 64:16:20 DPPC/DPPS/chol bilayer exhibits lipid phase separation at ambient temperature (Fig. 3a, b), but with a smooth percolating phase that likely presents a cholesterol-rich liquid ordered (Lo) phase. The Lo phase was ~ 1.2 nm higher than the surrounding lipid phase (Fig. 3c) and well visible in the AFM phase contrast images as darker shade (Fig. 3b, e). After incubation with 0.1 μg/ml Hsp70-1A for 30 min, the protein was found to insert only into the Lo phase and in form of loose but localized clusters, as opposed to a uniform distribution of isolated molecules (Fig. 3d, e). The height of the protein in the Lo phase was 1.5 nm (Fig. 3f) just as in the DPPS domains of the DPPC/DPPS bilayer (Fig. 2f). In contrast to the cholesterol-free bilayer, protein insertion was also observed at ambient temperature, possibly as a result of increased membrane fluidity of the Lo phase due to cholesterol (Fritzsching et al. 2013). Nonetheless, in subsequent experiments on DPPC/DPPS/chol bilayers, incubation was continued at 41 °C for consistency of the temperature protocol and comparability with DPPC/DPPS bilayers.
Fig. 3.
Phase separated DPPC/DPPS/chol 64:16:20 bilayer on mica at ambient temperature before (a–c) and after incubation with 0.1 μg/ml Hsp70-1A for 30 min (d–f). Topography images (a, d), corresponding overlay of topography and AFM phase shift images (b, e) as well as height profiles along the indicated cross sections (c, f) are presented. Cholesterol inserts between lipid molecules orienting the hydroxyl group near the lipid–water interface and the fatty acid tail aligned with the fatty acid chains of the lipids increasing the order of the lipid bilayer. The percolating cholesterol-rich liquid ordered phase (Lo) exhibits a height increase between 1.0 and 1.5 nm to the surrounding liquid disordered phase (Ld). The Lo phase appears as dark contrast in the AFM phase shift images (b, e). Note that Hsp70-1A molecules inserted only into specific domains within the Lo phase
Hsp70-1A causes blebbing in DPPC/DPPS/chol bilayers
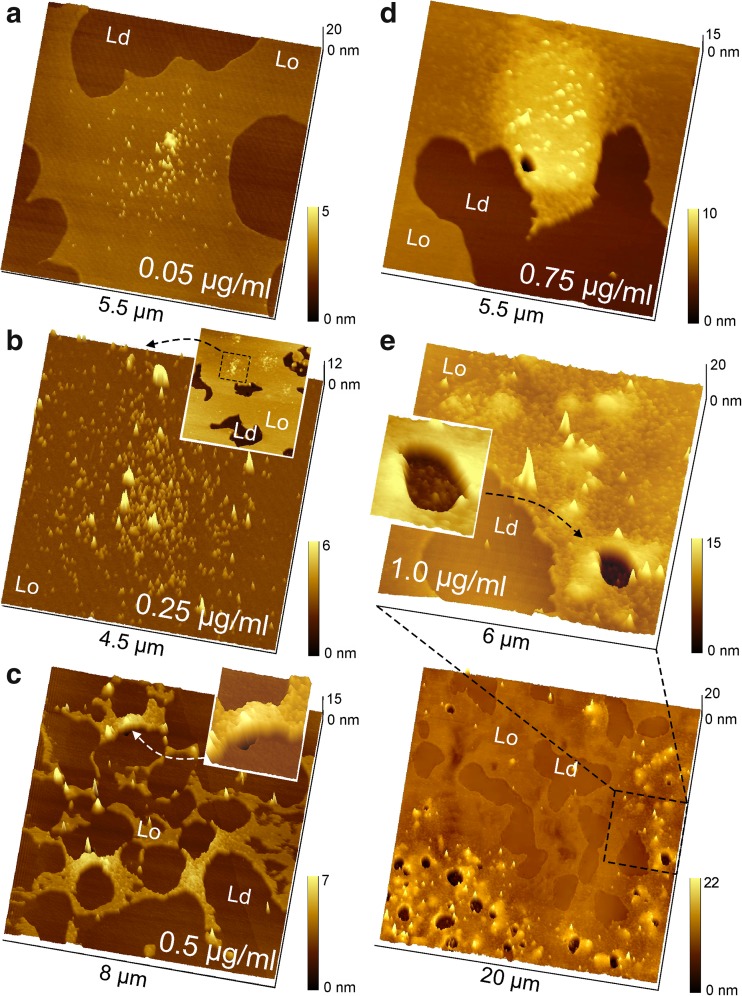
Figure 4a–e shows 64:16:20 DPPC/DPPS/chol bilayers after incubation with increasing concentrations of Hsp70-1A from 0.05 to 1.0 μg/ml for 30 min at 41 °C. At higher protein concentration from 0.5 μg/ml (Fig. 4c) to 0.75 μg/ml (Fig. 4d) and 1.0 μg/ml (Fig. 4e), the formation of rather localized circular protein clusters became more and more evident and the packing density within the clusters increased. Most notably, at a concentration of ~ 0.5 μg/ml, Hsp70-1A clusters started to bulge from the flat bilayer (Fig. 4c, inset). This is particularly interesting, as in cholesterol-free DPPC/DPPS bilayers, the packing density of Hsp70-1A in DPPS domains reached a maximum at ~ 0.5 μg/ml and membrane defects started to emerge (Fig. 2c). However, whereas 80:20 DPPC/DPPS bilayers were saturated with protein at 0.5 μg/ml in 64:16:20 DPPC/DPPS/chol bilayers, the cluster size still slightly increased with higher concentration. At 1.0 μg/ml, massive ‘blebbing’ was observed that left large circular membrane defects of up to 2 μm (Fig. 4e, overview scan and zoom-in section with inset)
Fig. 4.
Representative AFM images of phase separated DPPC/DPPS/chol bilayers after incubation with 0.05 to 1.0 μg/ml Hsp70-1A. The Lo and Ld phases are labeled for ease of distinction. Hsp70-1A selectively parted into the Lo phase and formed circular clusters with increasing protein density towards the center of a cluster. With increasing concentration, cluster size and packing density of molecules grew. a Hsp70-1A concentration of 0.05 μg/ml. b Protein concentration of 0.25 μg/ml. The inset shows the corresponding overview scan, and the dashed square marks the area of the zoom-in. c At a concentration of 0.5 μg/ml, clusters started to bulge out of plane and formed blebs. The inset shows a magnification of one of the blebs. d Hsp70-1A concentration of 0.75 μg/ml. At higher concentration, membrane blebs became prone to rupturing. e Hsp70-1A concentration of 1.0 μg/ml. The bottom panel shows a 20 × 20-μm2 overview scan. Numerous membrane blebs had formed and many of them had burst, leaving holes that were filled with protein as illustrated by the zoom-in in the top panel and the magnification of a hole in the inset image
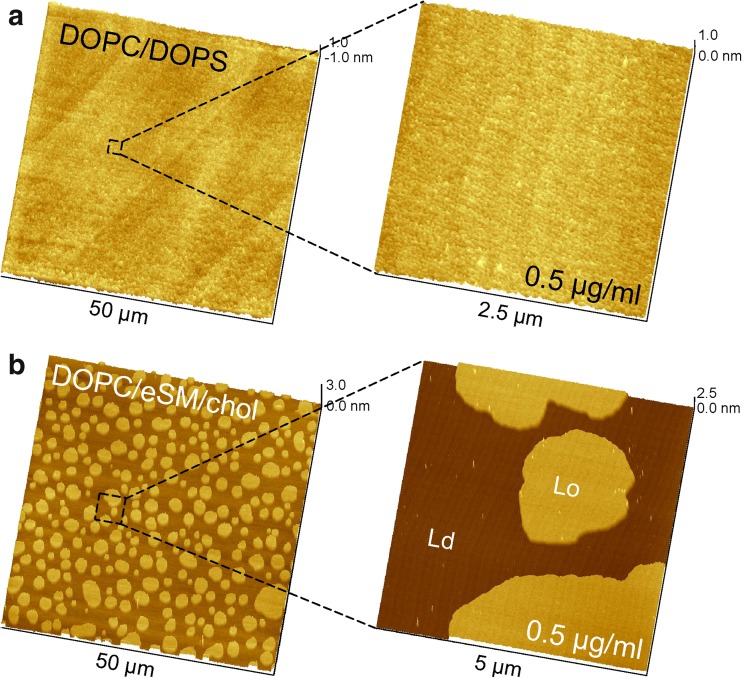
Control experiments: Hsp70-1A does not insert into DOPC/DOPS (80:20 mol%) and DOPC/eSM/chol (40:40:20 mol%) bilayers
Using lipid vesicle sedimentation (LVS) assays, researchers showed that Hsp70-1A associates much weaker with bilayers that contain DOPS with two unsaturated fatty acid chains (Armijo et al. 2014; McCallister et al. 2016). We thus prepared 80:20 DOPC/DOPS bilayers and incubated them with various concentrations of Hsp70-1A as control samples and to underline the specificity of the insertion of Hsp70-1A into domains of saturated DPPS lipids. Figure 5a displays a representative overview scan and a small area scan after incubation with 0.5 μg/ml Hsp70-1A. As opposed to 80:20 DPPC/DPPS, DOPC and DOPS did not segregate at ambient temperature, since both lipids have very low transition temperatures of − 17 °C (DOPC) and − 11 °C (DOPS) and remain well-mixed in a liquid disordered phase (Ld). More importantly, there was no protein on the bilayer, whereas in 80:20 DPPC/DPPS, the same concentration led to saturation of all DPPS domains with Hsp70-1A molecules.
Fig. 5.
Control membranes illustrate the selective association of Hsp70-1A with DPPS. a Representative overview and zoom-in images of a DOPC/DOPS 80:20 bilayer after incubation with 0.5 μg/ml Hsp70-1A for 30 min. Like all other samples, the bilayer was gently washed after incubation to remove unbound protein. Imaging then revealed the absence of Hsp70-1A all over the sample. Note the vertical range of 2 nm in the overview image and 1 nm in the small scan area image. This lipid mixture does not segregate at ambient temperature, but remains in a mixed disordered phase with a smooth topography. b A DOPC/eSM/chol 40:40:20 bilayer crudely approximates the extracellular leaflet of a eukaryotic cell. This model membrane typically separates into domains of eSM- and cholesterol-rich Lo phase in a ‘sea’ of cholesterol-depleted DOPC-rich Ld phase. Incubation with 0.5 μg/ml Hsp70-1A for 30 min was followed by gentle washing steps. The zoom-in scan shows very few molecules that associated only non-specifically with both Lo and Ld
At last, we tested an overall uncharged bilayer with a sphingolipid component (egg sphingomyelin, eSM) that consists to over 70% of PSM (16:0 SM, N-palmitoylsphingomyelin), which has two saturated fatty acid chains comparable to DPPS (Ramstedt et al. 1999). We chose a 40:40:20 ratio of DOPC/eSM/chol, which is often employed to crudely imitate the exoplasmic leaflet of eukaryotic cells (Ira et al. 2009; Simons and Vaz 2004). At ambient temperature, the SLB exhibits phase separation into a cholesterol-depleted DOPC-rich Ld phase and a cholesterol-rich SM Lo phase (Fig. 5b). Some proteins preferentially interact with the Lo phase, for instance to exploit a precise hydrophobic match with the fatty acid chains or because of a high affinity for cholesterol-rich regions (Garcia-Saez et al. 2007; Sullan et al. 2010; van Meer et al. 2008; Windschiegl et al. 2009). Incubation of Hsp70-1A to this SLB was done at a physiological temperature of 37 °C. Imaging of several samples and multiple locations revealed only unspecific binding of a few individual molecules on the bilayer with no preference for Lo or Ld domains (Fig. 5b, zoom-in).
Discussion
In tumor cells, Hsp70-1A exhibits a multitude of activities that go beyond the conventional role of a chaperone and contribute to enhanced cell growth, suppressed senescence, resistance to stress-induced apoptosis, and the development of resistances to radiation therapy (Juhasz et al. 2013; Rohde et al. 2005; Zorzi and Bonvini 2011). Twenty years ago, Hsp70-1A has been first identified also in a plasma membrane associated form as mHsp70 on the surface of human cancer cells (Multhoff et al. 1995). The reason for this cancer exclusive association with the exoplasmic leaflet of the plasma membrane and the mechanism of membrane anchorage are still under debate (Mahalka et al. 2014; Multhoff and Hightower 2011). Different roles of mHsp70 on the surface of tumor cells have been proposed including a stimulation of clathrin-independent endocytosis (Nimmervoll et al. 2015), its presence as an intermediate step in a non-classical secretory pathway (Arispe and De Maio 2000; Armijo et al. 2014), or a protective role against radiation therapy (Gehrmann et al. 2005; Multhoff 2007).
Schilling et al. (2009) found that hypoxia in cancer cells initiates the synthesis and export of Hsp70-1A across the plasma membrane in conjunction with translocation of PS from the inner leaflet to the cell surface. Armijo et al. (2014) and McCallister et al. (2016) then demonstrated the direct interaction of Hsp70-1A with PS and discovered a preferential association with saturated PS lipids such as DPPS. Based on these findings, we further investigated and visualized the association of Hsp70-1A with model membranes on the molecular level using AFM imaging.
Interaction of Hsp70-1A with DPPS
In control experiments on DOPC/DOPS bilayers, we confirmed the conclusion of McCallister and co-workers (McCallister et al. 2016) that the negative charge of the PS lipid head-group alone is not sufficient to facilitate membrane insertion of Hsp70-1A. This was further proven by our observation that Hsp70-1A only inserted into the cholesterol-free DPPC/DPPS membrane at temperatures above 41 °C as a consequence of the high transition temperature of DPPS. At higher temperatures, the protein had associated and did not dissociate during image scanning with the AFM cantilever, pointing towards insertion into the membrane. We thus presume that electrostatic interaction is essential for the initial association with the membrane and that anchoring is driven by the alignment of protein domains with the dipalmitoyl chains of DPPS. Such a mechanism has been proposed to govern membrane anchorage of a number of lipids (Resh 2016) and has recently been suggested for the interaction of Hsp70-1A with intracellular membranes (Mahalka et al. 2014).
Cluster formation and blebbing
Our experiments showed that the capacity for binding of Hsp70-1A to the membrane was limited only by the amount of DPPS lipid in the bilayer. The DPPS content of our model membranes was 20% of the total lipid, which compares well with the physiological amount of PS in the cytosolic leaflet of the plasma membrane in human tumor cells (Kiessling et al. 2009). Fluorescence leakage assays have indicated formation of membrane pores in unilamellar lipid vesicles containing 20% PS when incubated with 1.0 μg/ml Hsp70-1A and higher concentrations (Armijo et al. 2014). However, concentrations below 1.0 μg/ml were not tested, perhaps due to the detection limit of this bulk method. With the advantage of single molecule resolution in AFM imaging on planar model membranes, we were able to explore the concentration range between 0.05 and 1.0 μg/ml. We found that in DPPC/DPPS bilayers, all DPPS domains were saturated with Hsp70-1A molecules at a concentration of 0.5 μg/ml (7 nM); higher concentrations lead to an erosion of the bilayer. We further observed that the protein arranged in dense clusters rather than specific pores as suggested by others (Arispe and De Maio 2000; Armijo et al. 2014). This oligomerized state has been shown to inhibit substrate binding and release and suspend the chaperone activity (Aprile et al. 2013).
When cholesterol was present in the membrane, we observed the onset of membrane blebbing at protein concentrations above ~ 0.5 μg/ml. Cholesterol increases the fluidity and elasticity of the model membrane (Marquardt et al. 2016) and has a stabilizing effect which might explain the formation of blebs as opposed to the erosion of DPPS domains in the cholesterol-free DPPC/DPPS bilayer. However, cholesterol also has a significantly lower miscibility in DPPS than DPPC (Bach and Wachtel 2003; Sergelius et al. 2013). We thus assume that DPPS arranged in form of cholesterol depleted island within the Lo phase, which would explain the formation of rather spherical Hsp70-1A clusters (see also Fig. S3). Taken together, these results seem to support the idea of a non-classical secretory pathway of Hsp70-1A in tumor cells (Arispe and De Maio 2000; Armijo et al. 2014).
Role of Hsp70-1A in radio resistance of tumor cells
Multhoff and co-workers were the first to suspect a protective role of plasma membrane Hsp70-1A against radiation therapy (Gehrmann et al. 2005; Multhoff 2007). They investigated a connection between hypoxia—an important factor in the development of radio resistance in tumors (Sovik et al. 2007)—and the synthesis and export of Hsp70-1A in tumor cells (Schilling et al. 2009). Co-expression of Hsp70-1A and PS on the cell surface in response to hypoxia was demonstrated in vivo. Under normal conditions, charged lipids such as PS are predominantly restricted to the intracellular leaflet of the plasma membrane. Once they translate to the surface, recognition processes are triggered that result in removal of the cell by macrophages and production of autoantibodies (Verhoven et al. 1995). Masking of surface PS, however, inhibits these mechanisms (Asano et al. 2004) leading to cell survival.
Our experiments demonstrated the strong association of Hsp70-1A with DPPS and a saturation of DPPS domains already at low protein concentration. Together with the findings of Schilling et al., the following scenario might explain the presence of Hsp70-1A on the cell surface of certain tumor cells and the co-occurrence of radio resistance in these cells. Enrichment of cancer cells with ionic saturated lipids such as DPPS (Beloribi-Djefaflia et al. 2016) in combination with over-excessive production of Hsp70-1A due to stress as a result of radiation and possibly chemotherapy leads to accumulation of the protein in the inner leaflet of the plasma membrane and the collective transition to the cell surface. Masking of these ionic lipids by Hsp70-1A impedes detection by the immune system and the development of tumor resistance. Schilling et al. also observed a significant enhancement of radiation-induced tumor cell killing after addition of extracellular Hsp70-1A at a concentration of 50 μg/ml, which is well in agreement with our results that show substantial membrane blebbing at higher protein concentration.
Summary
We investigated the interaction of Hsp70-1A with saturated phospatidylserine DPPS on the molecular level by AFM imaging. A strong concentration-dependent association was observed that led to saturation of DPPS membrane domains already at nano molar concentration (7 nM) of Hsp70-1A. Higher concentrations of the protein caused membrane blebbing, supporting previous considerations of a non-classical secretion pathway for Hsp70-1A in cancer cells. Based on our results and in conjunction with the current literature, we proposed a conceivable link between the anchorage of Hsp70-1A in the plasma membrane via charged saturated lipids and the involvement of mHsp70 in the development of tumor resistances against radiation and chemotherapy. We suggest that this hypothesis should to be investigated in more detail in the future. Finally, our study highlights once more the significance of a possible correlation between cancer-specific membrane proteins and cancer-specific lipid profiles.
Electronic supplementary material
(GIF 251 kb)
(GIF 376 kb)
(GIF 261 kb)
(GIF 374 kb)
(DOCX 6980 kb)
Acknowledgments
This project received funding from the European Union’S Framework Programme for Research and Innovation Horizon 2020 (2014-2020) under the Marie Sklodowska-Curie Grant Agreement No. 656842 and the Carl-Zeiss-Stiftung (Carl Zeiss Foundation) (Az. 0563-2.8/685/4). The work has been supported in part by the German Federal Ministry of Education and Research (BMBF) in the framework of the EU ERASynBio project SynGlycTis (031A464), by the Ministry of Science, Research and the Arts of Baden-Württemberg (Az: 33-7532.20) and by the Excellence Initiative of the German Research Foundation (EXC 294).
Abbreviations
- AFM
atomic force microscopy
- chol
cholesterol
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPS
1,2-dioleoyl-sn-glycero-3-phospho-L-serine
- DPPC
1,2-dipalmitoylphosphatidylcholine
- DPPS
1,2-dioleoyl-sn-glycero-3-phospho-L-serine
- eSM
sphingomyelin from chicken egg yolk
- GUV
giant unilamellar vesicle
- Hsp70-1A
heat shock protein 70-1A
- Lα
liquid crystalline phase
- Lβ
solid or gel phase
- Ld
liquid disordered phase
- Lo
liquid ordered phase
- PC
phosphatidylcholine
- SLB
supported lipid bilayer
- SUV
small unilamellar vesicle
Author’s contributions
CL and MG designed the experiments. CL and JM conducted the experiments. CL analyzed the data. AE, WR, GM, and MG helped to conceive the project and discuss results. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0879-0) contains supplementary material, which is available to authorized users.
References
- Aprile FA, Dhulesia A, Stengel F, Roodveldt C, Benesch JLP, Tortora P, Robinson CV, Salvatella X, Dobson CM, Cremades N. Hsp70 oligomerization is mediated by an interaction between the Interdomain linker and the substrate-binding domain. PLoS One. 2013;8(6):e67961. doi: 10.1371/journal.pone.0067961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, De Maio A (2000) ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J Biol Chem 275(40):30839–30843. 10.1074/jbc.M005226200 [DOI] [PubMed]
- Arispe N, Doh M, De Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7(4):330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18(14):1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Armijo G, Okerblom J, Cauvi DM, Lopez V, Schlamadinger DE, Kim J, Arispe N, de Maio A. Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones. 2014;19(6):877–886. doi: 10.1007/s12192-014-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200(4):459–467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D, Wachtel E. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim Biophys Acta Biomembr. 2003;1610(2):187–197. doi: 10.1016/S0005-2736(03)00017-8. [DOI] [PubMed] [Google Scholar]
- Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5(1):e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas B, et al. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13:147–152. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Fritzsching KJ, Kim J, Holland GP. Probing lipid-cholesterol interactions in DOPC/eSM/Chol and DOPC/DPPC/Chol model lipid rafts with DSC and C-13 solid-state NMR. Bba-Biomembranes. 2013;1828(8):1889–1898. doi: 10.1016/j.bbamem.2013.03.028. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Chiantia S, Schwille P. Effect of line tension on the lateral organization of lipid membranes. J Biol Chem. 2007;282(46):33537–33544. doi: 10.1074/jbc.M706162200. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jäättelä M, Zilch T, Multhoff G. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ. 2005;12(1):38–51. doi: 10.1038/sj.cdd.4401510. [DOI] [PubMed] [Google Scholar]
- Hantschel M, Pfister K, Jordan A, Scholz R, Andreesen R, Schmitz G, Schmetzer H, Hiddemann W, Multhoff G. Hsp70 plasma membrane expression on primary tumor biopsy material and bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5(5):438–442. doi: 10.1379/1466-1268(2000)005<0438:HPMEOP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira ZS, Ramirez DMC, Vanderlip S, Ogilvie W, Jakubek ZJ, Johnston LJ. Enzymatic generation of ceramide induces membrane restructuring: correlated AFM and fluorescence imaging of supported bilayers. J Struct Biol. 2009;168(1):78–89. doi: 10.1016/j.jsb.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Juhasz K, Lipp AM, Nimmervoll B, Sonnleitner A, Hesse J, Haselgruebler T, Balogi Z. The complex function of hsp70 in metastatic cancer. Cancers (Basel) 2013;6(1):42–66. doi: 10.3390/cancers6010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim Biophys Acta. 2009;1788(1):64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalka AK, Kirkegaard T, Jukola LT, Jaattela M, Kinnunen PK. Human heat shock protein 70 (Hsp70) as a peripheral membrane protein. Biochim Biophys Acta. 2014;1838(5):1344–1361. doi: 10.1016/j.bbamem.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Marquardt D, Kucerka N, Wassall SR, Harroun TA, Katsaras J. Cholesterol's location in lipid bilayers. Chem Phys Lipids. 2016;199:17–25. doi: 10.1016/j.chemphyslip.2016.04.001. [DOI] [PubMed] [Google Scholar]
- McCallister C, Kdeiss B, Nikolaidis N. Biochemical characterization of the interaction between HspA1A and phospholipids. Cell Stress Chaperones. 2016;21(1):41–53. doi: 10.1007/s12192-015-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G. Heat shock protein 70 (Hsp70): membrane location, export and immunological relevance. Methods. 2007;43(3):229–237. doi: 10.1016/j.ymeth.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Hightower LE. Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones. 2011;16(3):251–255. doi: 10.1007/s12192-010-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Murakami N, Kühnel A, Schmid TE, Ilicic K, Stangl S, Braun IS, Gehrmann M, Molls M, Itami J, Multhoff G. Role of membrane Hsp70 in radiation sensitivity of tumor cells. Radiat Oncol (Lond, Engl) 2015;10(1):149. doi: 10.1186/s13014-015-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nečas D, Klapetek P. Gwyddion: an open-source software for SPM data analysis. Cent Eur J Phys. 2012;10(1):181–188. [Google Scholar]
- Nimmervoll B, Chtcheglova LA, Juhasz K, Cremades N, Aprile FA, Sonnleitner A, Hinterdorfer P, Vigh L, Preiner J, Balogi Z. Cell surface localised Hsp70 is a cancer specific regulator of clathrin-independent endocytosis. FEBS Lett. 2015;589(19PartB):2747–2753. doi: 10.1016/j.febslet.2015.07.037. [DOI] [PubMed] [Google Scholar]
- Pfister K, Radons J, Busch R, Tidball JG, Pfeifer M, Freitag L, Feldmann HJ, Milani V, Issels R, Multhoff G. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer. 2007;110(4):926–935. doi: 10.1002/cncr.22864. [DOI] [PubMed] [Google Scholar]
- Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21(3):379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt B, Leppimäki P, Axberg M, Slotte JP. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur J Biochem. 1999;266(3):997–1002. doi: 10.1046/j.1432-1327.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fatty acylation of proteins: the long and the short of it. Prog Lipid Res. 2016;63:120–131. doi: 10.1016/j.plipres.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jaattela M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19(5):570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling D, Gehrmann M, Steinem C, de Maio A, Pockley AG, Abend M, Molls M, Multhoff G. Binding of heat shock protein 70 to extracellular phosphatidylserine promotes killing of normoxic and hypoxic tumor cells. FASEB J. 2009;23(8):2467–2477. doi: 10.1096/fj.08-125229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: an open platform for biomedical image analysis. Mol Reprod Dev. 2015;82(7-8):518–529. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergelius C, Yamaguchi S, Yamamoto T, Engberg O, Katsumura S, Slotte JP. Cholesterol's interactions with serine phospholipids — a comparison of N-palmitoyl ceramide phosphoserine with dipalmitoyl phosphatidylserine. Biochim Biophys Acta Biomembr. 2013;1828(2):785–791. doi: 10.1016/j.bbamem.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annu Rev Bioph Biom. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Sovik A, Malinen E, Skogmo HK, Bentzen SM, Bruland OS, Olsen DR. Radiotherapy adapted to spatial and temporal variability in tumor hypoxia. Int J Radiat Oncol Biol Phys. 2007;68(5):1496–1504. doi: 10.1016/j.ijrobp.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Sullan RMA, Li JK, Hao C, Walker GC, Zou S. Cholesterol-dependent Nanomechanical stability of phase-segregated multicomponent lipid bilayers. Biophys J. 2010;99(2):507–516. doi: 10.1016/j.bpj.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duyl BY, Ganchev D, Chupin V, de Kruijff B, Killian JA. Sphingomyelin is much more effective than saturated phosphatidylcholine in excluding unsaturated phosphatidylcholine from domains formed with cholesterol. FEBS Lett. 2003;547(1-3):101–106. doi: 10.1016/S0014-5793(03)00678-1. [DOI] [PubMed] [Google Scholar]
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;182(5):1597–1601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windschiegl B, Orth A, Römer W, Berland L, Stechmann B, Bassereau P, Johannes L, Steinem C. Lipid reorganization induced by Shiga toxin clustering on planar membranes. PLoS One. 2009;4(7):e6238. doi: 10.1371/journal.pone.0006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi E, Bonvini P. Inducible hsp70 in the regulation of cancer cell survival: analysis of chaperone induction, expression and activity. Cancers (Basel) 2011;3(4):3921–3956. doi: 10.3390/cancers3043921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(GIF 251 kb)
(GIF 376 kb)
(GIF 261 kb)
(GIF 374 kb)
(DOCX 6980 kb)