Abstract
Objective
Atherosclerosis is an autoimmune condition and the underlying cause of coronary artery disease (CAD). Circulating antibodies to self-antigens can have a pathogenic or protective function in atherosclerosis. The objective of the study was to understand the association of autoantibody levels with CAD and its correlation with circulating immune cells.
Methods
We assessed antigen concentration and antibodies to apolipoprotein B (ApoB) and heat shock protein (HSP)60 by ELISA in 252 acute coronary syndromes (ACS), 112 patients with stable angina (SA) and 203 healthy controls from Indian population. T cells in peripheral blood mononuclear cells (PBMC) were enumerated by flow cytometry. Cytokine concentrations were measured by multiplex assay.
Results
IgG and IgM antibodies to ApoB and HSP60 proteins were significantly lower in patients with ACS while only IgG levels to ApoB were lower in patients with SA, compared with control. Subjects in the highest tertile of antibodies showed significantly lower OR for ACS (IgG 0.52, 95% CI 0.31 to 0.88, p=0.02 and IgM 0.58, 95% CI 0.34 to 0.98, p=0.04), ApoB100 (IgG 0.52, 95% CI 0.31 to 0.88, p=0.02 and IgM 0.58, 95% CI 0.34 to 0.99, p=0.04) and HSP60, respectively. Interestingly, T helper 17 (TH17) cells showed an inverse relationship with ApoB and HSP60 IgG antibodies (r2=−0.17, p<0.001 and r2=−0.20, p<0.001, respectively), while interleukin 17 concentrations were negatively correlated with IgM antibodies to the proteins
Conclusion
This study shows that higher antibodies to ApoB and HSP60 proteins are less often associated with ACS and that these antibodies are inversely associated with inflammatory Th17 cells.
Keywords: coronary artery disease; autoimmune response; antibodies, heat shock proteins, apolipoprotein B, T helper cells
Introduction
Coronary artery disease (CAD) is a leading cause of death worldwide with Asians being a highly vulnerable ethnic group.1 Recent studies suggest that the Asians—especially the south Asians—and Indians differ markedly from their Caucasian counterparts with respect to risk factors and severity of the disease.2 3 The levels of biomarkers are also known to differ in different ethnic groups.4
Atherosclerosis, the underlying cause of CAD, is now considered as an autoimmune disease initiated by antigens such as oxidised low-density lipoprotein (Ox-LDL), apolipoprotein B (ApoB) and heat shock protein (HSP)60.5–8 Several studies have reported a positive association between anti-Ox-LDL antibodies and CAD,9 10 while others find no such associations.11 12 In a recent study, lower levels of antibodies to malondialdehyde-modified ApoB100 peptides p45 and p210 were reported to be associated with higher risk of coronary events.13
Apart from their role as molecular chaperones, HSPs are also immunodominant antigens.14 Higher levels of anti-HSP65 antibodies were reported to be associated with cardiovascular severity and mortality.15 These antibodies were also found to mediate endothelial cytotoxicity, further strengthening their pathogenic role.16 On the contrary, studies in the last two decades have reported an immunoregulatory phenotype for HSP60, suggesting that these autoantigens also have the potential to induce a regulatory immune response to control inflammation in atherosclerosis.8 17 18
Detailed analysis of antibody levels to different antigens has not been reported for the Indian population. We have earlier reported a protective role for peptides derived from ApoB and HSP60 proteins in the murine model of atherosclerosis. Protection was mediated by increasing antigen-specific regulatory T cells (Tregs) and reducing the inflammatory T helper 17 (Th17) cells. In another clinical study, we observed an increase in Th17 cells in the peripheral blood of patients with CAD. In order to understand the role of ApoB and HSP60 antigens and antibodies in CAD and their relationship to Th17 and Treg cells, we enumerated the plasma antibody levels to ApoB100 and HSP60 proteins, and two peptides derived from them and the percentage of proinflammatory T cells and regulatory subtype in the peripheral blood. The present investigations were carried out in an Indian population who have an increased susceptibility to CAD at a much younger age and are also reported to have higher pathogen burden and antigen load.19–22
Methods
Study design
The investigation and biological sample collections were approved by the institutional ethics committees of Thrombosis Research Institute and Narayana Hrudayalaya Hospital and conformed to the Declaration of Helsinki and the Indian Council of Medical Research (India) guidelines. An informed consent was taken from all the participants prior to enrolment. The study included patients in the age group 18–60 years, admitted to the cardiology department of Narayana Hrudayalaya Hospital, with no previous report of any cardiac problem. Patients were further categorised into stable angina (SA) if they were admitted for coronary angiography with chest pain spreading to the left shoulder and arm upon exertion which could be relieved by rest, and their angiogram showed more than 50% block in the coronary arteries. Patients admitted with acute myocardial infarction require emergency treatment with a positive test for creatine kinase MB and troponin I (acute coronary syndrome, ACS). Healthy individuals with no previous record or symptoms of cardiac diseases with a normal ECG report from the community and with the same ethnic background as patients were included as the control population. Samples were collected within 24 hours from admission to the hospital. Plasma and serum samples were stored at −80°C until use. A standardised questionnaire was used to record demographic data.
Biological measurements in plasma
Total cholesterol, triglycerides and high-density lipoprotein cholesterol (Bayer Diagnostics, Randox Laboratories, Dade-Behring, UK) were determined using the Cobas Fara II Clinical Chemistry autoanalyser (F Hoffman La Roche, Switzerland), following manufacturer’s instructions. LDL cholesterol was calculated using Friedwald’s formula.23
Measurement of autoantibodies to antigens
Antibodies were determined by coating ApoB100, HSP60 (1 µg/mL), in ELISA plates. Plasma from patients and control samples were compared at a dilution of (1:100). Antihuman IgG or IgM conjugated to peroxidase and TMB substrate were used to develop the plates and absorbance was measured at 450 nm with a reference wavelength of 570 nm. Two peptides derived from human ApoB100 (peptide sequence: CI688EIGLEGKGFEPTLEALFGK707, numbered including signal peptide) equivalent to p45 reported in the literature24 25 and an epitope from HSP60 (peptide sequence: CA153ELKKQSKPVT163)26 were taken for ELISA based on earlier data from the lab.17 27 Peptides (1 µg/mL) were coated on Maleimide-activated 96-well plates (Pierce, Thermo Scientific, USA). Cysteine (10 µg/mL) was used to block excess maleimide and was developed and measured as mentioned earlier. Normal pooled human plasma was used as a control in all the plates. About 10% of the samples were repeated at random and few samples were repeated at different positions in the same assay plate to increase the reliability of the antibody measurement. Interassay and intra-assay coefficients of variation were lower than 8% and 10%, respectively.
Measurement of antigen levels in plasma
The antigen concentration of HSP60 in serum was measured by ELISA as per the manufacturer’s instructions (Wuhan Fine Biological Technology, Wuhan, China). ApoB levels were analysed by clinical chemistry analyser as described earlier.
Cytokine detection
Plasma concentrations of cytokines interleukin (IL)6 and IL17 were measured by multiplex assay by Milliplex kits using the Luminex platform (Merck Millipore, USA).
Enumeration of immune cells of adaptive immune response
The T cell subsets were determined by flow cytometry as described earlier.28 Briefly, PBMCs were labelled with anti-CD3-APC H7, anti-CD4-FITC, anti-IL17-APC for Th17 cells and anti-CD25-APC, Fox P3-PE in a separate tube for Tregs staining. Samples were acquired using FACS Canto II flow cytometer analysed using FLOWJO V.7.6.5 (Tree Star, Oregon, USA).
Statistical analysis
Quantitative data were tested for normality using Kolmogorov-Smirnov test and were log transformed for analysis, wherever a deviation from normality was observed (lipid levels, body mass index (BMI), waist-hip ratio (WHR) and cytokine levels). The qualitative and categorical data were analysed using frequencies/proportions. Baseline data were compared between controls and patients with SA or ACS using Χ2 tests for categorical variables and independent t-test for quantitative data. Numerical results are expressed as mean±SD. Antibody levels in control and cases were compared using multivariate analysis using epidemiological risk factors such as age, gender, current smoking, BMI, WHR, diabetes and hypertension as covariates. To estimate the OR of association of lower levels of antibodies, the values are categorised into tertiles. The associations of higher tertiles of antibodies with CAD were assessed by logistic regression models using the first tertile as the reference. In the logistic regression models with multiple biomarkers, associations were identified taking the first tertiles of both as the reference. ORs and their 95% CI were obtained as estimates of the association between antibodies and CAD. Correlation analysis of the antibody level to the classical risk factors, antigen level and T cell subsets and cytokine level was performed using Pearson bivariate correlation. All statistical analyses were performed using SPSS V.17.0 for Windows and a p value <0.05 was considered statistically significant.
Results
Demographic characteristics of the study population
The study included 252 patients with ACS and 112 patients with SA compared with 203 controls. The prevalence of classical risk factors (diabetes, hypertension and current smoking) was significantly higher among patients. Lipid levels were in the normal range and lower in patients compared with controls, which could be attributed to statin use in patients. Other prevalent medications in patients included beta blockers, ACE inhibitors and aspirin. In patients, the percentage of TH17 cells and serum concentration of IL17 was significantly higher while that of regulatory T cells was significantly lower (table 1).
Table 1.
Demographic characteristic of study population
| Variables | Control (n=203) | ACS (n=252) | SA (n=112) |
| Age (years) | 46.67±8.3 | 47.7±8.7 | 50.86±6.3*** |
| Gender (M/F) | 175/28 | 233/19 | 100/12 |
| Risk factors | |||
| Diabetes, n (%) | 23 (11.3) | 109 (43.2)*** | 51 (45.5)*** |
| Hypertension, n (%) | 22 (10.8) | 71 (28.1)*** | 45 (40.1)*** |
| Smoking, n (%) | 50 (24.6) | 124 (49.2)*** | 39 (34.8)* |
| BMI | 25.15±3.78 | 25.39±3.69 | 25.38±3.49 |
| WHR | 0.92±0.06 | 0.93±0.07 | 0.93±0.09 |
| Lipid levels (mmol/L) | |||
| TC | 5.03±0.010.25 | 4.32±1.05*** | 3.68±1.13*** |
| TG | 1.9±0.010.25 | 1.78±0.92 | 1.88±0.96 |
| HDL | 1.02±0.32 | 0.84±0.24*** | 0.76±0.23*** |
| LDL | 3.15±0.91 | 2.72±0.93*** | 2.1±1.02*** |
| FBS | 6.35±2.26 | 9.27±3.77*** | 7.72±4.97*** |
| Circulating T cells and cytokines | |||
| Th17 | 3.71±1.15 | 4.91±1.61*** | 4.56±1.91*** |
| Treg | 4.01±1.34 | 3.29±01.38*** | 3.18±01.25*** |
| IL17 (pg/mL) | 12.07±1.71 | 21.43±2.18** | 14.25±1.71* |
| Medications, n (%) | |||
| Beta-blockers | 4 (1.9) | 194 (76.9)*** | 82 (73.2)*** |
| ACE inhibitors | 2 (0.9) | 131 (51.9)*** | 19 (16.9)*** |
| Statin | 3 (1.4) | 229 (90.8)*** | 104 (92.8)*** |
| Aspirin (antiplatelet) | 3 (1.4) | 230 (91.2)*** | 101 (90.1)*** |
| Number of affected vessels, n (%) | |||
| 1 | 120 (47.6) | 27 (24.1) | |
| 2 | 49 (19.4) | 29 (25.8) | |
| 3 | 25 (9.9) | 42 (37.5) | |
The values are expressed as mean±SD or numbers (%). All the values were log transformed prior to analysis. A comparison was made between age and gender-matched controls and patients.
P value <0.05 was considered as significant. *P<0.05; **P<0.01; ***P<0.001.
ACS, acute coronary syndrome; BMI, body mass index; FBS, fasting blood sugar; HDL, high-density lipoprotein; IL, interleukin; LDL, low-density lipoprotein; SA, stable angina; TC, total cholesterol; TG, triglycerides; WHR, waist-hip ratio.
Autoantibody levels in patients and control
Both IgM and IgG antibodies to the antigens were lower in patients compared with controls. The antibodies showed an interesting trend, with statistically significant difference in ApoB100 IgG (p=0.01) and IgM (p=0.03), HSP60 IgG (p=0.04) and IgM (p=0.02), in patients with ACS, while only anti-IgG against ApoB100 (p=0.01) was significantly lower in patients with SA. Antibodies reacting to HSP60 peptide were found to be significantly lower in only patients with ACS, while no difference was observed for the ApoB peptide (table 2).
Table 2.
Antibody levels in patients and control
| Variable | Control (n=203) | ACS (n=252) | SA (n=112) |
| ApoB100 IgG | 1.99±0.05 | 1.76±0.04* | 1.7±0.07* |
| ApoB100 IgM | 0.81±0.04 | 0.68±0.03* | 0.71±0.05 |
| HSP60 IgG | 2.23±0.05 | 2.05±0.05* | 2.03±0.07 |
| HSP60 IgM | 0.92±0.04 | 0.8±0.04* | 0.8±0.05 |
| ApoB100 IgG (peptide) | 1.45±0.05 | 1.35±0.05 | 1.42±0.09 |
| HSP60 IgG (peptide) | 2.21±0.09 | 1.94±0.08* | 2.0±0.19 |
Antibody levels were measured by ELISA. Absorbance values at a dilution of 1:100 are compared between control and patient group using multivariate analysis by taking age, gender, body mass index (BMI), current smoking, hypertension and diabetes as covariates.
P value <0.05 was considered as significant. *P<0.05.
ACS, acute coronary syndrome; ApoB, apolipoprotein B; HSP, heat shock protein; SA, stable angina.
Regression analysis
Regression analysis was carried out for ACS and SA groups individually and by taking patients with CAD by combining both the groups. The antibody levels were divided into three equal tertiles for the analysis. As seen in table 3, higher autoantibody levels were less often associated with ACS. Subjects in the highest tertile of IgG and IgM antibodies showed a lower OR for both ApoB (IgG 0.52, 95% CI 0.31 to 0.88, p=0.02 and IgM 0.58, 95% CI 0.34 to 0.98, p=0.04) and HSP60 (IgG 0.52, 95% CI 0.31 to 0.88, p=0.02 and IgM 0.59, 95% CI 0.34 to 0.99, p=0.04). The OR for anti-ApoB antibodies (IgG+IgM) taken together was 0.45 (95% CI 0.25 to 0.79, p=0.01), and that for HSP60 was 0.51 (95% CI 0.28 to 0.92, p=0.02) (table 3). The highest tertile of HSP60 peptide was associated with a significantly lower OR of 0.036 (95% CI 0.002 to 0.87, p=0.04) for ACS and 0.44 (95% CI 0.22 to 0.86, p=0.016) for CAD, while the combination of ApoB and HSP60 peptides showed an OR of 0.18 (95% CI 0.03 to 0.88, p=0.03) and 0.7 (95% CI 0.52 to 0.95, p=0.021) for ACS and CAD, respectively. The patterns of ORs were similar for patients with CAD (ACS+SA), while only ApoB IgG antibodies showed a significant association with SA.
Table 3.
Logistic regression analysis
| Variable | OR (95% CI), P values | ||
| ACS (n=252) | SA (n=112) | CAD (n=364) | |
| ApoB100 IgG | 0.52 (0.31 to 0.88), 0.02 | 0.47 (0.25 to 0.89), 0.02 | 0.53 (0.33 to 0.84), 0.008 |
| ApoB100 IgM | 0.58 (0.34 to 0.98), 0.04 | 0.85 (0.45 to 1.61), NS | 0.66 (0.42 to 1.06), 0.089 |
| HSP60 IgG | 0.52 (0.31 to 0.88), 0.02 | 0.57 (0.3 to 1.07), NS | 0.57 (0.35 to 0.92), 0.02 |
| HSP60 IgM | 0.58 (0.34 to 0.99), 0.04 | 0.61 (0.31 to 1.21), NS | 0.59 (0.36 to 0.95), 0.031 |
| ApoB IgG+M | 0.45 (0.25 to 0.79), 0.01 | 0.65 (0.32 to 1.29), NS | 0.54 (0.33 to 0.89), 0.016 |
| HSP60 IgG+M | 0.51 (0.28 to 0.92), 0.02 | 0.67 (0.33 to 1.39), NS | 0.59 (0.35 to 1.01), 0.052 |
| ApoB+HSP60 IgM | 0.67 (0.38 to 1.16), NS | 0.51 (0.26 to 1), 0.05 | 0.51 (0.32 to 0.83), 0.007 |
| ApoB HSP60 IgG | 0.44 (0.23 to 0.85), 0.014 | 0.59 (0.3 to 1.16), NS | 0.56 (0.35 to 0.91), 0.018 |
| ApoB peptide | 0.11 (0.005 to 2.46), NS | 0.44 (0.01 to 15.41), NS | 0.53 (0.18 to 1.53), NS |
| HSP60 peptide | 0.036 (0.002 to 0.87), 0.04 | 0.28 (0.03 to 2.73), NS | 0.44 (0.22 to 0.86), 0.016 |
| ApoB+HSP60 peptide | 0.187 (0.039 to 0.88), 0.03 | 0.73 (0.28 to 1.9), NS | 0.7 (0.52 to 0.95), 0.021 |
The antibody levels were divided into tertiles. The association of the antibody level in the third tertile in comparison to the first tertile was assessed using logistic regression analysis taking age, gender, body mass index (BMI), waist-hip ratio (WHR), current smoking, hypertension and diabetes as covariates.P value <0.05 was considered significant.
ACS, acute coronary syndrome; ApoB, apolipoprotein B; CAD, coronary artery disease; HSP, heat shock protein; NS, not significant; SA, stable angina.
Circulating antigen levels in patients and control
Next, we looked at the circulating antigen levels in patients with ACS and control. The antigen concentration of HSP60 (33.77±3.52 vs 49.6±3.43, p=0.002) was significantly higher in patients compared with control. On the contrary, ApoB100 (104.26±1.26 vs 90.04±1.28, p<0.001) levels were lower in patients (figure 1).
Figure 1.
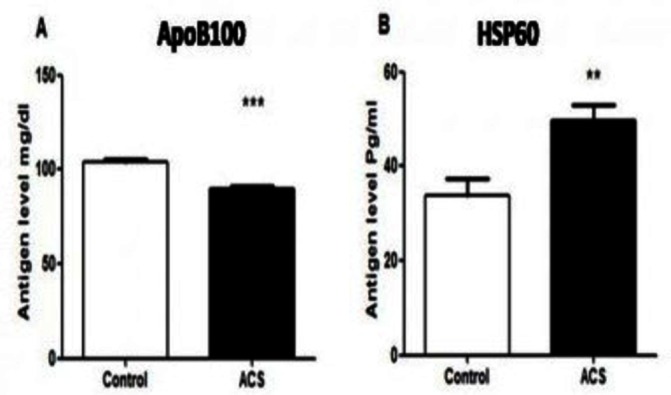
Antigen concentration in control and patients. The antigen concentrations in the serum of control and patients with acute coronary syndrome (ACS) were measured by commercial kits as mentioned in the Methods section. (A) Apolipoprotein (Apo)B100. (B) Heat shock protein (HSP)60.
Correlation analysis
IgG antibodies to ApoB and HSP60 proteins showed an inverse relationship with Th17 cells (r2=−0.17 and r2=−0.21, p<0.001 for ACS and r2=−0.12 and r2=−0.16, p<0.001 for CAD) (table 4). The inflammatory cytokines IL17 and IL6 showed a negative correlation with anti-IgM antibodies to ApoB (r2=−0.30, p<0.001 and r2=−0.21, p<0.05) and HSP60 (r2=−0.27, p<0.001 and r2=−0.25, p<0.05), respectively. IL17 levels were negatively correlated with antibodies to HSP60 peptide (r2=−0.43, p<0.001), while IL6 levels showed negative correlation to antibodies to ApoB peptide (r2=−0.24, p<0.05 for ACS (table 4)). IL6 levels were inversely correlated with anti-IgM antibodies in patients with SA and CAD (SA+ACS), but IL17 showed a negative correlation with antibodies only in patients with ACS. Antibodies to all the antigens showed a significant negative association with smoking (−0.09 to −0.18, p<0.05), while IgM antibodies to ApoB and HSP60 (r2=−0.11 to −0.13, p<0.05) showed an inverse correlation with prevalence of hypertension. Prevalence of diabetes showed inverse correlation with IgG antibodies to ApoB (r2=−0.10, p<0.05) (table 4). The pattern of correlation was very similar for ACS and CAD groups, but in the SA group anti-IgM antibodies showed a negative correlation with hypertension and IL6 levels and an inverse correlation was observed between anti-IgG antibodies and diabetes.
Table 4.
Correlation between autoantibody levels and cardiovascular risk factors and cells of adaptive immune response and cytokines
| Antibody | ApoB100 | HSP60 | HSP60 peptide | ApoB100 peptide | ||
| Variables | IgG | IgM | IgG | IgM | IgG | IgG |
| Adaptive immune cells and cytokines | ||||||
| Th17 | ||||||
| ACS | −0.17** | −0.06 | −0.20** | −0.05 | −0.029 | −0.082 |
| SA | −0.016 | 0.001 | −0.023 | 0.043 | −0.057 | −0.125 |
| CAD | −0.121** | −0.043 | −0.161** | −0.032 | −0.042 | −0.064 |
| Tregs | ||||||
| ACS | 0.04 | −0.06 | 0.01 | −0.08 | −0.143 | −0.155 |
| SA | 0.064 | 0.009 | 0.034 | −0.025 | −0.248* | −0.0323** |
| CAD | 0.038 | −0.024 | −0.010 | −0.058 | −0.175* | −0.187* |
| IL17 | ||||||
| ACS | −0.1 | −0.30** | −0.04 | −0.27** | −0.092 | −0.427** |
| SA | 0.044 | 0.111 | 0.056 | 0.094 | 0.034 | 0.002 |
| CAD | 0.009 | −0.015 | 0.022 | −0.022 | −0.236* | −0.135 |
| IL6 | ||||||
| ACS | −0.2 | −0.21* | −0.12 | −0.25* | −0.242* | −0.147 |
| SA | −0.068 | −0.265** | −0.132 | −0.246* | −0.189 | −0.163 |
| CAD | −0.090 | −0.197* | −0.104 | −0.221** | −0.138 | −0.363** |
| Risk factors | ||||||
| Smoking | ||||||
| ACS | −0.14** | −0.11* | −0.13** | −0.09* | −0.088 | −0.232** |
| SA | −0.055 | −0.107 | −0.039 | −0.088 | −0.097 | −0.060 |
| CAD | −0.085* | −0.105* | −0.082 | −0.087* | −0.093 | −0.192* |
| Hypertension | ||||||
| ACS | −0.07 | −0.11* | −0.05 | −0.12* | −0.099 | −0.068 |
| SA | −0.099 | −0.139* | −0.078 | −0.196** | 0.003 | −0.043 |
| CAD | −0.068 | −0.099* | −0.062 | −0.133** | −0.043 | −0.007 |
| Diabetes | ||||||
| ACS | −0.099* | −0.08 | −0.07 | −0.06 | −0.077 | 0.069 |
| SA | −0.186** | −0.104 | −0.190** | −0.157** | −0.083 | −0.040 |
| CAD | −0.142** | −0.072 | −0.140** | −0.091* | −0.054 | 0.047 |
| FBS | ||||||
| ACS | −0.1 | −0.05 | −0.07 | −0.04 | −0.112 | −0.133 |
| SA | −0.110 | −0.047 | −0.106 | −0.086 | −0.088 | −0.084 |
| CAD | −0.091 | −0.035 | −0.091 | −0.041 | −0.113 | −0.138 |
Pearson bivariate correlation was used for correlation of IgG and IgM antibodies with current smoking (CS), hypertension (HPT), diabetes (DBT), fasting blood sugar (FBS), cells of adaptive immune response Th17 and atherogenic antigens.
P value <0.05 was considered significant. *P<0.05; **P<0.01.
ACS, acute coronary syndrome; ApoB, apolipoprotein B; CAD, coronary artery disease; FBS, fasting blood sugar; HSP, heat shock protein; IL, interleukin; LDL, low-density lipoprotein; n; SA, stable angina; Th17, T helper 17 cell; Tregs, regulatory T cells.
Discussion
In the current study, we investigated the serum levels of antibodies to ApoB and HSP60 proteins and peptides, free antigens, cytokine levels and proportion of Th17 cells in the peripheral blood of patients with CAD and healthy controls in a high-risk Indian population. Contrary to earlier reports, we observed a lower level of antibodies to HSP60 and ApoB proteins in patients with ACS, compared with healthy individuals, while circulating antigen levels to HSP60 were significantly higher in these patients. An inverse correlation was observed between antibody levels and Th17 cells and IL17 concentration in serum. Surprisingly, antibodies were not significantly different in patients with SA.
Natural cross-reactive IgM antibodies recognising phospholipids were reported to have a protective effect on atherosclerosis.29 30 Later, IgG antibodies specific for an Ox-LDL epitope was found to be protective in animal models, although it did not translate in human clinical trials.7 31 IgG antibodies are considered proatherogenic except for a few recent studies, where B cell depletion was associated with decreased atherosclerosis with a reduction in Ox-LDL-specific IgG antibodies.32 HSP60 is a dominant antigen that is recognised during infection and displays immunomodulatory effects during autoimmune diseases.33–35 In atherosclerosis, high level of antibodies to HSP60 is generally described to play a pathogenic role.36 37 We observed a lower level of autoantibodies to HSP60 protein as well as a peptide epitope (CA153ELKKQSKPVT163) derived from it. This is in contrast to the above-mentioned studies associating HSP60 with a pathological complication of atherosclerosis.
Higher levels of IgG and IgM antibodies to ApoB and HSP60 proteins were associated with 48% and 41% lower odds of ACS while total antibodies to ApoB and HSP60 (IgG+IgM) lowered the odds by 65% and 49%, respectively. Presence of antibodies to HSP60 peptide was also associated with a lower OR for ACS. In a study on immune tolerance to prevent atherosclerosis, we observed an increase in antibody levels to HSP60 protein and peptide in mice treated with an immunomodulatory molecule in association with atheroprotection (unpublished observation), which supports a beneficial role for antibodies in atherosclerosis.
Similar to our results, higher level of antibodies to p210 and p45 from ApoB protein was shown to be associated with lower risk of ACS and severity of coronary atherosclerosis.25 In a large population of more than 5000 subjects with 15 years of follow-up, subjects who developed ACS had a lower basal level of IgM antibodies to ApoB-derived peptides (p45 and p210) and lower IgG to p210.13 Antibodies to p210 peptide were also associated with lower risk of postoperative mortality, myocardial infarction and severity of lesions in diabetic and carotid endarterectomy patients.38 39 In a recent study, Khamis et al reported that subjects in the highest tertile of total IgG had 60% lower risk of CAD compared with those in the lowest tertile,40 suggesting that antibodies could have a protective function in cardiovascular disease (CVD).41 Antibodies as therapeutics are gaining importance as in a recent study, a novel monoclonal antibody targeting lipoprotein-bound human apolipoprotein C-III (Apo-III) was found to promote the clearance of circulating ApoC-III thereby reducing circulating triglyceride levels. These findings suggest that an antibody to ApoC-III would be an effective therapeutic approach to protect against coronary heart disease.42
We observed an inverse correlation between IgG antibodies to ApoB100 and HSP60 with circulating Th17 cells. Abnormalities in specific subsets of T cells including Th17 and CD4+ CD28 cells have been shown to be involved in plaque instability and ACS by our group as well as several other studies.28 43 TH17 cells were found to help in B cell class switch to produce autoantibodies which induce arthritis.44 It is likely that when the Th17 cells are amplified there is a reduction in antibodies, which prevents the sequestering of free antigens in circulation. The cytokines IL17 and IL6 also showed an inverse correlation with antibody levels, further suggesting that the antibody levels are reduced in the presence of an inflammatory milieu. Smoking was inversely correlated with autoantibodies in our study. Smoking is suggested to be a predisposing factor for autoimmune diseases45 and is a strong contributing factor for the progression and fatal outcomes in CVD. Smoking increases oxidative stress and endothelial dysfunction and induces a procoagulant and inflammatory environment.46 These results suggest an inverse correlation between proinflammatory conditions and antibodies in the studied population.
As oxidative stress increases due to risk factors, there is an increase in oxidation of LDL and increased amount of HSP60 which may be released in circulation. Under these conditions, the circulating antibodies can sequester the antigens, which can explain the lower level of antibodies in patients compared with healthy controls. In corroboration with this hypothesis, we observed higher concentrations of HSP60 in the patients.
Currently, immunomodulatory treatment for CVD is gaining momentum. Reduction in inflammation by inducing a regulatory immune response to atherogenic antigens has shown considerable success in animal models.47 48 We have earlier reported that peptides derived from ApoB and HSP60 proteins induce immune regulation and increase regulatory T cells while decreasing Th17 cells in animal models.47 We also reported an increase in circulating Th17 cells in the Indian population.28 The results have shown an inverse correlation between Th17 cells and antibody levels, suggesting that lower serum antibodies could probably be developed as a surrogate biomarker to understand reduced inflammatory status.
In summary, our study suggests that serum antibody levels to HSP60 and ApoB100 are less often associated with the development of ACS. A negative correlation of these antibodies to Th17 cells and inflammatory cytokines suggests inflammatory response reduce the level of antibodies. Risk stratification in CVD requires screening and surveillance of a large population for 10–15 years to authenticate the usefulness of a biomarker as a risk predictor. Alternately, biomarkers associated with inflammation can be used as a marker to identify individuals responding to an anti-inflammatory therapy, which is a major challenge in immunomodulatory treatments. Enumerating T cell subtypes in peripheral blood is tedious and expensive. An important outcome of our study is the inverse correlation between TH17 cells and antibody levels which can be exploited to identify individuals responding to novel anti-inflammatory therapies. Screening of a larger population for antibodies to either the complete protein or the peptide epitope will help in authenticating the use of antibodies as a subclinical marker for understanding the effectiveness of immunomodulatory treatment.
Key messages.
What is already known about this subject?
Circulating antibodies to self-antigens can have either pathogenic or protective function in atherosclerosis.
What does this study add?
This study shows that higher antibodies to ApoB and HSP60 proteins are less often associated with acute coronary syndrome and that these antibodies are inversely associated with Th17 cells.
How might this impact on clinical practice?
Antibodies to HSP60 and ApoB proteins may be developed as a subclinical marker to understand the effectiveness of immunomodulatory/anti-inflammatory therapies for atherosclerosis.
Acknowledgments
We are extremely grateful to Late Professor Vijay V Kakkar, Scientific Chairman, Thrombosis Research Institute, Bangalore, for his constant encouragement and support. We gratefully acknowledge the support of the trustees of Thrombosis Research Institute, London and Bangalore, Mary and Gary Weston Foundation, research grants from Tata Social Welfare Trust, India (TSWT/IG/SNB/JP/Sdm) and the support from Bharathi Foundation for PhD students.
Footnotes
Dr Vijay V Kakkar died on 5 November 2016.
Contributors: The study was designed by SKV and LAM. SKV (cardiologist) authenticated the sample identification. MR was the clinical coordinator and helped in the collection of demographic and clinical data. All experiments were performed by TP. The first draft was written by TP, reviewed and corrected by LAM and SKV. All the authors approved the final draft.
Funding: The study is supported by the Indian Council of Medical Research (ICMR), Government of India (5/4/1-4/11-NCD-II), and research grants from Tata Social Welfare Trust, India (TSWT/IG/SNB/JP/Sdm).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Institutional ethics committees of Thrombosis Research Institute and Narayana Hrudayalaya Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. WHO Global status report on noncommunicable diseases 2010. 2011;176.
- 2. Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005;111:1313–20. 10.1161/01.CIR.0000157730.94423.4B [DOI] [PubMed] [Google Scholar]
- 3. Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000;356:279–84. 10.1016/S0140-6736(00)02502-2 [DOI] [PubMed] [Google Scholar]
- 4. Gijsberts CM, den Ruijter HM, Asselbergs FW, et al. Biomarkers of coronary artery disease differ between asians and caucasians in the general population. Glob Heart 2015;10:301–311. 10.1016/j.gheart.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 5. Lichtman AH, Binder CJ, Tsimikas S, et al. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J Clin Invest 2013;123:27–36. 10.1172/JCI63108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006;6:508–19. 10.1038/nri1882 [DOI] [PubMed] [Google Scholar]
- 7. Schiopu A, Frendéus B, Jansson B, et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(-/-)/low-density lipoprotein receptor(-/-) mice. J Am Coll Cardiol 2007;50:2313–8. 10.1016/j.jacc.2007.07.081 [DOI] [PubMed] [Google Scholar]
- 8. Wick G, Jakic B, Buszko M, et al. The role of heat shock proteins in atherosclerosis. Nat Rev Cardiol 2014;11:516–29. 10.1038/nrcardio.2014.91 [DOI] [PubMed] [Google Scholar]
- 9. Maggi E, Finardi G, Poli M, et al. Specificity of autoantibodies against oxidized LDL as an additional marker for atherosclerotic risk. Coron Artery Dis 1993;4:1119–22. 10.1097/00019501-199312000-00014 [DOI] [PubMed] [Google Scholar]
- 10. Inoue T, Uchida T, Kamishirado H, et al. Clinical significance of antibody against oxidized low density lipoprotein in patients with atherosclerotic coronary artery disease. J Am Coll Cardiol 2001;37:775–9. 10.1016/S0735-1097(00)01199-2 [DOI] [PubMed] [Google Scholar]
- 11. Rossi GP, Cesari M, De Toni R, et al. Antibodies to oxidized low-density lipoproteins and angiographically assessed coronary artery disease in white patients. Circulation 2003;108:2467–72. 10.1161/01.CIR.0000097122.19430.48 [DOI] [PubMed] [Google Scholar]
- 12. Uusitupa MI, Niskanen L, Luoma J, et al. Autoantibodies against oxidized LDL do not predict atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 1996;16:1236–42. 10.1161/01.ATV.16.10.1236 [DOI] [PubMed] [Google Scholar]
- 13. Björkbacka H, Alm R, Persson M, et al. Low levels of apolipoprotein B-100 autoantibodies are associated with increased risk of coronary events. Arterioscler Thromb Vasc Biol 2016;36:765–71. 10.1161/ATVBAHA.115.306938 [DOI] [PubMed] [Google Scholar]
- 14. Kaufmann SH. Heat shock proteins and the immune response. Immunol Today 1990;11:129–36. 10.1016/0167-5699(90)90050-J [DOI] [PubMed] [Google Scholar]
- 15. Birnie DH, Holme ER, McKay IC, et al. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J 1998;19:387–94. 10.1053/euhj.1997.0618 [DOI] [PubMed] [Google Scholar]
- 16. Schett G, Xu Q, Amberger A, et al. Autoantibodies against heat shock protein 60 mediate endothelial cytotoxicity. J Clin Invest 1995;96:2569–77. 10.1172/JCI118320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mundkur L, Mukhopadhyay R, Samson S, et al. Mucosal tolerance to a combination of ApoB and HSP60 peptides controls plaque progression and stabilizes vulnerable plaque in Apob(tm2Sgy)Ldlr(tm1Her)/J mice. PLoS One 2013;8:e58364 10.1371/journal.pone.0058364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mundkur L, Mukhopadhyay R, Deshpande V, et al. Comparison of oral tolerance to ApoB and HSP60 peptides in preventing atherosclerosis lesion formation in Apob48 − /Ldlr − Mice. Journal of Vaccines 2013;2013:1–13. 10.1155/2013/212367 [DOI] [Google Scholar]
- 19. Mundkur LA, Rao VS, Hebbagudi S, et al. Pathogen burden, cytomegalovirus infection and inflammatory markers in the risk of premature coronary artery disease in individuals of Indian origin. Exp Clin Cardiol 2012;17:63–8. [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta R. Recent trends in coronary heart disease epidemiology in India. Indian Heart J 2008;60(2 Suppl B):B4–18. [PubMed] [Google Scholar]
- 21. Enas EA, Singh V, Munjal YP, et al. Reducing the burden of coronary artery disease in India: challenges and opportunities. Indian Heart J 2008;60:161–75. [PubMed] [Google Scholar]
- 22. Joshi P, Islam S, Pais P, et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007;297:286–94. 10.1001/jama.297.3.286 [DOI] [PubMed] [Google Scholar]
- 23. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 24. Fagerberg B, Prahl Gullberg U, Alm R, et al. Circulating autoantibodies against the apolipoprotein B-100 peptides p45 and p210 in relation to the occurrence of carotid plaques in 64-year-old women. PLoS One 2015;10:e0120744 10.1371/journal.pone.0120744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fredrikson GN, Schiopu A, Berglund G, et al. Autoantibody against the amino acid sequence 661-680 in apo B-100 is associated with decreased carotid stenosis and cardiovascular events. Atherosclerosis 2007;194:e188–e192. 10.1016/j.atherosclerosis.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 26. van Puijvelde GH, van Es T, van Wanrooij EJ, et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:2677–83. 10.1161/ATVBAHA.107.151274 [DOI] [PubMed] [Google Scholar]
- 27. Lu X, Chen D, Endresz V, et al. Immunization with a combination of ApoB and HSP60 epitopes significantly reduces early atherosclerotic lesion in Apobtm2SgyLdlrtm1Her/J mice. Atherosclerosis 2010;212:472–80. 10.1016/j.atherosclerosis.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 28. Ponnusamy T, Srikanth KV, Manjunatha R, et al. Circulating Th17 and Tc17 cells and their imbalance with regulatory T cells is associated with myocardial infarction in young Indian patients. World J Cardiovasc Dis 2015;05:373–87. 10.4236/wjcd.2015.512043 [DOI] [Google Scholar]
- 29. Lewis MJ, Malik TH, Ehrenstein MR, et al. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 2009;120:417–26. 10.1161/CIRCULATIONAHA.109.868158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Binder CJ, Shaw PX, Chang MK, et al. The role of natural antibodies in atherogenesis. J Lipid Res 2005;46:1353–63. 10.1194/jlr.R500005-JLR200 [DOI] [PubMed] [Google Scholar]
- 31. Lehrer-Graiwer J, Singh P, Abdelbaky A, et al. FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc Imaging 2015;8:493–4. 10.1016/j.jcmg.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 32. Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med 2010;207:1579–87. 10.1084/jem.20100155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stevens TR, Winrow VR, Blake DR, et al. Circulating antibodies to heat-shock protein 60 in Crohn's disease and ulcerative colitis. Clin Exp Immunol 1992;90:271–4. 10.1111/j.1365-2249.1992.tb07941.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abulafia-Lapid R, Elias D, Raz I, et al. T cell proliferative responses of type 1 diabetes patients and healthy individuals to human hsp60 and its peptides. J Autoimmun 1999;12:121–9. 10.1006/jaut.1998.0262 [DOI] [PubMed] [Google Scholar]
- 35. Zügel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 1999;12:19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu J, Quyyumi AA, Rott D, et al. Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation 2001;103:1071–5. 10.1161/01.CIR.103.8.1071 [DOI] [PubMed] [Google Scholar]
- 37. Prohászka Z, Duba J, Horváth L, et al. Comparative study on antibodies to human and bacterial 60 kDa heat shock proteins in a large cohort of patients with coronary heart disease and healthy subjects. Eur J Clin Invest 2001;31:285–92. 10.1046/j.1365-2362.2001.00819.x [DOI] [PubMed] [Google Scholar]
- 38. McLeod O, Silveira A, Fredrikson GN, et al. Plasma autoantibodies against apolipoprotein B-100 peptide 210 in subclinical atherosclerosis. Atherosclerosis 2014;232:242–8. 10.1016/j.atherosclerosis.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 39. Asciutto G, Dias NV, Edsfeldt A, et al. Low levels of IgG autoantibodies against the apolipoprotein B antigen p210 increases the risk of cardiovascular death after carotid endarterectomy. Atherosclerosis 2015;239:289–94. 10.1016/j.atherosclerosis.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 40. Khamis RY, Hughes AD, Caga-Anan M, et al. High serum immunoglobulin G and M levels predict freedom from adverse cardiovascular events in hypertension: a nested case-control substudy of the anglo-scandinavian cardiac outcomes trial. EBioMedicine 2016;9:372–80. 10.1016/j.ebiom.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilsson J. Can antibodies protect us against cardiovascular disease? EBioMedicine 2016;9:29–30. 10.1016/j.ebiom.2016.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khetarpal SA, Zeng X, Millar JS, et al. A human APOC3 missense variant and monoclonal antibody accelerate apoC-III clearance and lower triglyceride-rich lipoprotein levels. Nat Med 2017;23:1086–1094. 10.1038/nm.4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma T, Gao Q, Zhu F, et al. Th17 cells and IL-17 are involved in the disruption of vulnerable plaques triggered by short-term combination stimulation in apolipoprotein E-knockout mice. Cell Mol Immunol 2013;10:338–48. 10.1038/cmi.2013.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hickman-Brecks CL, Racz JL, Meyer DM, et al. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun 2011;36:65–75. 10.1016/j.jaut.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shoenfeld Y, Zandman-Goddard G, Stojanovich L, et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases--2008. Isr Med Assoc J 2008;10:8–12. [PubMed] [Google Scholar]
- 46. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–15. 10.1161/ATVBAHA.113.300156 [DOI] [PubMed] [Google Scholar]
- 47. Mundkur L, Ponnusamy T, Philip S, et al. Oral dosing with multi-antigenic construct induces atheroprotective immune tolerance to individual peptides in mice. Int J Cardiol 2014;175:340–51. 10.1016/j.ijcard.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 48. Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 2001;21:1876–90. 10.1161/hq1201.100220 [DOI] [PubMed] [Google Scholar]


