Abstract
Background and objectives
Transcatheter aortic valve-in-valve implantation (ViV) has emerged as a valuable technique to treat failed surgical bioprostheses (BPs) in patients with high risk for redo surgical aortic valve replacement (SAVR). Small BP size (≤21 mm), stenotic pattern of degeneration and pre-existing prosthesis–patient mismatch (PPM) have been associated with worse clinical outcomes after ViV. However, no study has evaluated the actual haemodynamic benefit associated with ViV. This study aims to compare haemodynamic status observed at post-ViV, pre-ViV and early after initial SAVR and to determine the factors associated with worse haemodynamic outcomes following ViV, including the rates of high residual gradient and ‘haemodynamic futility’.
Methods
Early post-SAVR, pre-ViV and post-ViV echocardiographic data of 79 consecutive patients who underwent aortic ViV at our institution were retrospectively analysed. The primary study endpoint was suboptimal valve haemodynamics (SVH) following ViV defined by the Valve Academic Research Consortium 2 as the presence of high residual aortic mean gradient (≥20 mm Hg) and/or at least moderate aortic regurgitation (AR). Haemodynamic futility of ViV was defined as <10 mm Hg decrease in mean aortic gradient and no improvement in AR compared with pre-ViV.
Results
SVH was found in 61% of patients (57% high residual gradient, 4% moderate AR) after ViV versus 24% early after SAVR. Pre-existing PPM and BP mode of failure by stenosis were independently associated with the primary endpoint (OR: 2.87; 95% CI 1.08 to 7.65; p=0.035 and OR: 3.02; 95% CI 1.08 to 8.42; p=0.035, respectively) and with the presence of high residual gradient (OR: 4.38; 95% CI 1.55 to 12.37; p=0.005 and OR: 5.37; 95% CI 1.77 to 16.30; p=0.003, respectively) following ViV. Criteria of ViV haemodynamic futility were met in 7.6% overall and more frequently in patients with pre-existing PPM and stenotic BP (18.5%) compared with other patients (2.0%). ViV restored haemodynamic function to early post-SAVR level in only 34% of patients.
Conclusion
Although ViV was associated with significant haemodynamic improvement compared with pre-ViV in >90% of patients, more than half harboured SVH outcome. Furthermore, only one-third of patients had a restoration of valve haemodynamic function to the early post-SAVR level. Pre-existing PPM and stenosis pattern of BP degeneration were the main factors associated with SVH and haemodynamic futility following ViV. These findings provide strong support for the prevention of PPM at the time of initial SAVR and careful preprocedural patient screening.
Keywords: transcatheter aortic valve-in-valve, bioprosthesis dysfunction, hemodynamics, hemodynamic futility, prosthesis-patient mismatch
Key questions.
What is already known about this subject?
Transcatheter aortic valve-in-valve implantation (ViV) is a valuable alternative to redo surgery for the treatment of failed bioprosthesis (BP) in patients with high surgical risk. However, high residual gradient is frequent following ViV and is associated with worse outcomes.
What does this study add?
This is the first study to assess the valve haemodynamic benefit of ViV compared with pre-ViV status and also the degree of restoration of valve haemodynamic function compared with that achieved by initial surgical aortic valve replacement (SAVR). ViV improved significantly the valve haemodynamic status compared with pre-ViV in 92% of patients and was ‘haemodynamically futile’ in only 8%. However, aortic valve haemodynamics was suboptimal according to Valve Academic Research Consortium 2 criteria (high residual gradient and/or moderate aortic regurgitation) in 61% post-ViV versus 100% pre-ViV and 24% early post-SAVR. ViV procedure was able to restore valve function to the post-SAVR level in only 34% of patients. Pre-existing prosthesis–patient mismatch (PPM) of the surgical BP as well as BP failure by stenosis were the main factors associated with higher rates of high residual gradient, suboptimal valve haemodynamics and haemodynamic futility following ViV.
How might this impact on clinical practice?
These findings provide strong support for the prevention of PPM at the time of initial SAVR and for the systematic assessment of PPM as well as the BP mode of failure (stenosis) for the selection of the candidates for ViV and for the consideration of concomitant procedures such as BP stent fracturing at the time of ViV in those patients being at risk for high residual gradients.
Introduction
Transcatheter valve-in-valve (ViV) implantation has emerged as a valuable alternative to treat failed surgical bioprostheses (BPs) in patients with prohibitive or high risk for redo surgical aortic valve replacement (SAVR).1 2 However, high residual gradients (mean aortic gradient ≥20 mm Hg) are frequently observed after ViV procedure. In previous registries,3–7 small surgical valve label size (≤21 mm), stenotic pattern of BP degeneration and pre-existing prosthesis–patient mismatch (PPM) of the BP were associated with the persistence of high residual gradient, less improvement in functional capacity and increased risk of mortality following ViV. However, there are relatively few data on the degree of valve haemodynamic improvement following ViV and on how this procedure eventually restores the valve haemodynamic function to that observed early after initial SAVR. This study aims: (1) to compare the aortic valve haemodynamic status at post-ViV versus pre-ViV and versus early post-SAVR and (ii) to determine the factors associated with worse haemodynamic outcomes following ViV, including the rates of high residual gradient and ‘haemodynamic futility’ (ie, absence of significant valve haemodynamic improvement).
Methods
Seventy-nine (79) consecutive patients presenting with a failed surgical BP and deemed unsuitable for redo surgery underwent transcatheter ViV at the Quebec Heart and Lung Institute between 2009 and 2017 and were included in this study.
Doppler echocardiographic measurements
For each patient included in the study, transthoracic echocardiograms (TTE) performed early (1–3 months) after SAVR (post-SAVR), prior to ViV (pre-ViV) and 1–3 months after ViV (post-ViV) were retrospectively analysed in the echocardiography core laboratory of our institution. The stroke volume was measured in the left ventricular outflow tract (LVOT) with the use of the diameter and velocity measured just underneath the prosthesis stent. The effective orifice area (EOA) was calculated as the LVOT stroke volume divided by the aortic jet velocity time integral and was indexed for body surface area.8 The mean aortic pressure gradient (MG) was measured using the modified Bernoulli formula. An integrative, semiquantitative approach was used to grade the severity of aortic regurgitation (AR) as mild, moderate or severe.8 9
At pre-ViV TTE, significant stenosis was defined by a MG ≥20 mm Hg with an increase ≥10 mm Hg in MG and decrease >0.3 cm2 in EOA compared with post-SAVR TTE.8 10 11 Patients with stenotic pattern of BP degeneration but less than moderate AR were classified in the stenosis group. Patients with ≥moderate AR without significant stenosis were classified in the AR group. Patient combining both patterns of BP degeneration were classified as having mixed valve dysfunction.
PPM was defined as not clinically significant (ie, mild or no PPM) if the EOA indexed for body surface area (EOAi) was >0.85 cm2/m2, moderate if it was >0.65 cm2/m2 but ≤0.85 cm2/m2 and severe if it was ≤0.65 cm2/m2.
Post-SAVR TTE was not available in 20 patients of the study population, and for them, we used the predicted EOAi to define pre-existing PPM.8
Study endpoints
The primary study endpoints were the presence of suboptimal valve haemodynamic (SVH) function as defined by the Valve Academic Research Consortium (VARC) 2: that is, high residual gradient (MG ≥20 mm Hg) and/or ≥moderate AR.9 The secondary endpoints were: (1) haemodynamic ‘futility’ of the ViV procedure defined as <10 mm Hg decrease in MG and no improvement in AR or worsening compared with pre-ViV; (2) restoration of the valve haemodynamic function to that observed at early post-SAVR TTE, defined as a post-ViV MG within ±10 mm Hg of the post-SAVR MG and a post-ViV AR grade less or equivalent to the post-SAVR AR grade.
Statistical analysis
Continuous data are presented as mean±SD or SE and were compared between groups using Student’s t-test or analysis of variance (ANOVA) and Tukey post hoc test as appropriate. Continuous variable at post-ViV versus pre-ViV or post-SAVR were compared using paired t-tests or two-way repeated measures ANOVA with Holm-Sidak post hoc test. Categorical variables are presented as proportions and were compared using χ2 test or Fisher’s exact test as appropriate. Multivariable stepwise forward logistic regression was performed to determine the adjusted correlates for primary and secondary endpoints. Statistical analyses were performed using JMP 13.0 (SAS Institute, USA) and SigmaPlot 11 (Systat Software, USA), and a p value <0.05 was considered statistically significant.
Results
Baseline and ViV procedural characteristics
Mean age of the patients at the time of ViV was 74.5±11.0 years; 66% were men; 58% were in New York Heart Association class 3, and the average EuroSCORE in this series was 10.2%±2.7% (table 1). The surgical BPs were predominantly stented pericardial valves (72% of stented BPs among which 67% were pericardial) and BP label size ranged from 19 mm to 29 mm with a mean internal orifice diameter (IOD) of 20.3±2.4 mm. The time from SAVR to BP failure requiring ViV was 11±4 years (table 2). BP mode of failure was stenosis in 40.5%, regurgitation in 31.7% and mixed in 27.8%. Balloon-expandable (SAPIEN first generation/SAPIEN XT/SAPIEN 3) transcatheter heart valve (THV) type was more frequently (62%) used than CoreValve type for ViV in this series (table 3). THV sizes ranged from 20 mm to 31 mm, the majority of them being 23 mm valves (70%), and transfemoral approach was the predominant access (60%). The predominant utilisation of balloon-expandable THVs for ViV in this study is mainly related to the fact that our centre started the transcatheter aortic valve replacement programme with SAPIEN THVs, and for several years, this was the only type of THV used for these procedures. The ratio of balloon expandable versus self-expanding THVs for ViV has however decreased markedly and shifted during the past 5 years, and nowadays >65% of the THVs used for ViV are CoreValve devices (online supplementary figure 1).
Table 1.
Baseline clinical and Doppler echocardiographic data at the time of initial surgical aortic valve replacement according to the presence or absence of suboptimal aortic valve haemodynamics after ViV
| Whole cohort n=79 |
Valve haemodynamics after ViV | P values | ||
| Adequate: MG <20 mm Hg and AR ≤mild n=31 (39.2%) |
Suboptimal: MG ≥20 mm Hg and/or AR ≥moderate n=48 (60.8%) |
|||
| Age, years | 74.5±11.0 | 73.6±12.6 | 75.0±9.9 | 0.587 |
| BSA, m2 | 1.87±0.24 | 1.79±0.25 | 1.92±0.22 | 0.027 |
| Males, n (%) | 52 (65.8) | 18 (58.1) | 34 (70.8) | 0.243 |
| EuroSCORE, % | 10.2±2.7 | 10.4±2.7 | 10.1±2.7 | 0.721 |
| LVEF, % | 61.5±9.9 | 59.5±13.1 | 62.7±7.2 | 0.237 |
| Stroke volume, mL | 69.3±14.0 | 67.4±12.0 | 70.6±15.3 | 0.392 |
| Surgical BP type, n (%) | 0.141 | |||
| Stented porcine | 19 (24.1) | 8 (25.8) | 11 (22.9) | |
| Stented pericardial | 38 (48.1) | 11 (35.5) | 27 (56.3) | |
| Stentless | 22 (27.8) | 12 (38.7) | 10 (20.8) | |
| Surgical BP model, n (%) | 0.059 | |||
| Mitroflow | 17 (21.5) | 6 (19.4) | 11 (22.9) | |
| Mosaic | 15 (18.9) | 7 (22.6) | 8 (16.7) | |
| Homograft | 13 (16.5) | 5 (16.1) | 8 (16.7) | |
| Magna | 9 (11.4) | 1 (3.2) | 8 (16.7) | |
| Freestyle | 8 (10.1) | 6 (19.4) | 2 (4.2) | |
| Magna Ease | 6 (7.6) | 0 (0.0) | 6 (12.5) | |
| Perimount | 5 (6.3) | 3 (9.7) | 2 (4.2) | |
| Intact | 2 (2.5) | 0 (0.0) | 2 (4.2) | |
| Hancock | 1 (1.3) | 0 (0.0) | 1 (2.1) | |
| Solo | 1 (1.3) | 1 (3.2) | 0 (0.0) | |
| Trifecta | 1 (1.3) | 1 (3.2) | 0 (0.0) | |
| Epic Supra | 1 (1.3) | 1 (3.2) | 0 (0.0) | |
| Surgical BP size (≤21 mm), n (%) | 26 (32.9) | 8 (25.8) | 18 (37.5) | 0.280 |
| Internal orifice diameter*, mm | 20.3±2.4 | 20.8±2.2 | 20.0±2.5 | 0.131 |
| MG, mm Hg | 14.1±6.0 | 11.0±5.4 | 16.3±5.6 | 0.0007 |
| EOA, cm2 | 1.59±0.47 | 1.70±0.47 | 1.52±0.45 | 0.087 |
| EOAi, cm2/m2 | 0.86±0.26 | 0.96±0.27 | 0.80±0.23 | 0.006 |
| Doppler velocity index | 0.38±0.09 | 0.40±0.11 | 0.37±0.08 | 0.428 |
| Pre-existing PPM, n (%) | 0.032 | |||
| None | 32 (40.5) | 18 (58.1) | 14 (29.2) | |
| Moderate | 29 (36.7) | 9 (29.0) | 20 (41.7) | |
| Severe | 18 (22.8) | 4 (12.9) | 14 (29.2) | |
| AR | 0.450 | |||
| None | 25 (48.1) | 8 (40.0) | 17 (53.1) | |
| Trace | 23 (44.2) | 11 (55.0) | 12 (37.5) | |
| Mild | 4 (7.7) | 1 (5.0) | 3 (9.4) | |
*According to the Bapat’s ViV application.
AR, aortic regurgitation; BP, bioprosthesis; BSA, body surface area; EOA, effective orifice area; EOAi, indexed EOA; LVEF, left ventricular ejection fraction; MG, mean transvalvular pressure gradient; PPM, prosthesis–patient mismatch; ViV, valve-in-valve implantation.
Table 2.
Doppler echocardiographic data prior to ViV procedure according to the presence or absence of suboptimal aortic valve haemodynamic performance after ViV
| Whole cohort n=79 |
Valve haemodynamics after ViV | P values | ||
| Adequate: MG <20 mm Hg and AR ≤mild n=31 (39.2%) |
Suboptimal: MG ≥20 mm Hg and/or AR ≥moderate n=48 (60.8%) |
|||
| Time to failure, years | 11.1±4.2 | 11.3±4.0 | 10.9±4.3 | 0.724 |
| BP mode of failure, n (%) | 0.035 | |||
| AS | 32 (40.5) | 9 (29.0) | 23 (47.9) | |
| AR | 25 (31.7) | 15 (48.4) | 10 (20.8) | |
| Mixed | 22 (27.8) | 7 (22.6) | 15 (31.3) | |
| LVEF, % | 58.9±10.9 | 55.8±13.1 | 60.8±8.9 | 0.047 |
| Stroke volume, mL | 82.2±20.7 | 79.6±18.7 | 83.8±21.9 | 0.398 |
| MG, mm Hg | 36.6±20.2 | 28.0±17.4 | 42.2±20.1 | 0.002 |
| EOA, cm2 | 1.04±0.52 | 1.21±0.60 | 0.95±0.44 | 0.031 |
| EOAi, cm2/m2 | 0.57±0.29 | 0.68±0.34 | 0.50±0.23 | 0.009 |
| Doppler velocity index | 0.27±0.13 | 0.33±0.18 | 0.24±0.09 | 0.008 |
| AR, n (%) | 0.368 | |||
| None | 7 (9.0) | 1 (3.3) | 6 (12.5) | |
| Trace | 15 (19.2) | 5 (16.7) | 10 (20.8) | |
| Mild | 11 (14.1) | 4 (13.3) | 7 (14.6) | |
| Moderate | 25 (32.1) | 9 (30.0) | 16 (33.3) | |
| Severe | 20 (25.6) | 11 (36.7) | 9 (18.8) | |
| AR ≥moderate, n (%) | 45 (57.7) | 20 (66.7) | 25 (52.1) | 0.205 |
AR, aortic regurgitation; AS, aortic stenosis; BP, bioprosthesis; BSA, body surface area; EOA, effective orifice area; EOAi, indexed EOA; LVEF, left ventricular ejection fraction; MG, mean transvalvular pressure gradient; Mixed, mixed dysfunction; PPM, prosthesis–patient mismatch; ViV, valve-in-valve implantation.
Table 3.
Procedural data and Doppler echocardiographic data post-ViV according to the presence or absence of suboptimal aortic valve haemodynamic performance after ViV
| Whole cohort | Valve haemodynamics after ViV | P values | ||
| n=79 | Adequate: MG <20 mm Hg and AR ≤mild n=31 (39.2%) |
Suboptimal: MG ≥20 mm Hg and/or AR ≥moderate n=48 (60.8%) |
||
| Procedural data | ||||
| Balloon-expandable THV, n (%) | 49 (62.0) | 19 (61.3) | 30 (62.5) | 0.914 |
| THV size (≤23), n (%) | 55 (69.6) | 19 (61.3) | 36 (75.0) | 0.196 |
| THV model, n (%) | 0.484 | |||
| SAPIEN/XT/3 | 49 (62.0) | 19 (61.3) | 30 (62.5) | |
| CoreValve/Evolut R | 28 (35.4) | 12 (38.7) | 16 (33.3) | |
| Portico | 2 (2.5) | 0 (0.0) | 2 (4.2) | |
| Access, n (%) | 0.224 | |||
| Transfemoral | 47 (59.5) | 16 (51.6) | 31 (64.6) | |
| Transapical | 23 (29.1) | 12 (38.7) | 11 (22.9) | |
| Transcarotid | 6 (7.6) | 1 (3.2) | 5 (10.4) | |
| Transaortic | 3 (3.8) | 2 (6.5) | 1 (2.1) | |
| Procedural success*, n (%) | 62 (78.5) | 24 (77.4) | 38 (79.2) | 0.854 |
| Coronary occlusion, n (%) | 1 (1.3) | 0 (0) | 1 (2.1) | 1.0 |
| More than one THV, n (%) | 8 (10.1) | 3 (9.7) | 5 (10.4) | 1.0 |
| Need of pacemaker, n (%) | 3 (3.8) | 0 (0.0) | 3 (6.3) | 0.276 |
| New onset of atrial fibrillation, n (%) | 5 (6.8) | 2 (6.7) | 3 (6.8) | 1.0 |
| Major vascular complication, n (%) | 2 (2.5) | 1 (3.2) | 1 (2.1) | 1.0 |
| Major bleeding, n (%) | 3 (3.8) | 1 (3.2) | 2 (4.2) | 1.0 |
| THV malposition or embolisation, n (%) | 2 (2.5) | 0 (0.0) | 2 (4.2) | 0.517 |
| Hospital stay, days | 6.9±4.9 | 7.7±5.5 | 6.3±4.4 | 0.214 |
| Doppler echocardiographic data post-ViV | ||||
| MG, mm Hg | 22.2±9.3 | 14.1±3.9 | 27.3±8.0 | <0.0001 |
| EOA, cm2 | 1.15±0.38 | 1.29±0.29 | 1.06±0.40 | 0.009 |
| EOAi, cm2/m2 | 0.62±0.21 | 0.73±0.18 | 0.56±0.20 | 0.0003 |
| Doppler velocity index | 0.30±0.08 | 0.34±0.08 | 0.27±0.07 | <0.0001 |
| PPM, n (%) | <0.0001 | |||
| None | 11 (13.9) | 8 (25.8) | 3 (6.3) | |
| Moderate | 14 (17.7) | 12 (38.7) | 2 (4.2) | |
| Severe | 54 (68.4) | 11 (35.5) | 43 (89.6) | |
| LVEF, % | 57.5±11.7 | 55.8±10.7 | 58.7±11.7 | 0.584 |
| Stroke volume, mL | 70.2±18.5 | 72.0±24.0 | 67.9±14.2 | 0.684 |
| AR, n (%) | 0.035 | |||
| None | 26 (33.3) | 8 (26.7) | 18 (37.5) | |
| Trace | 39 (50.0) | 16 (53.3) | 23 (47.9) | |
| Mild | 10 (12.8) | 6 (20.0) | 4 (8.3) | |
| Moderate | 3 (3.9) | 0 (0.0) | 3 (6.3) | |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Suboptimal valve function | ||||
| MG ≥20 mm Hg, n (%) | 45 (57.0) | 0 (0.0) | 45 (93.8) | – |
| AR ≥moderate, n (%) | 3 (3.9) | 0 (0.0) | 3 (6.3) | – |
| Change from pre-ViV | ||||
| ΔMG, mm Hg | −14.4±18.3 | −13.7±16.6 | −14.8±19.5 | 0.806 |
| ΔEOA, cm2 | +0.10±0.47 | +0.07±0.62 | +0.12±0.36 | 0.646 |
| ΔEOAi, cm2/m2 | +0.05±0.26 | +0.04±0.35 | +0.06±0.19 | 0.698 |
| ΔAR, grade | −1.8±1.4 | −2.1±1.4 | −1.6±1.3 | 0.104 |
| Change from post-SAVR | ||||
| ΔMG, mm Hg | +7.8±7.9 | +3.5±4.9 | +10.8±8.3 | 0.0003 |
| ΔEOA, cm2 | −0.44±0.40 | −0.42±0.40 | −0.46±0.40 | 0.668 |
| ΔEOAi, cm2/m2 | −0.24±0.21 | −0.23±0.22 | −0.24±0.21 | 0.873 |
| ΔAR, grade | +0.28±0.91 | +0.13±0.66 | +0.38±1.02 | 0.359 |
*Procedural success was defined as the absence of any major adverse cardiovascular and cerebrovascular event (stroke, myocardial infarction, in-hospital death) and device success (no more than one transcatheter prosthesis, no procedural death and no vascular complication).
AR, aortic regurgitation; EOA, effective orifice area; EOAi, indexed EOA; LVEF, left ventricular ejection fraction; MG, mean transvalvular pressure gradient; PPM, prosthesis–patient mismatch; SAVR, surgical aortic valve replacement; THV, transcatheter heart valve; ViV, valve-in-valve implantation.
openhrt-2018-000854supp001.docx (129.5KB, docx)
Forty-seven (47; 59.5%) patients had pre-existing PPM of the surgical BP: 29 of them had moderate (36.7%) and 18 patients had severe (22.8%) PPM. These patients received stented BPs more frequently than patients free of pre-existing PPM (p<0.0001) and the percentage of BP with ≤21 mm label size was numerically (but not statistically) higher in patients with pre-existing PPM (50% in patients with severe PPM, 31% in patients with moderate PPM and 25% in patients free of PPM; p=0.19) (online supplementary table 1). BPs with pre-existing severe or moderate PPM failed more frequently by stenosis with a shorter time from SAVR to BP failure and were more frequently treated with small THVs (THV ≤23 mm) compared with patients free of pre-existing PPM (94.4%, 82.8% and 43.8%, respectively, p=0.0001) (online supplementary tables 2 and 3).
ViV haemodynamic outcomes
Forty-eight (61%) patients met the VARC2-based primary endpoint of SVH after ViV procedure. Among them, the vast majority (45 patients) had high residual gradient and only three had moderate AR after ViV.
Compared with patients with adequate haemodynamic performance after ViV, patients with SVH had larger body surface area and MG, smaller EOAi and more pre-existing PPM at the time of initial SAVR (table 1), as well as more BPs with stenosis pattern of degeneration (table 2). Regarding echocardiographic Doppler data pre-ViV (table 2) and post-ViV (table 3), patients with SVH had higher MG and smaller EOA, EOAi, and Doppler velocity index. Finally, these patients had a larger increase in MG from post-SAVR to post-ViV (table 3).
High residual gradient after ViV was more frequently met in patients with severe (78%) or moderate (69%) pre-existing PPM compared with those without PPM (34%) (p<0.01) (online supplementary figure 2, online supplementary table 3). High residual gradient after ViV was also more frequent in patients with stenosis (72%) and combined degeneration pattern of the BP (68%) compared with patients with regurgitant pattern (28%) (p<0.002) (online supplementary figure 2).
Comparison of valve haemodynamic status at post-SAVR, pre-ViV and post-ViV
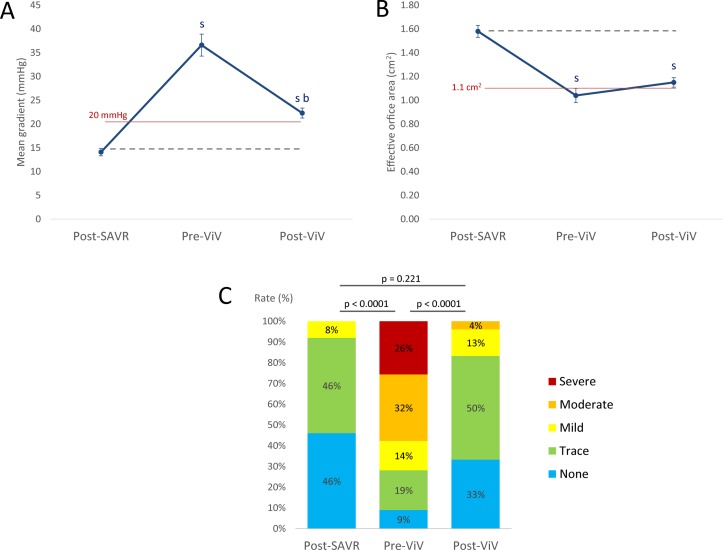
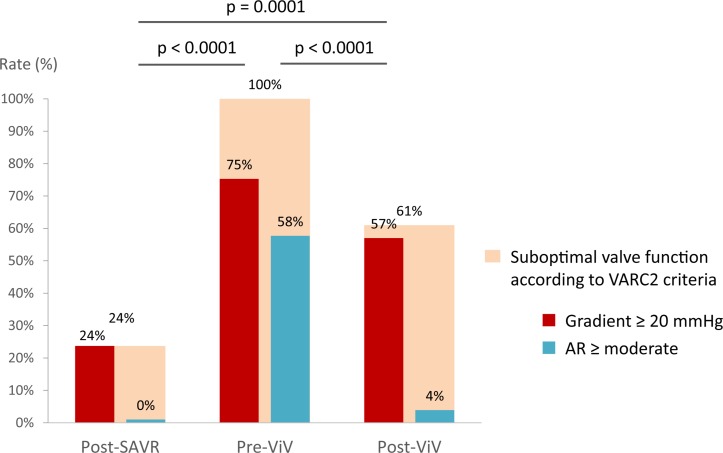
As expected, EOA decreased and MG and rate of ≥moderate AR increased from post-SAVR to pre-ViV TTEs (tables 1 and 2, figures 1 and 2). After ViV, MG and rate of ≥moderate AR decreased significantly compared with pre-ViV, but they nonetheless remained higher compared with early post-SAVR (tables 2 and 3, figures 1 and 2, online supplementary figure 3). SVH function was present in 24% (high residual gradient: 24%, AR: 0%) of patients post-SAVR, in 100% of the patients pre-ViV (high residual gradient: 75%, AR: 58%) and in 61% (high residual gradient: 57%, AR: 4%) of patients post-ViV (p<0.0001) (figure 2).
Figure 1.
Haemodynamic performance of the aortic bioprosthesis early after SAVR, before and after ViV. Panel A shows the mean transvalvular pressure gradient, panel B shows the effective orifice area and panel C shows the distribution of aortic regurgitation grades. sStatistical difference versus post-SAVR; bStatistical difference versus pre-ViV. AR, aortic regurgitation; SAVR, surgical aortic valve replacement; ViV, valve-in-valve.
Figure 2.
Suboptimal aortic valve haemodynamics according to the VARC2 criteria (MG ≥20 mm Hg or AR ≥moderate) early post-SAVR, pre-ViV and post-ViV. AR, aortic regurgitation; SAVR, surgical aortic valve replacement; VARC 2, Valve Academic Research Consortium 2; ViV, valve-in-valve.
The criteria of haemodynamic futility of ViV were observed in 7.6% (n=6) of the whole cohort (figure 3). ViV restored valve haemodynamic function to that observed at early post-SAVR in only 34% of patients in this series (figure 3).
Figure 3.
ViV haemodynamic futility and restoration of SAVR valve function. Panel A shows haemodynamic futility of the ViV procedure, defined as no improvement in AR and <10 mm Hg decrease in mean aortic gradient. Panel B shows the rate of restoration of the valve haemodynamic function to that observed early after SAVR. AR, aortic regurgitation; AS, aortic stenosis; BP, bioprosthesis; n, number of patients; PPM, prosthesis–patient mismatch; SAVR, surgical aortic valve replacement; ViV, valve-in-valve.
Factors associated with ViV haemodynamic outcomes
In univariable analysis, the factors associated with SVH after ViV procedure were: BP mode of failure (AS and mixed vs AR) and moderate or severe pre-existing PPM after SAVR (table 4). The factors associated with isolated high residual gradient after ViV were: BP type (pericardial stented vs stentless), smaller BP IOD, mode of BP failure (AS and mixed vs AR), moderate or severe pre-existing PPM after SAVR and THV size ≤23 mm (table 4). In multivariable analysis, pre-existing PPM and BP mode of failure (AS and mixed) remained significantly associated with SVH and with higher risk of high residual gradient after ViV (table 4).
Table 4.
Univariable and multivariable analyses of the factors associated with post-ViV high residual gradient and/or ≥moderate aortic regurgitation
| High gradient (MG ≥20 mm Hg) |
High gradient and/or AR ≥moderate |
|||||||
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | |||||
| OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | OR (95% CI) |
P values | |
| Male | 1.37 (0.54 to 3.50) |
0.509 | – | – | 1.75 (0.68 to 4.52) |
0.245 | – | – |
| BP type (stented vs stentless) | 4.29 (1.50 to 12.28) |
0.007 | – | – | 2.40 (0.88 to 6.55) |
0.087 | – | – |
| BP type | ||||||||
| Stented pericardial vs stentless | 5.26 (1.69 to 16.42) |
0.004 | – | – | 2.95 (0.99 to 8.79) |
0.053 | – | – |
| Stented porcine vs stentless | 2.95 (0.82 to 10.58) |
0.098 | – | – | 1.65 (0.48 to 5.69) |
0.428 | – | – |
| BP size (≤21 mm) | 2.17 (0.80 to 5.84) |
0.127 | – | – | 1.73 (0.64 to 4.66) |
0.283 | – | – |
| BP IOD, mm | 0.72 (0.58 to 0.91) |
0.005 | – | – | 0.86 (0.71 to 1.05) |
0.135 | – | – |
| BP mode of failure (AS or mixed vs AR) | 6.11 (2.14 to 17.46) |
0.001 | 5.37 (1.77 to 16.30) |
0.003 | 3.56 (1.32 to 9.59) |
0.012 | 3.02 (1.08 to 8.42) |
0.035 |
| Pre-existing PPM (≥moderate) | 4.99 (1.89 to 13.17) |
0.001 | 4.38 (1.55 to 12.37) |
0.005 | 3.36 (1.31 to 8.67) |
0.012 | 2.87 (1.08 to 7.65) |
0.035 |
| THV type (balloon expandable vs self-expanding) | 1.27 (0.51 to 3.17) |
0.611 | – | – | 1.05 (0.42 to 2.67) |
0.914 | – | – |
| THV design (intra-annular vs supra-annular) | 1.55 (0.61 to 3.93) |
0.356 | – | – | 1.26 (0.49 to 3.23) |
0.626 | – | – |
| THV size (≤23 mm) | 3.16 (1.17 to 8.55) |
0.024 | – | – | 1.90 (0.72 to 5.02) |
0.199 | – | – |
AR, aortic regurgitation; AS, aortic stenosis; BP, bioprosthesis; IOD, internal orifice diameter; MG, mean transaortic gradient; mixed, mixed dysfunction; PPM, prosthesis–patient mismatch; THV, transcatheter heart valve; ViV, valve-in-valve.
Criteria of haemodynamic futility of ViV were more frequently met in patients harbouring concomitant pre-existing PPM (moderate or severe) and stenosis pattern of BP degeneration (18.5%) compared with patients with no PPM and/or combined/regurgitant pattern of BP degeneration (2.0%) (figure 3).
The presence of a stented porcine BP was the only factor associated with restoration of valve haemodynamic function to that observed post-SAVR (OR: 4.58, 95% CI 1.15 to 18.28; p=0.03).
Discussion
The main findings of this study were: (i) ViV was associated with an improvement in valve haemodynamics compared with pre-ViV in 92% of patients. (2) However, SVH—as defined by VARC2 criteria—was observed in 61% of patients post-ViV versus 24% early post-SAVR. (3) ViV was able to restore valve function to that observed post-SAVR in only 34% of patients. (4) Pre-existing PPM of the surgical BP and BP mode of failure by stenosis were the main factors associated with higher rates of high residual gradient and SVH following ViV and of haemodynamic futility of this procedure.
Haemodynamic outcomes of ViV versus initial SAVR
About one quarter of patients had SVH (exclusively high residual gradient) early after initial SAVR. This is most likely related to the presence of severe PPM. However, following ViV, this rate of SVH increased by 2.5 folds compared with post-SAVR, and only one-third of the patients had a restoration of their valve haemodynamic function to the post-SAVR level following ViV (figures 2 and 3). These findings may be explained by the fact that the ViV procedure (ie, implanting a second valve within a pre-existing one) generally reduces the internal orifice area available for blood flow, unless the BP is expanded or fractured during the procedure. An analogy to this concept would be the Russian dolls, where the second doll is necessarily smaller than the first one. Hence, although the valve haemodynamic status of the failed BP is significantly improved (ie, gradient and/or AR are reduced compared with pre-ViV) in the vast majority of patients, the ViV procedure is generally not able to restore the baseline valve function post-SAVR.
The only factor associated with restoration of valve function to that observed early post-SAVR was the presence of stented porcine BP. In stented porcine BPs, the leaflets are mounted within the stent, whereas in most stented pericardial BPs (eg, Mitroflow) included in this study, the leaflets were mounted outside the stent. Hence, in pericardial BPs, ViV generally leads to a worsening of valve haemodynamics, whereas in stented porcine BPs, the radial forces exerted by the THV during ViV may compress the BP leaflet tissue and sutures and therefore expand the internal geometric orifice area of the BP. These factors may have resulted in less decrease, or even some increase, in the internal geometric orifice area and EOA of stented porcine BPs compared with stented pericardial BPs. These findings are consistent with recent in vitro studies that reported no increase in gradients after ViV implantation of the SAPIEN12 and the CoreValve13 within normally functioning porcine BPs.
Factors associated with ViV haemodynamic outcomes
Several studies reported that severe pre-existing PPM of the BP is associated with worse functional capacity, increased risk of mortality and increased rates of high residual gradient after ViV.6 7 However, the vast majority of previous studies have focused on the post-ViV haemodynamic status and did not compare with the pre-ViV and post-SAVR status. Furthermore, significant haemodynamic and clinical benefit may occur following ViV despite the presence of high residual gradients after the procedure. For example, a reduction in pre-ViV mean gradient of 60 mm Hg to post-ViV of 25 mm Hg will likely significantly improve the functional status of the patient.
The concept of treatment futility has been applied in the context of transcatheter valve therapy to functional and clinical outcomes, but the same principles may also be applied to haemodynamic outcomes.14–16 We thus defined haemodynamic futility as the absence of significant improvement in transprosthetic gradient (<10 mm Hg) and AR (<1 grade). In the present study, ViV was haemodynamically futile in 7.6% of the patients.
Pre-existing PPM of the surgical BP was associated with increased risk of high residual gradients after ViV and haemodynamic futility of ViV. Indeed, in a patient with severe pre-existing PPM, the EOAi is already small at the time of SAVR and a ViV generally further reduces the EOAi and worsens the haemodynamic status (ie, fitting a second doll in an already small doll). Patients with no-pre-existing PPM nonetheless harboured less improvement in EOA and gradients compared with those with pre-existing PPM (online supplementary table 3, online supplementary figure 3). Patients with no pre-existing PPM also often are those in whom regurgitation or mixed dysfunction is the predominant mechanism of BP failure. In such patients, the valve haemodynamic improvement following ViV is essentially related to the reduction in transprosthetic regurgitation, and there is thus no or minimal decrease in gradients per se.
Overall, these findings further emphasise the paramount importance of: (1) avoiding PPM, especially severe PPM, at the time of initial SAVR and (2) performing systematic screening for the presence of pre-existing PPM and/or acquired BP stenosis at the time of pre-ViV assessment. The new BP generations implanted in a supra-annular position allow to surgeon to achieve the prevention of severe PPM in most patients. Another option would be to perform a transcatheter aortic valve replacement in place of SAVR. Indeed, PPM is less frequent with transcatheter aortic valve replacement than with SAVR, and ViV within a THV is associated with better haemodynamics results than within a surgical BP.17
BP mode of failure by stenosis is also associated with higher risk of high residual gradients and haemodynamic futility following ViV. This may be explained by the fact that the thickened and calcified leaflets of the failed surgical BP may limit the expansion of the THV within the BP during ViV.
Hence, the haemodynamic utility versus futility ratio of the ViV procedure should be carefully evaluated in patients with severe pre-existing PPM and/or BP stenosis. In patients with severe PPM, one may consider fracturing the BP stent with a non-compliant balloon.18 19 Further studies are however needed to assess the risk–benefit ratio and long-term outcomes of this procedure. The findings of this study also provide support to the development of new generations of surgical BPs with expansible stents to allow the implantation of a larger THV at the time of ViV.
Several studies reported that THV with supra-annular design are associated with lower rates of high residual gradient after VIV compared with THV with intra-annular design, especially in patients with small BPs and/or BPs with severe PPM.4 7 20 In the present study, the THV design was not found to be associated with worse haemodynamic outcomes after ViV. Intra-annular THVs were used in the vast majority of the patients included in this series, and the supra-annular THVs were used specifically in small BPs.
Conclusions
ViV is associated with a significant improvement in valve haemodynamics compared with pre-ViV in >90% of patients. However, SVH as defined by VARC2 criteria occurred in 61% (57% high residual gradient) of patients post-ViV versus 24% early post-SAVR. Furthermore, ViV was able to restore valve function to the post-SAVR level in only 34% of patients. Pre-existing PPM and BP failure by stenosis were the main factors associated with higher rates of high residual gradient, SVH and haemodynamic futility following ViV. These findings provide strong support for the prevention of PPM at the time of initial SAVR and for the consideration of BP stent fracturing at the time of ViV if severe pre-existing PPM is present.
Acknowledgments
We would like to thank Céline Boutin, Martine Parent and Danielle Tardif for their help in echocardiographic reading and data gathering.
Footnotes
Contributors: The study was planned and conducted by A-SZ and PP. AD, ES, JR-C, MC, RDL, J-MP, DD, SM, ER, CC and TR-G were involved in clinical and/or echocardiographic data acquisition. A-SZ, AD, ES, M-AC and PP performed and/or supervised data management and statistical analyses. A-SZ, AD, ES, M-AC, GO, EG, RR and PP contributed to the interpretation of the data. The manuscript was drafted by A-SZ. ES, M-AC, GO, EG and PP made critical revisions. All authors read and approved the final manuscript.
Funding: This study was funded by a Foundation Scheme Grant (#FDN-143225) from Canadian Institutes of Health Research (Ottawa, Ontario, Canada). PP holds the Canada Research Chair in Valvular Heart Disease. JR-C holds the Research Chair on the Development of Interventional Therapies for Structural Heart Diseases – Family Jacques Larivière Foundation.
Competing interests: PP and JR-C report research grant from Edwards Lifesciences and Medtronic.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–89. 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease: the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 3. Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014;312:162–70. 10.1001/jama.2014.7246 [DOI] [PubMed] [Google Scholar]
- 4. Simonato M, Webb J, Kornowski R, et al. Transcatheter replacement of failed bioprosthetic valves: large multicenter assessment of the effect of implantation depth on hemodynamics after aortic valve-in-valve. Circ Cardiovasc Interv 2016;9 10.1161/CIRCINTERVENTIONS.115.003651 [DOI] [PubMed] [Google Scholar]
- 5. Webb JG, Mack MJ, White JM, et al. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: partner 2 valve-in-valve registry. J Am Coll Cardiol 2017;69:2253–62. 10.1016/j.jacc.2017.02.057 [DOI] [PubMed] [Google Scholar]
- 6. Deeb GM, Chetcuti SJ, Reardon MJ, et al. 1-year results in patients undergoing transcatheter aortic valve replacement with failed surgical bioprostheses. JACC Cardiovasc Interv 2017;10:1034–44. 10.1016/j.jcin.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 7. Pibarot P, Simonato M, Barbanti M, et al. Impact of pre-existing prosthesis-patient mismatch on survival following aortic valve-in-valve procedures. JACC Cardiovasc Interv 2018;11:133–41. 10.1016/j.jcin.2017.08.039 [DOI] [PubMed] [Google Scholar]
- 8. Lancellotti P, Pibarot P, Chambers J, et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:589–90. 10.1093/ehjci/jew025 [DOI] [PubMed] [Google Scholar]
- 9. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg 2012;42:S45–S60. 10.1093/ejcts/ezs533 [DOI] [PubMed] [Google Scholar]
- 10. Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:3382–90. 10.1093/eurheartj/ehx303 [DOI] [PubMed] [Google Scholar]
- 11. Dvir D, Bourguignon T, Otto CM, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation 2018;137:388–99. 10.1161/CIRCULATIONAHA.117.030729 [DOI] [PubMed] [Google Scholar]
- 12. Kuehnel RU, Hartrumpf M, Erb M, et al. Hemodynamic performance of endovascular valves as valve-in-valve in small stented bioprosthesis. Thorac Cardiovasc Surg 2017;65 10.1055/s-0036-1586492 [DOI] [PubMed] [Google Scholar]
- 13. Zenses AS, Evin MA, Stanová V, et al. Effect of size and position of self-expanding transcatheter valve on haemodynamics following valve-in-valve procedure in small surgical bioprostheses: an in vitro study. EuroIntervention 2018;14:282-289 10.4244/EIJ-D-17-00875 [DOI] [PubMed] [Google Scholar]
- 14. Puri R, Iung B, Cohen DJ, et al. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J 2016;37:2217–25. 10.1093/eurheartj/ehv756 [DOI] [PubMed] [Google Scholar]
- 15. Lindman BR, Alexander KP, O’Gara PT, et al. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc Interv 2014;7:707–16. 10.1016/j.jcin.2014.01.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanz J, Pilgrim T. Transcatheter aortic valve replacement in patients with chronic lung disease: utile or futile? JACC Cardiovasc Interv 2017;10:2294–6. 10.1016/j.jcin.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 17. Pibarot P, Weissman NJ, Stewart WJ, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort--a analysis. J Am Coll Cardiol 2014;64:1323–34. 10.1016/j.jacc.2014.06.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johansen P, Engholt H, Tang M, et al. Fracturing mechanics before valve-in-valve therapy of small aortic bioprosthetic heart valves. EuroIntervention 2017;13:e1026–e31. 10.4244/EIJ-D-17-00245 [DOI] [PubMed] [Google Scholar]
- 19. Nielsen-Kudsk JE, Andersen A, Therkelsen CJ, et al. High-pressure balloon fracturing of small dysfunctional Mitroflow bioprostheses facilitates transcatheter aortic valve-in-valve implantation. EuroIntervention 2017;13:e1020–e5. 10.4244/EIJ-D-17-00244 [DOI] [PubMed] [Google Scholar]
- 20. Simonato M, Azadani AN, Webb J, et al. In vitro evaluation of implantation depth in valve-in-valve using different transcatheter heart valves. EuroIntervention 2016;12:909–17. 10.4244/EIJV12I7A149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2018-000854supp001.docx (129.5KB, docx)