Abstract
The non-digestible oligosaccharide fraction of maternal milk represents an important of carbohydrate and energy source for saccharolytic bifidobacteria in the gastrointestinal tract during early life. However, not all neonatal bifidobacteria isolates can directly metabolise the complex sialylated, fucosylated, sulphated and/or N-acetylglucosamine-containing oligosaccharide structures present in mothers milk. For some bifidobacterial strains, efficient carbohydrate syntrophy or crossfeeding is key to their establishment in the gut. In this study, we have adopted advanced functional genomic approaches to create single and double in-frame deletions of the N-acetyl glucosamine 6-phosphate deacetylase encoding genes, nagA1 and nagA2, of B. breve UCC2003. In vitro phenotypic analysis followed by in vivo studies on co-colonisation, mother to infant transmission, and evaluation of the relative co-establishment of B. bifidum and B. breve UCC2003 or UCC2003ΔnagA1ΔnagA2 in dam-reared neonatal mice demonstrates the importance of crossfeeding on sialic acid, fucose and N-acetylglucosamine-containing oligosaccharides for the establishment of B. breve UCC2003 in the neonatal gut. Furthermore, transcriptomic analysis of in vivo gene expression shows upregulation of genes associated with the utilisation of lactose, sialic acid, GlcNAc-6-S and fucose in B. breve UCC2003, while for UCC2003ΔnagA1ΔnagA2 only genes for lactose metabolism were upregulated.
Introduction
Bifidobacteria are among the earliest and most abundant bacterial colonisers of the neonatal gut where their presence is associated with a myriad of benefits to the host intestinal, metabolic and immune health1. While the genus Bifidobacterium comprises more than 50 species/subspecies, the dominant infant associated species include Bifidobacterium longum subsp. longum, Bifidobacterium longum subsp infantis, Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium pseudocatenulatum, Bifidobacterium. catenulatum, Bifidobacterium kashiwanohense, and Bifidobacterium adolescentis2,3. The dominance of bifidobacteria in breastfed infants has been attributed to their ability to utilise human milk oligosaccharides (HMOs). In particular, strains of B. longum subsp. infantis and B. bifidum have been studied extensively for their ability to utilise host derived carbohydrates and have been found to harbour dedicated, yet distinct, metabolic capabilities for the utilisation of HMOs4,5 while more recently specific strains of B. longum subsp. longum, B. pseudocatenulatum and B. kashiwanohense have been investigated for their ability to utilise fucosyllactose, the dominant oligosaccharide in human milk6,7. Intriguingly, the ability to utilise HMOs would seem to be a variable trait among infant-derived strains of B. breve, with some strains exhibiting good growth on purified HMOs under in vitro conditions, while other B. breve strains exhibit no appreciable growth2. The presence and isolation of B. breve strains that do not directly metabolise HMOs would suggest that these strains likely adopt carbohydrate syntrophy to allow their establishment in the infant gut. We hypothesise that B. breve, and perhaps other Bifidobacterium sp, can exploit the extracellular glycosyl-hydrolase activity of other (bifido)bacterial members of the infant gut microbiome as a source of fermentable carbohydrates to support growth in the intestine.
We have previously shown that B. breve UCC2003, a nursling infant stool isolate, can efficiently utilise sialic acid, but not host derived 3′ sialyllactose, as sole carbohydrate source8. However, B. breve UCC2003 can crossfeed on released sialic acid derived from the extracellular metabolism of 3′ sialyllactose by B. bifidum PRL2010 during an in vitro sequential co-culture experiment8. Similarly B. breve UCC2003 cannot directly utilise the most abundant HMO in mother’s milk, fucosyllactose (2′FL or 3′FL), but can crossfeed on monosaccharides, including fucose, that are released during co-culture with B. bifidum PRL2010 in mMRS medium supplemented with porcine mucin9.
N-acetylglucosamine deacetylase (NagA) activity is central to the metabolism of HMOs and host derived carbohydrates, whereby NagA catalyses the conversion of N-acetylglucosamine 6-phosphate to glucosamine 6-phosphate. The genome of B. breve UCC2003, ate, harbours 2 genes, namely Bbr_0846 and Bbr_1247 (designated nagA1 and nagA2, respectively) whose protein products, NagA1 and Nag A2, respectively, share 74% identity and are predicted to encode N-acetyl glucosamine deacetylase activity. Expression of nagA1is significantly upregulated during growth of B. breve UCC2003 in mMRS medium supplemented with the host derived sulphated carbohydrate N-acetyl glucosamine-6-sulphate (GlcNAc-6-S) or Lactosamine-HCl, while expression of nagA2 is significantly upregulated during growth of B. breve UCC2003 in mMRS medium supplemented with sialic acid, LNT, LNnT, GlcNAc-6-S and also when B. breve UCC2003 is grown in co-culture with B. bifidum PRL2010 in medium supplemented with mucin8–11. Previously a B. breve UCC2003-nagA2 insertion mutant strain was found to exhibit growth comparable to that of the parent strain, B. breve UCC2003, in medium supplemented with sialic acid as sole carbohydrate source suggesting that NagA1 may compensate in the absence of NagA2 activity8.
To extend our understanding of bifidobacterial mutualism and carbohydrate syntrophy in the gut we adopted advanced functional genomics to create single- and double-deletion isogenic strains of the NagA-encoding genes of B. breve UCC2003. The resulting strains were examined, as compared to the parent strain, for their ability to metabolise particular host-derived carbohydrates. In addition, the B. breve strains were examined for their cross-feeding capability and ability to establish, in the presence of B. bifidum, in the gut of dam fed neonatal mice.
Results
Phenotypic analysis of B. breve UCC2003 strains harbouring deletions of the N-acetyl glucosamine deacetylase encoding genes, nagA1 and nagA2
To establish if N-acetyl glucosamine deacetylase activity is essential for the metabolism of sialic acid and other host derived carbohydrates isogenic B. breve UCC2003 derivative strains harbouring inframe deletions of nagA1 or nagA2, and a double (nagA1nagA2) deletion strain were created, and designated B. breve UCC2003ΔnagA1, UCC2003ΔnagA2 or UCC2003ΔnagA1ΔnagA2, respectively. These three mutants were compared to B. breve UCC2003 for their ability to utilise lactose, sialic acid, Lacto-N-tetraose (LNT), Lacto-N-neotetraose (LNnT), or N-Acetyl-D-glucosamine-6-O-sulfate (GlcNAc-6-S) as the sole carbohydrate source. All strains achieved final optical densities (OD 600nm) greater than 2.0 in mMRS medium supplemented with lactose (positive control). In mMRS medium supplemented with sialic acid, LNT, LNnT or GlcNAc-6-S B. breve UCC2003, UCC2003ΔnagA1, UCC2003ΔnagA2 achieved comparable final optical density values for each carbohydrate substrate, while growth of B. breve UCC2003ΔnagA1ΔnagA2 was impaired (Fig. 1).
Figure 1.
Final optical densities (OD 600 nm) of B. breve UCC2003, UCC2003ΔnagA1, UCC2003ΔnagA2 or UCC2003ΔnagA1ΔnagA2 following 24 hours growth in mMRS medium supplemented with lactose, sialic acid, LNT, LNnT or GlcNAc-6-S at 0.5% w/v final concentration.
Carbohydrate syntrophy enhances the establishment of Bifidobacterium breve UCC2003 in the neonatal gut
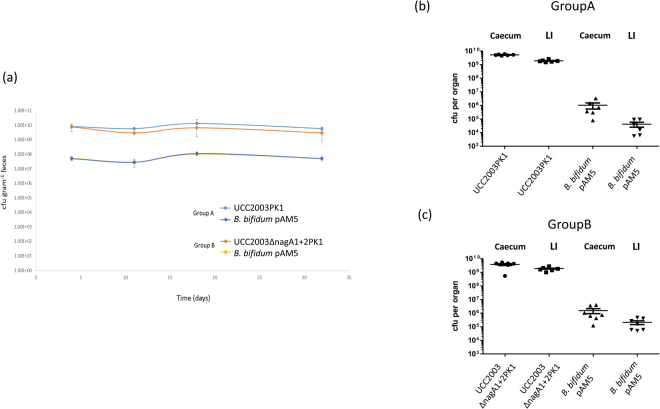
In order to establish if the ability to metabolise host derived carbohydrates enhances the numbers of B. breve UCC2003 in the gastrointestinal tract of Dam-reared neonatal murine pups, a co-association study was performed. Groups of 7 pregnant C57BL/6 Germ free mice were administered a single dose of 1 × 109 cfu of each B. bifidum PAM5 and B. breve UCC2003PK1, or B. bifidum PAM5 and UCC2003∆nagA1 + 2PK1. Fecal samples were collected weekly during the trial period to enumerate bifidobacterial shedding and determine the relative colonisation ability of B. bifidum PAM5, B. breve UCC2003PK1 or B. breve UCC2003∆nagA1 + 2PK1. Interestingly, despite administration at equal levels, B. breve UCC2003 or B. breve UCC2003∆nagA1 + 2PK1 colonised the pregnant mice at approximately 100 fold higher level as compared to B. bifidum PAM5 (Fig. 2a). This difference in colonisation ability between B. breve UCC2003 derivatives and B. bifidum PAM5 was also reflected in the numbers of each strain recovered from the caecum and large intestine of the adult mice at the end of the trial period (Fig. 2b and c). The first litters of pups were born 9 days after administration of the Bifidobacterium strains, with all pups born within a period of 5 days. For Group A, administered B. bifidum PAM5 and B. breve UCC2003PK1, just three of the seven mothers produced litters of pups, while for Group B administered B. bifidum PAM5 and B. breve UCC2003∆nagA1 + 2PK1 five of the seven mothers gave birth to litters of pups (Table 1). All pups were allowed to feed from their mothers and at 2 time points postpartum, and while the pups were exclusively dam reared, half of each group was culled for enumeration of each Bifidobacterium strain in the caecum or large intestine based by plate counting with selection based on antibiotic resistance. For Group A, where pregnant mothers were administered B. bifidum PAM5 and B. breve UCC2003PK1, mother-to-pup transmission of both Bifidobacterium strains was observed with B. breve UCC2003PK1 present at a level that was almost 100-fold higher as compared to that achieved by B. bifidum PAM5 in the large intestine of the pups at each time point (p ≤ 0.001on cull day 1 and 2), achieving average levels of 1 × 106 cfu and 1.3 × 104 cfu, respectively, on cull day 1, while levels of 2.59 × 107 cfu and 6.3 × 105 cfu, respectively were recovered from the large intestine on cull day 2 (Fig. 3a and b). Similarly, for Group B, where mothers were administered B. bifidum PAM5 and B. breve UCC2003∆nagA1 + 2PK1, mother-to-pup transmission of both bifidobacterial strains was observed. However, the numbers of B. breve UCC2003∆nagA1 + 2PK1 and B. bifidum PAM5 were not significantly different on either cull day. Average levels of B. breve UCC2003∆nagA1 + 2PK1 and B. bifidum PAM5 recovered from the large intestines were 6.5 × 105 and 3.1 × 105 respectively, on the first cull day, with levels of each strain increasing to 2.3 × 106 cfu and 1.77 × 106 cfu, respectively on cull day 2 (Fig. 3a and b).
Figure 2.
Co-colonisation of B. breve UCC2003PK1 and B. bifidum PAM5 or B. breve UCC2003ΔnagA1 + 2′ PK1 and B. bifidum PAM5 in pregnant germ free C57BL/6 mice (a) Recovery of B. breve UCC2003PK1 (pale blue) and B. bifidum PAM5 (dark blue) or B. breve UCC2003ΔnagA1ΔnagA2 (orange) and B. bifidum PAM5 (yellow) from murine fecal samples of C57BL/6 co-associated mice over 4 week trial period. (b) Comparison of numbers of B. breve UCC2003PK1 and B. bifidum PAM5 or (c) B. breve UCC2003ΔnagA + 2PK1 and B. bifidum PAM5 and recovered from the caecum and the large intestine of co–associated animals.
Table 1.
Number of pups born to each C57Bl/6 mother and number of dam-reared pups culled at each timepoint.
| Mother/litter | Number of pups in litter | Cull day: 1 | Cull day: 2 | |
|---|---|---|---|---|
| Number of pups (age in days) | Number of pups (age in days) | |||
| Group A: administered B. bifidum PAM5 and UCC2003PK1 | A1 | 8 | 4 (10 days) | 4 (14 days) |
| A2 | 0 | |||
| A3 | 3 | 2 (11 days) | 1 (15 days) | |
| A4 | 0 | |||
| A5 | 0 | |||
| A6 | 0 | |||
| A7 | 7 | 3 (11 days) | 4 (15 days) | |
| Total 9 animals | Total 9 animals | |||
| Group B: administered B. bifidum PAM5 and UCC2003∆nagA1 + 2PK1 | B1 | 5 | 2 (8 days) | 3 (12 days) |
| B2 | 6 | 3 (12 days) | 3 (16 days) | |
| B3 | 8 | 4 (10 days) | 4 (14 days) | |
| B4 | 0 | |||
| B5 | 7 | 3 (10 days) | 4 (14 days) | |
| B6 | 4 | 2 (9 days) | 2 (13 days) | |
| B7 | 0 | |||
| Total 14 animals | Total 16 animals |
Figure 3.
Enumeration of Bifidobacteria from the intestine of dam reared mice. Enumeration of B. breve UCC2003PK1 and B. bifidum PAM5 (shaded black), or B. breve UCC2003ΔnagA1 + 2PK1 and B. bifidum PAM5 (shaded grey) on cull day 1 (a) or 2 (b) from the intestine or caecum of dam reared neonatal mice.
B. breve UCC2003 or UCC2003∆nagA1 + 2 transcriptome during colonisation of dam-reared neonatal mice
To determine the B. breve UCC2003 or UCC2003∆nagA1 + 2 genes that are differentially transcribed in the gut of the dam-reared neonatal mice relative to the transcriptional profile under laboratory conditions, total bacterial RNA was isolated from the large intestines of neonatal mice harbouring B. breve UCC2003PK1 and B. bifidum PAM5 or B. breve UCC2003∆nagA1 + 2PK1 and B. bifidum PAM5. The RNA was reverse transcribed and the cDNA used to determine the in vivo transcriptome of each strain as compared to an exponential phase culture B. breve UCC2003 grown in mMRS supplemented with ribose. A total of 74 B. breve UCC2003 genes were significantly upregulated in vivo (≥3 fold; p < 0.001) and 95 downregulated (≥8 fold; p < 0.001), while for B. breve UCC2003∆nagA1 + 2PK1 23 genes were significantly upregulated in vivo (≥3 fold; p < 0.01) and 88 downregulated (≥8 fold; p < 0.001) relative to the control. The most highly upregulated genes were in loci dedicated to carbohydrate metabolism. In particular genes Bbr_1551 and Bbr_1552, dedicated to lactose transport and metabolism were significantly upregulated in both B. breve UCC2003PK1 and B. breve UCC2003∆nagA1 + 2PK1 under in vivo conditions, while genes in the sialic acid metabolism locus (Bbr_0160-Bbr_0172), the fucose metabolism locus (Bbr_1741–1745), and the nag and sulphatase locus (Bbr_0846- Bbr_0853) were significantly upregulated in B. breve UCC2003PK1 but not in B. breve UCC2003∆nagA1 + 2PK1 under in vivo conditions (Table 2). In addition, genes predicted to be remnants of an N-acetylglucosamine PTS system (Bbr_1878-Bbr_1880) were also upregulated in B. breve UCC2003PK1. Expression of the bile salt hydrolase encoding gene (BBr_1520) and several genes encoding hypothetical membrane proteins were upregulated in both B. breve UCC2003PK1 and B. breve UCC2003∆nagA1 + 2PK1under in vivo conditions indicating that B. breve UCC2003 expresses specific sets of genes in response to the in vivo environment and during feeding on mother’s milk (Table S1).
Table 2.
Differential expression of carbohydrate utilisation gene loci in B. breve UCC2003PK1 or B. breve UCC2003ΔnagA1ΔnagA2PK1 under in vivo conditions in dam-reared neonatal mice.
| Locus tag | Function | Fold upregulation UCC2003 | Fold upregulation UCC2003ΔnagA1ΔnagA2 |
|---|---|---|---|
| Sialic acid metabolism | |||
| Bbr_0160 | Conserved hypothetical protein | 2.80a | —b |
| Bbr_0161 | Conserved hypothetical protein in ROK family | — | — |
| Bbr_0162 | N-acetylmannosamine-6-phosphate 2-epimerase | 4.52 | 2.39 |
| Bbr_0163 | Hydrolase | — | — |
| Bbr_0164 | Substrate binding protein | 11.89 | — |
| Bbr_0165 | ABC transport system permease protein | 5.36 | — |
| Bbr_0166 | ABC transport system ATP-binding protein | 7.60 | 3.11 |
| Bbr_0167 | ABC transport system ATP-binding protein | 7.57 | — |
| Bbr_0168 | nanA N-acetylneuraminate lyase | 20.35 | 4.7 |
| Bbr_0169 | Glucosamine-6-phosphate isomerase | 9.00 | — |
| Bbr_0171 | Sialidase A | 4.49 | — |
| Bbr_0172 | ATPase | — | — |
| nag genes and metabolism of sulphated sugars | |||
| Bbr_0846 | nagA1 N-acetylglucosamine-6-phosphate deacetylase | 2.0 | — |
| Bbr_0847 | nagB2 Glucosamine-6-phosphate isomerase | 3.78 | — |
| Bbr_0851 | Glucose/fructose transport protein | 5.55 | 2.95 |
| Bbr_0852 | Sulfatase family protein | 2.04 | — |
| Bbr_0853 | atsB Arylsulfatase regulator (Fe-S oxidoreductase) | — | — |
| Bbr_0854 | Conserved hypothetical | 2.12 | — |
| Bbr_0855 | Hypothetical protein | 5.14 | — |
| Lactose metabolism | |||
| Bbr_1550 | Hypothetical protein | 6.24 | 4.94 |
| Bbr_1551 | lacS Galactoside symporter | 4.50 | 3.86 |
| Bbr_1552 | lacZ6 Beta-galactosidase | 4.41 | 4.25 |
| Fucose metabolism | |||
| Bbr_1741 | Conserved hypothetical protein | 4.91 | — |
| Bbr_1742 | L-fucose permease | 3.16 | — |
| Bbr_1743 | Short chain dehydrogenase | 3.35 | — |
| Bbr_1744 | Mandelate racemase | 3.33 | — |
| Bbr_1745 | Transcriptional regulator | — | — |
| Remnants of Putative N-acetylglucosamine PTS system | |||
| Bbr_1878 | Hypothetical protein | 3.27 | — |
| Bbr_1879 | PTS system, glucose-specific IIABC component | 6.15 | — |
| Bbr_1880 | PTS system, N-acetylglucosamine-specific IIBC component | 6.77 | 3.78 |
aFold upregulation ≥2 and p value ≤ 0.001. bValues below threshold.
Discussion
The predominant carbohydrate in human milk is lactose which is found at a concentrations ranging from of 60–70 g L−1. The concentration of HMOs, with a degree of polymerisation ≥3, in human milk varies considerably, with values of 22–24 g L−1 in colostrum, and 12–13 g L−1 in mature milk12. HMOs comprise more than 200 oligosaccharide structures that are classified as Type I or Type II oligosaccharides based the disaccharide lacto-N-biose or N-acetyllactosamine, respectively, at their reducing end. Fucosyllactose, Lacto-N-tetraose and Lacto-N-fucopentaose are the most abundant HMOs, and these 3 oligosaccharides can comprise up to 55% of the HMO content of human milk12–14. The predominance of Type I oligosaccharides is a feature of human milk, also found in mother’s milk of chimpanzees and elephants, however, among other mammals and marsupials Type II oligosaccharides tend to dominate15.
In this study we sought to establish the role of the B. breve N-acetylglucosamine deacetylases, NagA1 and NagA2, in utilising host-derived carbohydrates including HMOs. In contrast to the parental strain, B. breve UCC2003, and the single deletion strains, B. breve UCC2003∆nagA1or UCC2003∆nagA2, B. breve UCC2003∆nagA1∆nagA2 was incapable of utilising sialic acid, LNT, LNnT or GlcNAc-6-S as sole carbohydrate source while this double nagA deletion strain exhibits growth comparable to the parent strain, and the single deletion strains, in medium supplemented with lactose.
We next wished to establish if the ability to metabolise host-derived oligosaccharides confers B. breve with a growth advantage in the neonatal gut. There has been a recent surge in publications describing the oligosaccharide composition of mother’s milk from various animal sources, however despite laboratory mice being used extensively for research purposes, deciphering the oligosaccharide fraction of murine milk has received relatively little attention. This is likely due to the difficulty in obtaining sufficient volumes of milk for analysis. Despite this obstacle, Prieto et al.16 successfully determined the oligosaccharide composition of murine milk and established that in addition to lactose, murine milk contains oligosaccharides including sialyllactose (3′SL and 6′SL) and fucosyllactose. Since B. breve UCC2003 does not directly utilise these milk oligosaccharides we adopted a co-inoculation strategy whereby two groups of 7 pregnant germ free mice were gnotobiotically co-colonised with B. breve UCC2003PK1 and B. bifidum PAM5 or B. breve UCC2003∆nagA1 + 2PK1 and B. bifidum PAM5. Interestingly, the B. breve strains colonised the pregnant adult mice at an approximately 2 log higher level as compared to B. bifidum PAM5. This is likely due to the ability of UCC2003 PK1 or UCC2003∆nagA1 + 2PK1 to metabolise starch, a major component of the adult rodent diet17. The higher numbers of either B. breve strain as compared to B. bifidum PAM5 were also reflected in the bacterial numbers in the caecum and large intestine of the adult mice at the end of the trial period. Despite being housed in the same animal room and under the same conditions just 3 of the 7 mothers in group A produced viable litters, while 5 of the 7 mothers in group B produced viable litters. We attribute this relatively low number of mothers producing litters to the C57Bl/6 mice being first time mothers, and had we used established C57Bl/6 breeding mice more viable litters may have been obtained. Despite this, we obtained sufficient pups to have two timepoints for analysis while the pups were exclusively dam-reared. For each group of mice we observed mother-to-pup transmission of the Bifidobacterium strains. Enumeration of B. breve UCC2003PK1 and B. bifidum PAM5 from the caecum or large intestine of the pups on either cull day showed an almost 2 log higher level of B. breve UCC2003PK1 as compared to B. bifidum PAM5, while the numbers of B. breve UCC2003∆nagA1 + 2PK1 or B. bifidum PAM5 were not significantly different.
This data clearly indicates that the ability to cross-feed on host-derived carbohydrates provides B. breve UCC2003 with a competitive advantage, as compared to it’s isogenic double mutant or B. bifidum PAM5, that allows this strain to establish to a higher level in the gut of the dam-reared neonatal mice. To validate our findings we determined the in vivo transcriptome of B. breve UCC2003PK1 or UCC2003∆nagA1 + 2 as compared to UCC2003 grown under in vitro conditions. The in vivo transcriptome of both strains shows significant upregulation of genes involved in lactose metabolism demonstrating that both B. breve UCC2003PK1 or B. breve UCC2003∆nagA1 + 2PK1 are utilising lactose as a carbohydrate source in the neonatal gut. However, gene loci involved in metabolism of sialic acid, fucose and sulphated sugars were significantly upregulated in the in vivo transcriptome of B. breve UCC2003, yet not in that of B. breve UCC2003∆nagA1 + 2PK1, demonstrating that sialic acid, fucose and sulphated sugars are a valuable carbohydrate source, and metabolised by B. breve in the neonatal gut. Interestingly, transcription of the bile salt hydrolase-encoding gene was upregulated in both strains under these in vivo conditions, as well as a number of genes encoding uncharacterised membrane spanning hypothetical protein, suggesting that they may mediate microbe-microbe, or microbe-host dialogue.
Collectively these data demonstrate that bifidobacterial mutualism and carbohydrate syntrophy occurs in the neonatal gut, thereby allowing B. breve strains that to not directly metabolise dominant host-derived oligosaccharides to efficiently cross-feed and achieve high numbers. In addition, these data suggest that the intracellular levels amino sugars N-acetylglucosamine and N-acetylgalactosamine or glucosamine 6-phosphate may be key intermediates regulating the expression of B. breve loci dedicated to metabolising host-derived oligosaccharides.
Methods
Bacterial strains, plasmids and culture conditions
Bacterial strains and plasmids used in this study are listed in Table 3. Bifidobacterial strains were routinely cultured in reinforced clostridial medium (RCM; Oxoid Ltd, Basingstoke, Hampshire, United Kingdom). Carbohydrate utilization by bifidobacteria was examined in de Man Rogosa and Sharpe Medium (MRS) prepared from first principles18. Prior to inoculation MRS was supplemented with cysteine-HCl (0.05% w/v) and a particular carbohydrate source (0.5% w/v). The carbohydrates used were lactose and sialic acid (purchased from Sigma), N-Acetyl-D-glucosamine-6-O-sulfate (purchased from Dextra laboratories, Reading, UK), Lacto-N-tetraose (purchased from Elicityl, Crolles, France) and Lacto-N-neotetraose (obtained from Glycom, Lyngby, Denmark). Bifidobacterial cultures were incubated at 37 °C under anaerobic conditions which were maintained using an anaerobic hood (Davidson and Hardy, Belfast, Ireland). Escherichia coli was cultured in Luria Bertani broth (LB)19 at 37 °C with agitation. Where appropriate growth media contained tetracycline (Tet; 15 μg ml−1), chloramphenicol (Cm; 5 μg ml−1 for E. coli or 2.5 μg ml−1 for B. breve), Spectinomycin (Spec; 100 μg ml−1 for E. coli or B. breve) or kanamycin (Km; 50 μg ml−1 for E. coli). Recombinant E. coli cells containing pBS423∆rep were selected on LB agar containing Spec.
Table 3.
Bacterial Strains and Plasmids used in this study.
| Strain or plasmid | Relevant features | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli strains | ||
| E. coli EC101 | Cloning host, repA+ kmr | 29 |
| E. coli EC101-pNZ-M.BbrII + M.BbrIII | EC101 harbouring pNZ8048 derivative containing bbrIIM and bbrIIIM, Cmr | 25 |
| Bifidobacterium sp. strains | ||
| B. breve UCC2003 | Isolate from nursling stool | 21 |
| B. breve UCC2003-nagA1-(I) | UCC2003 pBS423Δrep first crossover integrant via nagA1 deletion fragment I, Specr | This study |
| B. breve UCC2003-nagA1-(II) | UCC2003 pBS423Δrep first crossover integrant via nagA1 deletion fragment II, Specr | This study |
| B. breve UCC2003-nagA2-(I) | UCC2003 pBS423Δrep first crossover integrant via nagA2 deletion fragment I, Specr | This study |
| B. breve UCC2003-nagA1-(I)-pRTB101 | B. breve UCC2003-nagA1-(I) harbouring pRTB101, Specr, Cmr | This study |
| B. breve UCC2003-nagA1-(II)-pRTB101 | B. breve UCC2003-nagA1-(II) harbouring pRTB101 Specr, Cmr | This study |
| B. breve UCC2003-nagA2-(I)-pRTB101 | B. breve UCC2003-nagA2-(I) harbouring pRTB101 Specr, Cmr | This study |
| B. breve UCC2003ΔnagA1 | nagA1 846 bp inframe deletion mutant of UCC2003 | This study |
| B. breve UCC2003ΔnagA2 | nagA2 969 bp inframe deletion mutant of UCC2003 | This study |
| B. breve UCC2003ΔnagA1ΔnagA2 | nagA1, nagA2 double deletion mutant of UCC2003 | This study |
| UCC2003PK1 | B. breve UCC2003 harbouring pPKCM Cmr | 21 |
| UCC2003ΔnagA1 + 2PK1 | B. breve UCC2003ΔnagA1ΔnagA2 harbouring pPKCM Cmr | This study |
| B. bifidum ATCC29521(PRL2010) | B. bifidum type strain | ATCC |
| B. bifidum PAM5 | B. bifidum ATCC29521 harbouring pAM5 Tetr | This study |
| Plasmids | ||
| pBS423Δrep | 4.4 kb, E. coli- vector, ΔpMB1, ori pTB4 ori repA Specr | 30 |
| pRTB101 | 7.3 kbp, E. coli-Bifidobacterium shuttle vector, pMB1 ori pTB4 ori repA | 30 |
| pPKCM | pCIBA089-pSK-Cmr | 31 |
| pAM5 | pBC1-puC19-Tetr | 32 |
ATCC’ American type culture collection.
Nucleotide sequence analysis
Sequence data were obtained from the Artemis-mediated20 genome annotations of the B. breve UCC200321. Database searches were performed using non-redundant sequences accessible at the National Centre for Biotechnology Information internet site (http://www.ncbi.nlm.nih.gov) using Blast.
DNA manipulations
Chromosomal DNA was isolated from B. breve UCC2003 as previously described22. Minipreparation of plasmid DNA from E. coli or B. breve was achieved using the Qiaprep spin plasmid miniprep kit (Qiagen GmBH, Hilden, Germany). For B. breve an initial lysis step was included whereby cells are incubated in lysis buffer containing 30 mg ml−1 of lysozyme for 30 minutes at 37 °C. Single stranded oligonucleotide primers used in this study were synthesized by Eurofins (Ebersberg, Germany). Standard PCRs were performed using TaqPCR mastermix (Qiagen), while high fidelity PCR was achieved using Q5 DNA polymerase (New England Biolabs, Ipswich, MA, United States). B. breve colony PCRs were performed as described previously (O’Connell Motherway et al.17). PCR fragments were purified using the Roche high pure PCR purification kit (Roche, Hilden, Germany). Electroporation of plasmid DNA into E. coli was performed as described by Sambrook et al.19 and into B. breve UCC2003 as described by Maze et al.23.
Construction of B. breve UCC2003 deletion mutants
Isogenic non-polar deletion mutants of nagA1 (Bbr_0846), nagA2 (Bbr_1247) or a double deletion strain harbouring deletions in both nagA1 and nagA2, with 846 bp of the 1245 bp of nagA1, or 969 bp of the 1278 bp of nagA2 deleted, were created using pBS423Δrep constructs and generated by the splicing by overlap extension (SOEing) PCR procedure24. In each case primers SOE AB and SOE CD (Table 4) were used to amplify regions flanking the sequence to be deleted using genomic DNA of B. breve UCC2003 as template. The resulting products, designated I or II were purified, mixed in a 1:1 ratio and used as template with primers SOE EF. The resulting product was digested with Pst1 and ligated to similarly digested pBS423Δrep prior to transformation into E. coli EC101 by electroporation. Transformants were selected based on resistance to Km and Spec, and screened by colony PCR using primers pBSF and pBSR to identify clones harbouring the correct insert. The presence of the correct insert in a number of positive clones was confirmed by plasmid isolation and restriction analysis, while the sequence integrity of the cloned DNA fragment and the orientation of the insert in the pBS423Δrep vector was confirmed by sequencing. First crossover insertion mutations were generated essentially as described previously25 to produce B. breve UCC2003 derivatives that were designated UCC2003-nagA1-(I), UCC2003-nagA1-(II), or UCC2003-nagA2-(I), respectively where I or II indicate that the first crossover occurred via fragment I or II (described above). Site-specific recombination in potential spec-resistant mutant isolates was confirmed by colony PCR using primer combinations specFw and specRv to verify Specr-encoding gene integration, and primers nagA1SOE A or nagA2SOE A (positioned upstream of the selected flanking regions of nagA1 or nagA2 respectively), each in combination with pBSR or to confirm integration at the correct chromosomal location. To promote pBS423Δrep plasmid excision in UCC2003-nagA1-(I), UCC2003-nagA1-(II), or UCC2003-nagA2-(I) the incompatible plasmid pRTB101-CM was introduced into each strain and transformants were selected on RCA supplemented with CM. CM resistant colonies were subcultured for eight transfers to promote loss of integrated pBS423Δrep. Cells which had excised pBS423Δrep and had either reverted to the wild type genotype, or harboured a nagA1 or nagA2 deletion, were selected based on Cmr Specs. Screening of Specs colonies for UCC2003 derivatives harbouring nagA1 or nagA2 deletion was performed by colony PCR using primer pairs nagA1SOEA and nagA1SOEB or nagA2SOEA and nagA2SOEB, respectively, and sequencing of the PCR products to confirm the inframe deletion. Curing of pRTB101-CM from B. breve deletion mutant strains was performed by subculturing at 42 °C for 8 transfers followed by plating on RCA and screening for CM sensitive mutant strains by replica plating. Construction of the double deletion strain B. breve UCC2003ΔnagA1ΔnagA2 was performed sequentially whereby the deletion strain UCC2003ΔnagA2 was constructed first and this strain was used as host for construction of a deletion of nagA1.
Table 4.
Oligonucleotide Primers used in this Study.
| Purpose | Primer | Sequencea |
|---|---|---|
| Construction of UCC2003 nagA1 deletion | nagA1SOE A | catctggtgctgctcgctttcg |
| nagA1SOE B | agtgagcagcacgtcggcggccgccacatcgatgccatccg | |
| nagA1SOE C | cggatggcatcgatgtggcggccgccgacgtgctgctcac | |
| nagA1SOE D | ctcaaggctgcgatcgacatg | |
| nagA1SOE E | cgctcactgcagcacccgcacgccacgatcatc | |
| nagA1SOE F | atctccctgcagctcaaggctgcgatcgacatg | |
| Construction of UCC2003 nagA2 deletion | nagA2SOE A | gcatccgcacgccacgattatc |
| nagA2SOE B | caccaaacctgttcgaccgtccaatagccaggagtcaggattcg | |
| nagA2SOE C | cgaatcctgactcctggctattggacggtcgaacaggtttggtg | |
| nagA2SOE D | gatctacggcatcaatgagc | |
| nagA2SOE E | aactgcctgcagcgctacgcatacacccacaag | |
| nagA2SOE F | atctccctgcagatcattcgttcctgtgcgctttg | |
| pBS423 multiple cloning site primers | pBSF | cgttacgttattagttat |
| pBSR | gtaatacgttcgtgtcgcg | |
| Amplification of spectinomycin resistance cassette | specFw | gtcgtcgtatctgaacc |
| specRv | gataactacgaactgctaac |
aRestriction sites incorporated into oligonucleotide primer sequences are indicated in bold.
Mother-to-pup transmission of B. bifidum and B. breve
All experiments using mice were approved by the University College Cork animal ethics committee and all methods were performed in accordance with the relevant guidelines and regulations. Twelve-week-old pregnant female, germ-free C57BL/6 mice were housed in flexible film gnotobiotic isolators under a strict 12 h light cycle. Mice were fed an autoclaved standard polysaccharide-rich mouse chow diet. At approximately 11 days of gestation two groups of mice (n = 7 per group) were inoculated with 1 × 109 cfu of B. bifidum PAM5 and B. breve UCC2003PK1, or B. bifidum PAM5 and B. breve UCC2003∆nagA1 + 2PK1 in 20 μl of PBS by oral pipetting whereby the inoculums are delivered by positioning a micropipette tip immediately behind the incisors. Five mice were maintained as uninoculated controls to monitor the germ free status of the facility. Fecal pellets were collected weekly to determine the density of bacterial colonisation of each strain based on colony forming units. At approximately 9 days after inoculation the first litter of pups was born and all litters were born within the following 5 days. Half of each dam-reared litter was sacrificed at each of two time points, while the mice were exclusively dam-fed, the first timepoint was at postnatal day 8–11, with the second four days later when mice aged between 12 and 16 days (Table 1). The stomachs of all culled pups were inspected to ensure no chow particles were present. Enumeration of bifidobacterial strains in the caecum and large intestine of each pup was performed using antibiotic selection and plate counting. Enumeration of the bifidobacterial strains in the caecum and large intestine of each mother was also determined at the end of the trial period.
Statistical analysis
Results are presented as mean +/− SEM. Findings were statistically evaluated using one-way Anova with Dunnett’s post-test. The p value, p < 0.0001, p < 0.001 or p < 0.01 is indicated by three, two or one stars (*), respectively as appropriate. ns indicates non-significant.
Transcriptional profiling of B. breve UCC2003 and UCC2003∆nagA1 + 2PK1
Large intestine samples from each group of neonatal pups were snap frozen in liquid nitrogen. After thawing in RNA protect solution each tissue sample was homogenised to separate the luminal contents from the mucosa, and centrifuged (1,200 × g for 10 sec) to remove the tissue fragments. The resulting bacterial supernatants were subjected to bacterial cell lysis and RNA isolation as described previously21,26. cDNA from bacterial mRNA was synthesized using the cDNA synthesis and labelling kit DSK-001 (Kreatech, Amsterdam, Netherlands) according to the manufacturer’s instructions and labelled with Cy3 or Cy5 using Cy3-ULS and Cy5-ULS from the cDNA synthesis and labelling kit DSK-001 (Kreatech). DNA-microarrays containing oligonucleotide primers representing each of the 1864 annotated genes on the genome of B. breve UCC2003 were designed by and obtained from Agilent Technologies (Palo Alto, Ca., USA). Labelled and amplified cDNA was hybridized using the Agilent Gene Expression hybridization kit (part number 5188–5242) as described in the Agilent Two-Color Microarray-Based Gene Expression Analysis (v4.0) manual (publication number G4140–90050). Notably, we do not see cross hybridisation of B. bifidum cDNA with the B. breve oligos on these arrays9. Following hybridization, microarrays were washed were washed in accordance with Agilent’s standard procedures and scanned using an Agilent DNA microarray scanner (model G2565A). Generated scans were converted to data files with Agilent’s Feature Extraction software (Version 9.5). DNA-microarray data were processed as previously described27. Differential expression tests were performed with the Cyber-T implementation of a variant of the t-test28. A gene was considered differentially expressed when p < 0.001 and an expression ratio of >2 or <0.33 relative to the control. The microarray data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE110077.
Electronic supplementary material
Acknowledgements
This work was supported by Science Foundation Ireland (SFI) (grant no. SFI/12/RC/2273) awarded to APC Microbiome Ireland, and HRB postdoctoral fellowship (grant no. PDTM/2011/9) awarded to M.O.C.M. The authors would like to thank Glycom A/S (Lyngby, Denmark) for the provision of purified HMO samples used in this study under their University donation program.
Author Contributions
M.O.C.M. concieved the experiments. M.O.C.M. conducted the experiments. F.O.B., T.O.D. and P.G.C. assisted with the animal experiments. All authors analysed the results and contributed to writing the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-29034-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hidalgo-Cantabrana, C. et al. Bifidobacteria and Their Health-Promoting Effects. Microbiol Spectr. 5(3) (2017). [DOI] [PMC free article] [PubMed]
- 2.Matsuki T, et al. A key genetic factor for fucosyllactose utilization affects infant gut microbiota development. Nat Commun. 2016;24(7):11939. doi: 10.1038/ncomms11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill CJ, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5(1):4. doi: 10.1186/s40168-016-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sela DA, et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci USA. 2008;105(48):18964–18969. doi: 10.1073/pnas.0809584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turroni F, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci USA. 2010;107(45):19514–19519. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido D, et al. A novel gene cluster allows preferential utilization of fucosylated milk oligosaccharides in Bifidobacterium longum subsp. longum SC596. Sci Rep. 2016;19(6):35045. doi: 10.1038/srep35045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunesova V, Lacroix C, Schwab C. Fucosyllactose and L-fucose utilization of infant Bifidobacterium longum and Bifidobacterium kashiwanohense. BMC Microbiol. 2016;16(1):248. doi: 10.1186/s12866-016-0867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan M, O’Connell Motherway M, Ventura M, van Sinderen D. Metabolism of sialic acid by Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2014;80(14):4414–4426. doi: 10.1128/AEM.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan M, et al. Cross-feeding by Bifidobacterium breve UCC2003 during co-cultivation with Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC Microbiol. 2014;14:282. doi: 10.1186/s12866-014-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James K, O’Connell Motherway M, Bottacini F, van Sinderen D. Bifidobacterium breve UCC2003 metabolises the human milk oligosaccharides lacto-N-tetraose and lacto-N-neo-tetraose through overlapping, yet distinct pathways. Sci Rep. 2016;6:38560. doi: 10.1038/srep38560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan M, Jiang H, O’Connell Motherway M, Oscarson S, van Sinderen D. Glycosulfatase-Encoding Gene Cluster in Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2016;82(22):6611–6623. doi: 10.1128/AEM.02022-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akkerman R, Faas MM, de Vos P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation. Crit Rev Food Sci Nutr. 2018;15:1–12. doi: 10.1080/10408398.2017.1414030. [DOI] [PubMed] [Google Scholar]
- 13.Smilowitz JT, Lebrilla CB, Mills DA, German JB, Freeman SL. Breast milk oligosaccharides: structure-function relationships in the neonate. Annu Rev Nutr. 2014;34:143–169. doi: 10.1146/annurev-nutr-071813-105721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacheco AR, Barile D, Underwood MA, Mills DA. The impact of the milk glycobiome on the neonate gut microbiota. Annu Rev Anim Biosci. 2015;3:419–445. doi: 10.1146/annurev-animal-022114-111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrecht S, et al. A comparative study of free oligosaccharides in the milk of domestic animals. Br J Nutr. 2014;111(7):1313–1328. doi: 10.1017/S0007114513003772. [DOI] [PubMed] [Google Scholar]
- 16.Prieto PA, et al. Remodeling of mouse milk glycoconjugates by transgenic expression of a human glycosyltransferase. J Biol Chem. 1995;270(49):29515–9. doi: 10.1074/jbc.270.49.29515. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell Motherway M, et al. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2008;74(20):6271–9. doi: 10.1128/AEM.01169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Man JC, Rogosa A, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- 19.Sambrook, J., Fritsch, E. F., & Maniatis, T. Molecular cloning a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y (1989).
- 20.Rutherford K, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;10:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 21.O’Connell Motherway M, et al. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA. 2011;108(27):11217–11222. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Riordan, K. Studies on antimicrobial activity and genetic diversity of Bifidobacterium species: molecular characterization of a 5.75 kb plasmid and a chromosomally encoded recA gene homologue from Bifidobacterium breve. Ph.D. National University of Ireland, Cork, Cork (1998).
- 23.Maze A, O’Connell-Motherway M, Fitzgerald GF, Deutscher J, van Sinderen D. Identification and characterization of a fructose phosphotransferase system in Bifidobacterium breve UCC2003. Applied and Environmental Microbiology. 2007;73:545–553. doi: 10.1128/AEM.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 25.O’Connell Motherway M, O’ Driscoll J, Fitzgerald GF, van Sinderen D. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003. Microb Biotechnol. 2009;2(3):321–332. doi: 10.1111/j.1751-7915.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokusaeva K, O’Connell-Motherway M, Zomer A, Fitzgerald GF, van Sinderen D. Characterization of two novel alpha-glucosidases from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2009;75:1135–1143. doi: 10.1128/AEM.02391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia De La Nava J, et al. Engene: the processing and exploratory analysis of gene expression data. Bioinformatics. 2003;19:657–658. doi: 10.1093/bioinformatics/btg028. [DOI] [PubMed] [Google Scholar]
- 28.Long AD, et al. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J Biol Chem. 2001;276:19937–19944. doi: 10.1074/jbc.M010192200. [DOI] [PubMed] [Google Scholar]
- 29.Law J, et al. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama Y, et al. Development of a double-crossover markerless gene deletion system in Bifidobacterium longum: functional analysis of the α-galactosidase gene for raffinose assimilation. Appl Environ Microbiol. 2012;78:4984–94. doi: 10.1128/AEM.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cronin M, Knobel M, O’Connell Motherway M, Fitzgerald GF, van Sinderen D. Molecular dissection of a bifidobacterial replicon. Appl Environ Microbiol. 2007;73:7858–7866. doi: 10.1128/AEM.01630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez-Martín P, O’Connell Motherway M, van Sinderen D, Mayo B. Functional analysis of the pBC1 replicon from Bifidobacterium catenulatum L48. Appl Microbiol Biotechnol. 2007;76:1395–1402. doi: 10.1007/s00253-007-1115-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.