Abstract
Mutations in amyloid precursor protein (APP) and presenilin1 result in overproduction and accumulation of β-amyloid (Aβ) peptide, which has been shown to play an important role in Alzheimer’s disease (AD) pathogenesis. Carvedilol, a nonselective β-adrenergic receptor blocker used for treatment for heart failure and hypertension, has displayed its neuroprotective capacity due to its antioxidant property. In this study, we investigated whether Carvedilol has a neuronal protective effect against endogenous Aβ neurotoxicity in mouse Neuro2a (N2a) cells transfected with Swedish amyloid precursor protein (Swe-APP) mutant and Presenilin exon9 deletion mutant (N2a/Swe.D9). Elevated levels of reactive oxygen species (ROS), protein carbonyls, and 4-HNE were found in N2a/Swe.D9 cells, which were ameliorated by administration of Carvedilol in a dose-dependent manner. In addition, the levels of ATP and mitochondrial membrane potential were reduced in N2a/Swe.D9 cells, which were restored by treatment with Carvedilol. N2a/Swe.D9 cells displayed increased vulnerability to H2O2-induced cell death and apoptosis, which could be attenuated by Carvedilol. Mechanistically, we found that Carvedilol prevented apoptosis signals through reducing cytochrome C release and the level of cleaved caspase-3. Taken together, our findings suggest a possible use of Carvedilol in AD treatment.
Electronic supplementary material
The online version of this article (10.1007/s12192-018-0881-6) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Amyloid β, Carvedilol, Oxidative stress, Apoptosis
Introduction
Alzheimer’s disease (AD), one of the major neurodegenerative disorders in the central nervous system (CNS), is pathologically characterized by the appearance of neurofibrillary tangles and senile plaques (SPs) (Selkoe 1999). The major component of SPs is β-amyloid (Aβ) peptide, which denotes peptides of 36–43 amino acids (Cotman and Su 1996). Aβ is derived from the amyloid precursor protein (APP) through sequential proteolysis by β-secretase and γ-secretase. Presenilin 1 (PS1) has been considered as the catalytic core of γ-secretase. Mutations in APP and PS1 are known to cause early onset familial AD (FAD) (LaFerla and Oddo 2005; Sherrington et al. 1995). These mutations alter proteolytic processing of APP, resulting in an overproduction and aggregation of neurotoxic forms of Aβ (Hardy and Higgins 1992; Mattson 2004). An important example is the Swedish APP mutation (Sw-APP, APPK670N, M671L) that causes FAD. Multiple lines of evidence show that more than 150 FAD-linked PS1 mutations have been identified to date and are suggested to affect γ-secretase activity to various extents. The PS1 ΔE9 (D9) variant has been reported to increase γ-secretase processing of APP as well as levels of secreted Aβ peptides. Increase in oxidative stress and mitochondrial dysfunction has been found in AD brains (Hirai et al. 2001; Butterfield et al. 2001; Smith et al. 1997). Notably, APP/PS-1 double mutant neurons have been reported to show a significant basal increase in reactive oxygen species (ROS) when compared with the wild-type neurons (Mohmmad Abdul et al. 2006). So far, many factors and several signaling pathways have been associated with the Aβ cascade hypothesis. Prevention of Aβ neurotoxicity has become an important therapeutic target in AD (Glat and Offen 2013).
Carvedilol, a nonselective β-adrenergic receptor blocker, has been widely prescribed for treating congestive heart failure and hypertension (Packer et al. 1996). Increasing evidence has shown that Carvedilol exerts neuroprotective effects due to its antioxidant property. Carvedilol protects PC12 cells against 6-OHDA-induced neurotoxicity possibly through increasing cell viability, decreasing reactive oxygen species (ROS), and activating the Akt and Nrf2/ARE signaling pathways (Wang et al. 2014). Another study demonstrated the neuroprotective potential of Carvedilol in aluminum chloride-induced cognitive dysfunction and oxidative damage by showing that chronic administration of Carvedilol daily to rats for a period of 6 weeks significantly improved the memory performance tasks of rats in the Morris water maze test, attenuated oxidative stress (reduced lipid peroxidation, nitrite concentration and restored reduced glutathione, superoxide dismutase, catalase, and glutathione S-transferase activity), decreased acetylcholinesterase activity, and aluminum concentration in aluminum-treated rats compared to control rats (Kumar et al. 2011). Interestingly, a recent study suggested that use of Carvedilol is associated with cognitive benefits in AD patients (Rosenberg et al. 2008). However, it is unknown whether Carvedilol has a neuro-protective effect against Aβ neurotoxicity. In the current study, we aimed to define the pharmacological function of Carvedilol against endogenous Aβ in neuroblastoma N2a cells. N2a cells stably overexpressing Swedish mutant APP and ΔE9-deleted presenilin-1 (N2a/Swe.D9) were used in this study. Our results indicate that treatment with Carvedilol ameliorated oxidative stress, improved mitochondrial function, and suppressed cell apoptosis. These results suggest a therapeutic potential of Carvedilol for the treatment of AD.
Materials and methods
Cell culture and treatment
Neuroblastoma N2a cells stably transfected with empty vector (N2a/Wt) or co-transfected with Swedish mutant APP and ΔE9-deleted presenilin-1 (N2a/Swe.D9) obtained from Dr. H.-X. Xu (The Burnham Institute, SD, USA) were used in this study. Cells were cultured in medium containing equal volumes of DMEM and OPTI–MEM supplemented with 5% fetal bovine serum (FBS) and supplemented with 200 μg/mL G418 and 0.1% antibiotics (penicillin and streptomycin, P/S). Cells were treated with 100 μM H2O2 in the presence or absence of 10 and 20 μM Carvedilol for 24 h.
Release of lactate dehydrogenase (LDH) determination
Lactate dehydrogenase (LDH) release was examined using a cytotoxicity detection kit (Roche Applied Science, Germany). N2a cells were plated in 24-well plates. Cells were treated with 100 μM H2O2 in the presence or absence of 10 and 20 μM Carvedilol for 24 h. After indicated treatment, reaction substrates were added and LDH experiments were performed. Fifty microliters of sample medium was collected and mixed with equal amount of reaction regents. After incubation in darkness at room temperature for 30 min, reactions were stopped using 50 μl stop solution. Absorbance measured at 490 nm was used to index the levels of LDH. Both the activity of LDH in the supernatant and total LDH activity were measured. The ratio of LDH activity in the supernatant to the total LDH activity was taken as the percentage of cell death according to the instructions.
Reactive oxygen species (ROS) determination
Levels of ROS in mitochondria were measured using DCFH-DA (Invitrogen, USA) according to the manufacturer’s instructions. Cells were treated with 100 μM H2O2 in the presence or absence of 10 and 20 μM Carvedilol for 24 h. After indicated treatment, cells were washed with HBSS three times and loaded with 5 μM DCFH-DA in Hank’s balanced salt solution (HBSS) for 30 min. Fluorescence signals were recorded using the IBE2000 inverted fluorescence microscope (Zeiss, Germany) with excitation at 510 nm and emission at 580 nm. The level of ROS was indexed by the average fluorescence intensity analyzed by Image-Pro Plus software.
Western blot analysis
Protein in N2a neuronal cells was extracted by using cell signaling lysis buffer. Protein concentrations were determined by the bicinchoninic acid (BCA) assay. Protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). PVDF membranes were blocked in 5% non-fat dry milk in tris-buffered saline containing 0.1% Tween 20 for 2 h at room temperature, followed by incubation overnight with primary antibody at 4 °C. Then, membranes were incubated with horse radish peroxidase (HRP)-conjugated secondary antibody for another 2 h. Blots were visualized using chemiluminescence technique (Santa Cruz Biotechnology, USA) according to the manufacturer’s instructions. The following antibodies were used in this study: rabbit polyclonal antibody against cleaved caspase-3 (Asp175) (#9661, Cell Signaling Technology, USA), rabbit monoclonal antibody against cytochrome C (#11940, Cell Signaling Technology, USA), and rabbit monoclonal antibody against β-actin (#4970, Cell Signaling Technology, USA). The blots were scanned and the sum optical density was quantitatively analyzed by Kodak Digital Science 1D software (Eastman Kodak Company, USA). Briefly, background was subtracted. Bands were selected and signal intensities were quantified. Data were exported for statistical analysis or graphical comparisons. Expression of target proteins was normalized to β-actin.
Protein carbonyl assay
Protein oxidation was determined by measuring protein carbonyls using a protein carbonyl ELISA Kit (#STA-310, Cell Biolabs, USA) in cells. Briefly, after indicated treatment, protein samples were extracted from cells and were adsorbed to wells of an ELISA plate and then reacted with dinitrophenylhydrazine (DNPH). After three washes, an anti-DNPH antibody was added and incubated for 3 h, followed by quantification with a second antibody conjugated with horseradish peroxidase. Oxidized BSA was used to calibrate the level of protein carbonyls.
4-Hydroxy-2-nonenal (4-HNE) immunofluorescence
Levels of 4-HNE in N2a cells were determined by immunofluorescence methods. Briefly, upon completion of indicated treatment, cells were fixed in 4% paraformaldehyde for 10 min at RT. After three washes, cells were permeabilized with 0.4% Triton X-100 for 15 min on ice, followed by blocking with 5% BSA and 2.5% FBS in phosphate-buffered saline with Tween 20 (PBST). Then, cells were sequentially probed with anti-4-hydroxynonenal mouse monoclonal antibody (ab48506, Abcam, USA) for 2 h, and Alexa-594 conjugated secondary antibodies (Invitrogen, USA) for another 1 h at RT. Staining signals were captured using the IBE2000 inverted fluorescence microscope (Zeiss, Germany). Immunofluorescence quantification was performed using the ImageJ software. Briefly, regions of interest (ROI) was defined and the average number of cells present in the previously defined ROI was determined. The integrated density value (IDV) in ROI was assessed. The IDV was divided by the average number of cells and was used to index average level of intracellular 4-HNE.
Measurement of mitochondrial membrane potential (MMP)
MMP in N2a cells was examined by tetramethylrhodamine methyl ester (TMRM) (Invitrogen, USA). Cells were treated with 100 μM H2O2 in the presence or absence of 10 and 20 μM Carvedilol for 24 h. Upon completion of indicated treatment, N2a cells were loaded with 20 nmol/L TMRM and incubated for 1 h at RT. After three washes, fluorescence signals were captured and recorded using the IBE2000 inverted fluorescence microscope (Zeiss, Germany). The level of MMP was indexed by the average fluorescence intensity analyzed by Image-Pro Plus software.
Determination of adenosine triphosphate (ATP) levels via bioluminescence assay
The level of intracellular ATP in N2a cells was determined by an ATP Bioluminescence assay kit (#A22066, thermo fisher scientific, USA) according to the manual instructions. Briefly, upon completion of indicated treatment, N2a cells were lysed with a lysis buffer and centrifuged at 10,000×g for 10 min at 4 °C. One hundred microliters of supernatant was collected and mixed with equal volume of the luciferin/luciferase reagent to catalyze the light production from ATP and luciferin. Light output from the reaction was immediately recorded by a microplate luminometer. Data were presented as “relative value” by normalizing the results to “untreatment” group, which represented “fold of control.”
Evaluation of cell viability
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich, USA) was used to determine cell viability of N2a cells. N2a cells were plated in 24-well plates. After necessary treatment, MTT (5 mg/mL) was added to each well and incubated for 4 h at 37 °C in darkness. The reaction product was dissolved in dimethyl sulfoxide (DMSO), and absorbance was read at 570 nm using an ELISA reader to index the viability percentage. Data were presented as relative value by normalizing the results to untreatment group.
TdT-mediated dUTP nick end labeling (TUNEL) assay
Cell apoptosis of cultured N2a cells was assessed by the TUNEL method using a commercial kit (Promega, USA). Briefly, N2a/Swe.D9 cells were treated with 100 μM H2O2 in the presence or absence of 20 μm Carvedilol. Cells were sequentially fixed with 4% formaldehyde for 20 min and 70% ethanol for 30 min at − 20 °C, followed by permeabilization with 0.1% Triton X-100 for minutes. Then, a terminal deoxynucleotidyl transferase enzyme (Promega, USA) was used to incubate with cells in darkness at 37 °C for 90 min. Nuclei of N2a cells were counterstained by 4′-6-diamidino-2-phenylindole (DAPI). Fluorescent signals were captured by a fluorescence microscope (Zeiss, Germany). A percentage of TUNEL-positive cells to the total number of cells was counted in eight tissue sections.
Statistical analysis
All experimental data are shown as mean ± SEM from at least three separate experiments. Statistical analysis was performed by analysis of variance (ANOVA) followed by Bonferroni post-test comparisons using Prism version 5 (GraphPad Software). P value less than 0.05 was considered statistically significant.
Results
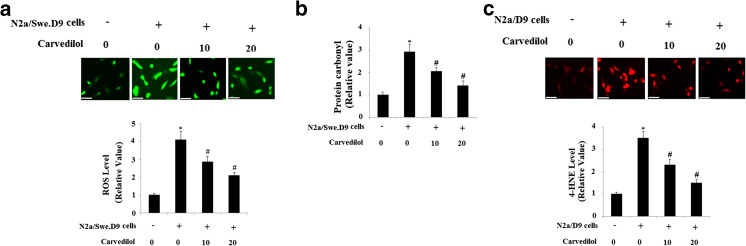
Firstly, we evaluated the effects of Carvedilol on cell viability by treating N2a/Swe.D9 cells with Carvedilol at the concentrations of 2 nm, 20 nM, 200 nM, 2 μM, 20 μM, 200 μM, and 2 mM. Results in supplementary Fig. 1 indicate that treatment with Carvedilol at the final concentration of 200 μM Carvedilol resulted in a significant reduction in mean cell viability. Therefore, Carvedilol at a concentration of 10 μM and 20 μM was used in this study to examine its effects on cytotoxicity induced by endogenous Aβ. Emerging evidences have shown that Aβ treatment induced oxidative stress in neuronal cells. Stable co-transfection with Swedish mutant APP and ΔE9-deleted presenilin-1 in N2a (N2a/Swe.D9) produced excessive endogenous Aβ (Sheng et al. 2009). Then, we used the fluorescence probe DCFH-DA to measure the production of intracellular ROS in N2a cells. As shown in Fig. 1a, ROS in N2a/Swe.D9 cells is significantly higher than that in controls, which can be suppressed by treatment with Carvedilol in a concentration-dependent manner. In addition, we found that the level of protein carbonyl in N2a/Swe.D9 cells was significantly higher than that in N2a/wt cells, which was attenuated by Carvedilol in a dose-dependent manner (Fig. 1b). Notably, N2a/Swe.D9 cells are found to have the highest basal levels of 4-HNE, which can be prevented by treatment with Carvedilol (Fig. 1c).
Fig. 1.
Carvedilol attenuates oxidative stress in AD cell models. a ROS was determined by DCFH-DA. b Protein carbonyl was determined by ELISA. c The level of intracellular 4-HNE was determined by immunofluorescence staining. Scale bars at 100 μM (ANOVA *P < 0.001 vs. vector control; #P < 0.01 vs. N2a/Swe.D9 non-treatment control)
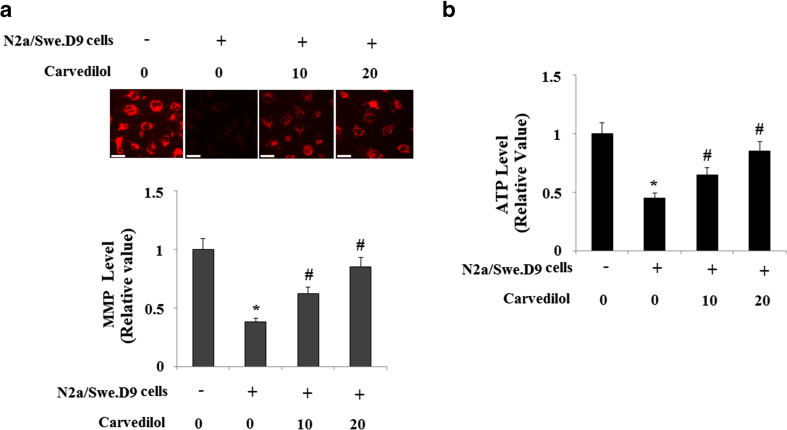
Increasing evidence has shown that mitochondria might be an important target of Aβ. Based on the observation above of oxidative stress, we speculated that Carvedilol might improve the impaired mitochondrial function induced by endogenous Aβ. To investigate whether Carvedilol improves mitochondria in our model cells, MMP was determined by using the fluorescence dye TMRM. Our results indicate that the level of MMP in N2a/Swe.D9 cells was significantly lower than that in N2a/wt cells, which was restored by treatment with Carvedilol (Fig. 2a). We also measured the level of ATP in N2a cells. Consistently, it was found that the levels of ATP in N2a/Swe.D9 cells were significantly decreased compared with those in N2a/wt cells. However, administration of Carvedilol rescued the reduced levels of ATP (Fig. 2b).
Fig. 2.
Effects of Carvedilol on endogenous Aβ-induced collapse of mitochondrial membrane potential (MMP) and reduction of Adenosine triphosphate (ATP) in AD cell models. a Representative fluorescence photos and quantitative analysis of MMP. Scale bars at 100 μM. b The levels of intracellular ATP were determined by a bioluminescence assay (ANOVA *P < 0.001 vs. vector control; #P < 0.01 vs. N2a/Swe.D9 non-treatment control)
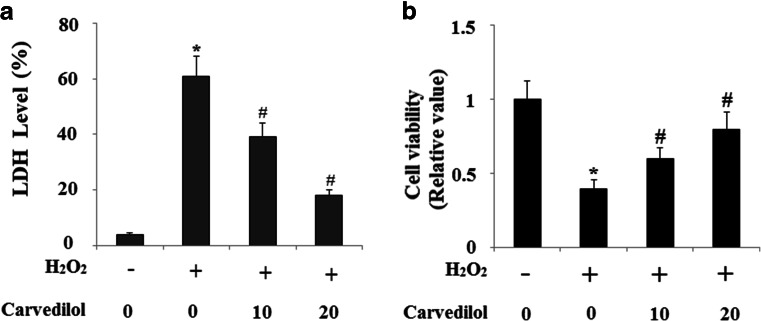
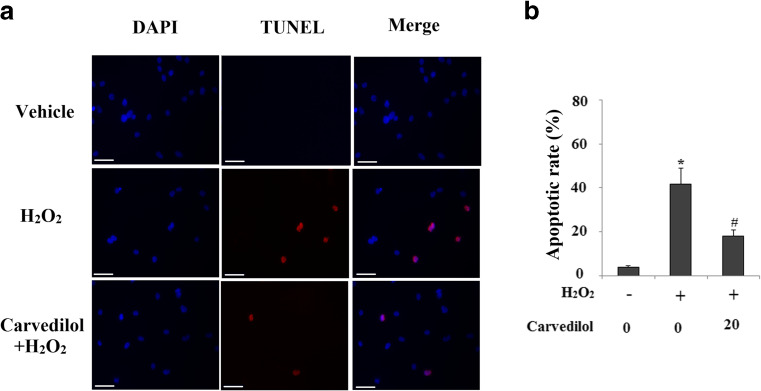
Carvedilol has displayed its anti-apoptotic capacity. Here, we investigated the effects of Carvedilol on endogenous Aβ-induced neuronal toxicity when cells were subject to a secondary insult from the oxidative damage agent H2O2. Patterns of cell death were determined by the LDH assay. Cells were treated with 100 μM H2O2 for 24 h. As shown in Fig. 3a, N2a/Swe.D9 cells exhibited obviously augmented LDH release into the medium as compared to the controls. However, H2O2-induced cell death was effectively prevented by Carvedilol treatment in a dose-dependent manner. Cell viability was determined by the MTT assay. Results in Fig. 3b indicate that H2O2-induced reduction of cell viability was prevented by treatment with Carvedilol in a dose-dependent manner. We also compared cell viability of N2a/Wt and N2a/Swe.D9 cells. Equal amounts (1 × 105) of cells were plated in each well of 6-well plates. Cell viability was determined after 24 and 48 h. Results in supplementary Fig. 2 indicate that the cell viability of N2a/Swe.D9 cells was significantly lower than N2a/Wt cells after 48 h incubation. The nuclear condensation characteristic of apoptosis was determined by the TUNEL staining. Results demonstrated that N2a/Swe.D9 cells displayed significant increases in the number of apoptotic cells after treatment with H2O2 compared with control ones, which was reduced by treatment with Carvedilol (Fig. 4).
Fig. 3.
Carvedilol protects against secondary oxidative stress insults induced by H2O2 in N2a/Swe.D9cells. a LDH release was measured by a commercial kit. b Cell viability determined by MTT assay (ANOVA *P < 0.001 vs. vector control; #P < 0.01 vs. H2O2 treatment control)
Fig. 4.
Carvedilol mitigates apoptosis induced by H2O2 in N2a/Swe.D9cells. N2a/Swe.D9 cells were treated with 100 μM H2O2 in the presence or absence of 20 μm Carvedilol. a Patterns of apoptosis were determined by TUNEL staining methods. Scale bars at 100 μM. b Quantitative analysis of apoptosis (ANOVA *P < 0.001 vs. vector control; #P < 0.01 vs. H2O2 treatment control)
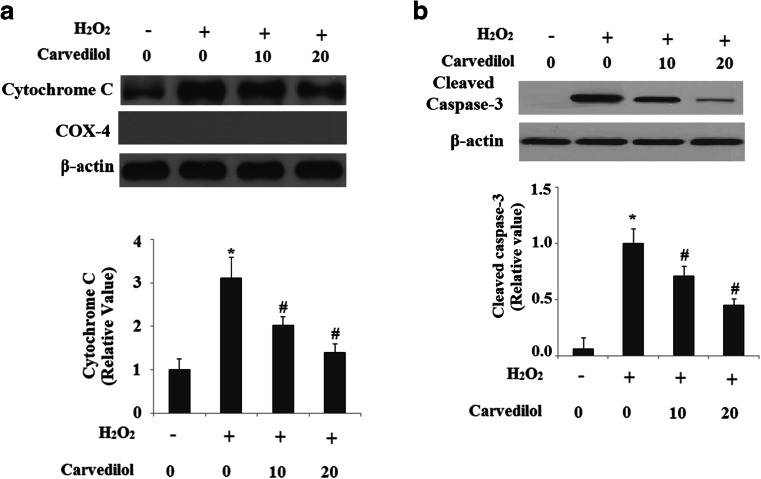
MMP disruption is an important cause to induce cytochrome C release and to trigger caspase-3 activation. Therefore, we next investigated the regulation of cytochrome C release from mitochondria into the cytoplasm. Our results indicated that levels of cytochrome C in cytosols were significantly increased in N2a/Swe.D9 cells after H2O2 treatment, which were markedly ameliorated by administration of Carvedilol. COX4 was used as the internal control for cytosolic fractions (Fig. 5a). Importantly, caspase-3 was activated in N2a/Swe.D9 cells after H2O2 treatment, and that the activation of caspase-3 was significantly inhibited by administration of Carvedilol (Fig. 5b).
Fig. 5.
Carvedilol attenuates the translocation of cytochrome C from the mitochondrial intermembrane space to the cytosol and the expression of cleaved Caspase-3. a Representative images of immunoblots for cytochrome C in the cytosolic fractions. COX-4 was used as a control for the fractionation efficiency. b Representative images of immunoblots for cleaved caspase-3 (ANOVA *P < 0.001 vs. vector control; #P < 0.01 vs. H2O2 treatment control)
Discussion
Genetic mutations in genes in APP and PS1 have been reported to increase the production of Aβ42 and lead to early onset of AD. In this study, we investigated the neuro-protective effects of Carvedilol on the neurotoxicity of AD-associated familial APP and PS1 gene mutations. Our data show that N2a/Swe.D9 cells have significantly increased oxidative stress, mitochondrial dysfunction, and increased vulnerability to H2O2, which can be rescued by treatment with Carvedilol.
Carvedilol is a third-generation, non-selective β-adrenoceptor antagonist used in chronic heart failure. Importantly, increasing evidence has shown that Carvedilol has displayed antioxidant, anti-apoptotic, anti-inflammatory, and anti-fibrotic properties (Li-Sha et al. 2013). A recent study has reported that the mitochondrial permeability transition (MPT) is triggered by a primary oxidative process, which can be effectively inhibited by Carvedilol (Oliveira et al. 2004a). Similarly, Oliveira and colleagues reported that the cardioprotective effects of Carvedilol against DOX-induced mitochondrial cardiotoxicity are due to its inherent antioxidant activity and not to its β-adrenergic receptor antagonism (Oliveira et al. 2004b). Ischemia has been reported to negatively affect mitochondrial function by inducing the mitochondrial permeability transition (MPT). Interestingly, Carvedilol protects ischemic mitochondria by preventing oxidative mitochondrial damage, and, by so doing, it may also inhibit the formation of the MPT pore (Carreira et al. 2004). Carvedilol improved mitochondrial function by elevating the levels of ATP and MMP. Collapse of MMP has been shown to result in the translocation of cytochrome C from the mitochondrial intermembrane space to the cytosol and to trigger caspase-3 activation to induce apoptosis (Green and Reed 1998). Importantly, Carvedilol suppresses apoptosis through inhibiting the translocation of cytochrome C from the mitochondrial intermembrane space to the cytosol, suggesting that Carvedilol protects against endogenous Aβ neuronal cytotoxicity from mitochondrial pathway. Consistent with our findings, Carvedilol reduced both cytochrome C release and alterations in membrane fluidity induced by toxin Chenodeoxycholate (Rolo et al. 2003).
Multiple lines of evidence have shown that Carvedilol displays neuroprotective properties. For example, Carvedilol has neuroprotective activity as a calcium channel blocker and as a non-competitive inhibitor at the NMDA receptor (Lysko et al. 1992). Furthermore, Carvedilol would be useful to inhibit neuronal cell death in the treatment of cerebrovascular stroke and neurodegenerative diseases in hypertensive patients (Yamagata et al. 2004). Carvedilol was also proved to protect neurons against death and suggested that suppression of PARP activity during reperfusion could be involved in this process (Strosznajder et al. 2005). In addition, neuroprotective effects of Carvedilol have been implicated in reestablishing basal synaptic transmission, enhancing neuronal plasticity, and suppressing neuronal hyperexcitability in AD animal models. Notably, Carvedilol treatment produced a significant increase in basal synaptic transmission and long-term potentiation (LTP) and suppressed spontaneous seizure activity in TgCRND8 mice as measured by the number of slices showing epileptic discharges (Arrieta-Cruz et al. 2010). Aβ has been shown to be responsible for the increased oxidative stress and mitochondrial dysfunction in AD brains. As a matter of fact, a variety of antioxidants have been reported to be efficient in AD in previous in vivo and in vitro studies. These drugs include vitamins, N-acetylcysteine, lipoic acid, polyphenols, Ginko biloba extract, and omega-3-fatty acid (Zuo et al. 2015). Additionally, other mitochondrial antioxidants such as MitoQ and plastoquinone could also prevent oxidative damage and mitochondrial dysfunctions in AD brains (Reddy and Reddy 2017). However, the therapeutic effects of antioxidants alone in clinical trials have been shown to be less promising. Therefore, the antioxidant bioavailability, mechanism of action, and specificity of Carvedilol should be fully explored in future studies. Our findings suggest a possible use of Carvedilol in AD treatment. Future study will provide us with a more complete picture of pharmacological capacity of Carvedilol in neurological disorders.
Electronic supplementary material
Effects of Carvedilol on the cell viability of N2a/Swe.D9 cells. N2a/Swe.D9 was treated with Carvedilol at the concentrations of 2 nm, 20 nM, 200 nM, 2 μM, 20 μM, 200 μM, and 2 mM for 24 h. Cell viability was determined by the MTT assay (*, P < 0.01; **, P < 0.0001 vs. untreatment group). (GIF 114 kb)
Comparison of cell viability of N2a/Wt and N2a/Swe.D9 cells. Equal amount of cells (1 × 105) were plated in each well of 6-well plates. Cell viability was determined after 24 h and 48 h incubation by the MTT assay (*, P < 0.01 vs. N2a/Wt cells). (GIF 86 kb)
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s12192-023-01399-w"
Change history
12/18/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1007/s12192-023-01399-w
References
- Arrieta-Cruz I, Wang J, Pavlides C, Pasinetti GM. Carvedilol reestablishes long-term potentiation in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;21(2):649–654. doi: 10.3233/JAD-2010-100225. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid β-peptide. Trends Mol Med. 2001;7(12):548–554. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- Carreira R, Duarte A, Monteiro P, Santos MS, Rego AC, Oliveira CR, Gonçalves LM, Providência LA. Carvedilol protects ischemic cardiac mitochondria by preventing oxidative stress. Rev Port Cardiol. 2004;23(11):1447–1455. [PubMed] [Google Scholar]
- Cotman CW, Su JH. Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol. 1996;6(4):493–506. doi: 10.1111/j.1750-3639.1996.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Glat MJ, Offen D. Cell and gene therapy in Alzheimer’s disease. Stem Cells Dev. 2013;22(10):1490–1496. doi: 10.1089/scd.2012.0633. [DOI] [PubMed] [Google Scholar]
- Green R, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Prakash A, Dogra S. Neuroprotective effect of Carvedilol against aluminium induced toxicity: possible behavioral and biochemical alterations in rats. Pharmacol Rep. 2011;63(4):915–923. doi: 10.1016/S1734-1140(11)70607-7. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med. 2005;11(4):170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Li-Sha G, Yi-He C, Na-Dan Z, Teng Z, Yue-Chun L. Effects of Carvedilol treatment on cardiac cAMP response element binding protein expression and phosphorylation in acute coxsackievirus B3-induced myocarditis. BMC Cardiovasc Disord. 2013;13(1):100. doi: 10.1186/1471-2261-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysko PG, Lysko KA, Webb CL, Feuerstein G. Neuroprotective effects of Carvedilol, a new antihypertensive, at the N-methyl-D-aspartate receptor. Neurosci Lett. 1992;148(1–2):34–38. doi: 10.1016/0304-3940(92)90798-C. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid β-peptide (1-42), H2O2 and kainic acid: implications for Alzheimer's disease. J Neurochem. 2006;96(5):1322–1335. doi: 10.1111/j.1471-4159.2005.03647.x. [DOI] [PubMed] [Google Scholar]
- Oliveira PJ, Esteves T, Rolo AP, Palmeira CM, Moreno AJ. Carvedilol inhibits the mitochondrial permeability transition by an antioxidant mechanism. Cardiovasc Toxicol. 2004;4(1):11–20. doi: 10.1385/CT:4:1:11. [DOI] [PubMed] [Google Scholar]
- Oliveira PJ, Bjork JA, Santos MS, Leino RL, Froberg MK, Moreno AJ, Wallace KB. Carvedilol-mediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol. 2004;200(2):159–168. doi: 10.1016/j.taap.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The U.S. Carvedilol heart failure study group. The effect of Carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Reddy AP, Reddy PH. Mitochondria-targeted molecules as potential drugs to treat patients with Alzheimer’s disease. Prog Mol Biol Transl Sci. 2017;146:173–201. doi: 10.1016/bs.pmbts.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Chenodeoxycholate induction of mitochondrial permeability transition pore is associated with increased membrane fluidity and cytochrome c release: protective role of Carvedilol. Mitochondrion. 2003;2(4):305–311. doi: 10.1016/S1567-7249(03)00007-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA, Breitner JC, Munger R, Lyketsos CG. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(11):883–892. doi: 10.1097/JGP.0b013e318181276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Sheng B, Gong K, Niu Y, Liu L, Yan Y, Lu G, Zhang L, Min H, Zhao N, Zhang X, Tang P, Gong Y. Inhibition ofγ-secretase activity reduces Aβproduction, reduces oxidative stress increases mitochondrial activity and leads to reduced vulnerability to apoptosis: implications for the treatment of Alzheimer’s disease. Free Radic Biol Med. 2009;46(10):1362–1375. doi: 10.1016/j.freeradbiomed.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer’s disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strosznajder RP, Jesko H, Dziewulska J. Effect of Carvedilol on neuronal survival and poly(ADP-ribose) polymerase activity in hippocampus after transient forebrain ischemia. Acta Neurobiol Exp (Wars) 2005;65(2):137–143. doi: 10.55782/ane-2005-1546. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang R, Jin M, Huang Y, Liu A, Qin J, Chen M, Wen S, Pi R, Shen W. Carvedilol attenuates 6-hydroxydopamine-induced cell death in PC12 cells: involvement of Akt and Nrf2/ARE pathways. Neurochem Res. 2014;39(9):1733–1740. doi: 10.1007/s11064-014-1367-2. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Ichinose S, Tagami M. Amlodipine and Carvedilol prevent cytotoxicity in cortical neurons isolated from stroke-prone spontaneously hypertensive rats. Hypertens Res. 2004;27(4):271–282. doi: 10.1291/hypres.27.271. [DOI] [PubMed] [Google Scholar]
- Zuo L, Hemmelgarn BT, Chuang CC, Best TM. The role of oxidative stress-induced epigenetic alterations in amyloid-β production in Alzheimer’s disease. Oxidative Med Cell Longev. 2015;2015:604658. doi: 10.1155/2015/604658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of Carvedilol on the cell viability of N2a/Swe.D9 cells. N2a/Swe.D9 was treated with Carvedilol at the concentrations of 2 nm, 20 nM, 200 nM, 2 μM, 20 μM, 200 μM, and 2 mM for 24 h. Cell viability was determined by the MTT assay (*, P < 0.01; **, P < 0.0001 vs. untreatment group). (GIF 114 kb)
Comparison of cell viability of N2a/Wt and N2a/Swe.D9 cells. Equal amount of cells (1 × 105) were plated in each well of 6-well plates. Cell viability was determined after 24 h and 48 h incubation by the MTT assay (*, P < 0.01 vs. N2a/Wt cells). (GIF 86 kb)