Abstract
Objective
The Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) clinical trial programme findings demonstrated that apremilast, an oral phosphodiesterase 4 inhibitor, is effective for treating psoriatic arthritis (PsA). Enthesitis and dactylitis are difficult-to-treat features of PsA leading to disability and affecting quality of life. PALACE 1, 2 and 3 data were pooled to assess the efficacy of apremilast on enthesitis and dactylitis outcomes in patients with these conditions at baseline.
Methods
Patients with enthesitis (n=945) or dactylitis (n=633) at baseline were analysed after receiving double-blind treatment with placebo, apremilast 30 mg two times per day or apremilast 20 mg two times per day up to 52 weeks and continuing up to 5 years. Data were analysed through 156 weeks. Enthesitis was evaluated by Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) and dactylitis via dactylitis count.
Results
At week 24, patients receiving apremilast 30 mg two times per day demonstrated a significantly greater mean change in enthesitis (−1.3 vs −0.9; p<0.05) and dactylitis (−1.8 vs −1.3; p<0.01) vs placebo. Patients in the 30 mg dose group showed significantly greater mean (−23.6% vs −7.0%; p<0.05) and median (−50.0% vs −21.1%; p<0.05) per cent changes in MASES; mean and median per cent changes in dactylitis count were numerically, but not significantly, different for either apremilast dose in patients with dactylitis. In the patient population remaining on apremilast, observed mean and median improvements in both conditions were sustained through 156 weeks.
Conclusion
Apremilast is effective for the treatment of active PsA, including improvements in enthesitis and dactylitis up to 3 years.
Trial registration numbers
Keywords: phosphodiesterase 4 inhibitors, apremilast, anti-rheumatic agents, arthritis, psoriatic
Key messages.
What is already known about this subject?
Enthesitis and dactylitis are core features of psoriatic arthritis (PsA) that are difficult to treat and impact the overall severity and burden of disease.
Apremilast, an oral small molecule that inhibits phosphodiesterase 4, has been shown to be effective in the treatment of PsA in the Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) clinical trial programme; however, the individual studies were not designed to obtain meaningful conclusions for patients with enthesitis and/or dactylitis.
What does this study add?
The findings of this pooled analysis of data from the PALACE studies provide long-term data demonstrating the effectiveness of apremilast in improving enthesitis and dactylitis in patients with active PsA.
How might this impact on clinical practice?
Apremilast provides an effective treatment option for the long-term treatment of enthesitis and dactylitis in patients with active PsA.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease that is remarkably diverse in presentation and course and is characterised by the presence of peripheral arthritis, psoriasis, enthesitis, dactylitis and spondylitis as well as skin and nail manifestations.1–3 Enthesitis and dactylitis are distinguishing features of PsA that may be associated with more severe disease; both conditions can be difficult to treat, lead to disability and negatively impact quality of life.2 4–8 Current therapeutic options for PsA include systemic therapy with conventional disease-modifying antirheumatic drugs (DMARDs) or biological agents; however, to date, there is little evidence of the efficacy of conventional DMARDs in enthesitis or dactylitis in PsA.9
Apremilast is an oral phosphodiesterase 4 inhibitor that helps regulate the aberrant immune response in PsA that causes joint symptoms, enthesitis, dactylitis, systemic inflammation and skin disease.1 10 The efficacy and safety of apremilast in PsA are being evaluated in the Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) clinical trial programme. The PALACE 1, 2 and 3 studies are assessing the efficacy and safety of apremilast in patients with active PsA despite prior conventional DMARDs and/or biological therapy. This programme has collected a large dataset for patients with PsA, allowing for comprehensive analyses of many aspects of treatment outcomes. Results from all three PALACE studies demonstrated that apremilast is effective in reducing the signs and symptoms of PsA and in improving physical function.11–14
Although the impact of apremilast treatment on enthesitis and dactylitis was evaluated in each of the PALACE studies, the presence of these conditions was not required for inclusion, and the studies were not specifically designed to obtain meaningful conclusions for patients with enthesitis and/or dactylitis. Therefore, to assess short-term and long-term outcomes in a broad population of patients with enthesitis and/or dactylitis who entered PALACE 1, 2 and 3, a pooled analysis of data from these patients in all three studies was conducted to evaluate the therapeutic benefit of apremilast for enthesitis and dactylitis over 156 weeks.
Methods
Study design
PALACE 1, 2 and 3 (NCT01172938, NCT01212757 and NCT01212770) are phase III, multicentre, randomised, double-blind, placebo-controlled clinical trials with similar designs, which were previously described in detail.11–14 Briefly, patients were randomised (1:1:1) to receive placebo, apremilast 30 mg two times per day or apremilast 20 mg two times per day, stratified by baseline DMARD use (yes/no) and, in PALACE 3 only, by baseline psoriasis involvement of the body surface area (<3%/≥3%; online supplementary figure 1). Patients whose swollen and tender joint counts had not improved by ≥20% at week 16 were considered non-responders and continued on their initial apremilast dose or, if initially randomised to placebo, were randomised (1:1) to receive apremilast 30 mg two times per day or 20 mg two times per day (early escape). At week 24, all remaining patients on placebo were switched to apremilast 30 mg or 20 mg two times per day. On completion of the 52 week, double-blind period, patients were eligible to enter a long-term treatment phase for a total follow-up of up to 5 years.
rmdopen-2018-000669supp001.pdf (106KB, pdf)
Study population
All patients provided written informed consent prior to study initiation. Detailed inclusion and exclusion criteria for the PALACE studies have been published elsewhere.11 13 14 Briefly, adult patients required a diagnosis of PsA for ≥6 months, met the Classification Criteria for Psoriatic Arthritis (CASPAR) at study entry and were required to have three or more swollen joints and three or more tender joints at baseline. All patients were required to have had current or prior therapy with DMARDs and/or biological agents; however, they were ineligible for study inclusion if they experienced therapeutic failure of more than three DMARDs or biologicals or more than one tumour necrosis factor blocker. In addition, one or more plaque psoriasis lesions that were ≥2 cm was required in PALACE 3. The focus of the current analysis is patients who had enthesitis or dactylitis at baseline. Limited analyses were conducted in patients without enthesitis or dactylitis at baseline to examine the development of these manifestations during 24 weeks.
Concomitant therapy of any combination of methotrexate (≤25 mg/week), leflunomide (≤20 mg/day), sulfasalazine (≤2 g/day) with a stable dose for at least 16 weeks prior to screening was permitted during the study, with one DMARD dose reduction allowed between weeks 24 and 52. Stable doses of non-steroidal anti-inflammatory drugs for ≥2 weeks or oral corticosteroids (prednisone ≤10 mg or equivalent) for ≥1 month prior to screening were allowed.
Efficacy assessments
Enthesitis and dactylitis were clinically evaluated at baseline and at weeks 16, 24, 52, 65, 78, 91, 104, 117, 130, 143 and 156 (or at early termination/withdrawal). The assessments of enthesitis and dactylitis in PsA were proven to be reliable in the INSPIRE study.15
Enthesitis in the current analysis was evaluated in patients who had enthesitis at baseline, defined as a Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) >0. The MASES determines the presence or absence of pain at 13 select entheses/tendon insertions,16 with scores ranging from 0 to 13. Enthesitis outcomes included the change and per cent change in MASES from baseline and achievement of a MASES of 0 (no pain at any assessed entheses).
Dactylitis was evaluated in patients with a dactylitis count >0 at baseline, reflecting the presence (score=1) or absence (score=0) of dactylitis in each of the 20 digits (the possible dactylitis count ranges from 0 to 20). Dactylitis outcomes included the change and per cent change from baseline in dactylitis count and achievement of a dactylitis count of 0, indicating the absence of dactylitis on all 20 digits.
Statistical analysis
Data from PALACE 1, 2 and 3 were pooled in a prespecified analysis, permitting a robust analysis of data for all randomised patients with pre-existing enthesitis (baseline MASES>0) and/or dactylitis (baseline dactylitis count >0) who received one or more doses of study medication. Changes and per cent changes from baseline in MASES and dactylitis count at week 24 were prespecified and analysed using an analysis of covariance model with treatment, baseline DMARD use (yes/no) and study as factors and the baseline value as a covariate. Per cent changes from baseline were also analysed using rank transformation, with the same analysis model. Missing values were handled using the last-observation-carried-forward (LOCF) methodology. Specifically, for patients who entered early escape at week 16, the week 16 value was carried forward to week 24; for other patients, the last postbaseline value was carried forward if the week 24 value was missing. Analyses at week 52 were prespecified and analyses at weeks 104 and 156 were posthoc; all long-term analyses are based on data as observed. Data from all patients with enthesitis or dactylitis at baseline were analysed descriptively through week 156, regardless of when patients started treatment with apremilast (baseline, week 16 or week 24).
Results
Patient characteristics
Across PALACE 1, 2 and 3, 63.3% (945/1493) of patients had enthesitis and 42.4% (633/1493) had dactylitis at baseline and were included in the current analysis. Baseline patient demographics, disease characteristics and prior and concurrent therapy were comparable across treatment groups (table 1). Baseline mean MASES (range 4.5–4.8) and dactylitis counts (range 3.2–3.4) were also similar across treatment groups among patients with enthesitis and dactylitis, respectively.
Table 1.
Baseline demographics and disease characteristics, by treatment group in patients with enthesitis or dactylitis at baseline
| Patients with enthesitis at baseline (n=945) | Patients with dactylitis at baseline (n=633) | |||||
| Placebo (n=311) |
Apremilast | Placebo (n=205) |
Apremilast | |||
| 30 mg two times per day (n=327) | 20 mg two times per day (n=307) | 30 mg two times per day (n=221) | 20 mg two times per day (n=207) | |||
| Age, mean, years | 50.2 | 50.8 | 49.7 | 49.2 | 49.5 | 47.8 |
| Females, n (%) | 174 (55.9) | 196 (59.9) | 175 (57.0) | 106 (51.7) | 105 (47.5) | 101 (48.8) |
| Body mass index, mean, kg/m2 | 30.5 | 30.1 | 30.4 | 30.6 | 29.5 | 29.6 |
| Duration of PsA, mean, years | 7.3 | 7.4 | 7.1 | 7.2 | 7.7 | 8.0 |
| Duration of psoriasis, mean, years | 18.0 | 17.6 | 17.9 | 17.1 | 17.3 | 17.6 |
| Swollen joint count (0–76), mean (SD) | 11.6 (8.3) | 12.3 (8.5) | 12.4 (9.3) | 13.3 (8.7) | 14.2 (9.8) | 14.0 (10.2) |
| Tender joint count (0–78), mean (SD) | 22.6 (15.9) | 25.1 (15.7) | 24.9 (17.9) | 22.4 (15.7) | 23.9 (15.0) | 24.7 (18.5) |
| MASES* (0–13), mean (SD) | 4.8 (3.3) | 4.5 (3.2) | 4.6 (3.3) | 4.8 (3.4) | 4.1 (2.7) | 4.8 (3.4) |
| Median (minimum, maximum) | 4.0 (1, 13) | 4.0 (1, 13) | 4.0 (1, 13) | 4.0 (1, 13) | 4.0 (1, 13) | 4.0 (1,13) |
| Dactylitis count† (0–20), mean (SD) | 3.4 (3.5) | 3.7 (3.6) | 3.8 (3.9) | 3.2 (3.3) | 3.3 (3.3) | 3.4 (3.4) |
| Median (minimum, maximum) | 2.0 (1, 20) | 3.0 (1, 19) | 2.0 (1, 20) | 2.0 (1, 20) | 2.0 (1, 19) | 2.0 (1, 20) |
| HAQ-DI score (0–3), mean (SD) | 1.3 (0.60) | 1.3 (0.62) | 1.3 (0.61) | 1.3 (0.62) | 1.3 (0.64) | 1.2 (0.68) |
| CRP (normal range: 0–0.499), mean, mg/dL | 1.10 (1.6) | 1.00 (1.5) | 0.94 (1.3) | 1.17 (1.5) | 1.21 (1.7) | 1.05 (1.3) |
| DAS-28 (CRP), mean (SD) | 4.8 (1.1) | 4.9 (1.0) | 4.8 (1.0) | 4.8 (1.1) | 4.9 (1.0) | 4.7 (1.2) |
| PASI score‡ (0–72), mean | 8.8 (9.7) | 8.4 (7.9) | 7.5 (7.1) | 8.1 (7.7) | 8.5 (8.6) | 7.8 (6.6) |
| Psoriasis body surface area, mean, % | 7.8 | 7.9 | 7.4 | 8.4 | 8.2 | 8.5 |
| Prior use of conventional DMARDs only, n (%) | 242 (77.8) | 252 (77.1) | 223 (72.6) | 157 (76.6) | 178 (80.5) | 167 (80.7) |
| Prior use of biologicals, n (%) | 62 (19.9) | 70 (21.4) | 81 (26.4) | 45 (22.0) | 41 (18.6) | 40 (19.3) |
| Prior biological failures, n (%) | 29 (9.3) | 28 (8.6) | 28 (9.1) | 20 (9.8) | 14 (6.3) | 10 (4.8) |
| Concomitant conventional DMARD use, n (%) | 201 (64.6) | 206 (63.0) | 192 (62.5) | 138 (67.3) | 145 (65.6) | 146 (70.5) |
| Methotrexate (mean dose, 15.3 mg/week*; 15.2 mg/week†) | 172 (55.3) | 172 (52.6) | 158 (51.5) | 118 (57.6) | 121 (54.8) | 127 (61.4) |
| Leflunomide (mean dose, 17.9 mg/day*; 17.7 mg/day†) | 20 (6.4) | 19 (5.8) | 20 (6.5) | 15 (7.3) | 19 (8.6) | 15 (7.2) |
| Sulfasalazine (mean dose, 2.03 g/day*; 1.94 g/day†) |
31 (10.0) | 31 (9.5) | 31 (10.1) | 19 (9.3) | 22 (10.0) | 22 (10.6) |
| Corticosteroid use (mean dose, 6.29 mg/day*; 6.27 mg/day†), n (%) | 29 (9.3) | 38 (11.6) | 59 (19.2) | 19 (9.3) | 23 (10.4) | 35 (16.9) |
| Baseline NSAID use, n (%) | 218 (70.1) | 233 (71.3) | 221 (72.0) | 137 (66.8) | 162 (73.3) | 154 (74.4) |
The n reflects the number of randomised patients who received at least one dose of study medication; actual number of patients available for each parameter may vary.
*Examined among patients who had enthesitis at baseline.
†Examined among patients who had dactylitis at baseline.
‡Patients with baseline psoriasis involvement of the body surface area of ≥3%.
§All converted to oral prednisone dose.
Note: The n reflects the number of randomised patients who received at least one dose of study medication; actual number of patients available for each parameter may vary.
CRP, C reactive protein; DAS-28, 28-joint count Disease Activity Score; DMARD, disease-modifying anti-rheumatic drug; HAQ-DI, Health Assessment Questionnaire-Disability Index; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; NSAID, non-steroidal anti-inflammatory drug; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis.
Efficacy results: enthesitis
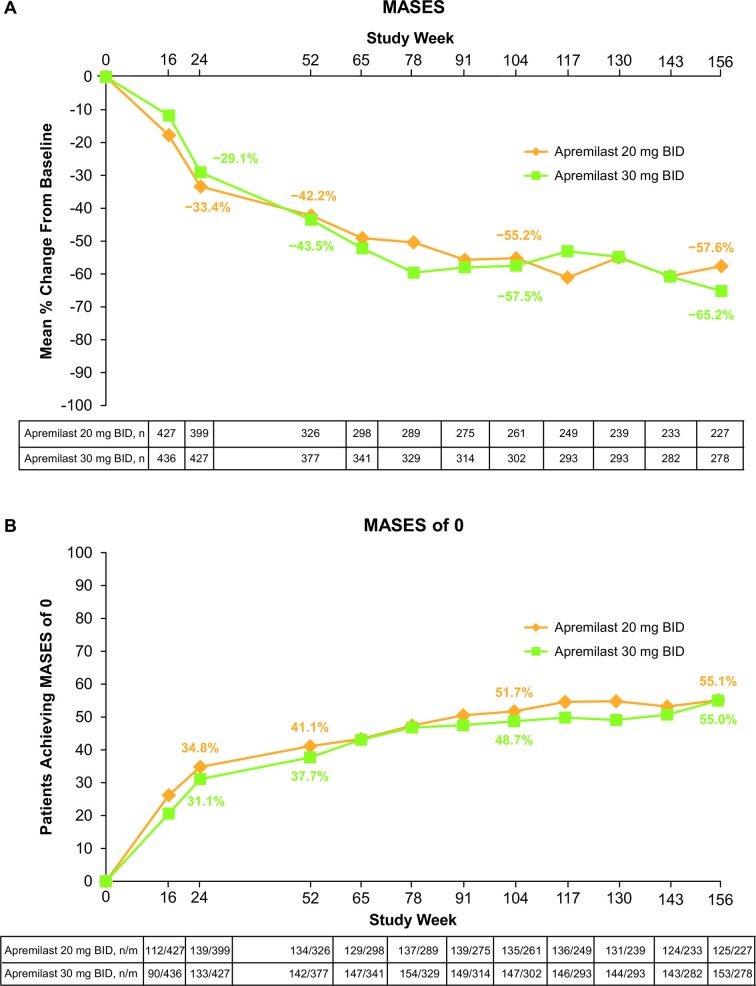
At week 24, patients treated with apremilast 30 mg two times per day demonstrated a significantly greater mean change from baseline in enthesitis compared with placebo (p=0.02) (table 2). Moreover, apremilast 30 mg two times per day resulted in significantly greater mean and median per cent changes in MASES from baseline compared with placebo (table 2). Among patients treated with either apremilast 30 mg twice daily or 20 mg twice daily, numerically greater improvements in enthesitis and in proportions of patients achieving a MASES of 0 were observed compared with placebo at week 24 (table 2). In the population of patients continuing apremilast 30 mg two times per day or 20 mg two times per day through 3 years, mean and median improvements in MASES were sustained. Specifically, at week 156, mean changes from baseline were −2.7 (30 mg two times per day) and −2.8 (20 mg two times per day) (table 2). Mean per cent changes from baseline were −65.2% (30 mg two times per day) and −57.6% (20 mg two times per day; figure 1A) and median per cent changes were −100% for both treatment groups (table 2). At week 156, 55.0% of patients treated with apremilast 30 mg two times per day and 55.1% treated with apremilast 20 mg two times per day achieved a MASES of 0 (figure 1B). When looking at the development of enthesitis over time in patients with a MASES of 0 at baseline, almost twice as many placebo patients (32.7%) with a MASES of 0 at baseline developed enthesitis at week 24 than patients treated with apremilast 30 mg two times per day (18.4%) or 20 mg two times per day (18.2%).
Table 2.
Enthesitis at week 24 (LOCF) and weeks 52, 104 and 156 (as observed)*
| MASES† | Week 24 | Week 52 | Week 104 | Week 156 | |||||
| Placebo (n=302) |
Apremilast | Apremilast | Apremilast | Apremilast | |||||
| 30 mg two times per day (n=315) | 20 mg two times per day (n=298) | 30 mg two times per day (n=377) | 20 mg two times per day (n=326) | 30 mg two times per day (n=302) | 20 mg two times per day (n=261) | 30 mg two times per day (n=278) | 20 mg two times per day (n=227) | ||
| Baseline, mean | 4.8 | 4.4 | 4.6 | 4.4 | 4.5 | 4.3 | 4.6 | 4.2 | 4.4 |
| Mean change from baseline | −0.9 | −1.3‡ | −1.2 | −2.0 | −2.2 | −2.6 | −2.6 | −2.7 | −2.8 |
| Mean per cent change from baseline | −7.0 | −23.6‡ | −19.3 | −43.5 | −42.2 | −57.5 | −55.2 | −65.2 | −57.6 |
| Median per cent change from baseline§ | −21.1 | −50.0‡ | −40.0 | −66.7 | −66.7 | −85.7 | −100.0 | −100.0 | −100.0 |
| Score of 0¶, n/m (%) | 70/311 (22.5) | 90/327 (27.5) | 84/307 (27.4) | 142/377 (37.7) | 134/326 (41.1) | 147/302 (48.7) | 135/261 (51.7) | 153/278 (55.0) | 125/227 (55.1) |
*The n at week 24 represents patients with a baseline value >0 and ≥1 postbaseline value at or before week 24. Data as observed for weeks 52, 104 and 156; the n represents the number of patients taking apremilast, regardless of when treatment started (baseline, week 16 or week 24), with a baseline value >0 and a value at the specific visit.
†MASES ranges from 0 to 13, with 0 indicating no pain at any assessed entheses and 13 indicating pain at all assessed entheses.
‡P<0.05 vs placebo.
§The per cent change noted occurred in >50% of patients.
¶Patients who did not have sufficient data (observed or imputed) for a definitive determination of response status at week 24 are counted as non-responders.
LOCF, last observation carried forward; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score.
Figure 1.
(A) Mean per cent change in MASES up to week 156 and (B) patients achieving a MASES of 0 up to week 156. Data as observed in patients with pre-existing enthesitis. Analyses include all patient data, including the placebo-controlled period, regardless of when patients started taking apremilast (baseline, week 16 or week 24). MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; n/m, number of responders/number of patients with sufficient data for evaluation.
Efficacy results: dactylitis
At week 24, mean dactylitis count was significantly improved from baseline in patients treated with apremilast 30 mg two times per day compared with placebo (p≤0.01). Mean and median per cent changes from baseline were not significantly different in either dose group compared with placebo; however, the median per cent change from baseline in the 30 mg dose group trended toward statistical significance vs placebo (p=0.06; table 3). The difference in the proportion of patients achieving a dactylitis count of 0 was not statistically significant (table 3).
Table 3.
Dactylitis at week 24 (LOCF) and weeks 52, 104 and 156 (as observed)*
| Dactylitis count† | Week 24 | Week 52 | Week 104 | Week 156 | |||||
| Placebo (n=194) |
Apremilast | Apremilast | Apremilast | Apremilast | |||||
| 30 mg two times per day (n=214) | 20 mg two times per day (n=202) | 30 mg two times per day (n=249) | 20 mg two times per day (n=225) | 30 mg t two times per day (n=200) | 20 mg two times per day (n=182) | 30 mg two times per day (n=181) | 20 mg two times per day (n=157) | ||
| Baseline, mean | 3.3 | 3.2 | 3.4 | 3.4 | 3.3 | 3.4 | 3.2 | 3.4 | 3.0 |
| Mean change from baseline | −1.3 | −1.8‡ | −1.6 | −2.5 | −2.3 | −2.9 | −2.4 | −3.0 | −2.4 |
| Mean per cent change from baseline | −38.2 | −48.6 | −43.2 | −67.9 | −70.2 | −80.0 | −75.6 | −83.6 | −73.4 |
| Median per cent change from baseline§ | −66.7 | −79.3 | −75.0 | −100.0 | −100.0 | −100.0 | −100.0 | −100.0 | −100.0 |
| Dactylitis count of 0¶, n/m (%) | 80/205 (39.0) | 102/221 (46.2) | 95/207 (45.9) | 168/249 (67.5) | 150/225 (66.7) | 155/200 (77.5) | 132/182 (72.5) | 144/181 (79.6) | 116/157 (73.9) |
*The n at week 24 represents patients with a baseline value >0 and ≥1 postbaseline value at or before week 24. Data as observed for weeks 52, 104 and 156. The n represents the number of patients taking apremilast, regardless of when treatment started (baseline, week 16 or week 24), with a baseline value >0 and a value at the specific visit.
†Dactylitis count is the sum of all scores for each of the 20 digits, with each digit scored as 0=absence or 1=presence of dactylitis.
‡P<0.01 vs placebo.
§The per cent change noted occurred in >50% of patients.
¶Patients who did not have sufficient data (observed or imputed) for a definitive determination of response status at week 24 are counted as non-responders.
LOCF, last observation carried forward.
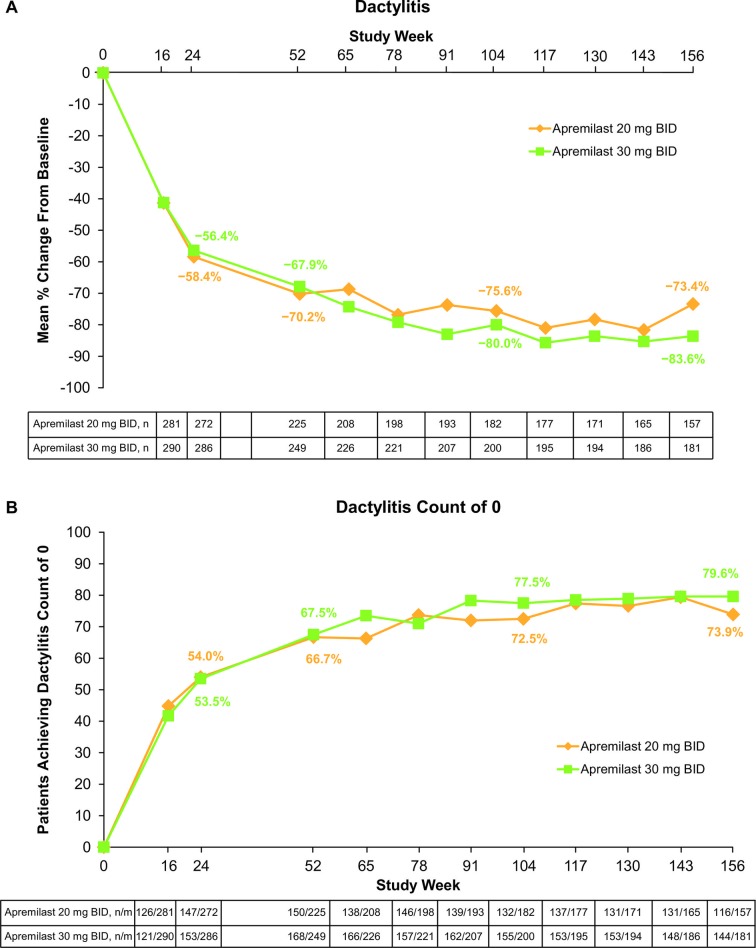
In the population of patients continuing apremilast treatment through 3 years, mean and median improvements in dactylitis count were sustained (figure 2A; table 3). Specifically, at week 156, mean changes from baseline were −3.0 (30 mg two times per day) and −2.4 (20 mg two times per day). Mean per cent changes in dactylitis count were −83.6% (30 mg two times per day) and −73.4% (20 mg two times per day; figure 2A) and median per cent changes from baseline at week 156 were −100.0% for both dose groups (table 3). At week 156, 79.6% of patients treated with apremilast 30 mg two times per day and 73.9% treated with apremilast 20 mg two times per day achieved a dactylitis count of 0 (figure 2B). Among patients with a dactylitis count of 0 at baseline, approximately twofold as many patients receiving placebo (15.8%) developed dactylitis versus patients treated with apremilast 30 mg two times per day (8.3%) or apremilast 20 mg two times per day (7.0%) at week 24.
Figure 2.
(A) Mean per cent change in dactylitis up to week 156 and (B) patients achieving a dactylitis count of 0 up to week 156. Data as observed in patients with pre-existing dactylitis. Analyses include all patient data, including the placebo-controlled period, regardless of when patients started taking apremilast (baseline, week 16 or week 24). n/m, number of responders/number of patients with sufficient data for evaluation.
Discussion
When assessing new therapies for the treatment of PsA, it is important to understand the impact treatment has on hallmark features of PsA such as enthesitis and dactylitis.9 17–21 These conditions often occur in the lower extremities and may cause tenderness or pain while standing and walking4 5 7 as well as limit the ability to hold or grasp objects and affect fine motor function.4 22 Overall, they are associated with impaired function, may negatively impact quality of life and are difficult to treat using conventional treatments.4–8 Both enthesitis and dactylitis also significantly contribute to the perceived burden of disease among patients with PsA.23 Dactylitis is actually considered a marker of PsA disease severity.7 24 25 It is also noteworthy that there is little evidence suggesting conventional DMARDs are effective in treating either enthesitis or dactylitis.9
The results from our pooled analysis of patients with enthesitis and/or dactylitis at baseline in the PALACE 1, 2 and 3 studies showed that apremilast is effective in reducing the severity of both conditions in the population of patients remaining on apremilast through 3 years of treatment. At baseline, nearly two-thirds of enrolled patients had enthesitis and approximately half had dactylitis in one or more digits. By week 24, statistically significant improvements in enthesitis (mean, mean per cent and median per cent changes) were observed after treatment with apremilast 30 mg two times per day. Although mean and median per cent improvements in dactylitis were not significant, apremilast 30 mg two times per day showed a strong trend towards improvement versus placebo (median per cent change, −79.3% vs −66.7%; p=0.06), and the mean change in dactylitis count at week 24 was significant and sustained through 156 weeks in the population of patients continuing treatment. Despite the pooling of the three studies, the number of patients in the analysis is relatively small.
It is also noteworthy that patients who were treated with apremilast showed improvements in enthesitis and dactylitis already present at baseline and demonstrated lower rates of onset of these conditions during the study. Specifically, among patients with a MASES of 0 at baseline, about twice as many patients receiving placebo developed enthesitis versus patients treated with apremilast 30 mg two times per day (32.7% vs 18.4%) at week 24. Among the subgroup of patients who entered early escape (failed to achieve ≥20% improvement in swollen and tender joint counts at week 16), a comparable number of placebo versus apremilast patients developed enthesitis (39.3% vs 41.5%). Among patients with a dactylitis count of 0 at baseline, almost twofold as many patients receiving placebo developed dactylitis versus patients treated with apremilast 30 mg two times per day (15.8% vs 8.3%) at week 24. Similarly, of those patients who entered early escape, nearly twice as many patients receiving placebo developed dactylitis versus patients treated with apremilast (21.5% vs 12.6%).
Previous analyses of PALACE data from weeks 16 and 24 did not detect consistent, significantly greater improvements in enthesitis or dactylitis with apremilast versus placebo in all studies.11 14 This might be partially due to insufficient numbers of patients with these conditions at baseline to yield adequate power to detect differences between treatment groups. We believe that this pooled analysis helps to address these challenges, yielding greater power to detect changes in enthesitis or dactylitis than in the individual PALACE studies; however, this still may not be enough.
This study had several limitations. First, long-term results were posthoc analyses of mean and median changes in a population of patients continuing treatment in the PALACE studies, which were not originally designed nor powered to investigate outcomes relating to enthesitis and dactylitis. Second, in recognising the heterogeneity in the instruments used to evaluate and interpret enthesitis and dactylitis measures,20 26 we understand that the MASES was designed for patients with ankylosing spondylitis where sites of interest are more central and may not be ideal for evaluating the more characteristically peripheral sites of interest in PsA.27 In future studies, scales such as the Spondyloarthritis Research Consortium of Canada enthesitis index or Leeds Enthesitis Index that evaluate peripheral enthesitis sites may be more appropriate for patients with PsA.15 27 Third, this study did not assess the impact of apremilast on patients with acute versus chronic dactylitis; rather, the analysis took a binary approach to determine whether the condition was merely present or absent. The mean dactylitis count at baseline was associated with a limited dynamic range (3.2–3.4), which may have limited our ability to detect differences between apremilast and placebo. This contrasts with methods based on the grading of dactylitis severity for each digit, as used in the GO-REVEAL study with golimumab (0=no dactylitis; 3=severe dactylitis; range 0–60)28 or based on the size and tenderness of each digit with dactylitis (Leeds Dactylitis Instrument),29 as used in the RAPID-PsA study.30 These methods may be associated with greater sensitivity to detect changes between active therapies and placebo.
Our analysis may have also been limited by the fact that improvements observed in placebo patients in the initial PALACE studies were somewhat greater than those observed in studies with other agents,31 32 potentially reducing the observed therapeutic effect of apremilast. The observed level of improvement in enthesitis and dactylitis among placebo patients was unexpected and not explained by baseline differences between groups in symptom severity, which was generally similar between treatment groups. The ability to detect enthesitis and dactylitis improvement following treatment may have been differentially impacted by the natural course and variation of the symptoms evaluated. For example, improvements among patients with enthesitis receiving placebo may be partially explained by previous evidence, suggesting this condition may naturally improve over time, regardless of treatment.6 8
In conclusion, enthesitis and dactylitis are core features of PsA that have an important impact on overall severity and burden of disease. In this analysis of patients continuing apremilast treatment, we observed improvements in the difficult-to-treat manifestations of PsA, enthesitis and dactylitis, up to 3 years.
Acknowledgments
The authors received editorial support in the preparation of this report from Peloton Advantage, LLC, funded by Celgene Corporation. The authors are fully responsible for all content and editorial decisions.
Footnotes
Contributors: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. In addition, all authors had full access to all of the data, and data are available on request. DDG takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design: DDG, AK, JJG-R, JW, MC, GS, EL, ND, BG, LT, CJE, CAB and PJM; acquisition of data: ND, LT and BG; analysis and interpretation of data: DDG, AK, JJG-R, JW, MC, GS, EL, ND, BG, LT, CJE, CAB and PJM.
Funding: These studies were sponsored by Celgene Corporation.
Competing interests: DDG has received grant/research support and has served as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, Celgene Corporation, Janssen, Novartis, Pfizer and UCB. AK has received grant/research support from Abbott, Amgen, AstraZeneca, BMS, Celgene Corporation, Centocor-Janssen, Pfizer, Roche and UCB. JJG-R has received grant/research support from Roche and Schering-Plough. JW has received research support from and served as a consultant for Abbott, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer and UCB. MC has received research support from and served as a consultant for Actelion, Bristol-Myers Squibb and Sanofi-Aventis. GS has received research support from and served as a consultant for Abbott, Celgene Corporation, Roche and UCB. EL has received research support from and served on a speakers bureau for Amgen, Eli Lilly, Novartis and Servier. ND, BG and LT are employees of Celgene Corporation. CJE has received research support from Celgene Corporation, Pfizer, Roche and Samsung and has served on a speakers bureau for Abbott, GlaxoSmithKline, Pfizer and Roche. CAB has received research support from Amgen, Bristol-Myers Squibb, Incyte, Eli Lilly, Merck and Pfizer. PJM has received research support from and served as a consultant for Abbott, Amgen, Biogen Idec, Bristol-Myers Squibb, Celgene Corporation, Eli Lilly, Genentech, Janssen, Novartis, Pfizer, Roche and UCB and has served on a speakers bureau for Abbott, Amgen, Biogen Idec, Bristol-Myers Squibb, Eli Lilly, Genentech, Janssen, Pfizer and UCB.
Patient consent: Obtained.
The Institutional Review Board/Ethics Committee at each site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Lloyd P, Ryan C, Menter A. Psoriatic arthritis: an update. Arthritis 2012;2012:1–6. 10.1155/2012/176298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, et al. . Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–17. 10.1136/ard.2004.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 4. Sakkas LI, Alexiou I, Simopoulou T, et al. . Enthesitis in psoriatic arthritis. Semin Arthritis Rheum 2013;43:325–34. 10.1016/j.semarthrit.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 5. Carneiro S, Bortoluzzo A, Goncalves C. Effect of enthesitis on 1505 Brazilian patients with spondyloarthritis. J Rheumatol 2013;40:1725. [DOI] [PubMed] [Google Scholar]
- 6. Gladman DD, Ziouzina O, Thavaneswaran A, et al. . Dactylitis in psoriatic arthritis: prevalence and response to therapy in the biologic era. J Rheumatol 2013;40:1357–9. 10.3899/jrheum.130163 [DOI] [PubMed] [Google Scholar]
- 7. Brockbank JE, Stein M, Schentag CT, et al. . Dactylitis in psoriatic arthritis: a marker for disease severity? Ann Rheum Dis 2005;64:188–90. 10.1136/ard.2003.018184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Polachek A, Li S, Chandran V, et al. . Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome. Arthritis Care Res 2017;69:1685–91. 10.1002/acr.23174 [DOI] [PubMed] [Google Scholar]
- 9. Nash P, Clegg DO. Psoriatic arthritis therapy: NSAIDs and traditional DMARDs. Ann Rheum Dis 2005;64(Suppl 2):ii74–7. 10.1136/ard.2004.030783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schafer PH, Parton A, Gandhi AK, et al. . Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol 2010;159:842–55. 10.1111/j.1476-5381.2009.00559.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. . Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020–6. 10.1136/annrheumdis-2013-205056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. . Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol 2015;42:479–88. 10.3899/jrheum.140647 [DOI] [PubMed] [Google Scholar]
- 13. Cutolo M, Myerson GE, Fleischmann RM, et al. . A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol 2016;43:1724–34. 10.3899/jrheum.151376 [DOI] [PubMed] [Google Scholar]
- 14. Edwards CJ, Blanco FJ, Crowley J, et al. . Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis 2016;75:1065–73. 10.1136/annrheumdis-2015-207963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gladman DD, Inman RD, Cook RJ, et al. . International spondyloarthritis interobserver reliability exercise--the INSPIRE study: II. Assessment of peripheral joints, enthesitis, and dactylitis. J Rheumatol 2007;34:1740–5. [PubMed] [Google Scholar]
- 16. Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A, et al. . Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. 10.1136/ard.62.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gottlieb A, Korman NJ, Gordon KB, et al. . Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol 2008;58:851–64. 10.1016/j.jaad.2008.02.040 [DOI] [PubMed] [Google Scholar]
- 18. Gossec L, Smolen JS, Ramiro S, et al. . European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 19. Coates LC, Tillett W, Chandler D, et al. . BSR clinical affairs committee & standards, audit and guidelines working group and the BHPR. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology 2013;52:1754–7. 10.1093/rheumatology/ket187 [DOI] [PubMed] [Google Scholar]
- 20. Orbai AM, Weitz J, Siegel EL, et al. . Systematic review of treatment effectiveness and outcome measures for enthesitis in psoriatic arthritis. J Rheumatol 2014;41:2290–4. 10.3899/jrheum.140878 [DOI] [PubMed] [Google Scholar]
- 21. Rose S, Toloza S, Bautista-Molano W, et al. . Comprehensive treatment of dactylitis in psoriatic arthritis. J Rheumatol 2014;41:2295–300. 10.3899/jrheum.140879 [DOI] [PubMed] [Google Scholar]
- 22. Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Rheumatol Ther 2016;3:91–102. 10.1007/s40744-016-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dandorfer SW, Rech J, Manger B, et al. . Differences in the patient's and the physician's perspective of disease in psoriatic arthritis. Semin Arthritis Rheum 2012;42:32–41. 10.1016/j.semarthrit.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 24. McGonagle DG, Helliwell P, Veale D. Enthesitis in psoriatic disease. Dermatology 2012;225:100–9. 10.1159/000341536 [DOI] [PubMed] [Google Scholar]
- 25. Ritchlin CT, Kavanaugh A, Gladman DD, et al. . Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 2009;68:1387–94. 10.1136/ard.2008.094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramiro S, Smolen JS, Landewé R, et al. . How are enthesitis, dactylitis and nail involvement measured and reported in recent clinical trials of psoriatic arthritis? A systematic literature review. Ann Rheum Dis 2018;77:782–3. 10.1136/annrheumdis-2017-211447 [DOI] [PubMed] [Google Scholar]
- 27. Wong PC, Leung YY, Li EK, et al. . Measuring disease activity in psoriatic arthritis. Int J Rheumatol 2012;2012:1–10. 10.1155/2012/839425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kavanaugh A, McInnes IB, Mease PJ, et al. . Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study. Ann Rheum Dis 2013;72:1777–85. 10.1136/annrheumdis-2012-202035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helliwell PS, Firth J, Ibrahim GH, et al. . Development of an assessment tool for dactylitis in patients with psoriatic arthritis. J Rheumatol 2005;32:1745–50. [PubMed] [Google Scholar]
- 30. Mease PJ, Fleischmann R, Deodhar AA, et al. . Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48–55. 10.1136/annrheumdis-2013-203696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kavanaugh A, Mease P. Treatment of psoriatic arthritis with tumor necrosis factor inhibitors: longer-term outcomes including enthesitis and dactylitis with golimumab treatment in the Longterm Extension of a Randomized, Placebo-controlled Study (GO-REVEAL). J Rheumatol Suppl 2012;89:90–3. 10.3899/jrheum.120254 [DOI] [PubMed] [Google Scholar]
- 32. Kavanaugh A, McInnes IB, Gottlieb AB, et al. . SAT0271 Continued Improvement of Signs and Symptoms in Ustekinumab-Treated Patients with Active Psoriatic Arthritis: Week 52 Results of a Phase 3, Multicenter, Double-Blind, Placebo-Controlled Study. Ann Rheum Dis 2013;72(Suppl 3):674–675. 10.1136/annrheumdis-2013-eular.1996 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2018-000669supp001.pdf (106KB, pdf)