Key messages.
What is already known about this subject?
BAFF/BLyS is a key cytokine in systemic lupus erythematosus (SLE) and targeting BAFF by belimumab is used to treat patients with SLE.
Antiphospholipid syndrome (APS) is a major cause of thrombosis and pregnancy morbidity in patients with rheumatic diseases and immunomodulatory treatments are novel drug candidates to treat APS.
What does this study add?
BAFF levels are elevated in patient with primary APS and correlate with higher adjusted global antiphospholipid syndrome scores.
In SLE, but not in APS, the expression of BAFF receptors is altered on peripheral blood B cells.
How might this impact on clinical practice?
A subset of patients with APS may benefit from BAFF- targeting therapies such as belimumab.
Antiphospholipid syndrome (APS) is a systemic autoimmune disease characterised by antiphospholipid antibodies (aPL), thrombosis and obstetric complications. Other manifestations seen in APS include thrombocytopenia, heart valve disease and leg ulcers. APS was first described in patients suffering from systemic lupus erythematosus (SLE). However, APS may also affect patients not suffering from SLE or another underlying disease, termed primary APS (PAPS). Current treatment strategies for APS relying on anticoagulants are suboptimal, particularly for non-thrombotic manifestations and immunomodulatory drugs have been suggested as potential novel drug candidates in APS.1 2
B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS), is an important growth factor for B cells. The importance of BAFF in SLE is supported by animal models as mice transgenic for BAFF spontaneously develop SLE-like autoimmunity including the production of antibodies against double-stranded DNA (anti-dsDNA).3 In humans, patients with SLE have elevated levels of BAFF which correlate with disease activity.4 5 Moreover, belimumab, a monoclonal antibody against BAFF, is approved for the treatment of SLE and several other BAFF-targeting therapies are under development for the treatment of SLE.4
In contrast to SLE, little is known on the role of BAFF in APS. Case reports suggest a beneficial effect of belimumab in the treatment of non-thrombotic manifestations of patients with PAPS6 and blockade of the BAFF receptor (BAFF-R) prevents the development of APS in a murine model for APS.7 Post hoc analyses of the belimumab trials reveal that belimumab reduces anticardiolipin antibodies in patients with SLE.8 As a result, belimumab is a promising drug candidate for APS. However, no studies are available that compare the expression of BAFF and its receptors in patients with SLE and APS.
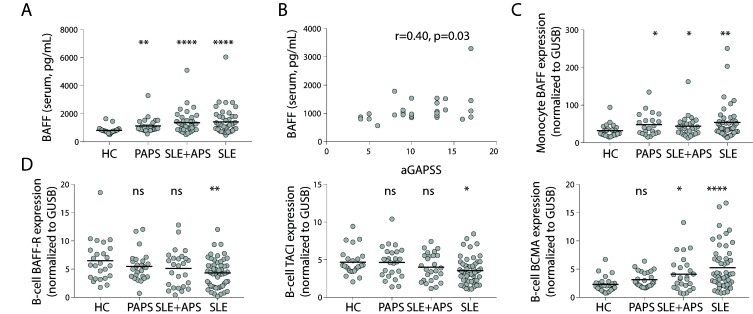
We measured BAFF levels by ELISA in sera of patient with SLE, SLE+APS and PAPS (table 1) and found increased serum levels of BAFF in patients with PAPS compared with healthy controls (HC), similar as in patients with SLE and SLE+APS (all p<0.05, figure 1A). Setting a threshold using the mean plus two SDs of HC, the prevalence of increased BAFF levels in PAPS was 7/29 (24%, p=0.07) and 21/54 (39%, p=0.002) and 17/40 (43%, p=0.001) in patients with SLE and SLE+APS, respectively, as compared with 2/29 (7%) of HC.
Table 1.
Clinical characteristics
| HC (n=29) | PAPS (n=29) | SLE+APS (n=40) | SLE (n=54) | |
| Clinical manifestations | ||||
| Age | 43 (34–50) | 40 (33–50) | 45 (37–53) | 36 (28–48) |
| Female (%) | 93 | 97 | 95 | 96 |
| SELENA-SLEDAI | – | 4 (1–5) | 4 (2–6) | |
| Malar rash (%) | 0 | 55 | 65 | |
| Discoid rash (%) | 0 | 18 | 19 | |
| Photosensitivity (%) | 0 | 53 | 43 | |
| Oral ulcers (%) | 0 | 35 | 32 | |
| Arthritis (%) | 0 | 65 | 72 | |
| Serositis (%) | 0 | 20 | 32 | |
| Lupus nephritis (%) | 0 | 45 | 69 | |
| Neurologic disorder (%) | 10* | 18 | 4 | |
| Haematologic disorder (%) | 35† | 85 | 67 | |
| Arterial thrombosis (%) | 59 | 43 | 9 | |
| Venous thrombosis (%) | 38 | 60 | 2 | |
| Obstetric morbidity (%) | 31 | 23 | 6 | |
| Current drug use | ||||
| Hydroxychloroquine (%) | 21 | 55 | 76 | |
| Prednisone (%) | 0 | 45 | 67 | |
| Azathioprine (%) | 0 | 41 | 33 | |
| Mycophenolate mofetil (%) | 0 | 10 | 19 | |
| Oral anticoagulant (%) | 62 | 75 | 0 | |
| Aspirin (%) | 48 | 28 | 20 | |
| Serology | ||||
| Anti-dsDNA (IU/mL) | – | 14 (5–58) | 27 (6–99) | |
| C3 | – | 0.79 (0.68–0.92) | 0.86 (0.70–1.03) | |
| C4 | – | 0.14 (0.10–0.22) | 0.15 (0.12–0.20) | |
| Lupus anticoagulant (%) | 82 | 65 | 14 | |
| Anticardiolipin IgG (%) | 86 | 78 | 20 | |
| Anticardiolipin IgM (%) | 38 | 15 | 10 | |
| Anti-β2 glycoprotein I IgG (%) | 35 | 26 | 4 | |
| Anti-β2 glycoprotein I IgM (%) | 10 | 5 | 7 | |
| aGAPSS | 10 (8–13) | 10 (6–11) | – | |
| Hyperlipidaemia (%) | 35 | 24 | 21 | |
| Arterial hypertension (%) | 59 | 66 | 56 |
Medians with IQR or percentages of total.
*Seizures
†Thrombocytopenia
aGAPSS, adjusted global antiphospholipid syndrome score; APS, antiphospholipid syndrome; HC, healthy controls; PAPS, primary APS; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment; SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index.
Figure 1.
BAFF is increased in primary antiphospholipid syndrome and is associated with higher adjusted global antiphospholipid syndrome scores. (A) Serum levels of BAFF in patients with PAPS as compared with SLE+APS and PAPS. (B) Correlation of serum levels of BAFF with the aGAPSS in patients with PAPS. (C) mRNA expression of BAFF in purified monocytes of patients with PAPS, SLE+APS and SLE. (D) mRNA expression of BAFF-R, TACI en BCMA in purified CD19+ B-cells in patients with PAPS, SLE+APS and PAPS. Abbreviations: aGAPSS, adjusted global antiphospholipid syndrome score; APS, antiphospholipid syndrome; BCMA, B-cell maturation antigen; GUSB, Glucuronidase Beta; HC, healthy controls; ns, not significant; PAPS, primary APS; SLE, systemic lupus erythematosus; TACI, transmembrane activator and CAML interactor.
The adjusted global antiphospholipid syndrome score (aGAPSS) is a validated tool for risk stratification in patients with PAPS9 and serum levels of BAFF correlated with higher aGAPSS scores in patients with PAPS (r=0.40, p=0.03) (figure 1B). The aGAPSS includes data regarding cardiovascular risk factors (hypertension and hyperlipidaemia) and aPL status. In this regard, patients with PAPS positive for lupus anticoagulant had higher serum BAFF levels than patients without (p<0.05) whereas there were no significant differences between patients with or without anti-β2 glycoprotein I or anticardiolipin antibodies (data not shown). In patients with SLE, serum levels of BAFF significantly correlated with higher levels of anti-dsDNA antibodies (r=0.39, p<0.001) and lower levels of complement component C3 (r=−0.26, p=0.02, data not shown). Monocytes are major producers of BAFF, therefore we evaluated mRNA expression of BAFF in purified monocytes obtained from patients with SLE, SLE+APS and PAPS and found increased mRNA expression in all three patient groups as compared with HC (all p<0.05, figure 1C).
BAFF is recognised by one of its three receptors which are highly expressed by B cells: BAFF-R, transmembrane activator and CAML interactor (TACI) and B-cell maturation antigen (BCMA). Binding of BAFF to these receptors promotes B-cell survival and maturation through activation of NFκB.4 In purified B cells of patients with SLE we found a lower expression of BAFF-R and TACI mRNA, in line with previous studies that assessed protein expression of these receptors on circulating B cells by flow cytometry.5 10 However, in patients with APS, both in SLE+APS and PAPS, we found no changes in the mRNA expression of BAFF-R and TACI as compared with HC (all p>0.05, figure 1D). In contrast, patients with SLE and SLE+APS but not patients with PAPS had an increased expression of BCMA compared with HC (all p<0.05, figure 1D). BCMA is predominantly expressed by plasmablasts and plasma cells4 10 which are increased in patients with SLE, potentially explaining the difference in BCMA expression between PAPS and SLE. Therefore, the changes in mRNA expression of BAFF-R between patients with SLE, APS and HC may reflect changes in B-cell homeostasis in SLE or APS. Alternatively, alterations in the expression of BAFF-R may reflect internalisation after BAFF binding.11
In conclusion, similar to SLE, both mRNA and serum levels of BAFF are elevated in PAPS, in particular in patients with PAPS with higher aGAPSS which are at higher risk for thrombotic events.9 As the treatment response to belimumab in patients with SLE is associated with higher serum levels of BAFF,12 belimumab might be a therapeutic option in a subset of patients with PAPS with increased BAFF levels.
Footnotes
Contributors: All authors were involved in study design, interpretation of data, drafting of the work and gave final approval of the version published. LLvdH was involved in data collection and performed the analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This research was approved by the Medisch Ethische Toetsingscommissie (METC) UMC Utrecht.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Raw data are available from the corresponding author upon reasonable request.
References
- 1. Erkan D, Aguiar CL, Andrade D, et al. . 14th International congress on antiphospholipid antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014;13:685–96. 10.1016/j.autrev.2014.01.053 [DOI] [PubMed] [Google Scholar]
- 2. van den Hoogen LL, Fritsch-Stork RD, Versnel MA, et al. . Monocyte type I interferon signature in antiphospholipid syndrome is related to proinflammatory monocyte subsets, hydroxychloroquine and statin use. Ann Rheum Dis 2016;75:e81 10.1136/annrheumdis-2016-210485 [DOI] [PubMed] [Google Scholar]
- 3. Mackay F, Woodcock SA, Lawton P, et al. . Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999;190:1697–710. 10.1084/jem.190.11.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vincent FB, Morand EF, Schneider P, et al. . The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014;10:365–73. 10.1038/nrrheum.2014.33 [DOI] [PubMed] [Google Scholar]
- 5. Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A, et al. . Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 2016;25:582–92. 10.1177/0961203315608254 [DOI] [PubMed] [Google Scholar]
- 6. Yazici A, Yazirli B, Erkan D. Belimumab in primary antiphospholipid syndrome. Lupus 2017;26:1123–4. 10.1177/0961203316682102 [DOI] [PubMed] [Google Scholar]
- 7. Kahn P, Ramanujam M, Bethunaickan R, et al. . Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum 2008;58:2824–34. 10.1002/art.23764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vilas-Boas A, Morais SA, Isenberg DA. Belimumab in systemic lupus erythematosus. RMD Open 2015;1:e000011 10.1136/rmdopen-2014-000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sciascia S, Sanna G, Murru V, et al. . The global anti-phospholipid syndrome score in primary APS. Rheumatology 2015;54:134–8. 10.1093/rheumatology/keu307 [DOI] [PubMed] [Google Scholar]
- 10. Kim J, Gross JA, Dillon SR, et al. . Increased BCMA expression in lupus marks activated B cells, and BCMA receptor engagement enhances the response to TLR9 stimulation. Autoimmunity 2011;44:69–81. 10.3109/08916934.2010.509122 [DOI] [PubMed] [Google Scholar]
- 11. Sellam J, Miceli-Richard C, Gottenberg JE, et al. . Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjögren's syndrome and systemic lupus erythematosus. Ann Rheum Dis 2007;66:790–7. 10.1136/ard.2006.065656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth DA, Thompson A, Tang Y, et al. . Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab. Lupus 2016;25:346–54. 10.1177/0961203315604909 [DOI] [PMC free article] [PubMed] [Google Scholar]