Abstract
The use of highly inducible HSP promoters for exerting spatial and/or temporal control over the expression of therapeutic transgenes has long been discussed. Localized and time-limited induction of the heat shock response may potentially also be of medical interest. However, such applications would require targeted delivery of heat doses capable of activating HSP promoters in tissues or organs of interest. Accessible areas, including the skin and tissues immediately underneath it, may be most readily targeted. A few applications for heat-directed or heat-controlled therapy in the skin might involve expression of proteins to restore or protect normal skin function, protein antigens for vaccination/immunotherapy, vaccine viruses or even systemically active proteins, e.g., cytokines and chemokines. A review of the literature relating to localized heat activation of HSP promoters and HSP genes in the skin revealed that a multitude of different technologies has been explored in small animal models. In contrast, we uncovered few publications that examine HSP promoter activation in human skin. None of these publications has a therapeutic focus. We present herein two, clinically relevant, developments of heating technologies that effectively activate HSP promoters in targeted regions of human skin. The first development advances a system that is capable of reliably activating HSP promoters in human scalp, in particular in hair follicles. The second development outlines a simple, robust, and inexpensive methodology for locally activating HSP promoters in small, defined skin areas.
Keywords: Targeted heating, Skin, Human, Mammals, HSP promoters, HSP genes
Introduction
Therapies, including genetic therapies, are preferably constrained to the time required to produce the desired therapeutic effect and to the body region targeted by the intervention, should the nature of the therapy permit. Highly inducible promoters of HSP genes have long been considered for their potential to regulate gene therapies. While such promoters may be activated to some degree by various chemical compounds, heat tends to be the most effective activation modality. Furthermore, spatial control may not be readily achieved via chemical compound activation of an HSP promoter. Hence, reliable physical methods for delivering an activating heat dose (defined by temperature and duration of exposure) to the intended region are essential to employ HSP promoters in targeted therapies.
We focus herein on approaches that deliver an HSP promoter-activating heat dose to the skin, in particular human skin. Many applications can be envisaged that would require activation of HSP promoters in the skin. For example, regionally or systemically active polypeptides, including cytokines and hormones, may be expressed in the skin—either in cells of the skin or in cells introduced in the skin—from genes controlled by HSP promoters. For many of the latter active agents, control over the time during which they are produced or the location of their expression may be important. Other applications of HSP promoters may include expression of polypeptides for the treatment of various skin diseases. Yet other applications may involve the controlled expression of foreign antigens, i.e., subunit vaccines, or alternatively the controlled proliferation of vaccine viruses. Lastly, there may be skin conditions for which local activation of the heat shock response, i.e., the activation of endogenous HSP and/or other heat-inducible genes, may be beneficial.
In principle, heating of skin areas and immediately underlying tissue may be achieved using technology that is simpler than that required for targeted deep tissue heating. Still, the common obstacle for all heating applications is dissipation of heat. There are also important differences between rodent, e.g., mouse, skin, and human skin, which make efficient heat activation of HSP promoters more difficult to accomplish in human skin (Sundberg et al. 2012). Both human epidermis and dermis are much thicker than the corresponding mouse tissues (excluding areas of thick skin such as, e.g., footpads). Human dermis is also highly vascularized, containing rich papillary and deep dermal networks that play an essential role in thermoregulation by dissipating heat. In contrast, mouse dermis is poorly vascularized. Moreover, human skin, but not mouse skin (except for the footpads), is abundantly dotted with eccrine sweat glands. These glands are critically important for heat loss, as is underscored by the tendency for people without functional glands to develop hyperthermia and heat stroke. In summary, the rich dermal vascularization and the eccrine glands that are present body-wide cause heat dissipation to be much more effective in human skin than in mouse skin. Consequently, a method that successfully activates HSP promoters in mouse skin may not prove to be similarly effective in human skin. Furthermore, there are strong thermal effects on rates of perfusion. Local thermal exposure was found to increase perfusion in the human skin by as much as 10–20-fold (Dewhirst et al. 2003; Song et al. 1980, 1990; Houghton et al. 2006). Thus, achievement and maintenance of an elevated temperature in a targeted region of human skin is opposed by local heat dissipation, which in turn is boosted by the thermal exposure itself. Furthermore, the more efficient the local heat transfer, the steeper will be the thermal gradients. Adding to the complexity, a chosen activating heat dose may cause collateral thermal damage. Even if no damage is inflicted, the thermal exposure may exceed the threshold for pain sensation (Stoll and Greene 1959).
We begin by describing approaches that have been used in small animal models and discuss the few published accounts of experiments that detected HSP gene expression in heat-treated skin of human subjects. We then present our own work that was aimed at developing clinically applicable solutions for the effective regional / targeted activation of HSP promoters in human skin.
Experimental activation of HSP promoters in skin or in the subcutaneous space of small mammals
Skin may be heated using convective, conductive, or radiative heating approaches. By far the most studies employed a circulating temperature-controlled water bath for convective heating. Mice or rats are fitted with a floating device that allows them to maintain their heads above water, while the rest of their bodies is immersed in the water bath (Tolson and Roberts 2005). Core body temperature increases, and expression of HSP genes is observed in skin as well as in internal organs. Blake et al. (1990) induced HSP expression in skin and other organs by exposing adult rats in a positive forced air incubator to temperatures up to 40 °C for as long as 90 min. “Dry heat” (heated container) was employed for whole body heating of mice by Silverstein et al. (2014).
Region-specific heating of mice and rats was achieved by immersing their hind limbs in a temperature-controlled water bath, using appropriate restraining devices. Such regional heating was employed to heat skin and underlying tissues, which included muscles and subcutaneously implanted tumors in some experiments. Studies of this type are described, e.g., in Brade et al. (2000), Vilaboa et al. (2005), Che et al. (2007), Hall (2008), Fortin et al. (2014), Lee et al. (2015), and Bloom et al. (2015).
Conduction heating was employed in a study aimed at demonstrating HSP-dependent protection against chemotherapy-induced hair loss. Small patches of skin at the nape of the neck of young rats, as well as adult rodents, were heated via direct contact with the flat end of a metal cylinder (with a diameter of approximately 10 mm), which was internally heated by warm water circulating from a temperature-controlled water bath (Jimenez et al. 2008). Subsequent to a 46 °C heat application for 20 min to non-depilated skin of young rats, strong HSP70 staining in the epidermis and upper parts of hair follicles could be observed. Staining over the entire length of the follicles required exposure to 48.5 °C heat. Another study examined whether heat preconditioning could reduce skin damage from ruby laser irradiation (694.3 nm), which is a method used for depilation (Topping et al. 2001). Skin regions of adult mice were trimmed of hair and were heat-treated for 15 min at different temperatures with the use of a device that featured conduction heating with a thermostatically controlled 15-mm metal plate. Treatment at 45 °C produced strong HSP70 staining in the epidermis, and staining extended half-way down the hair follicles without producing recognizable damage. HSP70 staining over the entire length of the hair follicles required a 47 °C heat treatment.
Various studies explored the therapeutic potential of radiation heating applications in small mammal models. Smith et al. (2002) investigated the therapeutic utility of ultrasound-activated, HSP promoter-driven genes in mouse and rat models. One set of experiments utilized intradermal administration of an adenovirus vector that delivered a human HSPA7 promoter-controlled luciferase gene. One day after administration, the virus-infected skin region was subjected to irradiation using a portable, commercial, 1 MHz ultrasound device, which was equipped with a 2-cm2 applicator. Animals were euthanized 12 h later, and tissue samples were taken and processed for luciferase activity measurement. Under optimal conditions (20 min exposure; 50% duty cycle), luciferase expression was induced 212-fold over background.
A short diversion may be permitted to briefly discuss high-intensity focused ultrasound (HIFUS) which has received attention for its potential for heating deep-seated tissues. Acoustic energy can be concentrated into a focal volume within a few millimeters. Ultrasound systems have been combined with MRI, PET, or ultrasound imaging for image-guided dose exposure. A first demonstration that MRI-guided HIFUS could be employed to activate an endogenous HSP70 promoter in rat hind leg muscles was provided by Madio et al. (1998). Later work showed that the technology (improved by the introduction of an automated MR feedback) was also capable of inducing the expression of a GFP transgene which was under the control of a human HSPA7 promoter (Guilhon et al. 2003). The experiment involved focused heating in rats, which carried subcutaneous glioma xenografts that contained the latter transgene. Elevation of temperature in the target region to 44–50 °C for 180 s was sufficient for transgene induction. Expression of an adenovirus-delivered HSPA7-driven luciferase gene could also be activated by MRI-guided HIFUS in the prostate of beagles (Silcox et al. 2005). A more recent study was aimed at demonstrating the close correlation of MR temperature maps and transgene expression (Deckers et al. 2009). The latter study made use of a transgenic mouse model that harbored a mouse HSPA1B promoter-driven luciferase transgene. A close correspondence of local temperature and expression of the transgene assessed by in vivo bioluminescence was demonstrated. Mild heating protocols (2 min at 43 °C) that did not cause detectable tissue damage were sufficient for significant gene activation.
O’Connell-Rodwell et al. (2008) established a mouse model featuring a mouse HSPA1 promoter-driven firefly luciferase gene. Using this model, they demonstrated induction of luciferase expression in the skin following a 1 s CO2 laser pulse at 10,600 nm and radiant exposures of 1.8, 2.7, 3.5, or 4.4 J/cm2. The experiments suggested that this induction was energy dose-dependent, as only a weak induction in a narrow, central region occurred at 1.8 J/cm2. A more important induction in a larger region was observed at 2.7 J/cm2. At higher radiant exposures, toxicity dominated, and luciferase expression declined. See Wilmink et al. (2008) for a more elaborate study of the same model of induced reporter gene expression and mid-IR laser-induced tissue damage. In a follow-on study, a 6-mm diameter spot on the back of mice was irradiated under conditions (10,600 nm, 1 s pulse, 4.4 J/cm2) that were expected to cause some dermal damage (Mackanos et al. 2011). Seven hours after irradiation, in vivo bioluminescence was employed to define the zone of thermal damage and an adjacent zone in which luciferase was expressed. RNA was extracted from skin samples of the two zones, and microarray analyses were performed. In the adjacent zone, 69 genes were found to be upregulated (≥ 4-fold). Among these genes were HSPA1A, HSPA4, and HSPA8, although other genes including certain chemokine genes were more highly induced. The model was also used to further assess the influence of laser pulse duration and radiant exposure on HSP70 induction, with the intention of defining conditions that produce thermal stress without thermal damage to mouse skin (Mackanos and Contag 2011). The HSP70 response in vivo was evaluated using bioluminescence at 5 timepoints for pulse durations that ranged from 1 to 1000 ms. HSP70 signal was correlated with pulse duration, with the highest induction observed for the longest pulses (500 and 1000 ms).
NIR laser light has also been employed for focused heating of skin regions (Tolson and Roberts 2005). For example, Sajjadi et al. (2013) spot-irradiated the skin of mice using an Nd:YAG laser (1064 nm; 200 ns pulse width) for 15 s. Time-averaged power values varied between 3.74 and 7.42 W. Enhanced expression of both HSP70 and HSP47 occurred in the epidermis and the dermis of irradiated animals. The authors noted that HSP70 expression may delineate the thermal damage zone, whereas HSP47 expression may illustrate the process of recovery from thermally induced damage.
There is little absorption of NIR light in the 650–900 nm range by hemoglobin and water, a phenomenon termed “NIR tissue transmission window.” Consequently, NIR light can penetrate to a depth of up to 10 cm in soft tissue. While activation of HSP promoters in the skin and immediately below the skin can be achieved with this technology, little activation is induced in strata that lie just a few mm deeper (Rylander et al. 2011). The depth at which NIR light can efficiently activate HSP promoters has been increased by means of plasmonic nanoparticles that absorb in the NIR part of the spectrum. Such particles, developed for photothermal therapy applications, include gold nanorods, nanospheres, nanoshells and nanocages, and carbon nanotubes and nanohorns (Huang et al. 2008; Robinson et al. 2010). Conduction electrons of such particles can be photoexcited to induce surface plasmon oscillations. Upon surface plasmon formation, nonradiative relaxation occurs through electron−phonon and phonon−phonon coupling. Heat is efficiently produced on the nanoparticles and is then transferred to the surrounding tissue (Mackey et al. 2014). Several studies have demonstrated that this technology is highly effective in activating HSP promoters in the subcutaneous space (Miyako et al. 2012; Martin-Saavedra et al. 2014).
Fractional ablation therapies for skin resurfacing can impart heat and result in HSP induction. For example, Li et al. (2015) examined the histological response to dermal application of microplasma radio frequency to piglets using a roller tip. The roller tip consisted of six cogs, each with 38 metal pins. Both energy and speed of movement of the roller across the skin were varied: 40, 80, or 120 W at 6 cm/s, and 80 W at 2.5 cm/s. Heat generated by microsparks in the plasma between the skin and electrode spicules on the roller resulted in microlesions with upregulation of HSP70 and HSP47 expression in the surrounding epidermis and dermis as determined by immunohistochemical staining. Increased expression was evident by 7 days after treatment, mainly in the dermis. HSP72 and HSP47 expression peaked at 1 month and returned to normal at 6 months.
A recent study (Sandre et al. 2017) demonstrated the feasibility of using magnetic iron oxide nanoparticles in an alternating magnetic field to exert transcriptional control over an HSP promoter in skin. A colloidal dispersion in Matrigel of magnetic iron oxide nanoparticles was applied topically or injected subcutaneously in transgenic mice that expressed luciferase from a mouse HSPA1B promoter-driven gene. After dosing, mice were placed in a copper ring solenoid, and an alternating magnetic field was applied. A fiber optic temperature probe was placed in the “pseudotumor” created by the injected nanoparticles in Matrigel or, in the case of the dermally applied solution, in the droplet on the skin surface. The temperature probe was used to monitor temperature as well as to provide feedback control of the magnetic field generator with the goal of limiting local heating to 45 °C. Luciferase expression was evaluated using bioluminescence imaging 6 h after treatment. Both topical application and injection of magnetic iron nanoparticles, coupled with the presence of an alternating magnetic field, were capable of producing mild hyperthermia and increased luciferase expression.
Several studies investigating methods for improving skin flap survival exposed animals to heat treatments and examined expression of HSPs in the flap. In one such study, partially developed dorsal flaps in adult rats were heated for 30 min at 45 °C by means of a heating blanket (Koenig et al. 1992). Six hours later, the flaps were fully developed. Expression of HSP70 in the flaps of heat-treated animals but not control animals could be demonstrated immunohistochemically. This finding was corroborated by another study in which rats were subjected to general hyperthermia by immersion in a temperature-controlled water bath (at 45 °C until core body temperature reached 42 °C and then at 42 °C for 15 min) (Ghavami et al. 2002). Immunohistochemistry was performed 7 days after heat treatment and flap development. HSP70 staining of basal layer, hair follicles, eccrine glands, and endothelial cells was stronger in the heat-treated group than in the control group.
Microwave irradiation was used to activate HSP70 gene expression in the knee cartilage of rabbits (Tonomura et al. 2008). Region-specific heating was assured by covering not-targeted parts of the rabbits with aluminum foil. This approach may also be applicable to HSP promoter activation in the skin.
Experimental activation of HSP promoters in human skin
Trautinger et al. (1996) were concerned with protecting human skin from UVB damage via heat preconditioning. 25-cm2 areas of outer forearm skin were heated using a circular copper plate with a hollow copper heating coil affixed to the backside. The coil was connected to a temperature-controlled water bath. Heating was at 41 °C for 90 min. Biopsies were taken 4 h later and analyzed immunohistochemically. The authors reported that they detected enhanced expression of HSP70 (HSP72) in the epidermis and de novo expression in the dermis of heat-treated skin. Wilson et al. (2000) subjected 60-cm2 patches of buttock skin of human volunteers to 41.5 °C for 1 h using an unspecified electrical heating device. Punch biopsies of heated und unheated skin were taken 4, 8, and 24 h after heat treatment and were analyzed by immunohistochemistry and western blot. Immunohistochemistry revealed increased epidermal expression of HSP70 (HSP72), HSP60 and HSP27 4 and 8 h after heat treatment. HSP60 signals were noted in the dermis 24 h after heat treatment.
Kim et al. (2006) investigated possible effects of NIR exposure on the skin of human volunteers. Skin in a buttock region was irradiated for 65 min using a lamp emitting light in the NIR range of the spectrum (maximal intensity at about 1100 nm). Skin surface temperature rose to 42.1 ± 0.5 °C within about 10 min and remained at this level for the remainder of the exposure period. Punch biopsies were analyzed for HSP70 expression by semi-quantitative RT-PCR and western blot. A transient modest increase in HSP70 RNA and an accumulation of HSP70 protein were observed in the epidermis.
Several studies addressed effects of fractional photothermolysis, a “nonablative” laser modality for the rejuvenation of aged human skin (see, e.g., Laubach et al. 2006; Starnes et al. 2012). The method involves using IR laser light (at 1500 and 1550 nm, respectively, in the above-cited studies) to create many microscopic areas of skin necrosis (epidermis and underlying dermis). Perhaps not surprisingly, immunohistochemistry revealed enhanced HSP70 signals in areas between lesions. While the latter publications reported transiently increased HSP70 levels 1 day after treatment, which returned to background levels within a few days, two studies using CO2 lasers reported increased HSP70 (HSP72) immunostaining in epidermis and/or dermis that lasted for one to several months and apparently accompanied a protracted wound healing process (Hantash et al. 2007; Xu et al. 2011). The studies also found long-lasting increases in HSP47 immunostaining in the dermis.
Hantash et al. (2009) developed an RF device for fractional thermolysis. This device delivered bipolar RF energy to the dermis via five microneedle 30 G electrode pairs 6 mm in length and spaced 1.25 mm apart (inserted at an angle of 200). During the treatment of 4 s, dermal tissue temperature was maintained at 72 °C. The epidermis was cooled to prevent damage. The authors reported that the dermis stained positive for HSP70 (HSP72) 2 days after the procedure. This staining was no longer observed after 14 days. Staining for HSP47 was also noticeable at day 2. Elevated HSP47 levels were still observed 10 weeks after treatment.
Towards clinically applicable approaches for the effective activation of HSP promoters in the human skin
Activation of HSP promoters in hair follicles of the human scalp
We investigated whether it would be feasible to induce HSP genes in the root of hair follicles of the human scalp. Previous work in rodents had suggested the possibility that heat preconditioning of the hair follicles over their entire length would render them resistant to the toxicity of chemotherapy agents, preventing the alopecia (loss of scalp hair) that is caused by many chemotherapy regimens (Jimenez et al. 2008). Forced convection heating was considered the most appropriate method for this project (further elaborated in Discussion). A medical system was built whose centerpiece is a small round bath (“head bath”) appropriately sized to accommodate the scalp of a subject (Fig. 1a). Water of the desired temperature circulates through the bath at a rate of 19 l/min, entering through an opening at its bottom and exiting through outlets near its rim. A subject to be treated lays supine on an examination bed and immerses its scalp in the head bath. The subject’s head is supported by a cross of strings attached to positions on the rim of the bath (allowing hair to free-flow in the water to the maximum extent possible).
Fig. 1.
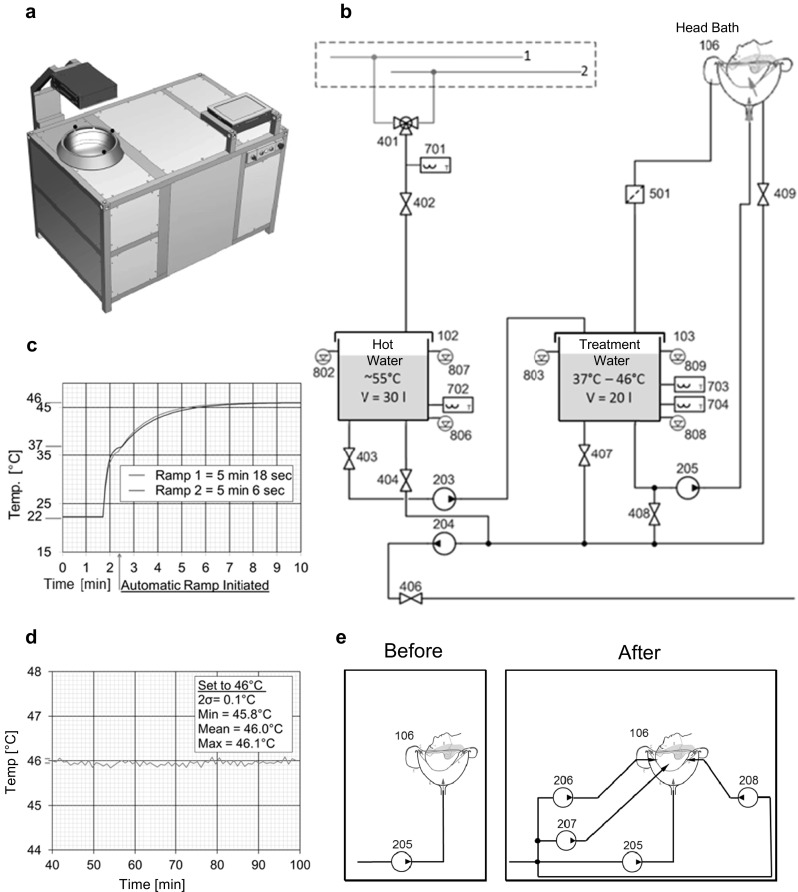
Forced convection heating of the human scalp for activation of HSP promoters. a Representation of medical system Thermather (excluding the external heating tower). Visible are the head bath, the touchscreen control unit, the lock, main and emergency interrupters, and an overhead display unit. b Hydraulic scheme (from which all elements relating to automated cleaning and disinfection of the system were omitted). 1, 2: building hot and cold tap water; 102, 103: hot water and treatment reservoirs; 106: head bath; 203–205: low-voltage diaphragm bilge pumps (membrane pumps); 401: motorized 3-way valve; 402–409: solenoid valves; 501: strainer; 701–704: Pt100 temperature sensors; 802, 803: analog ultrasound level sensors; 806–809: capacitance level sensors. Note that reservoir 103 is filled ahead of reservoir 102. c Ramp-up to the selected temperature, here 46 °C. The first part of the curve represents the filling of reservoir 103 with 37 °C–water. The ramp-up phase starts at the time indicated by the arrow. d Effective maintenance of the selected temperature in the head bath. e Drawing explaining how the system was modified to improve performance. Three additional membrane pumps (206–208) were introduced that operated in parallel to membrane pump 205 and delivered water directly to jets mounted in the head bath
The hydraulic scheme of the core system shows two reservoirs (102,103). The treatment water reservoir (103) is filled with warm water at about 37 °C produced by mixing hot and cold tap water from the building. The hot water reservoir (102) receives hot building water (Fig. 1b). Water from treatment water reservoir is pumped through the head bath (106). Temperature ramp-up in the head bath involves repeated addition of appropriate quantities of hot water from the hot water reservoir to the treatment water reservoir, and of removing excess water from the latter reservoir. Temperature maintenance in the head bath is also assured by the controlled transfer of small volumes of hot water to the treatment water reservoir. These and other operations are controlled by a processor. If desired or needed when the temperature of the hot building water is below that required, the core system is operated in conjunction with an auxiliary heating system. A typical ramp-up temperature profile is shown in Fig. 1c. Prior to ramp-up, the treatment water reservoir is filled with warm water. Ramp-up to the desired treatment temperature (here 46 °C) begins at the time point indicated by the arrow and takes about 5 min. As exemplified in Fig. 1d, the system maintains treatment temperature within narrow limits. Maxima and minima were 46.1 and 45.8 °C, respectively. Prior to exposure experiments, the system was tested for electrical safety and was found to conform to the relevant norm IEC 60601. The data presented below are from self-experiments in which the principals of the study participated.
In a typical experiment, a subject was donned earplugs and an ear-and-neck covering and then immersed its scalp in the warm water-filled head bath. After ramp-up, temperature in the head bath was maintained at 45.5 °C for 30 min. It is noted that the highest heat dose administered in any experiment was 46.5 °C for 30 min. This dose was known not to cause heat damage to skin from an earlier study by Moritz and Henriques (1947). While searching for a reason why HSP gene induction was not observed in a first series of experiments, we discovered that the temperature on the surface of a subject’s scalp (measured using a thermocouple) was several 0C lower than the water temperature in the head bath. To enhance the exchange of water on the scalp, the system was modified to include three additional pumps that operated parallel to pump 205 and delivered water from the treatment water reservoir to the sides and the back of the scalp through jets (increasing the overall flow rate to 76 l/min) (Fig. 1e). This improvement reduced the temperature difference between scalp surface and head bath to no more than about 0.2 °C. Core temperature was measured periodically and was found to remain unchanged.
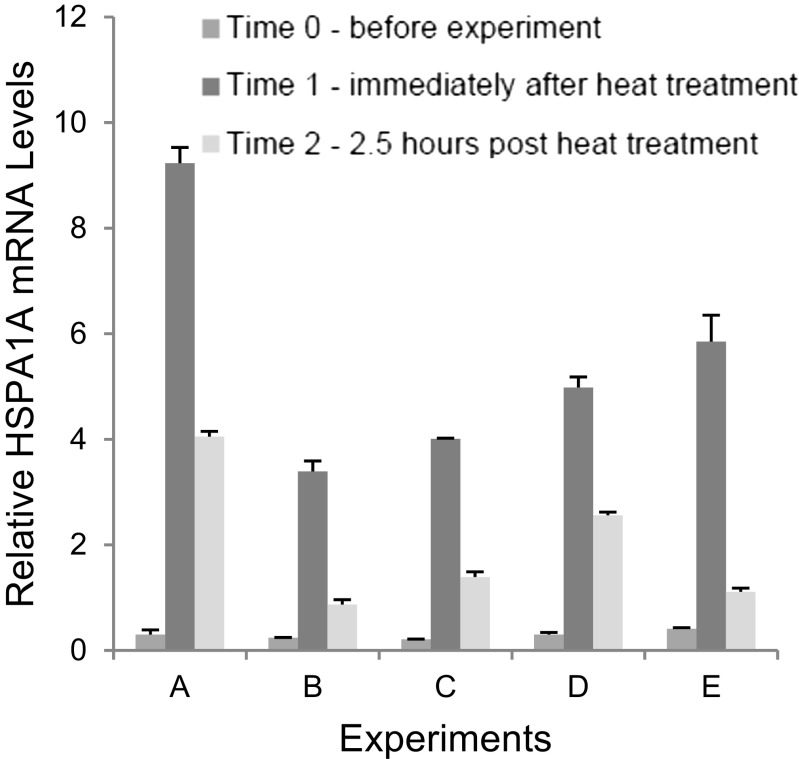
In the experiments performed with the modified system that are presented herein, hair plucks comprising about 50 hairs were taken before, immediately after and 2.5 h after the heat exposure. The lower portions of the hair plucks were quick-frozen in liquid nitrogen and stored at − 80 °C until analysis. RNA was extracted and analyzed by RT-qPCR for transcripts of the HSPA1A and HSPB1 genes. HSPA1A expression data from five experiments involving different individuals are shown in Fig. 2. Strong activation of the HSPA1A promoter was observed in all samples taken after heat treatment. Fold activation values were found to be between about 14 and 31 in determinations that employed the RPL13A gene for normalization. Substantial activation (> 1.5-fold) of the HSPB1 promoter was only seen in one of these experiments (not shown). In total, eight similar experiments were conducted. Elevated levels of HSPA1A transcripts were seen in all experiments, but levels of HSPB1 transcripts were only found substantially increased in three of the experiments. Generally similar results were obtained when the RPLP0 gene was employed for normalization of HSPA1A expression, except that heat activation was not evident in one of the eight experiments. Therefore, using forced convection heating, the HSPA1A promoter could be activated robustly in cells of hair follicle roots, which are located at a depth of approximately 4 mm in an abundantly vascularized part of the skin (Jimenez et al. 2011).
Fig. 2.
HSPA1A expression in hair follicles before, immediately after and 2.5 h after heat treatment. RT-qPCR data from five independent experiments are shown. HSPA1A RNA quantities were normalized using cellular RPL13A RNA and expressed as relative quantities. RNA was extracted from the biopsies using the RNeasy Fibrous Tissue Mini Kit (Qiagen) according to the supplier’s protocol. Final elution of RNA was with 30 μl water (from the kit). 500 ng RNA was reverse-transcribed employing the QuantiTect RT Kit (Qiagen). Quantitative PCR was with PowerSYBR-Green Mastermix (Applied Biosystems). Primers: HSPA1A: QT01671873; HSPB1: QT00233457; RPL13A: QT00089915
General method for activation of HSP promoters in the human skin
A heating method that is inexpensive, operative anywhere in the field as well as applicable without the need for medical assistance may employ pads that are heated by the crystallization of a supercooled solution. This technology is well known (see U.S. patent no. 3,951,127 awarded to Watson and Watson in 1976) and has long been used in commercial articles such as, e.g., heating pads for soothing muscle or joint aches.
In our proof-of-principle experiments, we aimed to deliver to human skin a heat dose near the upper end of the comfort zone but well below the threshold for skin damage (Moritz and Henriques 1947). We settled on a dose of about 45 °C/15 min. For the supercooled solution sodium thiosulfate pentahydrate was employed. This salt was chosen because it has a melting point of about 48 °C, is inexpensive and essentially nontoxic. 10 × 10 cm heating pads were made from 0.1 mm thick PVC film (double-layered on the contact side) and contained 150 ml of sodium thiosulfate pentahydrate solution (99% pure; Fox Chemicals GmbH, Germany) that had been stabilized by the inclusion of 3% (weight) of distilled water (Fig. 3a). We observed that the supercooled solution in the pad remained in the liquid state for several months when the pad was kept at room temperature. To ensure a tight contact between skin and heating pad, a water-based gel was applied to the area to be heated, here an area on an inner forearm of a subject. Crystallization was initiated, and the heating pad was placed on the forearm and was fastened using a sleeve having Velcro closures (Fig. 3b).
Fig. 3.
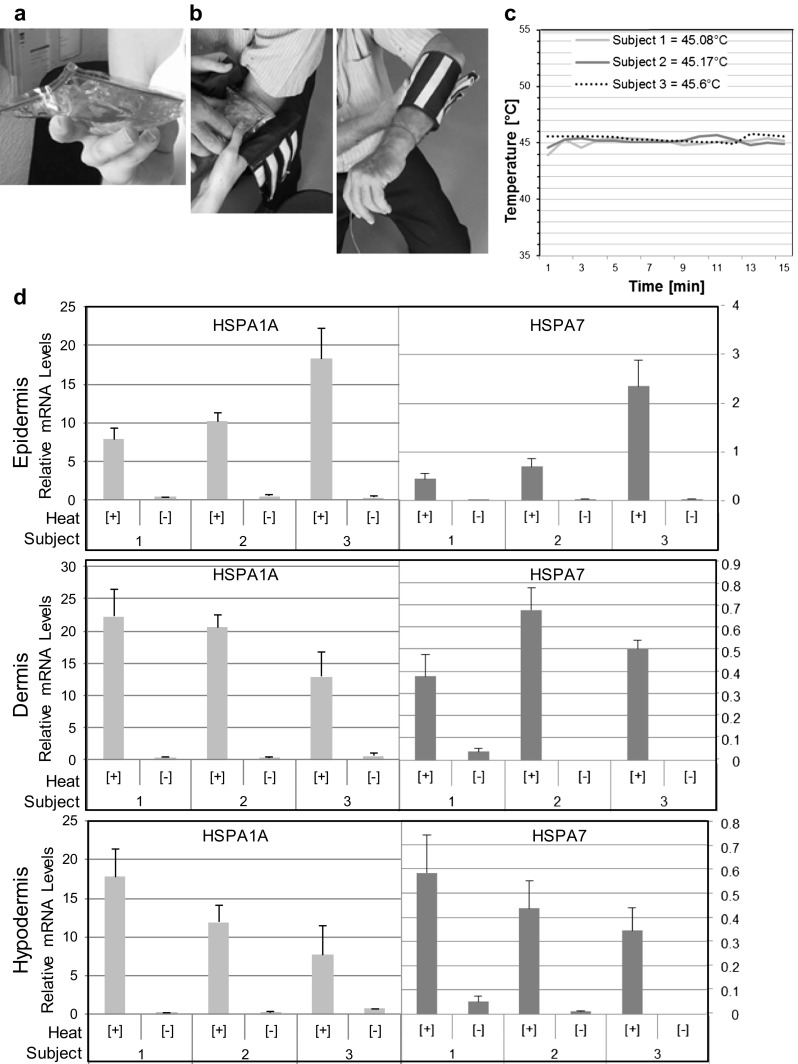
A general method for the activation of HSP promoters in the human skin. a Photograph of a heating pad. b Photographs showing how a heating pad is placed and fastened to a forearm of a subject. c Temperature on the skin surface during a 15-min heat treatment. d RT-qPCR data for three subjects. HSPA1A and HSPA7 RNA quantities were normalized using cellular β2-microglobulin RNA and expressed as relative quantities. Multiple tissue sections for each tissue compartment were pooled, and RNA was extracted using an RNeasy Mini Kit (Qiagen) and reverse-transcribed employing the QuantiTect RT Kit (Qiagen). RNA was quantified using a NanoDrop spectrophotometer. cDNA was amplified using a SYBR-Green RT-PCR kit from Qiagen. (For B2M primers see Cicinnati et al. 2008 and for HSPA1A and HSPA7 primers see Villa et al. 2015)
The data presented herein are from a self-experiment in which three principals of the study participated. The temperature evolution on the skin surface under the heating pad was measured by means of a calibrated thermocouple inserted between skin and heating pad. The intended operating temperature (45 ± 0.5 °C) was reached 1–2 min after the heating pad had been affixed and was maintained within narrow limits throughout the remainder of the 15-min exposure period (Fig. 3c). Core body temperature did not change. We found that a single prick with a fine needle reliably and rapidly triggered crystallization. More elaborate starter mechanisms such as the inclusion in the pad of a snap metal disc or small rigid objects such as glass beads were described in U.S. patents nos. 4,379,448 and 5,275,156. Thirty minutes after heat treatment, punch biopsies were taken from the center of the treated area as well as from a similar location on the contralateral arm. The cylindrical skin biopsies were embedded vertically in Tissue-Tek on metal holders, quick-frozen in liquid nitrogen and then stored at − 80 °C. Each sample was cut on a cryostat at a nominal section thickness of 40 μm. The texture of the sectioned tissue surfaces allowed differentiation between epidermis, dermis, and hypodermis. The metal knife was carefully cleaned (70% ethanol) when moving from one tissue compartment to the next. Multiple sections (8–12) of each compartment were pooled, and RNA was extracted using a standard method. Extracted RNAs were analyzed by RT-qPCR for HSPA1A and HSPA7 transcripts, using β2-microglobulin transcripts for normalization. We found that the heat treatment resulted in strong activation of the HSPA1A and HSPA7 promoters in all three subjects. Relative HSPA1A transcript levels in heat-treated epidermal tissue were 7.75, 10.1, and 18.0 for subjects 1–3, and levels in untreated tissue were 0.39, 0.42, and 0.33, respectively (Fig. 3d, top graph). Fold induction was 19.7, 23.6, and 54.4, respectively. For HSPA7 transcripts, corresponding relative values were 0.43, 0.68, and 2.31 for heat-treated tissue and 0.0071, 0.0007, and 0.013 for untreated tissue. Induction was 60.0-, 944-, and 171-fold. Similar findings were made for the other compartments, although lower fold induction values were also observed that were apparently due to elevated levels of uninduced expression in some tissue samples (Fig. 3d, middle and bottom graphs). It is noted that there was no obvious gradient of induced gene activity from epidermis to hypodermis. However, there appeared to be differences in the responses seen in the different subjects. For example, subject 3 expressed significantly higher levels of HSPA1A and HSPA7 transcripts in the epidermis than subject 1 but significantly lower levels in the hypodermis. Comparable results were obtained when HSP transcript levels were normalized relative to RPS13 gene transcripts (not shown), except that normalized levels were depressed for one of the subjects who expressed considerably more RPS13 RNA than the other two. We conclude that the general method presented herein, which employs heating pads that heat by crystallization of sodium thiosulfate pentahydrate, was capable of effectively activating HSPA1A and HSPA7 promoters in all skin layers.
Discussion and reflections
As elaborated above, various methods for localized heating were employed in small animals (mostly rodents). Convective heating, i.e., water bath heating, of rodent legs resulted in induction of HSP gene activity in skin and underlying tissues including muscles and subcutaneously implanted tumors. Such studies were described, e.g., in Brade et al. (2000), Vilaboa et al. (2005), Che et al. (2007), Fortin et al. (2014), Lee et al. (2015), and Bloom et al. (2015). Targeted conductive heating of mouse or rat skin activated endogenous HSP genes in epidermis and hair follicles (Topping et al. 2001; Jimenez et al. 2008). Application of ultrasound to the skin also induced HSP promoter-driven gene activity in the skin (Smith et al. 2002). Narrowly targeted irradiation using a NIR laser activated HSP gene expression in the skin and on the skin-proximal surface of a subcutaneously implanted tumor (Tolson and Roberts 2005; Rylander et al. 2011). Mid-IR irradiation has been employed to stimulate HSP gene expression in the skin (O’Connell-Rodwell et al. 2008; Wilmink et al. 2008; Sajjadi et al. 2013). Microwave irradiation may also be applicable to activation of HSP promoters in the skin (Tonomura et al. 2008).
Human skin was heated and HSP promoters activated by conductive methods (Trautinger et al. 1996; Wilson et al. 2000), irradiation with NIR (Kim et al. 2006) or fractional thermolysis using mid-IR (Laubach et al. 2006; Hantash et al. 2007; Starnes et al. 2012; Xu et al. 2011) or RF (Hantash et al. 2009). It is noted that the qualitative analysis methods employed in these studies make it impossible to assess how effectively HSP promoters were activated.
A priori, any of the above methods that have been used for heating the skin of animals or humans and activating HSP promoters in cells located therein may be considered for clinical applications. However, methods and/or equipment used in their practical implementation would need to be adapted to accommodate human anatomy as well as to fulfill requirements critical for the intended clinical application. The method for human hair follicle heating discussed above provides an example of such an adaptation. A key requirement was to heat the entire scalp of human subjects, taking into consideration the parameters of variant head size, different patterns of hair growth as well as the variety of length and density of hair. This requirement virtually excluded methods of conductive heating and of NIR irradiation. Other radiative methods such as focused ultrasound could have been used, except for the difficulty of designing a device/system that is capable of uniformly irradiating the scalp of any subject. What remained as the only apparent, practicable method was convection heating delivered with a water bath of sufficient size to permit the submersion of any subject’s scalp and that features high-velocity forced convection to exchange water molecules close to the scalp and to prevent the development of layers or pockets of (cooler) stagnant water on the scalp.
Convection heating, conduction heating using electrical heating equipment, as well as radiation heating approaches such as ultrasound, microwave, RF, or NIR or mid-IR laser may be employed to heat defined skin areas on human extremities (and, possibly, other body regions). All of these approaches require devices of some complexity and a hospital or medical practice environment. Even if they could be made portable, such devices would be dependent on electrical outlets, local energy generation, or a supply of charged batteries. Furthermore, physician or technician time may have to be allocated to each treatment. Hence, the expense of employing any of the latter approaches would be considerable. A heating method that is inexpensive, safe, operative anywhere in the field as well as capable of being used without medical assistance may employ pads that are heated by the crystallization of a supercooled solution. We have demonstrated that such a method can be successfully employed to reproducibly deliver a defined heat dose to a targeted skin area of a human subject. Having such a method available may facilitate therapy applications involving localized activation of a heat shock response as well as heat-controlled gene or cell therapies in skin or, possibly, also in the space below skin. Our own interest was to develop a robust and inexpensive method for activating replication-competent controlled vaccine viruses subsequent to their intradermal or hypodermal administration (Voellmy et al. 2015; Bloom et al. 2015).
For obvious reasons, our experiments were not capable of comprehensively characterizing the two methods for HSP activation in human skin presented herein. Thorough characterization will only be possible in the context of human clinical trials. We have chosen an inner forearm location for our experiments with the heating pads. The reason for this choice was that the inner forearms of most persons contain little terminal hair which was considered a potential confounding factor. At present, we do not know whether the HSPA1A and HSPA7 promoters were maximally activated under the chosen heat exposure conditions. If the promoters were in fact maximally activated, the heat dose administered may have been excessive, and it may be possible to reduce it. For instance, the duration of the heat exposure may be shortened. However, if full activation was not achieved (and full activation was expected to be required for the intended purpose), a larger heat dose would need to be applied. Unfortunately, temperatures above 45.5 °C are uncomfortable, and long exposures may not be desirable. In the context of genetic therapies or vaccination, a possible avenue for resolving such a problem would be modification of an HSP promoter to enhance its induced activity. Brade et al. (2000) added a short DNA segment containing three heat shock elements (HSE) upstream from the core human HSPA7 promoter. HSEs are binding sites for heat shock transcription factors (most notably HSF1) that control heat-induced expression from HSP promoters. The resulting modified promoter was as active following a 43 °C/45 min heat treatment as the original HSPA7 promoter after a 45 °C/45 min heat shock when assessed by reporter expression in transfected human MCF7 cells. Hence, the modification effectively reduced the activation temperature by about 2 °C. Ortner et al. (2015) built an artificial HSP promoter by adding to a basal promoter an array of six DNA segments each containing an idealized HSE. The engineered promoter was as active after a 41.5 °C/2 h heat exposure as the HSPA1A promoter after a 43 °C/2 h heat treatment in HEK 293 cells transfected with luciferase reporter genes driven by one or the other promoter. Another approach may consist of increasing HSF1 activity. A system involving expression of a constitutively active form of HSF1 under the control of an HSP promoter was described by Vilaboa et al. (2005). Wang et al. (2003) have used a similar approach to amplify the activity of a weak non-HSP promoter. Use of more elaborate designs that would allow for a reversible increase in HSF1 activity may also be contemplated. Finally, it is noted that our experiments specifically assessed heat-induced changes in transcript levels of HSP genes. Translation of transcripts of HSP promoter-driven genes may be subject to additional regulation, rendering the correspondence between transcript levels and expressed proteins less than perfect.
Acknowledgements
We would like to acknowledge the following colleagues for their contributions: Prof. Marco Celio and Dr. Walter Blum, Frimorfo Inc., Marly, Switzerland; Prof. Daniel Hohl, Dr. Marcel Huber and staff, Service de dermatologie et vénéréologie du CHUV, Lausanne, Switzerland; the mechanical engineers of the Haute école d’ingénierie et d’architecture de Fribourg, Switzerland; Lis Schärer; Prof. John Thome, Laboratory of heat and mass transfer, EPFL, Lausanne, Switzerland; and Dr. Nuria Vilaboa, University Hospital La Paz-IdiPAZ, Madrid and CIBER de Bioingeniería, Biomateriales y Nanomedicina, CIBER-BBN, Spain.
Funding information
The studies reported herein received funding from the Commission for Technology & Innovation CTI, a Swiss federal agency, and from HSF Pharmaceuticals SA.
RV is the founder of HSF Pharmaceuticals SA, a company with an exclusive focus on research and early development activities.
Contributor Information
Richard Voellmy, Email: rvoellmy@hsfpharma.com, Email: rvoellmy@ufl.edu.
Olivier Zürcher, Email: zurcherolivier@gmail.com.
Manon Zürcher, Email: zurchermanon@gmail.com.
Pierre A. de Viragh, Email: pierre.de.viragh@bluewin.ch
Alexis K. Hall, Email: alexiskh@phhp.ufl.edu
Stephen M. Roberts, Email: smroberts@ufl.edu
References
- Blake MJ, Gershon D, Fargnoli J, Holbrook NJ. Discordant expression of heat shock protein mRNAs in tissues of heat-stressed rats. J Biol Chem. 1990;265(25):15275–15279. [PubMed] [Google Scholar]
- Bloom DC, Feller J, McAnany P, Vilaboa N, Voellmy R. Replication-competent controlled herpes simplex virus. J Virol. 2015;89:10668–10679. doi: 10.1128/JVI.01667-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade AM, Ngo D, Szmitko P, Li PX, Liu FF, Klamut HJ. Heat-directed gene targeting of adenoviral vectors to tumor cells. Cancer Gene Ther. 2000;7(12):1566–1574. doi: 10.1038/sj.cgt.7700267. [DOI] [PubMed] [Google Scholar]
- Che J, Doubrovin M, Serganova I, Ageyeva L, Beresten T, et al. HSP70-inducible hNIS-IRES-eGFP reporter imaging: response to heat shock. Mol Imaging. 2007;6(6):404–416. doi: 10.2310/7290.2007.00036. [DOI] [PubMed] [Google Scholar]
- Cicinnati VR, Shen Q, Sotiropoulos GC, Radtke A, Gerken G, et al. Validation of putative reference genes for gene expression studies in human hepatocellular carcinoma using real-time quantitative RT-PCR. BMC Cancer. 2008;8:350. doi: 10.1186/1471-2407-8-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, et al. Image-guided, noninvasive, spatiotemporal control of gene expression. Proc Natl Acad Sci U S A. 2009;106(4):1175–1180. doi: 10.1073/pnas.0806936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst MW, Vigilanti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperth. 2003;19(3):267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- Fortin PY, Genevois C, Chapolard M, Santalucia T, Planas AM, et al. Dual-reporter in vivo imaging of transient and inducible heat-shock promoter activation. Biomed Opt Express. 2014;5(2):457–467. doi: 10.1364/BOE.5.000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami A, Nutt MP, Hardy SP. Heat shock protein and high dose aspirin: effects on random skin flap survival in a rat model. Ann Plast Surg. 2002;48(1):60–67. doi: 10.1097/00000637-200201000-00009. [DOI] [PubMed] [Google Scholar]
- Guilhon E, Voisin P, de Zwart JA, Quesson B, Salomir R, et al. Spatial and temporal control of transgene expression in vivo using a heat-sensitive promoter and MRI-guided focused ultrasound. J Gene Med. 2003;5:333–342. doi: 10.1002/jgm.345. [DOI] [PubMed] [Google Scholar]
- Hall A (2008) Harnessing the heat shock response to raise refined therapeutic outcomes. Open Access Dissertations. Paper 102. http://scholarlyrepository.miami.edu/oa_dissertations/102
- Hantash BM, Vikramaditya PH, Kapadia B, Rahman Z, Jiang K, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med. 2007;39(2):96–107. doi: 10.1002/lsm.20468. [DOI] [PubMed] [Google Scholar]
- Hantash BM, Anan AU, Chang H, Kafi R, Renton B. Bipolarfractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41(1):1–9. doi: 10.1002/lsm.20731. [DOI] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572(3):811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23(3):217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, Connelly EA, Chen Q, Zou J, Goldenberg C, Voellmy R. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13(1):31–38. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez F, Iseta A, Poblet E. Morphometric analysis of the human scalp hair follicle: practical implications for the hair transplant surgeon and hair regeneration studies. Dermatol Surg. 2011;37:58–64. doi: 10.1111/j.1524-4725.2010.01809.x. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim YK, Cho KH, Chung JH. Infrared exposure induces an angiogenic switch in human skin that is partially mediated by heat. Br J Dermatol. 2006;155(6):1131–1138. doi: 10.1111/j.1365-2133.2006.07510.x. [DOI] [PubMed] [Google Scholar]
- Koenig WJ, Lohner RA, Perdrizet GA, Lohner ME, Schweitzer RT, Lewis VL., Jr Improving acute skin-flap survival through stress conditioning using heat shock and recovery. Plast Reconstr Surg. 1992;90(4):659–664. doi: 10.1097/00006534-199210000-00016. [DOI] [PubMed] [Google Scholar]
- Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin response to fractional photothermolysis. Lasers Surg Med. 2006;38(2):142–149. doi: 10.1002/lsm.20254. [DOI] [PubMed] [Google Scholar]
- Lee J, Himori K, Tatebayashi D, Abe M, Yamada T. Response of heat shock protein 72 to repeated bouts of hyperthermia in rat skeletal muscle. Physiol Res. 2015;64(6):935–938. doi: 10.33549/physiolres.933084. [DOI] [PubMed] [Google Scholar]
- Li X, Fang L, Huang L. In vivo histological evaluation of fractional ablative microplasma radio frequency technology using a roller tip: an animal study. Lasers Med Sci. 2015;30:2287–2294. doi: 10.1007/s10103-015-1810-x. [DOI] [PubMed] [Google Scholar]
- Mackanos MA, Contag CH. Pulse duration determines the levels of Hsp70 induction in tissues following laser irradiation. J Biomed Opt. 2011;16:0708002. doi: 10.1117/1.3600013. [DOI] [PubMed] [Google Scholar]
- Mackanos MA, Helms M, Kalish F, Contag CH. Image-guided genomic analysis of tissue response to laser-induced thermal stress. J Biomed Opt. 2011;16(5):058001. doi: 10.1117/1.3573387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey MA, Moustafa R, Ali K, Austin LA, Near RD, El-Sayed MA. The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments. J Phys Chem B. 2014;118(5):1319–1326. doi: 10.1021/jp409298f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madio DP, van Gelderen P, DesPres D, Olson AW, de Zwart JA, Fawcett TW, Holbrook NJ, Mandel M, Moonen CTW. On the feasibility of MRI-guided focused ultrasound for local induction of gene expression. J Magn Reson Imaging. 1998;8(1):101–104. doi: 10.1002/jmri.1880080120. [DOI] [PubMed] [Google Scholar]
- Martin-Saavedra FM, Cebrian V, Gomez L, Lopez D, Arruebo M, et al. Temporal and spatial patterning of transgene expression by near-infrared irradiation. Biomaterials. 2014;35(28):8134–8143. doi: 10.1016/j.biomaterials.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyako E, Deguchi T, Nakajima Y, Yudasaka M, Hagihara Y, et al. Photothermic regulation of gene expression triggered by laser-induced nanohorns. Proc Natl Acad Sci U S A. 2012;109:7523–7528. doi: 10.1073/pnas.1204391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz A, Henriques F. Studies of thermal injuries II. The relative importance of time and surface temperature in the causation of thermal burns. Am J Pathol. 1947;23(5):695–720. [PMC free article] [PubMed] [Google Scholar]
- O’Connell-Rodwell CE, Mackanos MA, Simanovskii D, Cao YA, Bachmann MH, Schwettman HA, Contag CH. In vivo analysis of heat-shock-protein-70 induction following pulsed laser irradiation in a transgenic reporter mouse. J Biomed Opt. 2008;13(3):030501. doi: 10.1117/1.2904665. [DOI] [PubMed] [Google Scholar]
- Ortner V, Ludwig A, Riegel E, Dunzinger S, Czerny T. An artificial HSE promoter for efficient and selective detection of heat shock pathway activity. Cell Stress Chaperones. 2015;20(2):277–288. doi: 10.1007/s12192-014-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Welsher K, Tabakman SM, Sherlock SP, Wang H, Luong R, Dai H. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010;3(11):779–793. doi: 10.1007/s12274-010-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander MN, Stafford RJ, Hazle J, Whitney J, Diller KR. Heat shock protein expression and temperature distribution in prostate tumors treated with laser irradiation and nanoshells. Int J Hyperth. 2011;27(8):791–801. doi: 10.3109/02656736.2011.607485. [DOI] [PubMed] [Google Scholar]
- Sajjadi AY, Mitra K, Grace M. Expression of heat shock proteins 70 and 47 in tissues following short-pulse laser irradiation: assessment of thermal damage and healing. Med Eng Phys. 2013;35(10):1406–1414. doi: 10.1016/j.medengphy.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Sandre O, Genevois C, Garalo E, Adumeau L, Mornet S, Couillaud F. In vivo imaging of local gene expression induced by magnetic hyperthermia. Genes. 2017;8(2):61. doi: 10.3390/genes8020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox CE, Smith RC, King R, McDannold N, Bromley P, Walsh K, Hynynen K. MRI-guided ultrasonic heating allows spatial control of exogenous luciferase in canine prostate. Ultrasound Med Biol. 2005;31(7):965–970. doi: 10.1016/j.ultrasmedbio.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, et al. Inducing muscle heat shock protein 70 improves insulin sensitivity and muscular performance in aged mice. J Gerontol A Biol Sci Med Sci. 2014;70:800–808. doi: 10.1093/gerona/glu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Machluf M, Bromley P, Atala A, Walsh K. Spatial and temporal control of transgene expression through ultrasound-mediated induction of the heat shock protein 70B promoter in vivo. Hum Gene Ther. 2002;13(6):697–706. doi: 10.1089/104303402317322267. [DOI] [PubMed] [Google Scholar]
- Song CW, Kang MS, Rhee IG, Levitt SH. Effect of hyperthermia on vascular function in normal and neoplastic tissues. Ann N Y Acad Sci. 1980;335(1 Thermal Chara):35–47. doi: 10.1111/j.1749-6632.1980.tb50735.x. [DOI] [PubMed] [Google Scholar]
- Song CW, Chelstrom LM, Haumschild DJ. Changes in human skin blood flow by hyperthermia. Int J Radiat Oncol Biol Phys. 1990;18(4):903–907. doi: 10.1016/0360-3016(90)90415-G. [DOI] [PubMed] [Google Scholar]
- Starnes AM, Jou PC, Molitoris JK, Lam M, Baron ED, Garcia-Zuazaga J. Acute effects of fractional laser on photoaged skin. Dermatol Surg. 2012;38(1):51–57. doi: 10.1111/j.1524-4725.2011.02136.x. [DOI] [PubMed] [Google Scholar]
- Stoll A, Greene L. Relationship between pain and tissue damage due to thermal radiation. J Appl Physiol. 1959;14(3):373–382. doi: 10.1152/jappl.1959.14.3.373. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Nanney LB, Fleckman P, LE King J (2012) Skin and adnexa. In: Treuting P, Dintzis S, Fervert CW, Liggitt D, Montine KS (eds) Comparative anatomy and histology. A mouse and human atlas. Academic Press, Amsterdam, pp 433–455
- Tolson JK, Roberts SM. Manipulating heat shock protein expression in laboratory animals. Methods. 2005;35(2):149–157. doi: 10.1016/j.ymeth.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Tonomura H, Takahashi KA, Mazda O, Arai Y, Masaharu SY, et al. Effects of heat stimulation via microwave applicator on cartilage matrix gene and HSP70 expression in the rabbit knee joint. J Orthop Res. 2008;26(1):34–41. doi: 10.1002/jor.20421. [DOI] [PubMed] [Google Scholar]
- Topping A, Gault D, Grobbelaar A, Green C, et al. Successful reduction in skin damage resulting from exposure to the normal-mode ruby laser in an animal model. Br J Plast Surg. 2001;54(2):144–150. doi: 10.1054/bjps.2000.3501. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Knobler RM, Königsmann H, Mayr W, Kindas-Mügge I. Increased expression of the 72-kDa heat shock protein and reduced sunburn cell formation in human skin after local hyperthermia. J Invest Dermatol. 1996;107(3):442–443. doi: 10.1111/1523-1747.ep12365498. [DOI] [PubMed] [Google Scholar]
- Vilaboa N, Fenna M, Munson J, Roberts SM, Voellmy R. Novel gene switches for targeted and timed expression of proteins of interest. Mol Ther. 2005;12(2):290–298. doi: 10.1016/j.ymthe.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Villa F, Carrizzo A, Spinelli CC, Ferrario A, Malovini A, et al. Genetic analysis reveals a longevity-associated protein modulating endothelial function and angiogenesis. Circ Res. 2015;117:333–345. doi: 10.1161/CIRCRESAHA.117.305875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R, Bloom DC, Vilaboa N. A novel approach for addressing diseases not yielding to effective vaccination? Immunization by replication-competent controlled virus. Expert Rev Vaccines. 2015;14(5):637–651. doi: 10.1586/14760584.2015.1013941. [DOI] [PubMed] [Google Scholar]
- Wang J, Yao M, Zhang Z, Gu J, Zhang Y, Li B, Sun L, Liu X. Enhanced suicide therapy by chimeric tumor-specific promoter based on HSF1 transcriptional regulation. FEBS Lett. 2003;546(2-3):315–320. doi: 10.1016/S0014-5793(03)00606-9. [DOI] [PubMed] [Google Scholar]
- Wilmink GJ, Opalenik SR, Backham JT, Mackanos MA. In vivo optical imaging of hsp70 expression to assess collateral tissue damage associated with infrared laser ablation of skin. J Biomed Opt. 2008;13(5):054066. doi: 10.1117/1.2992594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N, McArdle A, Guerin D, Tasker H, Wareing P, Foster CS, Jackson MJ, Rhodes LE. Hyperthermia to normal human skin in vivo upregulates heat shock proteins 27, 60, 72i and 90. J Cutan Pathol. 2000;27(4):176–182. doi: 10.1034/j.1600-0560.2000.027004176.x. [DOI] [PubMed] [Google Scholar]
- Xu XG, Luo YJ, Wu Y, Chen JZ, Xu TH, et al. Immunohistological evaluation of skin responses after treatment using a fractional ultrapulse carbon dioxide laser on back skin. Dermatol Surg. 2011;37(8):1141–1149. doi: 10.1111/j.1524-4725.2011.02062.x. [DOI] [PubMed] [Google Scholar]