Abstract
The calcium-activated chloride channel anoctamin-2 (Ano2) is thought to amplify transduction currents in olfactory receptor neurons (ORNs), a hypothesis supported by previous studies in dissociated neurons from Ano2−/− mice. Paradoxically, despite a reduction in transduction currents in Ano2−/− ORNs, their spike output for odor stimuli may be higher. We examined the role of Ano2 in ORNs in their native environment in freely breathing mice by imaging activity in ORN axons as they arrive in the olfactory bulb glomeruli. Odor-evoked responses in ORN axons of Ano2−/− animals were consistently larger for a variety of odorants and concentrations. In an open arena, Ano2−/− animals took longer to approach a localized odor source than Ano2+/+ animals, revealing clear olfactory behavioral deficits. Our studies provide the first in vivo evidence toward an alternative or additional role for Ano2 in the olfactory transduction cascade, where it may serve as a feedback mechanism to clamp ORN spike output.
Introduction
Each subtype of olfactory receptor neuron (ORN) converges on a few locations in the olfactory bulb (OB), and therefore serves as a distinct input channel to the brain. ORNs generate electrical signals, in the form of action potentials (spikes), that are interpreted by postsynaptic cells in the OB1, including local and projection cells. The series of molecular events that coordinate olfactory transduction and spike generation have been well-delineated, yet much remains unknown about how each individual step in the transduction cascade contributes to overall ORN excitation and output. Specifically, the role of the calcium-activated chloride channel anoctamin-2 (Ano2; also called TMEM16B) remains controversial: many studies point toward its role in massively amplifying ORN transduction currents2–8, while paradoxically limiting ORN spike output9. In addition, there is conflicting evidence for its importance in olfactory behaviors8–10.
Odorants drawn into the nasal cavity bind to odorant receptors (ORs) on the cilia of ORNs. A large number (but not all) of these ORs are G-protein coupled receptors11–13, which trigger an intracellular signaling cascade leading to the opening of cyclic nucleotide-gated channels and a net inward flux of Na+ and Ca2+ ions. The increased intracellular abundance of Ca2+ then activates the calcium-activated chloride channel (CaCC) Ano29,14,15. As a result of the elevated intracellular Cl− concentration in ORNs and lower Cl− concentration extracellularly in the nasal epithelium16–18, negatively-charged Cl− anions flow outward16,18, resulting in an amplification of ORN membrane depolarizations19. As much as 90% of the total ORN transduction current may be mediated by Ano23,7, making it a critical component in the sensory transduction pathway that leads to OB input.
Surprisingly, despite its large contribution to the generation of olfactory transduction currents, recent work has suggested that Ano2 is not necessary for odor detection and discrimination8. Even the nature of the contribution of Ano2 to ORN activity has become uncertain, since a recent study has suggested that Ano2 may function to limit overall ORN excitability by contributing to a potent depolarization-block of Na+ channels9. Further investigation of the role that Ano2 plays in olfactory transduction is necessary to understand the precise mechanisms by which odor-evoked excitatory signals are transmitted to the brain.
An additional consideration in the function of Ano2 in olfactory transduction is its expression pattern within ORNs. There is clear evidence that, in addition to being expressed at their cilia11,20 within the olfactory epithelium, Ano2 is also abundantly expressed in ORN axons as they terminate in their respective glomeruli8. This raises the important possibility that differences between the extracellular Cl− concentration in nasal mucosa/epithelium and the brain may result in Ano2 playing distinct roles in olfactory transduction in different sub-cellular compartments.
How Ano2 contributes to ORN spike output in the native environment of an intact animal, especially at the axon terminals in the OB, has not been addressed. Here, we examine the role of Ano2 in the transmission of odor information to the brain by examining stimulus-evoked responses in ORN terminals in the olfactory bulb of mice with and without Ano2.
Results
Glomerular odor maps are unaltered in Ano2−/− animals
The axons of ORNs of a common subtype coalesce at a few glomeruli on the surface of the OB. The fasciculation of ORN axon bundles as they enter their respective glomeruli is an activity dependent process21–23 and could in principle be affected by changes in spontaneous, as well as overall ORN excitability in mice lacking Ano2. Past studies provide conflicting evidence as to whether Ano2 is required for proper targeting of ORNs to glomeruli, with one study reporting that glomerular positioning and number is unaffected in Ano2−/− mice for two ORN receptor subtypes8, while yet another study found an increase in the number of glomeruli incorporating axons of ORNs that express the I7 receptor9. We tested whether loss of Ano2 alters functional glomerular maps in the OB.
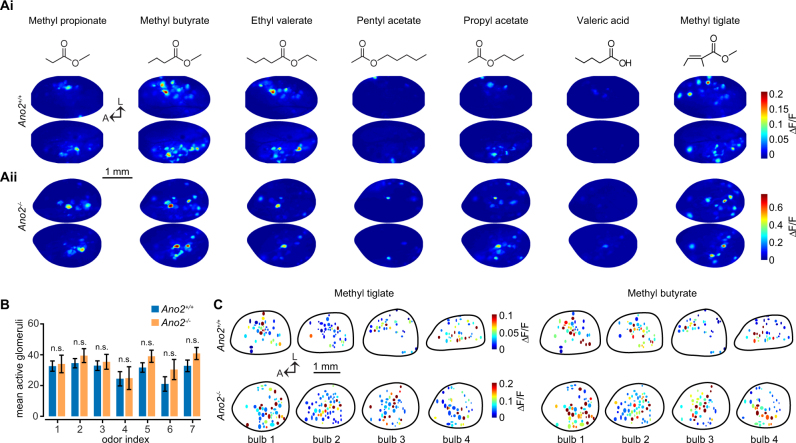
We crossed heterozygous Ano2−/+ mice24 with a mouse strain that expresses the Ca2+ indicator GCaMP3 in all ORNs (OMP-GCaMP)25 to obtain two groups of mice, Ano2−/−/OMP-GCaMP3 and Ano2+/+/OMP-GCaMP3. We refer to these two groups as Ano2−/− and Ano2+/+ for simplicity throughout this study. First we used wide-field epifluorescence imaging to obtain functional maps of activated glomeruli for seven monomolecular odors by measuring odor-evoked increases in GCaMP fluorescence at ORN axon terminals26. These odors (see Methods) were selected to activate a diverse number and range of glomeruli on the dorsal surface of the mouse OB27.
We compared the total number of glomeruli at each bulb that responded to each odor in our panel for Ano2+/+ and Ano2−/− animals. We used receiver operating characteristic (ROC) analysis to determine a threshold to define responsive glomeruli, by comparing the response distribution obtained from all glomerulus-odor pairs to a noise distribution obtained from interleaved blank odor trials in each experiment. An area under the receiver operating curve analysis was performed and the lowest threshold yielding 10 responses for every no odor response was chosen. (threshold = 0.005 ∆F/F; Supplemental Fig. 1). There was no significant difference between the number of active glomeruli per OB in Ano2+/+ and Ano2−/− animals for any of the odors (Fig. 1B; p > 0.05, Wilcoxon rank-sum test with Bonferroni multiple comparison correction).
Figure 1.
Functional maps of ORN activity. Ai-Aii. Example functional maps for seven unique odors in a representative Ano2+/+ (top) and Ano2−/− (bottom) animal. Molecular structures of the odors in the panel are depicted above. (B) Number of glomeruli per bulb (n = 11 Ano2+/+ bulbs, n = 5 Ano2−/− bulbs) responding to each of the seven odors above ROC threshold (threshold = 0.005 ΔF/F; p > 0.05, Wilcoxon rank-sum test with Bonferroni multiple comparison correction). (C) Example functional maps from four Ano2+/+ and Ano2−/− bulbs each for two different, but related odors. Glomeruli are represented as pseudocolored ellipses. The position of each ellipse reflects the location of a glomerulus within one olfactory bulb hemisphere. The black line designates the boundary of the imaged region of each olfactory bulb.
Although our experiments were not designed to identify specific glomeruli, we can nevertheless discount large scale glomerular duplication in Ano2−/− animals, for example due to a loss of targeting specificity and mixing of ORN axons within individual glomeruli28. We also cannot discount small-scale redistribution of glomerular positioning, which is unlikely to exceed expected animal-to-animal variance described previously in control animals26,28. Together, these results indicate ORN maps are largely conserved in Ano2−/− animals and confirm previous anatomical studies8.
ORN input to glomeruli is enhanced in Ano2−/− animals
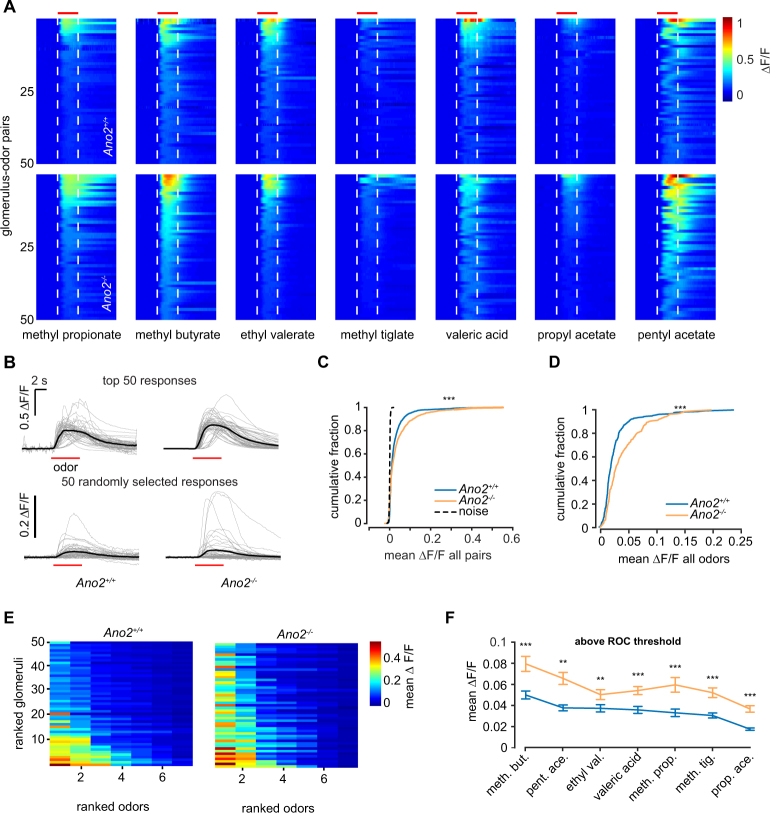
We next asked whether odor-evoked responses in individual glomeruli were altered in magnitude in Ano2−/− animals. From six Ano2+/+ animals (n = 9 bulbs) and three Ano2−/− animals (n = 5 bulbs), we identified 34.44 ± 3.48 and 43.60 ± 3.93 glomeruli per bulb, respectively (299 total glomeruli in Ano2+/+ animals and 218 total glomeruli in Ano2−/− animals). The 50 largest responses for each odor are shown in Fig. 2A. In addition, traces for the largest responses as well as for randomly chosen responses are shown in Fig. 2B. Our analysis revealed significantly larger odor-evoked responses in ORNs of Ano2−/− animals than Ano2+/+ animals (n = 2093 Ano2+/+ and 1526 Ano2−/− glomerulus-odor pairs, p < 0.001 Kolmogorov–Smirnov test; Fig. 2C). This difference was observed both when responses of each glomerulus were averaged across all seven odors (n = 299 Ano2+/+ glomeruli and 218 Ano2−/− glomeruli, p < 0.001, Kolmogorov–Smirnov test), and when responses of all glomeruli for each odor were averaged (Fig. 2D–F).
Figure 2.
Odor responses in Ano2−/− and Ano2+/+ mice. (A) The 50 largest odor-evoked Ca2+ signals across all animals for each of the seven odors in both groups. Dashed lines and red bar indicate odor delivery period. Data are sorted by the largest mean response during odor delivery. (B) Traces of the 50 largest (top) Ca2+ responses for Ano2+/+ and Ano2−/− animals across all odors and 50 randomly selected responses (bottom). (C) Cumulative distribution of the mean Ca2+ response in the odor period across all glomerulus-odor pairs (n = 2093 Ano2+/+ and 1526 Ano2−/− glomerulus-odor pairs, p < 0.001 Kolmogorov–Smirnov test). (D) Cumulative distribution of the mean Ca2+ response across all odors at each glomerulus (n = 299 Ano2+/+ glomeruli and 218 Ano2−/− glomeruli, p < 0.001, Kolmogorov–Smirnov test). (E) The top 50 glomeruli ranked by mean response across all odors and further ranked by individual odor responses for Ano2+/+ (right) and Ano2−/− (left) animals. (F) Mean response of all glomeruli responding above ROC-defined threshold (threshold = 0.005 ΔF/F, Wilcoxon rank-sum test with Bonferroni correction, *p < 0.05, **p < 0.01, ***p < 0.001).
Our data provide evidence that loss of Ano2 results in enhanced ORN input to OB glomeruli. We also observed that the number of glomeruli responding to an odor was similar in Ano2+/+ and Ano2−/− animals. These results are consistent with a scaling mechanism, where Ano2 may function as a negative feedback on ORN excitability and limit the number of action potentials generated in response to odor stimulation.
Loss of Ano2 does not impact respiration
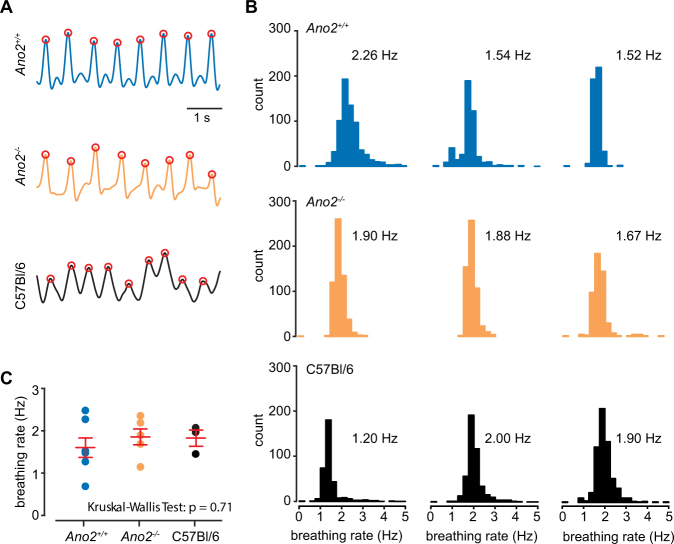
It is possible that the larger responses to odorants in Ano2−/− animals is due to faster respiration rates and temporal summation of responses29. Other ANO isoforms are expressed in smooth muscle tissue and may regulate the excitability of the diaphragm and the airway30–33, thereby altering normal breathing rhythms in Ano2−/− animals. We recorded the respiration rate of Ano2+/+, Ano2−/−, and C57BL/6 J mice using a thermocouple placed in front of the animal’s nose, under anesthesia conditions consistent with our previous experiments. We first validated the reliability of the external thermocouple for respiration tracking by comparing it to a well-established method, intranasal cannulas (Supplemental Fig. 2). Upon validation, we chose to record respiration using the non-invasive thermocouple to mitigate any effects of damaging the nasal cavity through cannula implantation.
Robust respiration signals could be recorded in anesthetized mice (n = 6 Ano2+/+, 7 Ano2−/−, 3 C57BL/6 J; Fig. 3A–B for examples). We included C57BL/6 J animals in our comparison to rule out any genetic background effects34. Despite slight animal-to-animal variability, we found that across all groups, there was no statistical difference in the overall respiration frequency (mean frequency: 1.60 ± 0.17 Hz Ano2+/+, 1.86 ± 0.23 Hz Ano2−/−, 1.77 ± 0.19 Hz C57BL/6 J, p = 0.71 Kruskal-Wallis test; Fig. 3C). The small, statistically insignificant increase in respiration rate we observed in Ano2−/− animals (16.3% increase) is insufficient to account for the larger Ca2+ responses obtained in our previous imaging experiments (p < 0.001, Kolmogorov–Smirnov test).
Figure 3.
Loss of Ano2 does not impact respiration rate. (A) Example respiration traces from a Ano2+/+, Ano2−/− and C57BL/6 J animals recorded with a thermocouple placed near the animal’s nose (see Supplemental Fig. 2 for technique validation). (B) Histograms of the instantaneous respiration frequency in a 5-minute window from three representative animals from each group. Mean instantaneous frequency is displayed next to each plot. (C) Mean instantaneous frequency for all animals in each group. Red bars denote mean and standard error across all animals (p = 0.71, Kruskal-Wallis test).
We conclude that anesthetized Ano2−/− animals do not breathe with increased frequency and that the larger Ca2+ signals we observed are not due to enhanced respiration rate.
Multiphoton imaging in Ano2−/− animals
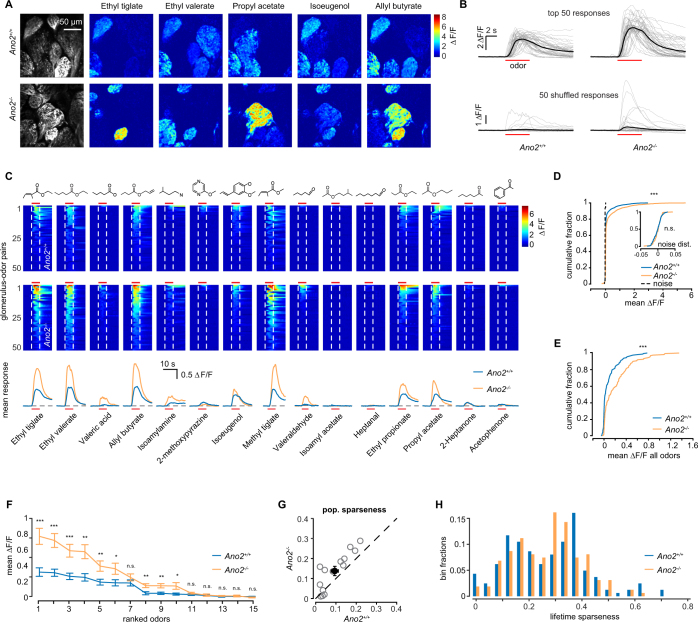
Due to the low resting fluorescence of GCaMP3 we were unable to identify glomeruli that did not respond to at least one of the seven odors in our panel using an epifluorescence microscope. We used multiphoton microscopy to overcome this limitation and were able to visualize all glomeruli, independent of their responsiveness (Fig. 4A). We also expanded our odor panel size to 15 odors to activate a wider range of glomeruli. The optical sectioning facilitated by multiphoton microscopy also allowed us to exclude any effects arising from the activity of en passant axons that could be detected using our epifluorescence setup. As a result of significant out-of-focus fluorescence in wide-field imaging, signal contamination could arise from Ca2+ activity in axons that pass above inactive glomeruli.
Figure 4.
Calcium responses in ORNs measured with multiphoton microscopy. (A) Example multiphoton-acquired images of glomeruli from an example Ano2+/+ and Ano2−/− animal, as well as example ΔF/F responses for five selected odors. Mean images from 20 frames preceding and during the odor delivery period were used. (B) Traces of the 50 largest (top) Ca2+ responses for Ano2+/+ and Ano2−/− animals across all odors and 50 randomly selected responses (bottom). (C) The 50 largest odor-evoked Ca2+ signals across all animals for each of 15 odors. Dashed line denotes odor onset and offset and red bar indicates odor duration. Molecular structures are depicted above. Bottom, mean response time course for each odor. (D) Cumulative distribution of the mean Ca2+ response in the odor period across all glomerulus-odor pairs (n = 2430 Ano2+/+ and 2415 Ano2−/− glomerulus-odor pairs, p < 0.001, Kolmogorov–Smirnov test). Inset, distribution of blank odor trial responses used to determine ROC threshold (threshold = 0.02 n = 162 Ano2+/+ and 161 Ano2−/− glomeruli, p > 0.05, Kolmogorov–Smirnov test). (E) Cumulative distribution of the mean Ca2+ response across all odors at each glomerulus (n = 162 Ano2+/+ and 161 Ano2−/− glomeruli, p < 0.001, Kolmogorov–Smirnov test). (F). Mean response of all glomeruli responding above threshold for each odor (Wilcoxon rank-sum test with Bonferroni correction, *p < 0.05, **p < 0.01, ***p < 0.001). (G) Scatter plot of population sparseness for each odor. Mean across all odors is the filled black circle (mean sparseness = 0.09 ± 0.02 in Ano2+/+ and 0.13 ± 0.02 in Ano2−/−, p = 0.002, Wilcoxon sign-rank test). (H) Histogram of lifetime sparseness across all glomeruli (mean sparseness = 0.28 ± 0.01 in Ano2+/+ and 0.28 ± 0.01 in Ano2−/−, p = 0.89, Wilcoxon rank-sum test.
Across 162 Ano2+/+ and 161 Ano2−/− glomeruli from five animals each, we found that ORNs in Ano2−/− animals responded with significantly larger Ca2+ transients (Fig. 4B–F), whether comparing individual glomerulus-odor pairs (n = 2430 Ano2+/+ and 2415 Ano2−/− glomerulus-odor pairs, p < 0.001, Kolmogorov–Smirnov test; Fig. 4D), or mean response across all odors (n = 162 Ano2+/+ and 161 Ano2−/− glomeruli, p < 0.001, Kolmogorov–Smirnov test; Fig. 4E). The 50 largest overall responses for each group are displayed in Fig. 4B (mean of all responses above threshold: 38.8 ± 0.02% ∆F/F in Ano2+/+ and 57.5 ± 0.03% ∆F/F in Ano2−/−). When considering each odor individually, in 9 out of 15 odors we observed significantly larger Ca2+ responses in Ano2−/− animals (Fig. 4H, Wilcoxon rank-sum test with Bonferroni correction).
We next compared the kinetics of the Ca2+ responses in Ano2+/+ and Ano2−/− animals. Due to the improved signal to noise ratio and optical sectioning of multiphoton microscopy, we were able to compare the responses of individual trials rather than the means of replicates from the same glomerulus. This procedure reduced the influence of respiration, which dictates the response onset. We first characterized the rise time. On average, Ano2−/− animals responded with a slightly faster rise time than Ano2+/+ animals (2.35 ± 0.05 s in Ano2+/+, n = 2614, and 2.25 ± 0.05 s in Ano2−/−, n = 3393, p < 0.001 Kolmogorov–Smirnov test). We also found that the odor responses in Ano2−/− animals decayed at a slightly faster rate than Ano2+/+ animals (decay time constant = 4.74 ± 0.08 s in Ano2+/+, n = 2376, and 4.51 ± 0.06 s in Ano2−/−, n = 3155, p = 0.004 Kolmogorov–Smirnov test). Our data indicate that Ano2 deletion may result in spike generation in ORNs within a narrower window following stimulus delivery, resulting in both a quicker rise and decay in Ca2+ responses. However, the slow timescale of GCaMP3 and our population imaging approach leave open the possibility that more dramatic differences may be observed on the single cell level.
Lastly, we compared sparsity of glomerular responses. We calculated the population sparseness (see Methods) to compare the fraction of activated glomeruli across all animals for each odor. Population sparseness is related to the fraction of glomeruli that are activated by a given odor stimulus, with values near one indicating uniform activity across all glomeruli and values near zero indicating highly selective responses across all observed glomeruli. We found that a larger fraction of glomeruli responded in Ano2−/− animals (population sparseness measure: 0.09 ± 0.02 in Ano2+/+ and 0.13 ± 0.02 in Ano2−/−, p = 0.002, Wilcoxon sign-rank test, Fig. 4G). We found no differences between Ano2+/+ and Ano2−/− animals in lifetime sparseness, which quantifies the extent to which a given glomerulus responds to different odor stimuli (lifetime sparseness measure: 0.28 ± 0.01 in Ano2+/+ and 0.28 ± 0.01 in Ano2−/−, p = 0.89, Wilcoxon rank-sum test, Fig. 4H). If all odors activate the observed glomerulus uniformly, the lifetime sparseness measure will be close to one, and if a glomerular response is specific to only a small number of odors, the measure will be close to zero. Together these results indicate that ORNs in Ano2−/− animals are indeed more sensitive to odor stimulation, but the breadth of their odor tuning is unchanged. Furthermore, the fact that we observed no difference in odor tuning further argues against the possibility that glomeruli in Ano2−/− animals receive heterogeneous innervation from multiple ORN subtypes. We also found no evidence for heterogeneous responses within individual glomerular regions of interest.
ORNs are more strongly excited by odors across a range of concentrations
What accounts for the larger fraction of activated glomeruli in Ano2−/− animals? One possibility is that the signal-to-noise ratio afforded by multiphoton microscopy allowed us to identify weak responses arising from odor-receptor binding in Ano2−/− animals that are sub-threshold for Ca2+ signal generation in Ano2+/+ animals. Conversely, given our previous results, another explanation for the increased Ca2+ signal magnitude in Ano2−/− animals is that in response to high odor concentrations, ORNs are able to maintain firing due to a reduction in depolarization induced Na+ inactivation driven by the amplifying current through Ano2. However, at low odor concentrations, ORNs in Ano2−/− animals may have weaker responses than ORNs in Ano2+/+ animals, since it has been shown that current amplification through Ano2 is most potent close to detection threshold24. We next investigated whether ORNs in Ano2−/− animals are more responsive to odors at different concentrations.
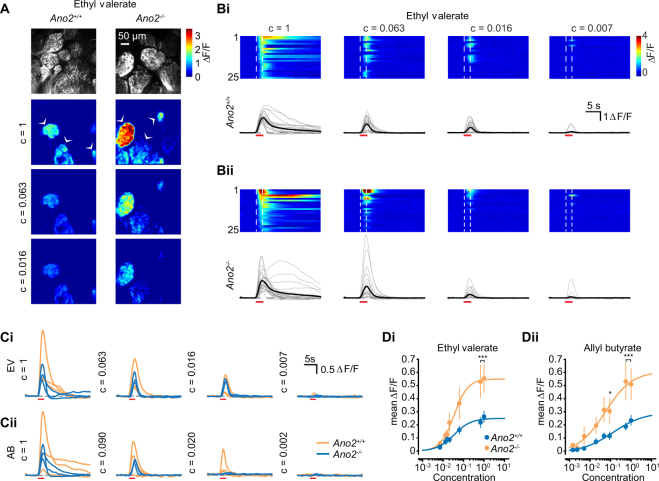
We used air dilution to alter odor concentration over four orders of magnitude for two odors, Ethyl valerate and Allyl butyrate, and decreased the odor delivery time to two seconds to prevent saturation of ORN responses at the highest concentrations. The relative concentration of each odor experienced by the animal was verified using a photoionization detector and odor concentrations were normalized to the lowest dilution (see Methods; Supplemental Fig. 3). At the strongest odor concentrations, glomerular ORN responses were again enhanced in Ano2−/− animals for both odors, as well as a mixture of the two (Fig. 5A–D, Wilcoxon rank-sum test). However, somewhat surprisingly, at low concentrations our analysis revealed no pair-wise differences. For low concentrations, the largest responses typically occurred in Ano2−/− animals, but the vast majority of response were typically within the range of those observed in Ano2+/+ animals, thereby limiting our ability to statistically differentiate the two groups (Supplemental Fig. 4). We were unable to compare the response amplitude of ORNs at their individual detection thresholds due to an inability to identify specific subtypes of ORNs (expressing a particular odorant receptor) across animals. We also note that Ca2+ signals in ORN axons report spike output rather than transduction current amplitudes. Therefore, our results indicate that while Ano2 may play a role in shunting ORN excitation following strong odor stimulation, it may have less of an effect on ORN activity following weak odor input.
Figure 5.
ORN responses in Ano2−/− animals are enhanced at high odor concentrations. (A) Example multiphoton-acquired images of glomeruli and ΔF/F responses at three odor concentrations. Odor concentrations were normalized to the highest concentration (~10% v/v) using a photoionization detector (see Supplemental Fig. 4). (Bi-ii) Examples of the 25 largest responses to the highest concentration of Ethyl valerate followed through four other concentrations for Ano2+/+ (top) and Ano2-/- (bottom) animals. Individual traces are displayed below. Dashed line denotes odor onset and offset, red bar indicates odor period. (Ci-ii) Example traces from three glomeruli in part A, identified by arrowheads followed through four concentrations of Ethyl valerate (EV; top) and Allyl butyrate (AB; bottom) Di-ii. Mean response (filled circles) at eight odor concentrations for both odors and sigmoidal fit to each (Wilcoxon rank-sum, *p < 0.05, **p < 0.01, ***p < 0.001).
Ano2 deletion increases latency to odor localization
Does increased ORN excitability alter odor detection capabilities of Ano2−/− animals? Recent studies provide evidence that Ano2−/− animals exhibit a greater latency to uncover a hidden food-object9,10, while another study was unable to find any difference in odor detection and discrimination using a learned behavior8. We decided to study innate odor-driven investigation to assess whether Ano2−/− animals displayed any sensory deficit independent of learned behavior.
We investigated the latency to explore odors as an indicator of how easily animals can detect odors9,10. To minimize experimenter-induced biases, our automated experimental apparatus consisted of a 56 cm diameter circular arena with four air inlets equally spaced around its circumference, as well as a vacuum in its center to balance air inflow and outflow. Under infrared illumination, mice were allowed to explore the arena space for 10 minutes, after which odorized air was delivered through one of the inlets. We then measured the latency and path taken by each animal to investigate the odor source, as determined by the animal approaching the odorized air inlet within 1 cm (Fig. 6A).
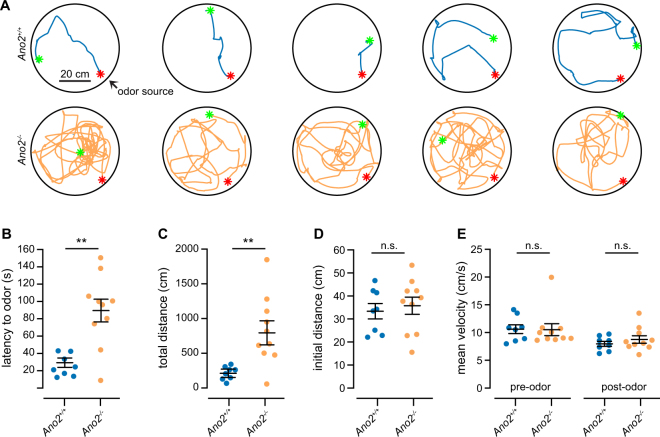
Figure 6.
Latency to odor localization is increased in Ano2−/− animals. (A) Examples of five Ano2+/+ and Ano2−/− animals tracking to the source of odorized air carrying a 1% dilution of peanut oil. Green and red asterisks mark the initial and final position of each animal. (B) Time latency for animals to locate the odor source (n = 8 Ano2+/+ and 10 Ano2−/− animals, p = 0.003, Wilcoxon rank-sum test). (C) Total distance traveled before finding the odor source across all animals (p = 0.002, Wilcoxon rank-sum test). (D) Initial distance from odor source (at odor onset) across all animals (p > 0.05, Wilcoxon rank-sum test). (E) Mean velocity of each animal prior to and following odor onset (p > 0.05, Wilcoxon rank-sum test).
We used the known appetitive odor peanut oil35 diluted to the same concentration as we used in our imaging experiments (1% in mineral oil). Consistent with previous reports9,10, across 8 Ano2+/+ and 10 Ano2−/− animals, we found that Ano2−/− animals required significantly more time to locate the odor source (Ano2+/+ = 29.28 ± 5.75 s, Ano2−/− = 94.71 ± 16.47 s, p = 0.003, Wilcoxon rank-sum test; Fig. 6B). Ano2−/− animals also traveled a significantly longer distance before ultimately arriving at the odor source (Ano2+/+ = 178.41 ± 28.46 cm, Ano2−/− = 822.43 ± 175.95 cm, p = 0.002, Wilcoxon rank-sum test; Fig. 6C) Because the odor onset occurred independently of the animal location in the arena, we calculated the initial starting distance from the odor source and observed no differences in their mean initial positions (Ano2+/+ = 30.71 ± 4.68 cm, Ano2−/− = 37.78 ± 3.58 cm, p = 0.002 Fig. 6D, p > 0.05, Wilcoxon rank-sum test). At the same time, we observed no differences in the locomotor activity of Ano2−/− animals as measured by their mean velocity both prior to and following odor delivery (Fig. 6E, p > 0.05, Wilcoxon rank-sum test). These behavioral data suggest a puzzling dissociation between the increased responses to odorants in Ano2−/− animals and the longer latency to locate the source of an appetitive odor.
Discussion
Our study presents direct evidence in freely-breathing mice that Ano2, despite its role in amplifying transduction currents in ORNs, limits their overall excitation and input to the OB in vivo. Our results are in agreement with recent in vitro measurements of ORN spike output in Ano2−/− animals9 and further point towards a dual functionality of Ano2 in ORN excitability whereby it both amplifies transduction currents and limits spike output.
Glomerular maps and respiration
Loss of Ano2 in mice could lead to more general changes that might be confounding factors that undermine conclusions about sensory transduction and coding. First, absence of Ano2 might alter the anatomical organization of glomerular maps in the OB. In particular, spontaneous activity in ORNs is known to play an important role in ORN fasciculation and glomerular emergence21–23. Any differences in spontaneous activity between Ano2+/+ and Ano2−/− animals might lead to disorganized glomerular organization and odor representation. Our results argue against a broad topographical reorganization of ORN inputs to the OB in Ano2−/− animals based on two factors. First, we found that the number of dorsal glomeruli responding above threshold to a given odor was unchanged and second, we found no difference in the lifetime sparseness of individual glomeruli from Ano2+/+ and Ano2−/− animals. Furthermore, glomeruli in Ano2−/− animals do not appear to receive heterogeneous ORN innervation since responding glomeruli were invariably homogeneous. Our in vivo imaging methods restricted us to imaging the dorsal portion of the OB, and we cannot rule out the possibility that ORN targeting is disrupted in the medial, lateral or ventral OB in Ano2−/− mice.
A second factor that might affect the data on glomerular imaging is the respiration rate. Faster respiration may lead to larger Ca2+ signals because of slow time course of axonal Ca2+ as well as indicator kinetics. Direct measurement of respiration, however, dispelled this concern – we found no significant change in respiration rate in Ano2−/− animals. On a methodological note, we also demonstrated that an externally-placed, non-invasive thermocouple is a reliable method to record and measure breathing responses in anesthetized mice. While this method could be valuable for experiments in anesthetized animals, we note that it rapidly loses fidelity when breathing rate increases, as in awake animals (Supplemental Fig. 2E,F).
Larger odor-evoked responses in Ano2−/− animals
The major finding of our study is that the magnitude of the ORN Ca2+ responses following odor stimulation was larger in Ano2−/− animals, with no observable change in the overall response duration. This was confirmed in two different modes of imaging – widefield microscopy that allowed larger regions to be imaged at lower resolution, and multiphoton microscopy that offered excellent optical sectioning and signal-to-noise ratio. We systematically varied the concentration of two different odorants and found that responses in ORNs from Ano2−/− animals were consistently larger at most concentrations. Interestingly, at lower concentrations of the two odors, the response amplitudes were similar between both groups. This result suggests that for low odor concentrations, ORN transduction currents may remain sufficiently modest, and limits further amplification through Ano2. In such a scenario, ORN transduction currents may be primarily carried through cyclic nucleotide-gated channels upstream of Ano2, thereby generating small membrane depolarizations without engaging significant Ano2-mediated amplification.
Biophysical studies in vitro, however, indicate that Ano2 currents are activated even for weak stimuli10,36. In vitro preparations allow for titration of odor concentrations for each neuron, thus allowing careful analysis of transduction currents in different response regimes, including threshold and sub-threshold responses. Perhaps glomerular imaging does not have enough sensitivity to detect responses to low concentrations of odors, and potential differences between Ano2+/+ and Ano2−/− animals were missed. In vivo, threshold odor concentrations are likely to activate only a subset ORNs due to their dispersion throughout the nasal epithelium37. The population imaging approach used in our studies further decreases the likelihood of detecting low-level signals because any responses arising a small number of ORNs are averaged across all ORNs that terminate at a given glomerulus. An alternative approach may include sparsely labeling ORNs with Ca2+ indicators to allow for recordings of individual optically isolated axon terminals; however, to date there is no reliable method available for such an approach.
Another key result is that responses saturated at lower amplitudes in Ano2+/+ than in Ano2−/− glomeruli, suggesting that the presence of Ano2 had a “clamping” effect, and its absence loosens the clamp to allow greater activity. Our findings and work of others9, suggest that in a high odor concentration regime, Ano2 may function as a feedback mechanism to limit the number of spikes generated by ORNs following odor stimulation. The proposed mechanism of action operates through a potent depolarization-induced inactivation of Na+ channels following transduction current amplification by Ano29. A potential caveat is that the larger responses we observe in Ano2−/− animals are the result of lower resting fluorescence due to a decrease in spontaneous ORN activity at some glomeruli9, thereby increasing the dynamic range available for Ca2+ indicator activity. Our experiments used odor concentrations that are generally thought to be sub-saturating for odor evoked responses in ORNs; however, it remains possible that at these concentrations, the largest signals observed in Ano2+/+ animals exceeded the range of our indicator due to a greater basal Ca2+ tone. Our results here argue against this possibility since the only observable differences occurred in response to strong odor stimulation – larger responses in Ano2−/− animals would not be observed if ORN responses in Ano2+/+ were saturated at these higher concentrations.
It is also possible that Ano2 alters neural excitability in other ways, especially since Ano2 is expressed in ORN terminals8,38. For example, in the thalamocortical39 system Ano2 functions to suppress neural excitability by enhancing the magnitude of action potential after-hyperpolarization. In the hippocampus40, Ano2 decreases the duration of individual action potentials by relying on a chloride gradient that favors outward membrane currents close to resting potential41. Although the chloride gradient in the nasal epithelium favors inward currents at near resting potentials16,18, the presumed chloride gradient at ORN axonal terminals could yield outward currents through Ano2, triggered by inward flux of Ca2+ during action potentials. A reduction in ORN transduction currents at the olfactory cilia of Ano2−/− animals could be offset by a reduction in the action potential after-hyperpolarization in the axonal compartments of ORNs.
Independent of the mechanisms involved, Ano2 seems to functionally compress the dynamic range of odor responses in individual ORNs by leaving weak responses unaffected (or enhancing them) and truncating the magnitude of responses to strong odors.
Role of Ano2 in olfactory coding
Our data suggests that currents through Ano2 may serve as a negative feedback mechanism to prevent excessive activation at higher concentrations. It remains possible that at low to moderate concentrations, Ano2 may act to amplify sensory signals and affect activity in ways undetected by our measurements. For instance, the latency to first spike (from the onset of inhalation) could be shorter in Ano2+/+ ORNs because of the amplification by Ano2. Such changes in timing could play an important role in odor coding42–44, but our imaging methods may not have the temporal resolution or sensitivity to detect such differences in latency. It is apparent that simply scaling up the activity in ORNs is insufficient enhance odor detection and may instead have deleterious effects.
Another potential role of Ano2 may arise from differences in expression of Ano2 in ORNs of a common subtype. Through varying expression levels, ORNs projecting to the same glomerulus could de-correlate their firing patterns in response to the same stimulus by shunting their spike output at different levels and thereby increasing their information carrying capacity as a population. Past studies demonstrate that intrinsic biophysical diversity between sister mitral cells functionally reduces correlations in their spike output45 and these observations are consistent in other systems including ganglion cells46 and M1 type ganglion cell photoreceptors47 in the retina. In our mouse line, all ORNs were labeled with GCaMP3 and we were therefore unable to study heterogeneity at the single cell level. However, future studies may seek to record from a small number of ORNs projecting to the same glomerulus to determine whether Ano2 plays a role in de-correlating their spike output. Furthermore, a decreased information carrying capacity of ORNs in Ano2−/− animals provides a potential explanation for the odor localization deficits observed in this study and others9,10. We observed that Ano2−/− animals, despite having larger odor-evoked ORN responses, require longer to locate a relatively low-concentration odor source. While our result is in agreement with recent behavioral analyses of Ano2−/− mice9,10, it stands in contrast to other reports that Ano2−/− mice have no detectable deficits in odor discrimination8. Notably, our paradigm takes advantage of innate odor-seeking behavior in mice rather than task performance following learning. One possibility is that Ano2−/− animals are able to overcome olfactory deficits through learning, thereby allowing for comparable performance when the experimental timescale is extended. Additional studies are necessary to explore potential differences in innate vs. learned olfactory behaviors, as well as any compensatory adaptations in Ano2−/− mice.
Our result suggests that deletion of Ano2 does not simply scale up the sensitivity of ORNs, but rather, results in a fundamental reformatting of how odor information is transmitted to the brain. At present it is not clear how odor information is restructured in ORNs of Ano2−/− animals, or if dysfunction in odor information processing is further compounded by downstream neurons.
Materials and Methods
Animal Care, General Statements
The Anoctamin-2 knock-out (Ano2−/−) mouse line was obtained from the PBmice project of Fudan University (see Li et al., for characterization)24. C57BL/6 J, Ano2+/+, Ano2−/−, and OMP-GCaMP325 mice were used in this study. The age of all animals at the time of the experiments was two to six months. All mice used in this study were housed in an inverted 12-hour light cycle and fed ad libitum. All the experiments were performed in accordance with the guidelines set by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at Harvard University.
In vivo imaging
Surgery
Adult mice were anesthetized with an intraperitoneal injection of ketamine and xylazine (100 and 10 mg/kg, respectively) and eyes were covered with petroleum jelly. The scalp was shaved and opened. After thorough cleaning and drying, the exposed skull was gently scratched with a blade, and a titanium custom-made headplate was glued on the scratches. The cranial bones over the OBs were then removed using a 3 mm diameter biopsy punch (Integra Miltex). The surface of the brain was cleared of debris and a glass coverslip was glued into the vacated cavity in the skull. Dental cement (Jet Repair, Lang Dental) was used to cover the headplate and form a well around the cranial window. Animals were allowed to recover for at least three days. Prior to each imaging session, animals were anesthetized with a mixture of ketamine and xylazine (90% of dose used for surgery) and body temperature was maintained at 37 °C by a heating pad.
Epifluoresence
Two photo lenses coupled front to front were used to image the OB surface onto the sensor of a CMOS camera (DFK 23GPO31, The Imaging Source GmbH). Images (960 × 600 pixels) were acquired at 8-bit resolution and 8 frames/s. Data from the camera were recorded to the computer via data acquisition hardware (National instruments) and custom software in Labview. A blue LED (CBT-90, Luminus) with a maximum output of 1.65 mW/mm2 was used for excitation.
Multiphoton
A custom-built two-photon microscope was used for in vivo imaging. Fluorophores were excited and imaged with a water immersion objective (20× , 0.95 NA, Olympus) at 920 nm using a Ti:Sapphire laser (Mai Tai HP, Spectra-Physics). Images were acquired at 16-bit resolution and 4 frames/s. The pixel size was 1.218 μm, and fields of view were typically 365 × 365 μm. The point-spread function of the microscope was measured to be 0.51 × 0.48 × 2.12 μm. Image acquisition and scanning were controlled by custom-written software in Labview. Emitted light was routed through two dichroic mirrors (680dcxr, Chroma and FF555- Di02, Semrock) and collected by a photomultiplier tube (R3896, Hamamatsu) using filters in the 500–550 nm range (FF01–525/50, Semrock).
Odor stimulation
Monomolecular odorants (Sigma) were used as stimuli and delivered by custom-built 8 channel (epifluorescence experiments) or 16 channel (2-photon experiments) olfactometer controlled by custom-written software in Labview (National Instruments)27. Odorants were maintained at a nominal volumetric concentration of 16% (v/v) in diethyl phthalate and further diluted 8 times with air for a final concentration of 2% for epifluorescence imaging. For multiphoton imaging odors were diluted in mineral oil at 16% (v/v) and diluted 16 times with air for a final concentration of 1%. For most experiments, odors were presented for 5 s with an interstimulus interval of at least 40 s.
The odor panel for epifluorescence imaging consisted of 1) Methyl propionate 2) Methyl butyrate 3) Ethyl Valerate 4) Pentyl acetate 5) Propyl acetate 6) Valeraldehyde 7) Methyl tiglate.
The odor panel for multiphoton imaging consisted of 1) Ethyl tiglate 2) Ethyl valerate 3) Valeric acid 4) Allyl butyrate 5) Isoamylamine 6) 2-Methoxypyrazine 7) Isugenol 8) Methyl tiglate 9) Valeraldehyde 10) Isoamyl acetate 11) Heptanal 12) mineral oil 13) Ethyl propionate 14) Propyl acetate 15) 2-Heptanone 16) Acetophenone.
For imaging glomerular responses to odor concentrations an additional 16 channel olfactometer outfitted with two odors, Ethyl valerate and Allyl butyrate, was used. The initial concentration series for each odor was 80%, 16%, 8%, 1.6%, 0.8%, 0.16%, 0.08% (v/v) in mineral oil and further diluted 16 times with air. Odors were presented for 2 s to prevent adaptation at the strongest concentrations. For all experiments, odors were delivered 3–5 times each.
Analysis
Calcium signals were extracted from raw images using custom-written scripts in MATLAB (MathWorks Inc.) and reported as ΔF/F signals, where F represents the average baseline fluorescence. Regions of interest were selected from average fluorescence projections for multiphoton imaging and ΔF/F projections for epifluorescence imaging. Response amplitude was measured from between three and five repeats of each odor as the mean response in the 5 seconds following odor onset. For analysis of response kinetics, measurements of the response rise time and decay time constant were taken from individual trials rather than reported as the mean to capture any intertribal variability. Rise time was measured as the time from when the signal first deviated 3.5 standard deviations from the mean of the baseline period to the peak of the response. Decay constants were obtained by fitting a single exponential to the signal, starting at the peak. Bleaching was corrected by fitting a single exponential to blank odor trials in multiphoton imaging and fitting a single exponential to the baseline period for epifluorescence experiments. For images of ΔF/F signals, the mean of an equal number of median filtered frames in the baseline and odor period was used. Traces of ΔF/F signals were smoothed for display. For figures where a threshold was applied to the data, thresholds were calculated based on the distribution of blank odor trials. An area under the receiver operating curve analysis was performed and the lowest threshold yielding ten responses for every one blank odor response was chosen. Sparseness measures were calculated as previously reported48,49. Population sparseness measures the fraction of glomeruli that are activated by a given odor, with values near one indicating uniform activity across all glomeruli and values near zero indicating a lack of activity in most glomeruli:
| 1 |
Where: = the number of glomeruli, = the response of glomerulus to odor .
Lifetime sparseness measures the extent to which a given glomerulus responds to different odor stimuli. Values near one indicate all odors uniformly activate a given glomerulus and values near zero indicate a high degree of odor selectivity:
| 2 |
Where: = the number of odors, = response of glomerulus to odor .
All statistical comparisons for imaging experiments were made as described in the text for each figure and values are given as mean + /− standard error of the mean.
Respiration measurements
Surgery
Animals were anesthetized with ketamine/xylazine as described above and a head plate was implanted in the skull as described previously in this article. For some mice, a small craniotomy was also made through the right nasal bone (1 mm anterior from the frontal/nasal fissure, 1 mm lateral from the midline), and a hollow cannula (#C313G; Plastics One Inc.) was lowered into the hole and glued to the skull. Finally, the whole exposed skull was covered with dental cement (Jet Repair, Lang Dental). The animals were given a week after the surgery to recover before any experiment was performed.
Respiration monitoring
Two strategies were used to monitor the breathing: measuring the intranasal pressure through an implanted cannula50,51, and measuring the temperature in front of the nose52.
For the intranasal pressure strategy, animals previously implanted with a cannula were head-fixed. Then, the cannula was connected to a pressure sensor (24PCEFJ6G; Honeywell International) through a piece of polyethylene tubing. The voltage signal generated by the sensor was amplified 1000×, low-pass filtered at 60 Hz, and digitized at 1000 Hz using custom software written in Labview.
For the temperature measurement, mice were head-fixed, and a thermocouple (5TC-TT-JI-40–1M, Omega Engineering) was placed ~2 mm in front of their nose. The voltage changes generated by the temperature variations were amplified 10000×, low-pass filtered at 60 Hz, and digitized at 1000 Hz using custom software written in Labview.
Analysis
Analysis of breathing signals and statistical tests were performed using custom software written in MATLAB. Two types of statistical tests were used: the Kruskal-Wallis test, and a bootstrap test to compare the means of two distributions (MATLAB function “bootstrp()” repeated 1,000,000 times for each bootstrapped statistics). The MATLAB toolbox CircStat was used to analyze circular data53. The critical p-value was set at 5% for all the tests, and Bonferroni correction was applied for multiple comparisons. On the figures, all the values are given as mean + /− standard error of the mean, unless otherwise stated.
Open Field Behavior
The arena consisted of 56 cm diameter circular inner chamber with four air inlets equally spaced around its circumference. The circular inner chamber was housed in light- and sound-proof outer chamber and illuminated using infrared LEDs. Throughout each experiment, airflow was maintained at a constant velocity for each inlet. After 10 minutes baseline exploration, air to one of the inlets was redirected through an odorized chamber while ensuring no change in its velocity. A vacuum was located at the center of the arena and its flow matched to the sum of all air inlets to prevent the accumulation of odor in the arena. Peanut oil was diluted in mineral oil as in imaging experiments. Each animal was only tested once and the order in which they were tested was randomized. After each trial the arena was thoroughly cleaned with ethanol to eliminate the presence of social cues. Images for tracking were acquired at 8 Hz using a USB camera (Grasshopper3, Point Grey Imaging) and custom-written Labview software. Images were processed using custom MATLAB routines to measure location and velocity. Animals with an initial position >10 cm from the odor source were excluded from our analysis. All statistical comparisons for behavior experiments were made with Wilcoxon rank-sum test and values are given as mean + /− standard error of the mean.
Data availability
All datasets in this manuscript are available from the corresponding author upon request.
Electronic supplementary material
Acknowledgements
We thank Vikrant Kapoor for technical assistance and Jessica Kim for helping collect behavioral data. We also thank members of the Murthy Lab and Prof. King-Wai Yau for helpful discussions. JDZ, JG, and VNM were partly supported by a grant from the NIH (R01 DC014454). JDZ was supported by NIH Fellowship F32 DC015938. R-CL and C-CL were supported by a grant from the NIH (R01 DC014941 to King-Wai Yau).
Author Contributions
JDZ and VNM designed the research. JDZ and JG collected and analyzed the data. R-CL and C-CL characterized and genotyped the Ano2−/− mouse line. JDZ and VNM wrote the manuscript with input from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28855-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol.Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- 2.Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J. Neurosci. 1991;11:3624–9. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–6. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- 4.Firestein S, Shepherd GM. Interaction of anionic and cationic currents leads to a voltage dependence in the odor response of olfactory receptor neurons. J Neurophysiol. 1995;73:562–567. doi: 10.1152/jn.1995.73.2.562. [DOI] [PubMed] [Google Scholar]
- 5.Kleene SJ. High-gain, low-noise amplification in olfactory transduction. Biophys. J. 1997;73:1110–7. doi: 10.1016/S0006-3495(97)78143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleene SJ, Pun RY. Persistence of the olfactory receptor current in a wide variety of extracellular environments. J. Neurophysiol. 1996;75:1386–1391. doi: 10.1152/jn.1996.75.4.1386. [DOI] [PubMed] [Google Scholar]
- 7.Boccaccio A, Menini A. Temporal development of cyclic nucleotide-gated and Ca2+-activated Cl− currents in isolated mouse olfactory sensory neurons. J. Neurophysiol. 2007;98:153–160. doi: 10.1152/jn.00270.2007. [DOI] [PubMed] [Google Scholar]
- 8.Billig GM, Pál B, Fidzinski P, Jentsch TJ. Ca2+-activated Cl− currents are dispensable for olfaction. Nat. Neurosci. 2011;14:763–769. doi: 10.1038/nn.2821. [DOI] [PubMed] [Google Scholar]
- 9.Pietra, G., Dibattista, M., Menini, A., Reisert, J. & Boccaccio, A. The Ca2+-activated Cl− channel TMEM16B regulates action potential firing and axonal targeting in olfactory sensory neurons. J. Gen. Physiol. 1–19, 10.1085/jgp.201611622 (2016). [DOI] [PMC free article] [PubMed]
- 10.Neureither F, Stowasser N, Frings S, Möhrlen F. Tracking of unfamiliar odors is facilitated by signal amplification through anoctamin 2 chloride channels in mouse olfactory receptor neurons. Physiol. Rep. 2017;5:e13373. doi: 10.14814/phy2.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischer J. Mammalian olfactory receptors. Front. Cell. Neurosci. 2009;3:1–10. doi: 10.3389/neuro.03.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberles SD. Trace amine-associated receptors: Ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015;34:1–7. doi: 10.1016/j.conb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer PL, et al. A Family of non-GPCR Chemosensors Defines an Alternative Logic for Mammalian Olfaction. Cell. 2016;165:1734–1748. doi: 10.1016/j.cell.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pifferi S, Dibattista M, Menini A. TMEM16B induces chloride currents activated by calcium in mammalian cells. Pflugers Arch. Eur. J. Physiol. 2009;458:1023–1038. doi: 10.1007/s00424-009-0684-9. [DOI] [PubMed] [Google Scholar]
- 15.Stephan AB, et al. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc. Natl. Acad. Sci. USA. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuter D, Zierold K, Schröder WH, Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J. Neurosci. 1998;18:6623–30. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko H, Nakamura T, Lindemann B. Noninvasive measurement of chloride concentration in rat olfactory receptor cells with use of a fluorescent dye. Am.J.Physiol.Cell Physiol. 2001;280:C1387–C1393. doi: 10.1152/ajpcell.2001.280.6.C1387. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko H. Chloride Accumulation in Mammalian Olfactory Sensory Neurons. J. Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengl T, et al. Molecular components of signal amplification in olfactory sensory cilia. Proc. Natl. Acad. Sci. USA. 2010;107:6052–6057. doi: 10.1073/pnas.0909032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkel B, et al. Co-expression of anoctamins in cilia of olfactory sensory neurons. Chem. Senses. 2015;40:73–87. doi: 10.1093/chemse/bju061. [DOI] [PubMed] [Google Scholar]
- 21.Lodovichi C, Belluscio L. Odorant receptors in the formation of the olfactory bulb circuitry. Physiology (Bethesda). 2012;27:200–212. doi: 10.1152/physiol.00015.2012. [DOI] [PubMed] [Google Scholar]
- 22.Yu CR, et al. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/S0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzon P, et al. Circuit Formation and Function in the Olfactory Bulb of Mice with Reduced Spontaneous Afferent Activity. J. Neurosci. 2015;35:146–160. doi: 10.1523/JNEUROSCI.0613-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, R.-C. et al. Ca2+ -activated Cl current predominates in threshold response of mouse olfactory receptor neurons. Proc. Natl. Acad. Sci. USA 201803443 10.1073/pnas.1803443115 (2018). [DOI] [PMC free article] [PubMed]
- 25.Isogai Y, et al. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 2009;12:210–20. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- 27.Rokni D, Hemmelder V, Kapoor V, Murthy VN. An olfactory cocktail party: figure-ground segregation of odorants in rodents. Nat. Neurosci. 2014;17:1225–1232. doi: 10.1038/nn.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou D, Chesler A, Firestein S. How the olfactory bulb got its glomeruli: a just so story? Nat. Publ. Gr. 2009;10:611–618. doi: 10.1038/nrn2666. [DOI] [PubMed] [Google Scholar]
- 29.Wachowiak M. All in a Sniff: Olfaction as a Model for Active Sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartzell HC, Yu K, Xiao Q, Chien L-T, Qu Z. Anoctamin/TMEM16 family members are Ca 2+ -activated Cl − channels. J Physiol. 2009;58710:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manoury B, Tamuleviciute A, Tammaro P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 2010;588:2305–14. doi: 10.1113/jphysiol.2010.189506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang CH, et al. The transmembrane protein 16A Ca2+ -activated Cl channel in airway smooth muscle contributes to airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2013;187:374–381. doi: 10.1164/rccm.201207-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein K, et al. Calcium-activated chloride channels anoctamin 1 and 2 promote murine uterine smooth muscle contractility. Am. J. Obstet. Gynecol. 2014;211:688.e1–688.e10. doi: 10.1016/j.ajog.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding S, et al. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Root, C. M., Denny, C. A., Hen, R. & Axel, R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature, 515(7526). 10.1038/nature13897. Nature 515, 269–273 (2014). [DOI] [PMC free article] [PubMed]
- 36.Li R-C, Ben-Chaim Y, Yau K-W, Lin C-C. Cyclic-nucleotide–gated cation current and Ca 2+ -activated Cl current elicited by odorant in vertebrate olfactory receptor neurons. Proc. Natl. Acad. Sci. 2016;113:11078–11087. doi: 10.1073/pnas.1613891113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamichi K. Continuous and Overlapping Expression Domains of Odorant Receptor Genes in the Olfactory Epithelium Determine the Dorsal/Ventral Positioning of Glomeruli in the Olfactory Bulb. J. Neurosci. 2005;25:3586–3592. doi: 10.1523/JNEUROSCI.0324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Schmelzeisen S, Parthier D, Frings S, Mohrlen F. Anoctamin calcium-activated chloride channels may modulate inhibitory transmission in the cerebellar cortex. PLoS One. 2015;10:1–23. doi: 10.1371/journal.pone.0142160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha GE, et al. The Ca2+ -activated chloride channel anoctamin-2 mediates spike-frequency adaptation and regulates sensory transmission in thalamocortical neurons. Nat. Commun. 2016;7:13791. doi: 10.1038/ncomms13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang WC, et al. Calcium-Activated Chloride Channels (CaCCs) Regulate Action Potential and Synaptic Response in Hippocampal Neurons. Neuron. 2012;74:179–192. doi: 10.1016/j.neuron.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghatpande AS, Reisert J. Olfactory receptor neuron responses coding for rapid odour sampling. J. Physiol. 2011;589:2261–2273. doi: 10.1113/jphysiol.2010.203687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J. Physiol. 2003;546:363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science (80-.). 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- 45.Tripathy SJ, Padmanabhan K, Gerkin RC, Urban NN. Intermediate intrinsic diversity enhances neural population coding. Proc. Natl. Acad. Sci. USA. 2013;110:8248–8253. doi: 10.1073/pnas.1221214110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitkow X, Meister M. Decorrelation and efficient coding by retinal ganglion cells. Nat. Neurosci. 2012;15:628–635. doi: 10.1038/nn.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emanuel AJ, Kapur K, Do MTH. Biophysical Variation within the M1 Type of Ganglion Cell Photoreceptor. Cell Rep. 2017;21:1048–1062. doi: 10.1016/j.celrep.2017.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallace JL, Wienisch M, Murthy VN. Development and Refinement of Functional Properties of Adult-Born Neurons. Neuron. 2017;96:883–896.e7. doi: 10.1016/j.neuron.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wienisch, M. & Murthy, V. N. Population imaging at subcellular resolution supports specific and local inhibition by granule cells in the olfactory bulb. 1–20 [DOI] [PMC free article] [PubMed]
- 50.Verhagen JV, Wesson DW, Netoff TI, White J. a & Wachowiak, M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat. Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- 51.Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS Biol. 2008;6:717–729. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lepousez G, Lledo PM. Odor Discrimination Requires Proper Olfactory Fast Oscillations in Awake Mice. Neuron. 2013;80:1010–1024. doi: 10.1016/j.neuron.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Berens P. CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 2009;31:1–21. doi: 10.18637/jss.v031.i10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets in this manuscript are available from the corresponding author upon request.