Abstract
All organisms and cells respond to various stress conditions such as environmental, metabolic, or pathophysiological stress by generally upregulating, among others, the expression and/or activation of a group of proteins called heat shock proteins (HSPs). Among the HSPs, special attention has been devoted to the mutations affecting the function of the αB-crystallin (HSPB5), a small heat shock protein (sHsp) playing a critical role in the modulation of several cellular processes related to survival and stress recovery, such as protein degradation, cytoskeletal stabilization, and apoptosis. Because of the emerging role in general health and disease conditions, the main objective of this mini-review is to provide a brief account on the role of HSPB5 in mammalian muscle physiopathology. Here, we report the current known state of the regulation and localization of HSPB5 in skeletal and cardiac tissue, making also a critical summary of all human HSPB5 mutations known to be strictly associated to specific skeletal and cardiac diseases, such as desmin-related myopathies (DRM), dilated (DCM) and restrictive (RCM) cardiomyopathy. Finally, pointing to putative strategies for HSPB5-based therapy to prevent or counteract these forms of human muscular disorders.
Keywords: Heat shock proteins, Disease, Physical activity, Chaperones, Crystallinopathies
Introduction
Organisms, to reduce their susceptibility to various stress conditions such as environmental, metabolic, or pathophysiological stress, have developed a first line of defense, of which taking part are a class of proteins called heat shock proteins (HSPs). Based on their approximate molecular mass, there have been identified five major and broadly conserved families such as HSPH (Hsp110s), HSPC (Hsp90s), HSPA (Hsp70s), HSPD (Hsp60s), DNAJ (Hsp40s), and small heat shock proteins (sHsps) (Richter et al. 2010).
The human family of sHsps contains ten members (HSPB1 to HSPB10), which are characterized by proteins of molecular mass ranging from 16.8 to 28.3 kDa (Kappé et al. 2003). Some are ubiquitously expressed (i.e., HSPB1, HSPB5, HSPB6, HSPB8) while others are only expressed in specific tissues, even in the absence of a stress (Garrido et al. 2012; Richter et al. 2010).
It is generally accepted that sHsps are a class of ATP-independent chaperones able to trap misfolded proteins through a so-called “holdase” activity and therefore avoiding aggregation. A cooperation with ATP-dependent chaperones, such as HSPD1 (Hsp60), HSP1A (Hsp70), and HSPC1 (Hsp90) is then required to bind unfolded or improperly folded proteins and promote either refolding using their “foldase” activity or the proteolytic elimination of the altered proteins (Carra et al. 2017; Mymrikov et al. 2011).
Among sHsps, the most prominent and also well-studied member of the family is the HSPB5 (human αB-crystallin), a protein playing a critical role in the modulation of several cellular processes related to survival and stress recovery, such as protein degradation, cytoskeletal stabilization, and apoptosis (Bakthisaran et al. 2015; Thornell and Aquilina 2015). Highlighting its importance in maintaining cellular function, either overexpression or deleterious mutations in HSPB5 are found in a number of known disorders (Cornford et al. 2000; Del Bigio et al. 2011; Fichna et al. 2016; Forrest et al. 2011; Inagaki et al. 2006; Liu et al. 2006; Pilotto et al. 2006; Reilich et al. 2010; Sacconi et al. 2012; Selcen and Engel 2003; Vicart et al. 1998). Moreover, differently from most sHsps, which are presumably not directly regulating or involved in diseases, mutations in the HSPB5 sequence are now recognized as causative of skeletal and cardiac myopathies (Del Bigio et al. 2011; Fichna et al. 2016; Forrest et al. 2011; Inagaki et al. 2006; Pilotto et al. 2006; Reilich et al. 2010; Sacconi et al. 2012; Selcen and Engel 2003; Vicart et al. 1998) (Fig. 1).
Fig. 1.
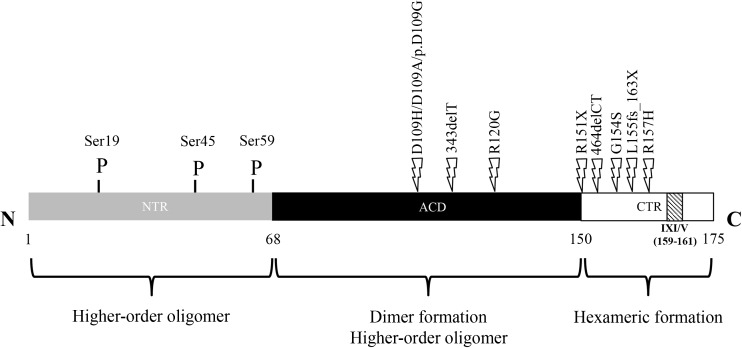
Full-length monomer structure of human HSPB5 protein. Gray box: N-Terminal Region (residues 1–68); light box: C-Terminal Region (residues 151–175); Striped box: highly conserved IXI/V sequence (residues 159–161); black box: alpha-crystallin domain (residues 69–150); P: phosphorylated serine residues. Positions of point mutations that are known to be responsible of specific muscle disorders (i.e., desmin-related myopathies, dilated, and restrictive cardiomyopathy) are indicated by thunderbolt. HSPB5 assembles into dimers through ACD-ACD interactions (building block). Higher-order oligomers are initially formed through CTR-ACD interactions (hexameric block) while the poorly defined NTR-ACD interactions drive the assembly of the final oligomer
For a long time, HSPB5 was considered as a lens-specific protein, where it plays an important role in maintaining the lens transparency (Dubin et al. 1990). This idea became obsolete when this protein was detected in muscle, heart, brain, and kidney as well as in extracellular fluids where it exhibits pleiotropic roles in several cellular processes (Bhat and Nagineni 1989; Gangalum et al. 2011; Rothbard et al. 2012; Thornell and Aquilina 2015).
HSPB5 is generally considered an intracellular protein; however, it has been detected at low level in extracellular fluids where it has been shown to bind inflammatory molecules and platelets (Enomoto et al. 2009; Gangalum et al. 2011; Rothbard et al. 2012). Since HSPB5 does not possess a signal sequence to be secreted through the normal secretory pathway, it might be released upon cell death or, as already demonstrated in human cells, via exosomes under specific stress conditions (Gangalum et al. 2011).
Because of the emerging role in general health and disease conditions, the main objective of this mini-review is to provide a brief account of the role of HSPB5 in human muscle pathophysiology. Here, we report the current status of the regulation and localization of HSPB5 in skeletal and cardiac tissue, making also a critical summary of all human HSPB5 mutations known to be strictly associated to specific skeletal and cardiac diseases, such as desmin-related myopathies (DRM) and dilated (DCM) and restrictive (RCM) cardiomyopathy (Brodehl et al. 2017; Inagaki et al. 2006; Vicart et al. 1998).
Finally, it will point to putative strategies for HSPB5-based therapy to prevent or treat these forms of muscular disorders.
The small heat shock protein αB-crystallin (HSPB5)
The human HSPB5 gene maps to 11q23.1 genome region and comprises three exons spanning 3.2 kb. It encodes a 175-aminoacid protein with a molecular mass of ~ 20 kDa (Dubin et al. 1990). As a monomeric subunit, HSPB5 is a protein organized in three regions: a conserved central domain, called “α-crystallin domain (ACD),” comprising residues 60–150, the flanking N-terminal region (NTR) and the C-terminal region (CTR) (Delbecq and Klevit 2013; Kappé et al. 2003; Kriehuber et al. 2010) (Fig. 1). Three-dimensionally, the ACD domain exhibits a β–sheet sandwich composed of eight anti-parallel strands connected by an inter-domain loop. A careful analysis of isolated ACDs, demonstrates that these form dimers that represent the basic “building blocks” of higher-order oligomers (Bagnéris et al. 2009; Baranova et al. 2011; Laganowsky et al. 2010). In particular, the dimer interface is formed by antiparallel alignment of the β6/β7 strands of the ACD. Structural information regarding the CTR domain highlights the presence of a three-residue isoleucine-proline-isoleucine/valine (IXI/V) motif that is typically found in many other sHsps. The intermolecular interaction between C-terminal IXI/V motif of one dimeric unit and the β4/β8 groove in an ACD of another dimer defines the secondary structure of an oligomer, called “hexameric block,” which is composed of three HSPB5 dimers (Delbecq et al. 2015). Finally, the NTR domain is the most divergent region among sHsps both in length and sequence (Kim et al. 1998). Although the interaction between NTR and/or with ACD is poorly defined, it is clear that the NTR is largely responsible for the assembly of higher-order HSPB5 oligomers and their dynamic distribution. Indeed, a model of a 24-mer with tetrahedral symmetry can be generated through extensive contacts between NTRs (Braun et al. 2011; Jehle et al. 2011). To date, the aforementioned structural model represents a considerable advancement in our understanding of HSPB5 architecture. However, a 24-mer represents only ~ 5% of the oligomeric population of HSPB5 that exists (Baldwin et al. 2011).
In common with all sHsps, HSPB5 shares the property to form large (molecular masses ranging from 50 to about 800 kDa), polydisperse (oligomers contain variable number of subunits), and structurally heterogeneous oligomers that undergo dynamic subunit exchange. The ability of HSPB5 to form different homo- and hetero-oligomers is modulated through addition or subtraction of subunits and seems to be tightly correlated with the regulation of its chaperone activity (Braun et al. 2011; Haslbeck et al. 2015; Zantema et al. 1992). Thus, not only the amount of HSPB5 per se but also the presence of specific types of oligomers is important and indicative for the states of cells and tissues.
Data on diverse interactions of sHsps with cellular proteins have been recently summarized (Arrigo 2013; Arrigo and Gibert 2014). These data indicate that human sHsps could bind either types of non-native protein “substrate” to stabilize the cell proteome or a sub-fraction of all substrates, called “client,” that is already bound under physiological conditions and where its binding and release give the cell the opportunity to regulate a specific cellular process (Arrigo 2013; Arrigo and Gibert 2014). Typical example for such client interactions is represented by procaspase-3, which is bound by HSPB1 and HSPB5 independently of general stress conditions. Only the phosphorylation of HSPB1 triggers the release of procaspase-3 and thus its activation and induction of apoptosis (WF et al. 2012; Voss et al. 2007). Further examples for clients are p53 and Bax for HSPB5, where binding inhibits their translocation to the mitochondria during apoptosis (Arrigo 2013; Arrigo and Gibert 2014). Besides the two above described modes of interaction, a third intracellular interaction is represented from the binding of co-chaperones that helps to target some sHsps or sHsp-substrate complex to other specific functional complexes (Arrigo 2013). The most prominent example for a co-chaperone of sHsps is Bag3, which binds to HSPB8 linking it to the Hsp70/ubiquitin ligation/proteasome machinery and the macroautophagy machinery (Gamerdinger et al. 2011). Additional data related to possible interaction of sHsps with cellular proteins can be found elsewhere (Arrigo 2013; Mymrikov et al. 2017).
To date, it remains unclear how sHsps might distinguish among different protein targets (i.e., substrate, client, or other sHsps). Even worse, the binding sites of sHsps that are involved in protein-target interactions have not been completely defined yet (Arrigo and Gibert 2013). Studies utilizing different strategies have identified short segment in the N-terminal sequence (Sharma et al. 1998), within ACD (Ghosh et al. 2005), and a part of ACD domain of HSPB5 (called “mini-αB-cristallin”) (Banerjee et al. 2015; Bhattacharyya et al. 2006), as well as in the C-terminal sequence (Treweek et al. 2010). Therefore, the emerging picture is that multiple binding sites throughout the molecule act together, presumably in a different manner for different substrate/client proteins.
Nevertheless, it is consolidated the idea that higher-order HSPB5 oligomers, where the potential substrate-binding sites are engaged in inter-subunit interactions, are likely to represent dormant storage forms, where smaller oligomers exposing hydrophobic patches might contribute, together with dissociated HSPB5 “building blocks,” to the pool of “binding-competent” species. The transition of HSPB5 from a low- to a high-affinity state presumably occurs through a remodeling of the ensemble composition by adjusting the dissociation/association rates of building blocks, determining the oligomer equilibrium according to the specific needs of the cell. Conditions that destabilize the oligomeric state can lead to an enhanced rate of dissociation of subunits, would raise populations of oligomers with higher binding capacity and thus increase the chaperone activity.
Serine phosphorylation in HSPB5, as well as in other human sHsps, is reported to shift the distribution of higher-order oligomers toward smaller species (often tetramers and hexamers) (Hayes et al. 2009; Peschek et al. 2013; Rogalla et al. 1999). Such predominance of these species upon phosphorylation is based on the localization of the phosphorylation sites in NTR and is in accordance with the hierarchical assembly of the oligomers (Peschek et al. 2013). Indeed, as already highlighted by Peschek and colleagues (122), NTR contributes decisively to the assembly and dynamic of oligomers and act as tunable conformation sensor in regulating HSPB5 activity.
The HSPB5 has three phosphorylation sites (serines 19, 45, and 59) (Fig. 1). The MAPKAPK2/3 kinases are responsible for the phosphorylation of S59 while p42/p44 MAPKinase phosphorylates S45 (Ito et al. 1997; Kato et al. 1998). The specific kinase of S19 is still unknown. Nevertheless, both unphosphorylated and phosphorylated forms of HSPB5 are reported to be equally effective in preventing in vitro assembly of glial fibrillary acidic protein (GFAP) and vimentin through their chaperone activity (Nicholl and Quinlan 1994). In fact, during physiological or pathological stress, both HSPB5 content and phosphorylation can be modulated (Adhikari et al. 2011; Fittipaldi et al. 2015; Morrison et al. 2003; Morrison et al. 2004; Reddy et al. 2015).
Depending on the type and/or duration of various stimuli, the fraction of phosphorylated HSPB5 ranges between 10 and 27% (Eaton et al. 2001; Ito et al. 1997). Different studies demonstrate that the phosphorylation of HSPB5 shows a dual role that leads to both beneficial or deleterious outcomes depending on the extent and duration of the stress and subsequent degree of phosphorylation: a phosphorylation at an initial stage of a stress is usually reversible and provides a beneficial outcome, while a prolonged stress can induce an irreversible phosphorylation which may lead to a deleterious outcome (Bakthisaran et al. 2015).
It is known that all aforementioned serine residues can be found phosphorylated after various stimuli (Ito et al. 1997), but only a few studies have reported their contemporary involvement in muscle tissues (den Engelsman et al. 2005; Li et al. 2011; Reddy et al. 2015). To date, most of the available data are related to HSPB5 expression and/or activation at Ser59 (Adhikari et al. 2011; Aggeli et al. 2008; Beltran Valls et al. 2015; Fittipaldi et al. 2015; Ivanov et al. 2008; Neppl et al. 2014; Pereira et al. 2015). Further details about the phosphorylation of HSPB5 in various physiological or pathological conditions can be found elsewhere (Bakthisaran et al. 2015).
Though phosphorylation might be the preferred regulation mechanism for human sHsps, several other post-translational modifications such as the deamidation (Gupta and Srivastava 2004), glycation (Satish Kumar et al. 2004), oxidation (Chalova et al. 2014; Chen et al. 2001), thiolation (Eaton et al. 2002), and the attachment of methylglyoxal (Oya-Ito et al. 2006) have been described to influence chaperone activity.
HSPB5 and skeletal muscle
An important aspect of muscle differentiation is the generation of multinucleated muscle fibers through fusion of mononucleated myoblasts. This process is orchestrated by many factors, including several members of the sHsp family, such as HSPB5 (Bucley and Konigsberg 1974).
The level of HSPB5 is elevated up to tenfold during skeletal muscle differentiation, suggesting a key role of the protein in the myogenic process. Indeed, the HSPB5 gene contains a skeletal-muscle preferred enhancer (− 427 to − 259), which includes at least four cis-acting regulatory elements (αBE-1, αBE-2, αBE-3, and MRF) (Dubin et al. 1990; Gopal-Srivastava and Piatigorsky 1993). It can modulate MyoD activity leading to delayed muscle differentiation (Golenhofen et al. 1999), as well as to protect skeletal muscle satellite cells, through an anti-apoptotic effect, during physiological or pathological changes associated with skeletal muscle regeneration and/or injury (Adhikari et al. 2011; Dimauro et al. 2014; Mercatelli et al. 2010). Moreover, experimental data have shown the presence of muscle abnormalities determined by the loss of HSPB5 function, hence confirming an important role of this protein during myogenesis (Brady et al. 2001).
HSPB5 is also highly expressed in slow and fast fibers of adult skeletal muscle where it is associated with actin microfilaments at level of Z-bands (Inagaki et al. 2006). Many different lines of evidence suggest that this sHsp protects mammalian skeletal muscle from heat, oxidative, and mechanical stresses produced during middle age and senescence or by physical activity (Dimauro et al. 2016a; Doran et al. 2007; Fittipaldi et al. 2014).
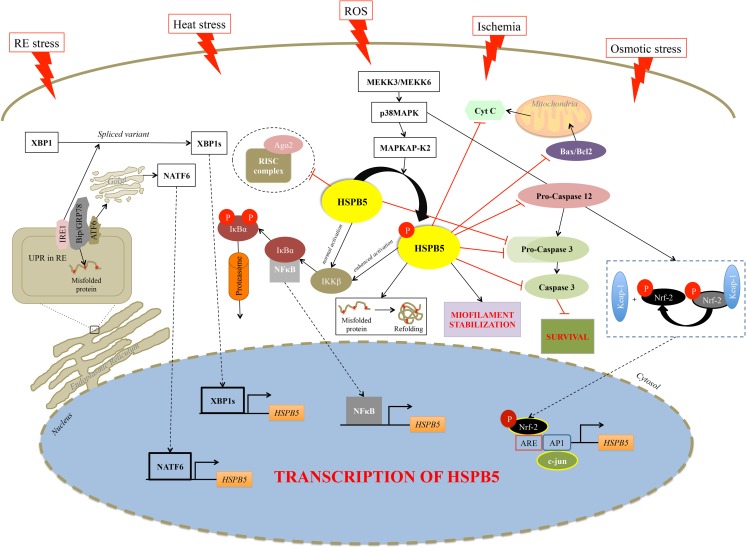
In particular, it has been demonstrated that the alteration of any of the three major components (i.e., microfilaments, microtubules, and intermediate filaments) results in a specific activation of p38MAPK and MAPKAP kinases 2 and 3 and the phosphorylation of HSPB5 (Launay et al. 2006). Our group also demonstrated that a reversible redox unbalance, which represents one of the main stimuli under different circumstances, induces HSPB5 expression through a JNK-mediated transcriptional mechanism in myogenic mammalian cells (Fittipaldi et al. 2015). The increased level of HSPB5 and its phosphorylation determine, on the one hand, its translocation to the myofilaments where it binds titin, desmin, vimentin, nebulette, and the inactive precursor of caspase 3, leading to the stabilization of the myofilament and to the inhibition of apoptosis (Adhikari et al. 2011; Webster 2003); on the other hand, it enhances NFκB activity, which translocates into the nucleus inducing the expression of genes involved in various biological events such as growth, differentiation, and cell death (Adhikari et al. 2011; Karin and Lin 2002; Perkins and Gilmore 2006) (Fig. 2).
Fig. 2.
Schematic representation of HSPB5 pathways present in skeletal and cardiac muscle tissue. Several stimuli such as heat shock, reactive oxygen species, ischemia, and osmotic and reticulum endoplasmic stress, lead to sustained activation of pathways, especially p38MAPK-MAPKAPK2, which result in progressive phosphorylation of HSPB5 at serine 59. HSPB5 is proposed to function at different levels of interrelated cellular pathways. HSPB5 can interfere with the mitochondrial pathway of apoptosis at various steps. In particular, it interacts with Bax and Bcl2 inhibiting their translocation into mitochondria, as well as with cytochrome-c preventing cytochrome-c-mediated interaction of Apaf-1 with procaspase-9 to form apoptosome. HSPB5 suppresses apoptosis also by binding to the pro-caspase 12 and pro-caspase-3 and preventing its maturation into the proteolytically active enzyme. HSPB5 interacts with IKKβ and enhances its kinase activity, which leads to phosphorylation and subsequent degradation of IκBα, a negative regulator of NFκB, facilitating the nuclear translocation of the transcription factor. Moreover, in unfavorable conditions such as redox unbalance and reticulum endoplasmic stress, a series of pathways are activated to induce the transcriptional activation of HSPB5 as well as its interaction with actin filaments. HSPB5 participates to homeostasis of skeletal muscle via modulation of Ago2, a potent endonuclease belonging to the central core of RISC complex, as well as at post-translational level; it controls all steps of the protein life cycle such as folding, aggregation, refolding, and degradation. Black lines indicate activation; red lines indicate inhibition; dash lines represent the translocation into the nucleus. Ago2, argonaute 2, RISC catalytic component; AP1, AP-1 promoter site; ARE, AU-rich elements; ATF6, activating transcription factor 6; Bax, Bcl-2-associated X protein; Bcl2, B-cell lymphoma 2; Bip/GRP78, binding immunoglobulin protein; HSPB5, αB-crystallin; CytC, cytochrome c; c-jun, Jun proto-oncogene, AP-1 transcription factor subunit; IRE1, endoplasmic reticulum to nucleus signaling 1; IKKB, inhibitor of nuclear factor kappa-B kinase subunit beta; IκBα, NF-kappa-B inhibitor alpha; Keap-1, Kelch-like ECH-associated protein 1; MEKK3, mitogen-activated protein kinase kinase kinase 3; MEKK6, mitogen-activated protein kinase kinase kinase 6; Nrf-2, nuclear factor, erythroid 2 like 2; p38MAPK, mitogen-activated protein kinase; MAPKAPK2, MAP kinase-activated protein kinase 2; NFκB, nuclear factor kappa B; RISC complex, RNA-induced silencing complex; XBP1, X-box binding protein 1; UPR, unfolded protein response
Moreover, HSPB5 appears to have a role in regulation of apoptosis in mammalian cells during heat shock, oxidative stress, and ischemia. This protein is able to prevent apoptosis by several mechanisms such as the inhibition of RAS-initiated RAF/MEK/ERK signaling pathway (Li et al. 2005), or downstream, blocking the BAX, and Bcl-2 translocation from the cytoplasm to the mitochondria (Mao et al. 2004), as well as interacting with p53 to retain it in the cytoplasm (Liu et al. 2007), or inhibiting autocatalytic maturation of caspase-3 (Kamradt et al. 2001).
A recent finding has established that HSPB5 is necessary for mammalian skeletal muscle homeostasis via modulation of Argonaute 2 (Ago2) activity (Neppl et al. 2014), a protein with endonuclease activity. It belongs to the central core of an RNA-induced silencing complex (RISC), which is capable of repressing the translation of mRNA into protein via a variety of mechanisms such as removal of the 5–7-methylguanylate cap (m7G), deadenylation of the 3′-poly(A) tail, and miRNA site-directed endonuclease cleavage of the mRNA (Bartel 2004; Djuranovic et al. 2011). This result indicates that HSPB5 functions as a positive allosteric regulator of Ago2/RISC. In fact, the absence of HSPB5 results in an imbalance of the hypertrophy-atrophy axis toward atrophy with an excessive miRNA loading into Ago2/RISC (Neppl et al. 2014) (Fig. 2).
HSPB5 and cardiac muscle
Early expression of some sHsps, including HSPB5, has been reported during the developmental phases of mammalian heart (Lutsch et al. 1997). The amount of this protein in heart reaches up to 3–5% of the total soluble protein. In the embryo, the expression of HSPB5 starts during the gastrulation stage, initially restricted to somites; it expands to the entire myotome, as well as heart and other tissues as development proceeds (Lutsch et al. 1997).
To date, the role of this sHsp in cardiogenesis is still unclear, although a HSPB5-KO mouse model shows a slightly dystrophic phenotype without alteration in cardiac development, morphology, or function (Morrison et al. 2004). Experimental data showed that HSPB5 depletion may compromise the correct folding of nascent myosin and thereby contribute to altered myofibrillogenesis (Smith et al. 2014).
Differently from skeletal muscle, the expression of HSPB5 in cardiac tissue requires an additional enhancer such as αB-E4, containing a control sequence 5′-GGAATCTTCC-3′ that resembles a reverse CArG box [5′-CC(A/T)6GG-3′], that is also found in other genes expressed in the heart (Gopal-Srivastava and Piatigorsky 1993). The intracellular localization of HSPB5 in cardiomyocytes has some very unusual characteristics. Rather than in the Z-disc, data from immunoelectron microscopy has shown that this sHsp localizes in a narrow region of the I-band under both normal and stress conditions. HSPB5 seems associated with cardiac titin in the N2B region and desmin filaments in order to either stabilize the conformation of these various filaments or to effectively prevent their tendency to form aggregates (Golenhofen et al. 2002; Morrison et al. 2004). It might be possible that HSPB5 translocates to the Z-line at an early phase, while a prolonged, irreversible damaging stress leads to extreme stretching of myofibrils and concomitant extension of HSPB5 localization to the I-bands (Golenhofen et al. 1999).
The interaction between cytoskeletal structures and HSPB5 in situ is fairly weak under normal condition; indeed, this protein is released into the water-soluble fraction upon heart homogenization (Longoni et al. 1990). However, a short period of ischemia in the heart can induce the redistribution of HSPB5 in the cell homogenate: the protein aggregates with the insoluble elements of the cells (Chiesi et al. 1990). Thus, during this stress, the affinity of this sHsp for some structural elements of the cell increases. This association seems to be dependent on the phosphorylation of both Ser45 and Ser59, since inhibition of their phosphorylation inhibits almost completely the interaction between this sHsp and the cardiac myofibrillar structures (Singh et al. 2007).
Similarly to skeletal muscle, the activation of p38MAPK via MKK3/MKK6 pathways under different stress conditions (i.e., ischemia, heat stress, oxidative stress) stimulates the MAP-activated protein kinase-2 (MAPKAPK2), which in turn phosphorylates HSPB5 at Ser59 residue (Kato et al. 1998; Maulik et al. 1996). However, in response to ischemia, HSPB5 is also phosphorylated on Ser45 by the ERK pathway (Morrison et al. 2003), but to date, it is accepted that Ser59 is selectively responsible for mediating the cytoprotection in cardiomyocytes (Hoover et al. 2000; Morrison et al. 2003) (Fig. 2).
Few reports also suggest that HSPB5 induction in cardiomyocytes of mammalians is an adaptive response to Endoplasmic Reticulum (ER) stress (Mitra et al. 2013). Under non-stress condition, Bip/glucose regulated protein 78 (GRP78/Bip), a master regulator of the Unfolded Protein Response (UPR), interacts with other ER resident proteins like PERK, ATF6, and IRE1 (Groenendyk et al. 2010). Upon ER stress and accumulation of misfolded proteins in the ER, GRP78/Bip dissociates from such protein aggregates to activate IRE1 and ATF6 dependent pathways (Groenendyk et al. 2010). IRE1 activation is responsible for the splicing of XBP1 mRNA to yield XBP1s splice variants, a potent transcription factor, which upregulates the expression of HSPB5 and other genes involved in ER associated degradation (ERAD) (Groenendyk et al. 2010). The ATF6 dependent pathway is activated when Bip/GRP78 dissociates from ATF6, which then translocate to the Golgi and is cleaved by S1P and S2P proteases. This process yields the release of the N-ATF6 transcription factor, that moves to the nucleus to activate HSPB5 and other UPR target genes (Ganguly et al. 2014; Groenendyk et al. 2010; Mitra et al. 2013) (Fig. 2).
HSPB5 and human muscle cristallinopathies
As the HSPB5 protein is known to play a role in the remodeling of the cytoskeleton during development and cell differentiation (as well as after stress stimuli), it is not surprising that several degenerative disorders of skeletal and cardiac muscle such as DRM, DCM, and RCM cardiomyopathy, are caused by mutations of the HSPB5 gene. In 2011, Houck and colleagues (Landsbury et al. 2011) demonstrated how three specific sequences of HSPB5 contributed to “substrate type” interaction between HSPB5 oligomers with desmin filaments to prevent their self-association and the formation of filament-filament aggregates. As a consequence of myopathy-associated HSPB5 mutations (e.g., R120G), the secondary, tertiary, as well as quaternary structures and chaperone activity of these molecules are compromised. This condition enhances the subunit dynamics driving the dissociation of monomers or dimers (Michiel et al. 2009) to mislead the oligomer equilibrium toward an excess of assemblies with dramatically increased substrate affinity and results in the formation of these abnormal aggregates featuring DRM (Bova et al. 1999). In fact, analysis of patient muscle biopsies showed morphological changes derived from disintegration of the sarcomeric Z disc and myofibrils, followed by abnormal ectopic accumulation of multiple proteins involved in the structure of the Z disc.
To date, the limited number of patients analyzed and the absence of studies with periodic follow-up that are able to exclude the late onset of other possible features, make it difficult to delineate a well-defined clinical and morphological phenotype for each mutated form of the protein.
Future studies should take into account the aforementioned issue and should provide detailed analysis of the different pathophysiological properties of each individual mutant allele with distinct phenotype in order to develop targeted therapeutic interventions.
Desmin-related myopathy
The desmin-related myopathy represents a subgroup of myofibrillar myopathy where myopathic manifestations of disease are caused mainly by mutations in desmin or HSPB5 (Dalakas et al. 2003).
The first description of a family with multisystemic involvement associated with a HSPB5 mutation (R120G) dates back to 1998 (Vicart et al. 1998) (Fig. 1). Vicart and colleagues (1998) (Liu et al. 2006) identified an arginine-to-glycine missense mutation at amino acid position 120 in HSPB5 implicated in the causation of DRM, an autosomal dominant myopathy characterized by weakness of the proximal and distal limb muscles and signs of cardiomyopathy and cataracts. This phenotype could be the consequence of an altered interaction between R120G HSPB5 and desmin due to changes in the molecular chaperone activity and/or to the tertiary structure of the HSPB5. Moreover, the pathogenic mutant tends to aggregate and forms toxic protein deposits containing desmin, amyloid oligomers, and fibrils (Sanbe et al. 2004).
Subsequently, two novel mutations (Q151X and 464delCT) that lead to DRM have been identified in the terminal part of the HSPB5 coding sequence (Selcen and Engel 2003). Both heterozygous truncating mutations showed symmetrical proximal and distal muscle weakness starting in adulthood, accompanied by muscle atrophy as well as respiratory involvement (Fig. 1).
In 2011, Forrest and colleagues (Forrest et al. 2011) identified a novel truncating HSPB5 mutation (S115fs129X) associated with autosomal recessive (AR) fatal hypertonic muscular dystrophy characterized by progressive limb and axial muscle stiffness, severe respiratory insufficiency, and death in infancy. Subjects affected by this form of myofibrillar myopathy are homozygous for a c.343delT (p.Ser115ProfsX14) mutation in exon 3 of the HSPB5 gene leading to a truncated protein of 127 amino acids (Fig. 1). The onset of symptoms occurs in the first few months of life (≈ 4 months), with evidence of muscle fiber necrosis at a structural level and phagocytosis with intense staining of desmin, myotilin, p62, and HSPB5.
The same year, Del Bigio and colleagues (Del Bigio et al. 2011) identified a similar fatal AR infantile hypertonic muscular dystrophy in Canadian aboriginals showing the pathological features of myofibrillar myopathy. All subjects affected were homozygous for the c.60C deletion mutation that predicts a Ser to Ala change at codon 21 and a stop codon after 23 missense residues (p.Ser21AlafsX24). The truncated HSPB5 gene produces a protein of 44 amino acids (Fig. 1).
Sacconi et al. (Sacconi et al. 2012), and a few years later Fichna et al. (Fichna et al. 2016), reported patients with clinical diagnosis of DRM associated with novel autosomal dominant (AD) HSPB5 mutations (D109H and D109A). Codon 109 is located in exon 3 of the gene (Fig. 1) and encodes an amino acid involved in the dimerization of the HSPB5 protein. In particular, the mutated proteins show modifications of hydrogen and ionic bonds responsible of interactions with residues from the same monomer as well as residues on the adjacent monomers. Therefore, both mutated proteins showed several unfolded β-sheets resulting in lower stability of the oligomer structure. In fact, the number of residues participating in β-sheets, which form the core of HSPB5, diminished by 4% for D109H and > 10% for D109A. These AD mutations induce not only classical symptoms involving skeletal muscle present in all patients with other HSPB5 mutations, such as myopathy, distal weakness, dysphonia, and dysphagia, but also cardiomyopathy and lens cataracts (Fichna et al. 2016; Sacconi et al. 2012).
Dilated cardiomyopathy
In addition to being involved in DRM, HSPB5 mutations have been identified in DCM. This disease is characterized by cardiac enlargement accompanied by systolic dysfunction, often manifested with congestive heart failure (Richardson et al. 1996). First Inagaki et al. (Inagaki et al. 2006) and then Pilotto et al. (Pipkin et al. 2003) reported subjects with late onset DCM pathology and occurrence of symptoms after the fourth decade that were heterozygous for HSPB5 mutations. They found two different AD missense mutations in exon 3: (1) Arg157His (R157H), due to a codon change 157 (CGC to CAC) replacing arginine with histidine; and (2) Gly154Ser (G154S), due to codon change 154 (GGC to AGC) replacing glycine with serine (Fig. 1). Both mutations showed different functional alteration from DRM-associated mutation. In particular, these HSPB5 mutations reduced the binding of the protein to the cardiac-specific N2B domain, but not to I26/I27 domain of titin/connectin expressed in both skeletal and cardiac muscle. Moreover, mutant proteins did not generate any aggregates. Since HSPB5 has been found translocated to a narrow region of I band during different stresses, it may suggest a protective role of the protein (Golenhofen et al. 2002). Therefore, the impaired localization of HSPB5 into the I-band region of cardiac muscle may predispose early progression to heart failure under stress condition. However, Pilotto and colleagues found in their case report study (G154S) a minimal increase of serum CPK, suggesting a potentially subclinical muscle involvement. Interestingly, a few year later, Reilich and colleagues (Reilich et al. 2010) reported the same HSPB5 mutation G154S in an old male patient showing a mild distal vacuolar myopathy with protein aggregates without associated cardiomyopathy, respiratory failure, and cataracts.
Restrictive cardiomyopathies (RCM)
It is a rare heart disease caused by genetic or nongenetic factors. During the last decade, RCM-associated mutations were identified in different genes (Arbustini et al. 2006; Brodehl et al. 2016; Wu et al. 2015) including HSPB5 (Brodehl et al. 2017). Particularly, Brodehl and colleagues were the first to report a HSPB5 missense mutation (D109G) in two German patients, which was associated with severe RCM and skeletal myopathy (Brodehl et al. 2017). The first diagnosis of RCM was received at the age of 19 and 28 years, respectively. It is known that the formation of two ionic bridges between D109 and R120 are essential for the stabilization of HSPB5 dimers, the “building block” of the oligomeric forms of HSPB5. Therefore, this mutation compromises the protein function leading to muscle crystallinopathies.
The morphological analysis of myocardial tissue showed Z-band structure partially disappeared with cytoplasmic protein aggregates positive for HSPB5 and desmin.
Therapeutic approaches
Protein misfolding and its pathogenic consequences have become an important issue over the last two decades. Indeed, it was estimated that protein misfolding could be involved in up to half of all human diseases (Bradbury 2003). Cellular molecular chaperones, including HSPs, are proteins that selectively recognize and bind non-native protein via non-covalent interactions, thus inhibiting irreversible aggregation of those proteins (Welch 2003).
Based on current knowledge, the pathogenic mechanism of all known HSPB5 mutations can be summarized in two ways: (1) limited ability to prevent aggregation of various proteins via dominant-negative effect on the chaperone function of oligomeric HSPB5 complex with other partners (including other HSP family members) and (2) the structural instability and propensity to aggregate of the mutated HSPB5 itself that results in a classical “loss of function” of the gene product.
To date, a possible theraputic approaches in HSPB5 mutation-related muscle diseases could be (1) restoring the HSPB5 functions through the enhanced expression of the corresponding wild-type protein; (2) inducing other sHsps such as HSPB1, HSPB6, and HSPB8, already known to be important in stabilizing the cytoskeleton and preserving contractile function, as well as to be normally present as a complex with HSPB5 in muscle tissues (Pipkin et al. 2003); and (3) improving the protein quality control (PQC) system (through HSPs) or enhancing both primary mechanisms for removing misfolded proteins from the cell, called “ubiquitin-proteasome system” (UPS) and “autophagy,” inhibiting the aggregate formation and thereby the accumulation of toxic deposits (Singh et al. 2010; Tyedmers et al. 2010).
Although no treatment is currently available for disorders related to HSPB5 mutations, studies from cellular and animal models have highlighted the possible effectiveness of different therapeutic options highlighted below.
HSPs as therapeutic compounds
Based on the premise that chaperones are protective from some of the deleterious effects of muscle disorders, many attempts have been made to increase their expression to induce endogenous chaperone genes by using chemical compounds. In particular, it was found in R120G-Transgenic (Tg) mice that the induction of HSPB1 and HSPB8 was a powerful inhibitor of amyloid oligomer and aggresomal formation (Sanbe et al. 2009). Particularly, Sanbe et al. (Sanbe et al. 2009) demonstrated that the treatment with geranylgeranylacetone (GGA), an inducer of HSPB1 and HSPB8, reduced the formation of amyloid oligomers as well as of insoluble aggregates in R120G-Tg mice. At a clinical level, this approach results in a reduction in heart size, inhibition of interstitial fibrosis, and recovery of cardiac function as well as improved survival (Sanbe et al. 2009). Although other chemical inducers of HSPs have been isolated (Ahmed et al. 2012), most of them have not been tested in these types of diseases. Moreover, particular care should be taken in the case of DMR caused by HSPB5 mutants, to avoid inducing the endogenous mutated (R120G) gene using, for instance, glucocorticoids (N’edellec et al. 2002).
These results imply that enhancing the induction of small HSPs could be beneficial in the treatment of crystallinopathies. Therefore, a plausible therapeutic option could be to administer pharmacological inducers of HSP response and/or possibly by directly delivering HSPB1 to human muscles using viral vectors.
Redox balance approach
Production of aggregates in muscle fibers, mitochondrial mislocalization induced by defects in Z-line structure, as well as the inability of HSPB5 to bind the redox-active Cu2+ may also generate more reactive oxygen species with a consequent increase of oxidative damage (Ahmad et al. 2008; Janu’e et al. 2007). In R120G-Tg mice, the inhibition of xanthine oxidase with oxypurinol restores mitochondrial function (Maloyan et al. 2009). However, cardiac contractile function and compliance do not improve (Maloyan et al. 2009). Other promising results had also been obtained by Banerjee-Mustafi et al. (Banerjee Mustafi et al. 2014). They found that in DRM, the thioredoxin system was altered at multiple levels (i.e., expression, activity, and regulation). This antioxidant system plays an important role in protein folding by reducing disulfide bond–oxidized cysteines and thereby could affect other proteins interacting with the mutant R120G and, subsequently, change their aggregation propensity. In vivo and in vitro experiments combined inhibition of TrxR1 and measurement of aggregate development in two different contexts, one being in hearts of R120G-Tg mice and the other in H9c2 myoblasts transfected with R120G, demonstrated that TrxR1 activity impacts aggregate growth. Therefore, it is tempting to speculate that augmentation of TrxR1 activity could be a potential therapeutic avenue for modifying disease onset and progression. Recently, two cellular studies on myoblasts seem to show that antioxidant or pro-autophagic compounds (i.e., NAC, trolox, pp 242) could reduce aggregation, linking antioxidant activity, and/or PQC system with modulation of protein aggregates (Cabet et al. 2015; Segard et al. 2013). Although additional studies are needed to test the efficacy of these compounds in existing animal models, the antioxidant treatment appears promising for counterbalancing the negative effects of accumulation of abnormal proteins and damaged mitochondria.
Improvement of proteasome and autophagy systems
The use of R120G-Tg mice indicates that PQC network becomes inadequate during the progression of DRM, perhaps because the amount of aggregates overloads PQC (Li et al. 2011; McLendon and Robbins 2011).
Under physiological conditions, PQC network is carried out by several classes of chaperones (i.e., holdases and foldases), which serve as sensors of misfolded proteins/aggregates and attempt either to repair or, when correct folding is not possible, to target abnormal proteins to disposal machinery, in a timely fashion (Balchin et al. 2016; Esser et al. 2004; Haslbeck et al. 2005). In particular, the role of most sHsps, including HSPB5, seems to be of an adaptor binding both the unfolded protein and being part of complexes involving poly-ubiquitylation (Barbash and Diehl 2008; Lin et al. 2006).
To date, no pharmacological inducer of the UPS are known, but autophagy constitutes a rescue system that is stimulated when UPS function is impaired (Ravikumar et al. 2010). In fact, R120G-Tg mice show more than twofold increase in muscle autophagic activity (Tannous et al. 2008). Increased level of basal autophagy in these mice decreases cardiac hypertrophy and intracellular aggregates that prolong survival (Bhuiyan et al. 2013). Therefore, another possible strategy might be to further stimulate autophagy using one among numerous compounds already described in the literature (e.g., AMPK, cyclosporine A, rapamycin) (Pauly et al. 2012; Rubinsztein et al. 2012).
Interestingly, McLendon and colleagues (McLendon et al. 2014) suggested, as a therapeutic strategy, the inhibition of a specific histone deacetylases (HDACs), the cytoplasmic HDAC6, able to affect cytoskeletal dynamics through deacetylation of α-tubulin and cortactin (Li et al. 2013). Since it is known that acetylated tubulin stabilizes microtubules and augments assembly of autophagic cargo along microtubules increasing autophagic degradation (Geeraert et al. 2010), the authors demonstrated that inhibiting HDAC6, and thus tubulin deacetylation, there was reduced aggregate formation and attenuated cardiac dysfunction of mutant R120G-Tg mice in vivo. Therefore, this protection is due in part to increased autophagy clearance of toxic protein accumulation induced by HSPB5 myopathy-mutants.
Over the years, physical activity has been shown to prevent and/or support conventional treatment of several pathological conditions through the induction of adaptive mechanisms at systemic or a tissue-specific level (Warburton et al. 2006). Regular participation to physical activity has been demonstrated to improve the homeostasis of macromolecules (i.e., DNA and proteins) involved in the physiological or pathological stress; the resulting beneficial effects were in terms of delaying the onset and progression of several diseases and aging-related biomarkers (Beltran Valls et al. 2014; Beltran Valls et al. 2015; Brunelli et al. 2012; Ceci et al. 2014; Cumming et al. 2014; Dimauro et al. 2016b; Dimauro and Sgura 2017; Pittaluga et al. 2015; Warburton et al. 2006). Moreover, evidences from animal and human studies demonstrated that acute or chronic exercise could represent a potent HSPs inducer in several human tissues (Dimauro et al. 2016a; Fittipaldi et al. 2014). The exercise-induced changes in HSPs seem to have multiple cytoprotective effects on mitochondria, sarcoplasmic reticulum and cytoskeleton components (Bornman et al. 1998; Sammut and Harrison 2003; Tupling et al. 2004), inhibitory effects on apoptosis (Gabai and Sherman 2002), as well as a role in the maintenance of enzymatic activity, insulin sensitivity, and glucose transport (Chung et al. 2008; Melkani et al. 2006).
Although published data clearly support a role for exercise-induced modulation of HSPB5 in the prevention of diseases caused by protein misfolding (Reddy and Reddy 2015), there are still no interventional studies in humans affected by DRM. Results obtained in mouse models show that long-term voluntary exercise reduces pre-amyloid toxic oligomer accumulation with a concomitant increase in lifespan (Maloyan et al. 2007). In particular, R120G-Tg mice housed in cages with running wheels exhibit a significant reduction in beta-amyloid oligomers with a concomitant increase in lifespan (Maloyan et al. 2007). After 22 weeks of exercise, amyloid oligomer levels were already 47% lower than in unexercised R120G-Tg mice, and after 6 months of voluntary exercise, R120G-Tg animals exhibited a 100% survival beyond the time point when all unexercised mice had died. Similar effect were observed by Bhuiyan et al. (Bhuiyan et al. 2013); they found that 50% of exercised R120G-Tg mice were alive by 7 months, while all sedentary control mice were already dead from heart failure. This percentage achieved 100% survival in R120G-Tg mice overexpressing Atg7, a non canonical E1-like enzyme necessary for autophagosome formation that recognizes two distantly related ubiquitin-like proteins, ATG8 and ATG12 (Komatsu et al. 2005).
To date, the molecular effectors of exercise are still unknown but the aforementioned studies suggest that both the modulation of apoptotic and autophagy pathways induced by regular exercise, reduce heart failure symptoms and rescuing the R120G-Tg mice from premature death (Bhuiyan et al. 2013; Maloyan et al. 2007). However, it cannot be excluded that the observed survival benefit results from an additive effect of multiple pathways induced by voluntary physical exercise such as induction of other HSPs.
Conclusion
To date, the number of reports that describe the role of HSPB5 in mammalian muscle tissue is increasing exponentially. Particularly, as sHsp is important not only during skeletal and cardiac development but also in the differentiated tissues, where it ensures the proper functioning of different cellular processes and the structural integrity of both muscle tissues either under normal or in stress conditions.
Indeed, from the discussion on the pathogenic mechanisms related to almost all mutant HSPB5-related myopathies (i.e., DRM, DCM, and RCM), it is clear that a loss of function of HSPB5 polypeptide chain due to specific gene mutations determines morphological changes in skeletal and cardiac muscles resulting from disintegration of the sarcomeric Z disc and myofibrils, with formation of structured aggregates being highly toxic for the cells.
To date, thanks to the use of in vitro and in vivo models, a large amount of data on functional aspects of HSPB5 is available. Nonetheless, several issues on regulatory mechanisms of this protein remain still unresolved. Therefore, it is imperative to plan further studies to improve our knowledge about sHsp and to develop new preventative and/or therapeutic approaches for those specific physiological and pathological conditions where the correct functioning of HSPB5 seems to be decisive.
Acknowledgements
We thank Dr. Timothy Pearson for the English revision.
Funding information
The work has been supported by grant from University of Rome Foro Italico, Research Grant 2015.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Ivan Dimauro, Email: ivan.dimauro@uniroma4.it.
Ambra Antonioni, Email: a.antonioni@studenti.uniroma4.it.
Neri Mercatelli, Email: mastroneri@hotmail.com.
Daniela Caporossi, Email: daniela.caporossi@uniroma4.it.
References
- Adhikari AS, Singh BN, Rao KS, Rao CM. αB-crystallin, a small heat shock protein, modulates NF-κB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-α induced cytotoxicity. Biochim Biophys Acta. 2011;1813(8):1532–1542. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Aggeli IK, Beis I, Gaitanaki C. Oxidative stress and calpain inhibition induce alpha B-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell Signal. 2008;20(7):1292–1302. doi: 10.1016/j.cellsig.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Ahmad MF, Singh D, Taiyab A, Ramakrishna T, Raman B, Rao CM. Selective Cu2+ binding, redox silencing, and cytoprotective effects of the small heat shock proteins alphaA- and alphaB-crystallin. J Mol Biol. 2008;382(3):812–824. doi: 10.1016/j.jmb.2008.07.068. [DOI] [PubMed] [Google Scholar]
- Ahmed K, Furusawa Y, Tabuchi Y, Emam HF, Piao JL, Hassan MA, Yamamoto T, Kondo T, Kadowaki M. Chemical inducers of heat shock proteins derived from medicinal plants and cytoprotective genes response. Int J Hyperth. 2012;28(1):1–8. doi: 10.3109/02656736.2011.627408. [DOI] [PubMed] [Google Scholar]
- Arbustini E, Pasotti M, Pilotto A, Pellegrini C, Grasso M, Previtali S, Repetto A, Bellini O, Azan G, Scaffino M, Campana C, Piccolo G, Viganò M, Tavazzi L. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur J Heart Fail. 2006;8(5):477–483. doi: 10.1016/j.ejheart.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Human small heat shock proteins: protein interactomes of homo- and hetero-oligomeric complexes: an update. FEBS Lett. 2013;587(13):1959–1969. doi: 10.1016/j.febslet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Gibert P. HspB1, HspB5 and HspB4 in human cancers: potent oncogenic role of some of their client proteins. Cancers. 2014;6(1):333–365. doi: 10.3390/cancers6010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo AP, Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int J Hyperth. 2013;29(5):409–422. doi: 10.3109/02656736.2013.792956. [DOI] [PubMed] [Google Scholar]
- Bagnéris C, Bateman OA, Naylor CE, Cronin N, Boelens WC, Keep NH, Slingsby C. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol. 2009;392(5):1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854(4):291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Hartl FU (2016) In vivo aspects of protein folding and quality control. Science 353: aac4354 [DOI] [PubMed]
- Baldwin AJ, Lioe H, Robinson CV, Kay LE, Benesch JLP. αBcrystallin polydispersity is a consequence of unbiased quaternary dynamics. J Mol Biol. 2011;413(2):297–309. doi: 10.1016/j.jmb.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Banerjee Mustafi S, Grose JH, Zhang H, Pratt GW, Sadoshima J, Christians ES, Benjamin IJ. Aggregate-prone R120GCRYAB triggers multifaceted modifications of the thioredoxin system. Antioxid Redox Signal. 2014;20(18):2891–2906. doi: 10.1089/ars.2013.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PR, Pande A, Shekhtman A, Pande J. Molecular mechanism of the chaperone function of mini-alpha-Crystallin, a 19-residue peptide of human alpha-Crystallin. Biochemistry. 2015;54(2):505–515. doi: 10.1021/bi5014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranova EV, Weeks SD, Beelen S, Bukach OV, Gusev NB, Strelkov SV. Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J Mol Biol. 2011;411(1):110–122. doi: 10.1016/j.jmb.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Barbash O, Diehl JA. SCF(Fbx4/alphaB-crystallin) E3 ligase: when one is not enough. Cell Cycle. 2008;7(19):2983–2986. doi: 10.4161/cc.7.19.6775. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Beltran Valls MR, Dimauro I, Brunelli A, Tranchita E, Ciminelli E, Caserotti P, Duranti G, Sabatini S, Parisi P, Parisi A, Caporossi D. Explosive type of moderate-resistance training induces functional, cardiovascular, and molecular adaptations in the elderly. Age (Dordr) 2014;36(2):759–772. doi: 10.1007/s11357-013-9584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran Valls MR, Wilkinson DJ, Narici MV, Smith K, Phillips BE, Caporossi D, Atherton PJ. Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia. J Gerontol A Biol Sci Med Sci. 2015;70(2):174–181. doi: 10.1093/gerona/glu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissue. Biochem Biophys Res Commun. 1989;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaBcrystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45(9):3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan MS, Pattison JS, Osinska H, James J, Gulick J, McLendon PM, Hill JA, Sadoshima J, Robbins J. Enhanced autophagy ameliorates cardiac proteinopathy. J Clin Invest. 2013;123(12):5284–5297. doi: 10.1172/JCI70877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornman L, Steinmann CML, Gericke GS, Polla BS. In vivo heat shock protects rat myocardial mitochondria. Biochem Biophys Res Commun. 1998;246(3):836–840. doi: 10.1006/bbrc.1998.8717. [DOI] [PubMed] [Google Scholar]
- Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, Horwitz J. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96(11):6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury J. Chaperones: keeping a close eye on protein folding. Lancet. 2003;361(9364):1194–1195. doi: 10.1016/S0140-6736(03)12975-3. [DOI] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42(12):2924–2934. [PubMed] [Google Scholar]
- Braun N, Zacharias M, Peschek J, Kastenmüller A, Zou J, Hanzlik M. Multiple molecular architectures of the eye lens chaperone αB-crystallin elucidated by a triple hybrid approach. Proc Natl Acad Sci U.S.A. 2011;108:20491–20496. doi: 10.1073/pnas.1111014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodehl A, Ferrier RA, Hamilton SJ, Greenway SC, Brundler MA, Yu W, Gibson WT, McKinnon ML, McGillivray B, Alvarez N, Giuffre M, Schwartzentruber J, FORGE Canada Consortium. Gerull B. Mutations in FLNC are associated with familial restrictive cardiomyopathy. Hum Mutat. 2016;37(3):269–279. doi: 10.1002/humu.22942. [DOI] [PubMed] [Google Scholar]
- Brodehl A, Gaertner-Rommel A, Klauke B, Grewe SA, Schirmer I, Peterschröder A, Faber L, Vorgerd M, Gummert J, Anselmetti D, Schulz U, Paluszkiewicz L, Milting H. The novel αB-crystallin (CRYAB) mutation p.D109G causes restrictive cardiomyopathy. Hum Mutat. 2017;38(8):947–952. doi: 10.1002/humu.23248. [DOI] [PubMed] [Google Scholar]
- Brunelli A, Dimauro I, Sgrò P, et al. Acute exercise modulates BDNF and pro-BDNF protein content in immune cells. Med Sci Sports Exerc. 2012;44(10):1871–1880. doi: 10.1249/MSS.0b013e31825ab69b. [DOI] [PubMed] [Google Scholar]
- Bucley PA, Konigsberg IR. Myogenic fusion and the duration of the post-mitotic gap (G1) Dev Biol. 1974;37(1):193–212. doi: 10.1016/0012-1606(74)90179-1. [DOI] [PubMed] [Google Scholar]
- Cabet E, Batonnet-Pichon S, Delort F, Gausser’es B, Vicart P, Lilienbaum A. Antioxidant treatment and induction of autophagy cooperate to reduce desmin aggregation in a cellular model of desminopathy. PLoS One. 2015;10(9):e0137009. doi: 10.1371/journal.pone.0137009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra S, Alberti S, Arrigo PA, Benesch JL, Benjamin IJ, Boelens W, Bartelt-Kirbach B, Brundel BJJM, Buchner J, Bukau B, Carver JA, Ecroyd H, Emanuelsson C, Finet S, Golenhofen N, Goloubinoff P, Gusev N, Haslbeck M, Hightower l, Kampinga HH, Klevit RE, Liberek K, Mchaourab HS, McMenimen KA, Poletti A, Quinlan R, Strelkov SV, Toth ME, Vierling E, Tanguay RM. The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones. 2017;22(4):601–611. doi: 10.1007/s12192-017-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci R, Beltran Valls MR, Duranti G, Dimauro I, Quaranta F, Pittaluga M, Sabatini S, Caserotti P, Parisi P, Parisi A, Caporossi D. Oxidative stress responses to a graded maximal exercise test in older adults following explosive-type resistance training. Redox Biol. 2014;2:65–72. doi: 10.1016/j.redox.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalova AS, Sudnitsyna MV, Semenyuk PI, Orlov VN, Gusev NB. Effect of disulfide crosslinking on thermal transitions and chaperone-like activity of human small heat shock protein HspB1. Cell Stress Chaperones. 2014;19(6):963–972. doi: 10.1007/s12192-014-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Sun TX, Akhtar NJ, Liang JJ. Oxidation of human lens recombinant alphaA-crystallin and cysteine-deficient mutants. J Mol Biol. 2001;305(4):969–976. doi: 10.1006/jmbi.2000.4348. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Longoni S, Limbruno U. Cardiac alpha-crystallin: involvement during heart ischemia. Mol Cell Biochem. 1990;97(2):129–136. doi: 10.1007/BF00221054. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;5:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60(24):7099–7105. [PubMed] [Google Scholar]
- Cumming KT, Raastad T, Holden G et al (2014) Effects of vitamin C and E supplementation on endogenous antioxidant systems and heat shock proteins in response to endurance training. Physiol Rep 2. pii: e12142. 10.14814/phy2.12142 [DOI] [PMC free article] [PubMed]
- Dalakas MC, Dagvadorj A, Goudeau B, Park KY, Takeda K, Simon-Casteras M, Vasconcelos O, Sambuughin N, Shatunov A, Nagle JW, Sivakumar K, Vicart P, Goldfarb LG. Progressive skeletal myopathy, a phenotypic variant of desmin myopathy associated with desmin mutations. Neuromuscul Disord. 2003;13(3):252–258. doi: 10.1016/s0960-8966(02)00271-7. [DOI] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of αB crystallin. FEBS Lett. 2013;587(8):1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecq SP, Rosenbaum JC, Klevit RE. A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry. 2015;54(28):4276–4284. doi: 10.1021/acs.biochem.5b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR, Chudley AE, Sarnat HB, Campbell C, Goobie S, Chodirker BN, Selcen D. Infantile muscular dystrophy in Canadian aboriginals is an αB-crystallinopathy. Ann Neurol. 2011;69(5):866–871. doi: 10.1002/ana.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Engelsman J, Gerrits D, de Jong WW, Robbins J, Kato K, Boelens WC. Nuclear import of {alpha}B-crystallin is phosphorylation-dependent and hampered by hyperphosphorylation of the myopathy-related mutant R120G. J Biol Chem. 2005;280(44):37139–37148. doi: 10.1074/jbc.M504106200. [DOI] [PubMed] [Google Scholar]
- Dimauro I, Grasso L, Fittipaldi S, Fantini C, Mercatelli N, Racca S, Geuna S, di Gianfrancesco A, Caporossi D, Pigozzi F, Borrione P. Platelet-rich plasma and skeletal muscle healing: a molecular analysis of the early phases of the regeneration process in an experimental animal model. PLoS One. 2014;9(7):e102993. doi: 10.1371/journal.pone.0102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimauro I, Mercatelli N, Caporossi D. Exercise-induced ROS in heat shock proteins response. Free Radic Biol Med. 2016;98:46–55. doi: 10.1016/j.freeradbiomed.2016.03.028. [DOI] [PubMed] [Google Scholar]
- Dimauro I, Scalabrin M, Fantini C, et al. Resistance training and redox homeostasis: correlation with age-associated genomic changes. Redox Bio. 2016;l10:34–44. doi: 10.1016/j.redox.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimauro I, Sgura A, Pittaluga et al (2017) Regular exercise participation improves genomic stability in diabetic patients: an exploratory study to analyse telomere length and DNA damage. Sci Rep 7:4137. doi: 10.1038/s41598-017-04448-4, 1. [DOI] [PMC free article] [PubMed]
- Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331(6017):550–553. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran P, Gannon J, O'Connell K, Ohlendieck K. Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur J Cell Biol. 2007;86(10):629–640. doi: 10.1016/j.ejcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dubin RA, Ally A, Chung S, Piatigorsky J. Human alpha-β-crystallin gene and preferential promoter function in lens. Genomics. 1990;7(4):594–601. doi: 10.1016/0888-7543(90)90204-8. [DOI] [PubMed] [Google Scholar]
- Eaton P, Fuller W, Bell JR, Shattock MJ. AlphaB crystallin translocation and phosphorylation: signal transduction pathways and preconditioning in the isolated rat heart. J Mol Cell Cardiol. 2001;33(9):1659–1671. doi: 10.1006/jmcc.2001.1418. [DOI] [PubMed] [Google Scholar]
- Eaton P, Fuller W, Shattock MJ. S-thiolation of HSP27 regulates its multimeric aggregate size independently of phosphorylation. J Biol Chem. 2002;277(24):21189–21196. doi: 10.1074/jbc.M200591200. [DOI] [PubMed] [Google Scholar]
- Enomoto Y, Adachi S, Matsushima-Nishiwaki R, et al. αB-crystallin extracellularly suppresses ADP-induced granule secretion from human platelets. FEBS Lett. 2009;583:2464e2468. doi: 10.1016/j.febslet.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Esser C, Alberti S, Hohfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta. 2004;1695(1-3):171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Fichna JP, Potulska-Chromik A, Miszta P, Redowicz MJ, Kaminska AM, Zekanowski C, Filipek S. A novel dominant D109A CRYAB mutation in a family with myofibrillar myopathy affects αB-crystallin structure. BBA Clin. 2016;7:1–7. doi: 10.1016/j.bbacli.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi S, Dimauro I, Mercatelli N, Caporossi D. Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Radic Res. 2014;48(1):52–70. doi: 10.3109/10715762.2013.835047. [DOI] [PubMed] [Google Scholar]
- Fittipaldi S, Mercatelli N, Dimauro I, Jackson MJ, Paronetto MP, Caporossi D. Alpha B-crystallin induction in skeletal muscle cells under redox imbalance is mediated by a JNK-dependent regulatory mechanism. Free Radic Biol Med. 2015;86:331–342. doi: 10.1016/j.freeradbiomed.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Forrest KM, Al-Sarraj S, Sewry C, et al. Infantile onset myofibrillar myopathy due to recessive CRYAB mutations. Neuromuscul Disord. 2011;21(1):37–40. doi: 10.1016/j.nmd.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92(4):1743–1748. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- Gamerdinger M, Carra S, Behl C. Emerging roles of molecular chaperones and co-chaperones in selective autophagy: focus on BAG proteins. J Mol Med. 2011;89(12):1175–1182. doi: 10.1007/s00109-011-0795-6. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. αB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261e3269. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S, Mitra A, Sarkar S. Role of α-crystallin B in regulation of stress induced cardiomyocyte apoptosis. Cardiovasc Hematol Agents Med Chem. 2014;12(2):60–65. doi: 10.2174/1871525713666150123151731. [DOI] [PubMed] [Google Scholar]
- Garrido C, Paul C, Seigneuric R, Kampinga HH. The small heat shock proteins family: the long forgotten chaperones. Int J Biochem Cell Biol. 2012;44(10):1588–1592. doi: 10.1016/j.biocel.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Geeraert C, Ratier A, Pfisterer SG, Perdiz D, Cantaloube I, Rouault A, Pattingre S, Proikas-Cezanne T, Codogno P, Poüs C. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem. 2010;285(31):24184–24194. doi: 10.1074/jbc.M109.091553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry. 2005;44(45):14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Arbeiter A, Koob R, Drenckhahn D. Ischemia induced association of the stress protein alpha B-crystallin with Iband portion of cardiac titin. J Mol Cell Cardiol. 2002;34(3):309–319. doi: 10.1006/jmcc.2001.1513. [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Htun P, Ness W, Koob R, Schaper W, Drenckhahn D. Binding of the stress protein alpha B-crystallin to cardiac myofibrils correlates with the degree of myocardial damage during ischemia/reperfusion in vivo. J Mol Cell Cardiol. 1999;31(3):569–580. doi: 10.1006/jmcc.1998.0892. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Piatigorsky J. The murine B-crystallin/small heat shock protein enhancer: identification of BE-1, BE-2, BE-3, and MRF control elements. Mol Cell Biol. 1993;13(11):7144–7152. doi: 10.1128/mcb.13.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107(10):1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- Gupta R, Srivastava OP. Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin. J Biol Chem. 2004;279(43):44258–44269. doi: 10.1074/jbc.M405648200. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Weinkauf S, Buchner J (2015) Regulation of the chaperone function of small Hsps. In: Hightower LE (ed) Tanguay RM. Springer International Publishing, Switzerland, The Small HSP World. Cham. isbn:978-3-319-16076-4
- Hayes D, Napoli V, Mazurkie A, Stafford WF, Graceffa P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J Biol Chem. 2009;284(28):18801–18807. doi: 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski CC. Alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000;275(31):23825–23833. doi: 10.1074/jbc.M003864200. [DOI] [PubMed] [Google Scholar]
- Houck SA1, Landsbury A, Clark JI, Quinlan RA (2011). Multiple sites in αB-crystallin modulate its interactions with desmin filaments assembled in vitro. PLoS One 6:e25859. doi: 10.1371/journal.pone.0025859. [DOI] [PMC free article] [PubMed]
- WF H, Gong L, Cao Z, et al. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr Mol Med. 2012;12:177–187. doi: 10.2174/156652412798889036. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Hayashi T, Arimura T, Koga Y, Takahashi M, Shibata H, Teraoka K, Chikamori T, Yamashina A, Kimura A. Alpha B-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342(2):379–386. doi: 10.1016/j.bbrc.2006.01.154. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H, Isobe T, Kato K. Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem. 1997;272(47):29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, Rademaker A, Gradishar WJ, Morrow M, Khan SA, Cryns VL. alphaB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008;111(3):411–417. doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- Janu’e A, Oliv’e M, Ferrer I. Oxidative stress in desminopathies and myotilinopathies: a link between oxidative damage and abnormal protein aggregation. Brain Pathol. 2007;17(4):377–388. doi: 10.1111/j.1750-3639.2007.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehle S, Vollmar BS, Bardiaux B, Dove KK, Rajagopal P, Gonen T, Oschkinat H, Klevit RE. N-terminal domain of alphaB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc Natl Acad Sci U S A. 2011;108(16):6409–6414. doi: 10.1073/pnas.1014656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates cytochrome c- and caspase-8- dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276(19):16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JAM, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8(1):53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Inaguma Y, Iwamoto I, Saga S. Phosphorylation of alpha beta-crystallin in mitotic cells and identification enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273(43):28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heatshock protein. Nature. 1998;394(6693):595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169(3):425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24(10):3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JLP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19(5):1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Expt. Cell Res. 2006;312(18):3570–3584. doi: 10.1016/j.yexcr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121(9):3689–3700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Mol Biol Cell. 2005;16(9):4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shin D, Kwon SH. Histone deacetylase 6 plays a role as a distinct regulator of diverse cellular processes. FEBS J. 2013;280(3):775–793. doi: 10.1111/febs.12079. [DOI] [PubMed] [Google Scholar]
- Lin DI, Barbash O, Kumar KG, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF (FBX4-alphaB crystallin) complex. Mol Cell. 2006;24(3):355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li J, Tao Y, Xiao X. Small heat shock protein alphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide induced apoptosis. Biochem Biophys Res Commun. 2007;354(1):109–114. doi: 10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A novel αB-crystallincmutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47(3):1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longoni S, James P, Chiesi M. Cardiac alpha-crystallin: isolation and identification. Mol Cell Biochem. 1990;97:113–120. [PubMed] [Google Scholar]
- Lutsch G, Vetter R, Offhauss U, Wieske M, Grone HJ, Klemenz R, Schimke I, Stahl J, Benndorf R. Abundance and location of the small heat shock proteins HSP25 and alphaB-crystallin in rat and human heart. Circulation. 1997;96(10):3466–3476. doi: 10.1161/01.cir.96.10.3466. [DOI] [PubMed] [Google Scholar]
- Maloyan A, Gulick J, Glabe CG, Kayed R, Robbins J. Exercise reverses preamyloid oligomer and prolongs survival in alphaB-crystallin-based desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104(14):5995–6000. doi: 10.1073/pnas.0609202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloyan A, Osinska H, Lammerding J, Lee RT, Cingolani OH, Kass d, Lorenz JN, Robbins J. Biochemical and mechanical dysfunction in a mouse model of desmin-related myopathy. Circ Res. 2009;104(8):1021–1028. doi: 10.1161/CIRCRESAHA.108.193516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB- crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11(5):512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Maulik N, Watanabe M, YL Z, et al. Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett. 1996;396(2-3):233–237. doi: 10.1016/0014-5793(96)01109-x. [DOI] [PubMed] [Google Scholar]
- McLendon PM, Ferguson BS, Osinska H, Bhuiyan MS, James J, McKinsey TA, Robbins J. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc Natl Acad Sci U S A. 2014;111(48):E5178–E5186. doi: 10.1073/pnas.1415589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon PM, Robbins J. Desmin-related cardiomyopathy: an unfolding story. Am J Physiol Heart Circ Physiol. 2011;301(4):H1220–H1228. doi: 10.1152/ajpheart.00601.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Cammarato A, Bernstein SI. αBcrystallin maintains skeletal muscle myosin enzymatic activity and prevents its aggregation under heat-shock stress. J Mol Biol. 2006;3:635–645. doi: 10.1016/j.jmb.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Mercatelli N, Dimauro I, Ciafré SA, Farace MG, Caporossi D. AlphaB-crystallin is involved in oxidative stress protection determined by VEGF in skeletal myoblasts. Free Radic Biol Med. 2010;49(3):374–382. doi: 10.1016/j.freeradbiomed.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Michiel M, Skouri-Panet F, Duprat E, Simon S, Férard C, Tardieu A, Finet S. Abnormal assemblies and subunit exchange of alphaB-crystallin R120 mutants could be associated with destabilization of the dimeric substructure. Biochemistry. 2009;48(2):442–453. doi: 10.1021/bi8014967. [DOI] [PubMed] [Google Scholar]
- Mitra A, Basak T, Datta K, Naskar S, Sengupta S, Sarkar S. Role of a-crystallin B as a regulatory switch in modulating cardiomyocyte apoptosis by mitochondria or endoplasmic reticulum during cardiac hypertrophy and myocardial infarction. Cell Death Dis. 2013;4(4):e582. doi: 10.1038/cddis.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of αB-Crystallin on Serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92(2):203–211. doi: 10.1161/01.res.0000052989.83995.a5. [DOI] [PubMed] [Google Scholar]
- Morrison LE, Whittaker RJ, Klepper RE, Wawrousek EF, Glembotski CC. Roles for alphaB-crystallin and HSPB2 in protecting the myocardium from ischemia-reperfusion-induced damage in a KO mouse model. Am J Physiol Heart Circ Physiol. 2004;286(3):H847–H855. doi: 10.1152/ajpheart.00715.2003. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J. The chaperone activity and substrate spectrum of human small heat shock proteins. J Biol Chem. 2017;292(2):672–684. doi: 10.1074/jbc.M116.760413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91(4):1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- N’edellec P, Edling Y, Perret E, Fardeau M, Vicart P. Glucocorticoid treatment induces expression of small heat shock proteins in human satellite cell populations: consequences for a desmin-related myopathy involving the R120G alpha B-crystallin mutation. Neuromuscul Disord. 2002;12(5):457–465. doi: 10.1016/s0960-8966(01)00306-6. [DOI] [PubMed] [Google Scholar]
- Neppl RL, Kataoka M, Wang DZ. Crystallin-αB regulates skeletal muscle homeostasis via modulation of argonaute2 activity. J Biol Chem. 2014;289(24):17240–17248. doi: 10.1074/jbc.M114.549584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. EMBO J. 1994;13(4):945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya-Ito T, Liu BF, Nagaraj RH. Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem. 2006;99(1):279–291. doi: 10.1002/jcb.20781. [DOI] [PubMed] [Google Scholar]
- Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol. 2012;181(2):583–592. doi: 10.1016/j.ajpath.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Pereira MB, Santos AM, Gonçalves DC, et al. Corrigendum: αB-crystallin interacts with and prevents stress-activated proteolysis of focal adhesion kinase by calpain in cardiomyocytes. Nat Commun. 2015;6:6508. doi: 10.1038/ncomms7508. [DOI] [PubMed] [Google Scholar]
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-κB. Cell Death Differ. 2006;13(5):759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Peschek J, Braun N, Rohrberg J, et al. Regulated structural transitions unleash the chaperone activity of aB-crystallin. Proc Natl Acad Sci U S A. 2013;110(E3780e):E3789. doi: 10.1073/pnas.1308898110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Marziliano N, Pasotti M, Grasso M, Costante AM, Arbustini E. alphaB-crystallin mutation in dilated cardiomyopathies: low prevalence in a consecutive series of 200 unrelated probands. Biochem Biophys Res Commun. 2006;346(4):1115–1117. doi: 10.1016/j.bbrc.2006.05.203. [DOI] [PubMed] [Google Scholar]
- Pipkin W, Johnson JA, Creazzo TL, Burch J, Komalavilas P, Brophy C. Localization, macromolecular associations, and function of the small heat shock-related protein HSP20 in rat heart. Circulation. 2003;107(3):469–476. doi: 10.1161/01.cir.0000044386.27444.5a. [DOI] [PubMed] [Google Scholar]
- Pittaluga M, Sgadari A, Dimauro I, Tavazzi B, Parisi P, Caporossi D. Physical exercise and redox balance in type 2 diabetics: effects of moderate training on biomarkers of oxidative stress and DNA damage evaluated through comet assay. Oxidative Med Cell Longev. 2015;2015:981242–981247. doi: 10.1155/2015/981242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Jakhotia S, Reddy PY, Reddy GB. Hyperglycemia induced expression, phosphorylation, and translocation of αB-crystallin in rat skeletal muscle. IUBMB Life. 2015;67(4):291–299. doi: 10.1002/iub.1370. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Reddy GB. Emerging role for αB-crystallin as a therapeutic agent: pros and cons. Curr Mol Med. 2015;15(1):47–61. doi: 10.2174/1566524015666150114112853. [DOI] [PubMed] [Google Scholar]
- Reilich P, Schoser B, Schramm N, Krause S, Schessl J, Kress W, Müller-Höcker J, Walter MC, Lochmuller H. The p. G154S mutation of the Alpha-B crystallin gene (CRYAB) causes late-onset distal myopathy. Neuromuscul Disord. 2010;20(4):255–259. doi: 10.1016/j.nmd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;22:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, Paul C, Wieske M, Arrigo AP, Buchner J, Gaestel M. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor a by phosphorylation. J Biol Chem. 1999;274(27):18947–18956. doi: 10.1074/jbc.274.27.18947. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Kurnellas MP, Brownell S, et al. Therapeutic effects of systemic administration of chaperone aB-crystallin associated with binding proinflammatory plasma proteins. J Biol Chem. 2012;287:9708e9721. doi: 10.1074/jbc.M111.337691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacconi S, Féasson L, Antoine JC, Pécheux C, Bernard R, Cobo AM, Casarin A, Salviati L, Desnuelle C, Urtizberea A. A novel CRYAB mutation resulting in multisystemic disease. Neuromuscul Disord. 2012;22(1):66–72. doi: 10.1016/j.nmd.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Sammut IA, Harrison JC. Cardiac mitochondrial complex activity is enhanced by heats hock proteins. Clin Exp Pharm Phys. 2003;30(1-2):110–115. doi: 10.1046/j.1440-1681.2003.03799.x. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Daicho T, Mizutani R, Endo T, Miyauchi N, Yamauchi J, Tanonaka K, Glabe C, Tanoue A. Protective effect of geranylgeranylacetone via enhancement of HSPB8 induction in desmin-related cardiomyopathy. PLoS One. 2009;4(4):e5351. doi: 10.1371/journal.pone.0005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, Robbins J. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci U S A. 2004;101(27):10132–10136. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish Kumar M, Mrudula T, Mitra N, Bhanuprakash Reddy G. Enhanced degradation and decreased stability of eye lens alpha-crystallin upon methylglyoxal modification. Exp Eye Res. 2004;79(4):577–583. doi: 10.1016/j.exer.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Segard BD, Delort F, Bailleux V, Simon S, Leccia E, Gausseres B, Briki F, Vicart P, Batonnet-Pichon S. N-acetyl-L-cysteine prevents stress-induced desmin aggregation in cellular models of desminopathy. PLoS One. 2013;8(10):e76361. doi: 10.1371/journal.pone.0076361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54(6):804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kumar GS, Murphy AS, Kester K. Identification of 1,1′-bi(4-anilino) naphthalene-5,5′-disulfonic acid binding sequences in alpha-crystallin. J Biol Chem. 1998;273(25):15474–15478. doi: 10.1074/jbc.273.25.15474. [DOI] [PubMed] [Google Scholar]
- Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao CM. Association of alphaB-crystallin, a small heat shock protein, with actin: role in modulating actin filament dynamics in vivo. J Mol Biol. 2007;366(3):756–767. doi: 10.1016/j.jmb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Singh BN, Rao KS, Rao CM. Ubiquitin–proteasome-mediated degradation and synthesis of MyoD is modulated by alphaB-crystallin, a small heat shock protein, during muscle differentiation. Biochim Biophys Acta. 2010;1803(2):288–299. doi: 10.1016/j.bbamcr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Smith DA, Carland CR, Guo Y, Bernstein SI. Getting folded: chaperone proteins in muscle development, maintenance and disease. Anat Rec (Hoboken) 2014;297(9):1637–1649. doi: 10.1002/ar.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A. 2008;105(28):9745–9750. doi: 10.1073/pnas.0706802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell E, Aquilina A. Regulation of αA- and αB-crystallins via phosphorylation in cellular homeostasis. Cell Mol Life Sci. 2015;72(21):4127–4137. doi: 10.1007/s00018-015-1996-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treweek TM, Rekas A, Walker MJ, Carver JA. A quantitative NMR spectroscopic examination of the flexibility of the C-terminal extensions of the molecular chaperones, alphaA- and alphaB-crystallin. Exp Eye Res. 2010;91(5):691–699. doi: 10.1016/j.exer.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Tupling AR, Gramolini AO, Duhamel TA, et al. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2þ-ATPase(SERCA1a) and prevents thermal inactivation. J Biol Chem. 2004;50:52382–52389. doi: 10.1074/jbc.M409336200. [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev. Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, Chateau D, Chapon F, Tomé F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20(1):92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Voss OH, Batra S, Kolattukudy SJ, Gonzalez-Mejia ME, Smith JB, Doseff AI. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J Biol Chem. 2007;282(34):25088–25099. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KA. Serine phosphorylation and suppression of apoptosis by the small heat shock protein alphaB-crystallin. Circ Res. 2003;92(2):130–132. doi: 10.1161/01.res.0000056967.51841.21. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Role of quality control pathways in human diseases involving protein misfolding. Semin Cell Dev Biol. 2003;15:31–38. doi: 10.1016/j.semcdb.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Wu W, Lu C, Wang Y et al (2015) Novel phenotype-genotype correlations of restrictive cardiomyopathy with myosin-binding protein C (MYBPC3) gene mutations tested by next generation sequencing. Journal of the American Heart Association 4. pii:e001879 [DOI] [PMC free article] [PubMed]
- Zantema A, Vries MVD, Maasdam D, Bol S, Eb A.v.d. Heat shock protein 27 and alphaB-cristallin can form a complex, which dissociates by heat shock. J Biol Chem. 1992;267(18):12936–12941. [PubMed] [Google Scholar]