Abstract
Objective
The American Diabetes Association and the European Association for the Study of Diabetes guidelines recommend to individualize treatment targets/strategies in inadequately controlled patients by lifestyle management and glucose-lowering drugs to decrease the burden of diabetes-related complications. This real-world practice study aimed to assess predictive factors for achieving the glycemic hemoglobin A1c (HbA1c) at 6 months as targeted by the treating physician in adults with type 2 diabetes who required initiation of basal insulin, initiation of bolus insulin, or modification from basal or premixed insulin to new insulin regimen containing insulin glargine and/or insulin glulisine.
Research design and methods
This was an international, multicenter, observational survey with 12-month follow-up time in adults with type 2 diabetes inadequately controlled conducted in 10 developing countries.
Results
Overall, 2704 patients (mean age: 54.6 years, body mass index: 28.7 kg/m2; Caucasian: 46.1%, type 2 diabetes duration: 10.1 years) with poor glycemic control (mean HbA1c: 9.7% (83 mmol/mol), fasting blood glucose: 196.8 mg/dL) were eligible. At 6 months, advanced age, Caucasian ethnicity, shorter type 2 diabetes duration (>10 vs 1 year, p<0.0001), lower baseline HbA1c (≥ 8.5% vs <7%, p<0.0001) and no intake of oral antidiabetic drug (OAD) (none vs 2, p=0.02) were predictive factors for achieving glycemic goal as targeted by the treating physician. Absolute changes in the mean HbA1c of −1.7% and −2% were observed from baseline to 6 and 12 months, respectively.
Conclusions
Along with some well-known predictive factors, this study suggested that early insulin regimen treatment initiation and/or intensification allowed patients to promote glycemic control.
Keywords: Type 2 Diabetes, Insulin, glycemic control, HbA1c
Significance of the study.
What is already known about this subject?
Adequate glycemic control and identification of risk factors may prevent the development of cardiovascular complications in type 2 diabetes.
What are the new findings?
Our study identified predictive factors for achieving glycemic goal as targeted by the treating physician and showed that patients with type 2 diabetes inadequately controlled starting or intensifying their insulin dosing regimen achieved a significant decrease in glycemic hemoglobin A1 at 6 and 12 months compared with baseline.
How might these results change the focus of research or clinical practice?
To avoid clinical inertia in clinical practice contributing to patients with type 2 diabetes living with suboptimal glycemic control for a long time, further potential approaches including fundamental changes in medical care should be studied.
Introduction
Diabetes is one of the major health priority of the 21st century1 causing 1.5 million deaths in 2012.2 It is expected that by 2025–2030, there will be 380 million people with type 2 diabetes,3 and this pathology will move up to the seventh leading cause of death worldwide.4 Diabetes is recognized as a well-known risk factor for cardiovascular diseases (CVDs), responsible for about a twofold excess risk for various vascular diseases such as coronary heart diseases (CHD), strokes, and other vascular deaths.5
Type 2 diabetes requires the adoption of a healthy diet, physical activity and maintenance of a normal body weight. But, the cornerstone of type 2 diabetes treatment includes oral antidiabetic drugs (OADs) and insulin use in the event of increased glycemia.
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) consensus6 for the management of type 2 diabetes recommends a glycemic target hemoglobin A1c (HbA1c) <7% (53 mmol/mol).7 The UK Prospective Diabetes Study (UKPDS) showed that each 1% reduction in HbA1c is associated with a risk reduction of complications that is clinically and statistically significant.8 After adjusting for multiple potential confounding factors, 1% rise in HbA1c was associated with a 7% increase in the risk of a first major CV event, a 12% rise in the risk of death, a 20% rise in the risk of heart failure.9 The goal of therapy for the majority of people with diabetes is to prevent CV events by achieving a sustained glycemic control (HbA1c<7% (53 mmol/mol)).6 The choice of the medications used to achieve glycemic goals must be individualized for each patient and adjusted as diabetes is a progressive disease. When lifestyle, metformin, and sulfonylurea or basal insulin are insufficient and do not lead to a glycemic control, the next step should be an initiation or intensification of insulin therapy including additional injections of short-acting or rapid-acting insulins.6 Despite an availability of a range of pharmacological agents and the stepwise approach to type 2 diabetes treatment advocated by ADA/EASD position statement,10 many patients with type 2 diabetes remain in poor glycemic control for prolonged periods of time.11
The aim of the present study was to assess the clinical and non-clinical predictive factors for achieving the glycemic goal HbA1c as targeted by the treating physician in adults with type 2 diabetes requiring insulin initiation, titration, and/or intensification.
Research design and methods
Study design
This was a 12 months real-world practice, multicenter, non-interventional, non-comparative study conducted in adults (>18 years) with type 2 diabetes inadequately controlled. In the context of the usual clinical practice, four visits were scheduled: at baseline (Visit 1 (V1)), and at 3 (V2), 6 (V3) and 12 months (V4) of follow-up (at the end of the study period). Participating physicians were, then, asked to record data for study endpoint assessments at baseline, every 3 months (±1 week) for the first 6 months, and then at 1 year through the observation period from treatment start and at the end of study. The estimated duration of the survey was 2 years.
Study setting
The study was carried out from October 2012 to January 2015 in 10 developing countries from Africa (Egypt, South Africa), Middle East (Israel, Saudi Arabia, United Arab Emirates, Iran and Lebanon) and South Asia (Bangladesh, India and Pakistan).
Physician selection
The number of physicians who participated in the study as well as their profile were determined on a country basis. HbA1c control was part of the center selection criteria. The target number of participating sites was 150 involving general practitioners (GPs) and diabetologists. Of the 198 participating physicians, 180 were active with the highest enrolment in South Asia (1509 patients and 58 physicians) and the lowest enrollment in Africa (710 patients and 42 physicians).
Patient selection
Each physician included patients until the targeted number of patients in his/her country was reached. A screening log was implemented to document this consecutive enrolment. As 1949 patients needed to be included, it was planned to recruit 2900 patients with a minimum of 100 patients by countries or region.
Study population
Patients meeting the following inclusion criteria were proposed to be included in this study: male and female, aged over 18 years, with a known and inadequately controlled type 2 diabetes because the glycemic goal targeted by the treating physician was not achieved, for whom treating physician had decided an initiation of adjunct therapy with basal insulin glargine or an adjustment of the current insulin therapy: either basal insulin or premixed insulin were switched to insulin glargine alone, or insulin glulisine and basal insulin, or insulin glargine and insulin glulisine. Conversely, patients with history of hypersensitivity to insulin glargine and/or insulin glulisine, pregnant women, or women with breast feeding, or of childbearing potential not using efficient contraception, patients with brittle diabetes, psychiatric or mental diseases, unable or unwilling to manage properly the basal bolus regimen were excluded from the study.
Study treatments
This was a product registry in which subcutaneous injectable solutions of insulin glargine±insulin glulisine were administered in routine clinical practice according to the approved indication. According to the physician’s opinion and independently from study entry, the patients who were enrolled in the study were decided to initiate a basal insulin therapy with insulin glargine, or to modify the existing basal insulin therapy by switching to insulin glargine, or to add insulin glulisine to basal insulin, or to initiate a new insulin dosing regimen with insulin glargine and insulin glulisine from premixed insulin. The dosage and timing of dose of insulin glargine was to be individually adjusted. The dose of insulin glargine was to be titrated based on the fasting (pre-breakfast) capillary plasma glucose (FPG) levels. In case of prescription of insulin glargine and insulin glulisine, the dosage of insulin glulisine was individually adjusted and titrated according to postprandial capillary plasma glucose level (PPG) and either to the carbohydrate content of the meal following calculation with carbohydrate (CHO) ratio. The estimated average treatment duration was 12 months.
Data collection
Data were reported on structured and strictly anonymous questionnaires (case report forms) for eligible patients. At inclusion, collected data included demographic and socioeconomic characteristics, relevant medical history, including diabetes complications, CV risk factors, glycemic control (blood glucose monitoring, self-monitoring blood glucose (SMBG), HbA1c, hypoglycemia), vital signs, reason(s) to add-on an insulin therapy to OADs/change in insulin therapy, patient’s glycemic goal targeted by the treating physician, life style, antidiabetic therapy at the time and at the end of the visit (ie, all type of antidiabetics including glucagon-like peptide-1 analog). HbA1c laboratory measurement was performed at each visit: at baseline, at 3 months, at 6 months, and at 12 months (end of study) from inclusion. At each follow-up visit (after 3, 6, or 12 months of treatment(s)), symptomatic hypoglycemic episodes (including time information, adverse drug reaction (ADR), vital signs, HbA1c, FPG and PPG monitoring (if available), and antidiabetic therapy at the time and at the end of the visit) were recorded by the physicians. Severe symptomatic hypoglycemia was reported as an event with clinical symptoms that were considered to result from hypoglycemia, needing third party help with plasma glucose (PG) <36 mg/dL (2 mmol/L) or countermeasure (oral carbohydrate/glucagon/intravenous glucose) leading to recovery attributable to the restoration of PG to normal. Serious hypoglycemia was defined as hypoglycemia with at least one of the following items: loss of consciousness, seizure, patients undergoing emergency department, or symptoms that fulfilled serious ADR criteria.
Statistical analysis
All data were analysed using SAS (V.8.2 or higher). Descriptive analyses and statistical tests (χ2 test, Fisher test, t-test, Wilcoxon Mann-Whitney test, Wilcoxon test) were used as appropriate. Statistical analyses were performed at the 5% global significance level using two-sided tests.
Univariate and multivariate logistic regression analyses were performed to identify clinical and non-clinical predictive factors for achieving glycemic control (HbA1c level targeted by the physician) at 6 and 12 months. Univariate OR was presented with its 95% CI. All significant variables in univariate statistical test at the 0.20 level were included in the multivariate initial model. A stepwise procedure was used with an entrance significance level at 20% and a removed significance level at 5%. The final model was constructed with the selected variables and adjusted OR with 95% CI were provided.
Ethical considerations
This registry was conducted in accordance with the ethical principles laid down by the Declaration of Helsinki, all international applicable guidelines, national laws and regulations of each country. The physician fully informed the patient using appropriate language about the aim and objectives of the study, and written informed consent was provided by participants. Appropriate measures were taken to ensure the confidentiality of the data. The study was coordinated by a leading diabetologist located in the following countries: Bangladesh, Iran, Israel, Lebanon, Pakistan, Saudia Arabia, and United Arab Emirates.
Results
During the recruitment period, 3173 patients were included in the study and 2704 eligible patients were analysed at baseline (figure 1). The study was completed by 2324 patients corresponding to 73.2% of included patients.
Figure 1.
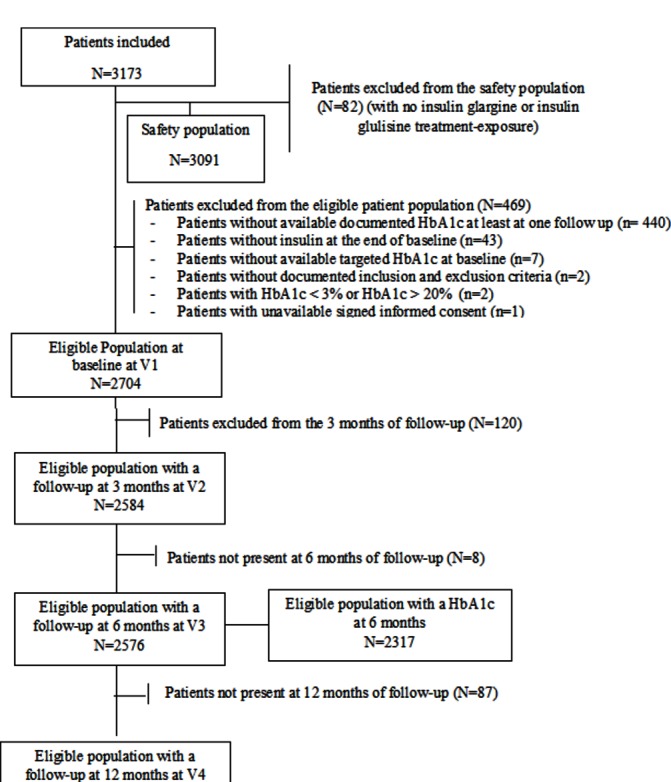
Disposition of patients time windows: follow-up at 3 months: (2–4.5 months) after baseline; follow-up at 6 months: (4.5–9 months) after baseline; follow-up at 12 months: >9 months after baseline. HbA1c, glycemic hemoglobin A1c.
Sociodemographic characteristics
More than half of the patients were males (53.5%) (table 1).
Table 1.
Sociodemographic characteristics of eligible patients with type 2 diabetes at baseline
| Demographics | Eligible patient population (N=2704) | |
| Age (years) | N (missing) | 2704 (0) |
| Mean (SD) | 54.6 (10.6) | |
| Male (n, %) | N (missing) | 2704 (0) |
| n (%) | 1446 (53.5%) | |
| Ethnicity (n, %) | N (missing) | 2704 (0) |
| Caucasian* | 1246 (46.1%) | |
| Non-Caucasian | 1458 (53.9%) | |
| Education level (n, %) | N (missing) | 2702 (2) |
| Illiterate | 172 (6.4%) | |
| Primary | 491 (18.2%) | |
| Secondary | 916 (33.9%) | |
| University/higher education | 1123 (41.6%) | |
| Medical health coverage (n, %) | N (missing) | 2703 (1) |
| n (%) | 1413 (52.3%) | |
| Employment status (n, %) | N (missing) | 2704 (0) |
| Full time | 1194 (44.2%) | |
| Part time | 131 (4.8%) | |
| Unemployed | 1379 (51.0%) | |
| Weight (kg) | N (missing) | 2688 (16) |
| m (SD) | 78.3 (16.2) | |
| BMI (kg/m2) | N (missing) | 2641 (63) |
| Mean (SD) | 28.7 (5.4) | |
| Duration of diabetes (year) | N (missing) | 2692 (12) |
| Mean (SD) | 10.1 (6.7) | |
| Diabetes-related complication (n, %) | N (missing) | 2704 (0) |
| n (%) | 1512 (55.9%) | |
| At least one macrovascular complication | N | 1512 |
| n (%) | 498 (32.9%) | |
| At least one microvascular complication | N | 1512 |
| n (%) | 1329 (87.9%) | |
| 10 years CV risk (%) | N (missing) | 1683 (1021) |
| Median | 14.6 | |
| Diagnosis of high blood pressure (n, %) | N (missing) | 2702 (2) |
| Yes | 1657 (61.3%) | |
| No / Unknown | 1045 (38.7%) | |
| Diagnosis of dyslipidemia (n, %) | N (missing) | 2702 (2) |
| Yes | 1603 (59.3%) | |
| No/Unknown | 1099 (40.7%) | |
| Smoking habits (n, %) | N (missing) | 2701 (3) |
| Never | 1960 (72.6%) | |
| Former | 403 (14.9%) | |
| Current | 338 (12.5%) | |
| Last HbA1c level in the last 3 months (%) | N (missing) | 2596 (108) |
| Mean (SD) | 9.7 (1.8) | |
| HbA1c level in the last 3 months in classes (%) | N (missing) | 2596 (108) |
| <7% | 58 (2.2%) | |
| 7–7.5% | 97 (3.7%) | |
| 7.5–8% | 226 (8.7%) | |
| 8–8.5% | 321 (12.4%) | |
| ≥8.5% | 1884 (73.0%) |
* Caucasian class included Caucasian and Oriental/Arab/Persian patients, as Caucasians were classified as individuals having origins from Europe, the Middle East, or North Africa.40
BMI, body mass index; CV, cardiovascular; HbA1c, glycemic hemoglobin A1c.
The mean age was 54.6±10.6 years with a range from 19 to 90 years. The majority (75.1%) of patients belonged to the 40–65 years age class. Most patients were of South Asian (48.8%) or Oriental, Arab, Persian (40.6%) ethnicities.
Medical history
On average, diabetes was diagnosed since a median time of 9 years and affected two or three generations in 48.4% of patients. The mean baseline BMI was 28.7±5.4 kg/m2. A total of 1512 patients (55.9%) had any diabetes-related CV complications; 87.9% suffering from microvascular and 32.9% from macrovascular CV complications. The most common diabetes-related complications were sensory neuropathy (53.8%) (online supplementary figure S1).
bmjdrc-2018-000519supp001.docx (29.1KB, docx)
CV risk factors
The 10-year CHD risk rate was estimated at 18.7%±14.5% (median: 14.6%) (table 1). Most patients had uncontrolled blood pressure (BP): 62.9% had systolic BP ≥130 mm Hg and 72.9% had diastolic BP ≥80 mm Hg.
Antidiabetic therapy
At the time of inclusion visit, 63.3% of patients were treated by OAD alone, 8.8% by insulin alone and 27.9% by OAD+insulin. Biguanide combined with sulfonylureas were the most frequently used OADs (23.2%). Among insulin-treated patients, 38.8%, 35.6% and 17.1% were receiving basal insulin alone, premix insulin and basal+prandial insulin respectively (online supplementary figure S2). At the end of the inclusion visit, antidiabetic therapy had been intensified by the physician if necessary. Thus, 85.4% of patients were prescribed both OAD and insulin, and 14.6% received insulin injectable alone. Regarding the insulin treatment prescribed, 71.2% received basal insulin alone; insulin glargine being the most frequent (96.0%).
Glycemic control and self-management
Among the 2596 patients with available tests performed in the last 3 months before the inclusion visit, the mean HbA1c level was 9.7%±1.8% (≈83 mmol/mol). Only 2.2% of the patients were achieving an HbA1c <7% (53 mmol/mol). Regarding the glycemic goals, the HbA1c targeted by the treating physician was <7% for 25.8% (n=698) of patients with a mean FPG of 116 mg/dL and a PPG of 157 mg/dL. For the rest of the population, the glycemic goals decided by the treating physician were 7%–7.5% in 60.5% of patients and ≥7.5% in 13.7%. About a quarter (26.8%) and 69.0% of patients did not have any follow-up visit by a diabetologist or a GP, respectively. Overall, 36.3% did not receive any diabetes education, 91.9% self-monitor their blood glucose regularly, and 51.5% followed a healthy diet and exercise plan.
Predictive factors for achieving HbA1c targeted by the physician at 6 months
The clinical variables relative to age, ethnicity, history of diabetes, presence of any diabetes-related complications, baseline level of HbA1c, insulin use as well as the non-clinical variables relative to diabetes education, frequency of HbA1c level measurement, and medical health coverage were significant and considered for multivariate analysis (table 2). The multivariate logistic regression analysis showed that higher age, Caucasian ethnicity, shorter duration of diabetes, and lower baseline HbA1c were predictors for achieving glycemic goal (HbA1c<7%) as targeted by the treating physician. Conversely, combination therapy of OADs (>2 OADs) and use of analog insulin were predictors of poor glycemic control.
Table 2.
Variables identified in the univariate and multivariate analyses for clinical and non-clinical factors as predictors of HbA1c goal as targeted by the treating physician at 6 months
| Effect | Univariate analysis* | Multivariate analysis* | |||||
| OR | 95% CI | P values | OR | 95% CI | P values | ||
| Clinical variables | |||||||
| Age | Age | 1.012 | 1.003 to 1.022 | 0.0050 | 1.024 | 1.012 to 1.036 | 0.0001 |
| Ethnicity | Non-Caucasian versus Caucasian | 0.628 | 0.513 to 0.768 | <0.0001 | 0.528 | 0.412 to 0.677 | <0.0001 |
| Diabetes duration in class (years) | >10 versus ≤1 | 0.440 | 0.304 to 0.639 | <0.0001 | 0.320 | 0.197 to 0.519 | <0.0001 |
| 5–10 versus ≤1 | 0.482 | 0.329 to 0.705 | 0.361 | 0.223 to 0.586 | <0.0001 | ||
| 1–5 versus ≤1 | 0.650 | 0.439 to 0.962 | 0.574 | 0.355 to 0.930 | 0.0240 | ||
| Diabetes affecting two or three generations | Yes versus no | 1.444 | 1.137 to 1.834 | 0.0023 | 1.556 | 1.173 to 2.065 | 0.0022 |
| Unknown versus no | 1.024 | 0.758 to 1.383 | 1.134 | 0.787 to 1.634 | 0.4991 | ||
| Any diabetes-related complications | Yes versus no | 0.926 | 0.751 to 1.141 | 0.0118 | 0.872 | 0.676 to 1.123 | 0.2887 |
| Unknown versus no | 1.835 | 1.153 to 2.922 | 2.046 | 1.137 to 3.681 | 0.0169 | ||
| HbA1c at baseline (five classes) | ≥8.5% versus <7% | 0.167 | 0.093 to 0.299 | <0.0001 | 0.173 | 0.091 to 0.326 | <0.0001 |
| 8–8.5% versus <7% | 0.254 | 0.135 to 0.477 | 0.270 | 0.135 to 0.539 | 0.0002 | ||
| 7.5–8% versus <7% | 0.487 | 0.256 to 0.923 | 0.511 | 0.254 to 1.029 | 0.0602 | ||
| 7–7.5% versus <7% | 0.612 | 0.298 to 1.256 | 0.799 | 0.362 to 1.764 | 0.5791 | ||
| Antidiabetics taken at the end of inclusion | |||||||
| Route of treatments | Oral+injectable versus injectable | 0.717 | 0.545 to 0.944 | 0.0288 | |||
| Analog insulin | Yes versus no | 0.481 | 0.243 to 0.951 | 0.0314 | 0.351 | 0.142 to 0.870 | 0.0238 |
| Number of OADs taken | >2 versus none | 0.390 | 0.246 to 0.620 | 0.0705 | 0.531 | 0.311 to 0.905 | 0.0200 |
| 2 versus none | 0.822 | 0.611 to 1.106 | 0.982 | 0.683 to 1.412 | 0.9235 | ||
| 1 versus none | 0.713 | 0.528 to 0.964 | 0.781 | 0.540 to 1.131 | 0.1913 | ||
| Reasons to add insulin therapy to OADs | |||||||
| HbA1c target not achieved | Yes versus no | 0.669 | 0.504 to 0.888 | 0.0053 | |||
| FPG target not achieved | Yes versus no | 0.777 | 0.622 to 0.971 | 0.0260 | |||
| Non-clinical variables | |||||||
| Receiving diabetes education | Yes versus no | 0.792 | 0.644 to 0.974 | 0.0269 | |||
| Frequency of tests during past year in class | ≥3 versus 0 | 1.121 | 0.299 to 4.202 | 0.0037 | |||
| 2 versus 0 | 0.717 | 0.191 to 2.688 | |||||
| 1 versus 0 | 0.705 | 0.188 to 2.637 | |||||
| Medical health coverage | Yes versus no | 1.277 | 1.043 to 1.562 | 0.0175 | |||
* All variables were significant in univariate statistical test at 0.20 level. ORs were estimated with two-sided 95% Cls with univariate logistic regressions. Stepwise selection; decision criteria to enter in the multivariate analysis: χ2 with significant level at 0.20, to stay: Wald χ2 with significant level at 0.05.
FPG, fasting plasma glucose; HbA1c, glycemic hemoglobin A1c; OAD, oral antidiabetic.
The univariate analysis performed at 12 months identified the same significant variables found at 6 months. In addition, patients diagnosed with high blood pressure (HBP) (OR 0.8; p=0.0207), taking at least one OAD (OR 0.8; p<0.0001), or following a healthy diet and exercise plan (OR 0.8; p=0.0012) were slightly more likely to fail to achieve HbA1c targeted by the physician at 12 months (online supplementary table S1).
Analysis of HbA1c
Table 3 reports the variables of interest the treating physician would have considered for the determination of that glycemic target. The glycemic control improved along the course of the study (figure 2). In average, after 6 and 12 months of treatment, a mean absolute change in HbA1c of −1.7% (95% CI −1.7% to −1.6%) and −2% (95% CI −2.1% to −2.0%) was observed from baseline, respectively. Similarly, the proportion of patients achieving HbA1c target<7% (53 mmol/mol) increased from 19.0% (n=440) at 6 months to 29.7% (n=684) at 12 months. Therefore, a large proportion of individuals (98.0%, 684/698) with a lower HbA1c goal achieved their HbA1c target at the end of study.
Table 3.
Baseline characteristics of the study population according to the glycemic goal as targeted by the treating physician
| Study populations | HbA1c level targeted by the physician | ||||
| <6.5% (N=117) | 6.5–7% (N=698) | 7–7.5% (N=1852) | >7.5% (N=421) | Unknown (N=3) | |
| Age (years) mean (SD) | 52.1 (9.3) | 51.6 (10.3) | 54.8 (10.5) | 57.2 (10.9) | 54.3 (12.2) |
| Gender, male (%) | 56.4 | 54.4 | 51.9 | 53.7 | 100 |
| Health coverage (%) | 45.3 | 46.7 | 52.3 | 53.4 | 66.7 |
| Duration of diabetes (years) mean (SD) | 7.3 (6.3) | 8.5 (6.6) | 10.4 (6.7) | 11.5 (7.3) | 5.7 (5.7) |
| BMI kg/m2 mean (SD) | 27.5 (5.2) | 28.4 (5.2) | 28.9 (5.6) | 28.7 (5.2) | 26.3 (2.2) |
| BMI ≥30 kg/m2 (%) | 29.8 | 35.0 | 37.0 | 37.4 | 0.0 |
| BMI 25–30 kg/m2 (%) | 36.0 | 38.1 | 38 | 39.3 | 50 |
| Any diabetes complications (%) | 33.3 | 52.9 | 56.4 | 57.2 | 33.3 |
| Microvascular complications (%) | 87.2 | 90.0 | 87.0 | 88.4 | 100 |
| Macrovascular complications (%) | 28.2 | 34.1 | 30.6 | 37.3 | 100 |
| HBP (%) | 49.6 | 52.9 | 62.5 | 66.9 | 66.7 |
| Dyslipidemia (%) | 49.6 | 57.0 | 57.0 | 64.0 | 66.7 |
| 10 year CHD mean (SD)/median (%) | 13.6 (10.9) | 16.6 (14.0) | 18.3 (14.2) | 24.0 (16.1) | 18.0 (21.9) |
| Diabetes education, (%) | 47.0 | 59.7 | 63.9 | 65.5 | 66.7 |
| Structured courses of diabetes education, yes (%) | 5.5 | 2.2 | 1.2 | 1.1 | 0.0 |
| Glucometer, yes (%) | 42.7 | 51.5 | 61.9 | 68.2 | 100 |
| SMBG use (%) | 94.0 | 88.0 | 92.7 | 89.9 | 100 |
| Routinely SMBG use (every day) (%) | 61.7 | 27.4 | 34.4 | 35.3 | 0.0 |
| Cost of strips is a limiting factor for SMBG use (yes/no) | 32.0 | 46.6 | 45.4 | 37.5 | 66.7 |
| Last HbA1c measurement (%) mean (SD) | 8.6 (1.5) | 9.3 (1.7) | 9.7 (1.8) | 10.7 (1.7) | 7.6 (2.1) |
| HbA1c<7% (%) | 16.5 | 3.6 | 1.4 | 0.2 | 33.3 |
| Number of people with severe hypoglycemia within last 3 months n (%) | 0.0 | 11 (21.2) | 31 (16.7) | 5 (10.2) | |
| Number of emergency room visits mean (SD)/median | 0.1 (0.3) / 0.0 | 0.1 (0.4) / 0.0 | 0.1 (0.6) / 0.0 | 0.1 (0.4) / 0.0 | – |
BMI, body mass index; CHD, coronary heart disease; HbA1c, glycemic hemoglobin A1c; HBP, high blood pressure; SMBG, self-monitoring blood glucose.
Figure 2.

Change in the glycemic hemoglobin A1c (HbA1c) during the 12-month study duration (mean±95% CI).
Hypoglycemia
Among the 3091 patients included in the safety population, 223 patients (7.2%) experienced a symptomatic episode of hypoglycemia. Overall, nocturnal or severe hypoglycemia (2.4% and 1.2%, respectively) was infrequent at the end of the study. According to the physician’s opinion, only three patients (0.1%) were exposed to a serious episode of hypoglycemia.
ADRs and deaths
A total of 18 patients experienced at least one ADR (0.6%); 14 out of 18 patients were treated with insulin glargine. A total of 20 events with ADR were reported; the most commonly reported ADR was hypoglycemia (0.3%). Among all ADR, six serious ADR were reported in six patients (0.2%): two hypoglycemias, one chest pain, one death of unspecified etiology, meningitis found in five patients receiving basal insulin glargine and one hyperglycemia reported in one patient taking insulin glulisine. Basal insulin glargine was discontinued in two patients (0.1%) because of the occurrence of ADR: one injection site reaction and one meningitis. In total, there were eight deaths including one fatal ADR.
There were no marked changes in weight, BMI, systolic and diastolic BP from baseline to the end of the survey.
Conclusions
The eligible study population was large including a high number of patients (n=2704) with 1 year of follow-up who remained uncontrolled despite the use of antidiabetics and also insulin. The baseline characteristics of the patients were similar to those observed in the International Diabetes Management Practices Survey (IDMPS) as far as age, gender, duration of diabetes, level of glycemic control, comorbidity (HBP and dyslipidemia), and exposure to any diabetes related complications were concerned12. In our study, people with type 2 diabetes had a poor glycemic condition with a mean (SD) baseline HbA1c of 9.7 (1.8)% (≈83 mmol/mol) and almost none of them achieved the HbA1c <7% target (53 mmol/mol) in the last 3 months before treatment initiation. Microvascular diabetes-related complications were observed in 87.9% of the study population. The median estimated 10 year risk of CHD was 14.6% and thus was comparable with those previously described in the UKPDS study (6.1%–16.5%)%) and another survey (11% in women to 21% in men).13 14 These findings were a call to action for achieving a better glycemic control and consequently expected better clinical outcomes on the long term. At the end of inclusion visit, all people were prescribed either insulin alone (14.6%) or in combination with OADs (85.4%); the preferred insulin dosing regimen was basal insulin alone (71.2%) and then the basal bolus regimen with basal plus prandial insulin (25.1%). Adherence to therapy was high with 85% of patients (1569/1849) still on basal insulin glargine at the end of the study period at 12 months. The study findings suggest that intensifying the treatment of patients with type 2 diabetes by basal insulin initiation and titration, or intensification resulted in a decreased HbA1c of 2% points over 12 months. These results can influence positively patients outcome as a linear relationship between absolute change in HbA1c and mortality has been identified in subjects with an index HbA1c>8% (64 mmol/mol) the greatest decline in HbA1c being associated with the lowest mortality.15
The reasons for having conducted the study were multiple. To date, there is still a large population of severely uncontrolled diabetes (45%–75% of uncontrolled patients worldwide16–20 despite several treatments and current (ADA/EASD position statement7 for the management of type 2 diabetes. Moreover, the insulin initiation or intensification by titration can be delayed in practice because of clinical inertia in patient follow-up defined as ‘failure of healthcare providers to initiate or intensify therapy when indicated’21; Khunti et al22 reported that only 30.9% of clinically eligible patients for intensification (HbA1c≥7.5% (58 mmol/mol)) received treatment intensified with a median time of 3.7 years from initiation of basal insulin until intensification. In addition, older age, a longer time since diabetes diagnosis, and more comorbidities could significantly increase the delay in the time to intensification.22 Another phenomenon termed ‘psychological insulin resistance’ IR) is linked to patient reluctance toward insulin regimen explaining the possible delay for starting and/or intensifying insulin therapy leading to prolonged periods of poor glycemic control.22 23 More than one-quarter of insulin-naive patients with type 2 diabetes reported to reject the insulin regimen initiation once it is prescribed.24 The fear of hypoglycemia as well as concerns relative to weight gain reported by the patients but also by some physicians after having increased doses of insulin could be barriers to insulin treatment.22–25 Non-adherence to treatment with insulins seemed to play also key role in the poor glycemic control due to several explanations. The patient does not necessarily accept injectable route of the insulin regimen and can experience needle anxiety. Compared with men and whites, women and Asians reported a greater fear of injection in the context of insulin initiation.25 The number of administrations, need of self-injections, necessity to monitor the blood glucose once a day or more and to have a clean place for storing products may lead to patient inconvenience and missing injections.22 Achieving optimal glycemic control in patients with type 2 diabetes by initiating insulin therapy can be also delayed due to socioeconomical barriers; high costs of medications can lead to a reduction of doses since access to treatments and healthcare systems are not the same everywhere.
Regarding the primary endpoint, age, Caucasian ethnicity, shorter duration of diabetes and lower HbA1c at baseline were predictors for achieving glycemic goal as targeted by the treating physician at 6 and 12 months. The same trends were already found in the literature for some of these factors such as longer duration of the disease positively associated with uncontrolled diabetes status,26 and initial HbA1c reported as one of the most important success predictor.27 Similarly another study conducted in Japan confirmed our data as the authors revealed that shorter diabetes duration (<1 year), lower HbA1c (<8.5% (69 mmol/mol)), and no retinopathy at baseline were significantly associated with a higher rate of achieving the target HbA1c in insulin-naive patients who started therapy with insulin glargine plus OADs.28 However, our findings differed from a previous report27 in which age showed no effects. In our survey, glycemic achievers were significantly older than non-achievers (median age: 57 vs 55 years, respectively), and age appeared to be a significant positive (but not a strong) predictive factor of glycemic control (OR 1.02; p=0.0001). Findings of this study suggest that poor diabetes education seemed to be a predictive factor of glycemic control. Data about education are conflicting.29 30 However, education program delivered by diabetes educators may promote medication adherence for better glycemic control by reducing hospital readmissions.29 Moreover, hypertension was identified as a significant predictor of poor glycemic control in this study. This finding was consistent with data found in the literature.30 31 Hypertension is a frequent comorbidity in patients with type 2 diabetes and should be managed by aggressive treatment as well as other comorbid disease conditions to increase the likelihood to achieve good glycemic control and to reduce the burden of CVD.32
We also looked at the characteristics of the patients for whom the treating physician was considering HbA1c target<6.5% (48 mmol/mol) instead of HbA1c target >7.5% (58 mmol/mol). Unsurprisingly, the severity of diabetes (long duration of disease, high rate of macrovascular complications) associated with a pejorative CV risk as illustrated by the 10-year CHD score was found as the physicians’ determinants for choosing a high HbA1c target (HbA1c>7.5%). This finding is aligned with the conclusions of the landmark33 34 studies claiming for individualized glycemic goals and precaution in terms of glycemic reduction for the patients who are exposed to a more severe condition.35
Therefore, in addition to the normalization of the possible identified risk factors, it appears necessary to treat early with insulin and intensify the treatment so that a large number of patients reach an optimal glycemic level, decreasing the risk for CVD according to the UKPDS study.9 To date, intensive glucose control by lowering the glycated hemoglobin value to 6.5% is understood to prevent microvascular36 and macrovascular complications34 in patients with type 2 diabetes. Evidence suggests that early treatment intensification helps a significant proportion of patients achieving a sustained glycemic control (glycemic goal of HbA1c ≤7.0% (53 mmol/mol))37 and may help lowering rates of diabetes-related complications, given that a 1 year delay in receiving intensification treatment was reported to be associated with significantly increased risk of macrovascular events such as stroke by 51% (HR, 95% CI 1.25 to 1.83) or heart failure by 64% (HR, 95% CI 1.40 to 1.91).38
Indeed, it seems urgent to improve the management of patients with type 2 diabetes. The more the time passesd the less powerful the tools. Now that the possible causes of this lack of attractiveness for initiating or continuing insulin therapy had been already identified,22 24 25 it seems necessary to set up several tools preventing the patient non-acceptability. Several authors23 39 investigated some barriers that can be easily overcome through an interprofessional medical approach using appropriate and new education tools, providing explanations on devices for injections, or the use of insulin-specific prescription forms to improve adherence to treatment. The healthcare providers should adopt a patient-centered approach11 and impose the patient to follow hygiene-dietetic rules and regular physical activity. Moreover, new therapies or combinations of existing therapies should aim to address the need for better type 2 diabetes management by demonstrating effective glycemic control using a physiological approach (targeting both FPG and PPG), with more patients achieving HbA1c targets, which may help mitigate the risk of diabetes-related microvascular and/or macrovascular complications.
These study observations suggest that safety findings were rather satisfactory under antidiabetic therapy with insulin therapy over 12 months. There were no new safety concerns regarding basal insulin glargine or insulin glulisine. These findings were consistent with data found in the literature showing a low risk of hypoglycemia and weight gain in patients with type 2 diabetes treated with basal insulin associated with 0/1 OAD37 as incidence of hypoglycemia was low (0.3%) in our survey, and no marked changes in body weight and vital signs were observed from baseline to the end of the survey (12 months).
Strength of this study are its large subset of adults (>18 years) with type 2 diabetes inadequately controlled (ie, more than 2000 eligible patients type 2 diabetes) included in many countries from a wide geographical area. The actual population reflects real-life clinical practice and partially avoids the selection bias that characterizes randomized clinical trials. The analysis of predictive factors of glycemic control was performed in taking into account many clinical and non-clinical variables, which improves its reliability. This study had few inclusion and exclusion criteria that enhances its applicability to broader populations. However, the present study has some limitations owing to its observational design. The HbA1c target level was established by the treating physician according to his/her clinical practice; this choice could be subjective and different between practitioners. Furthermore, regular HbA1c measurements were not available for about 22% of the total analysis population at 6 and 12 months of follow-up that may have a slight influence on the observed results.
In conclusion, in people with type 2 diabetes inadequately controlled starting or intensifying their insulin dosing regimen showed an important decrease in HbA1c. Insulin regimen therapy initiated and/or intensified allowed, then, patients to achieve a better glycemic control at 12 months compared with baseline. No specific warnings were identified concerning the safety of insulin glargine and insulin glulisine. The risk of hypoglycemia and weight gain was limited. This large study confirmed that some well-known predictive factors (shorter type 2 diabetes duration, lower baseline HbA1c, no hypertension) could influence the patient glycemic outcome and prognosis.
Acknowledgments
The authors would like to thank all investigators involved in the study, POPSICUBE-FOVEA CRO (Montigny-le-Bretonneux) for the logistics and data analyses, and Valérie Patin (Valor One) for her assistance in medical writing, funded by Sanofi.
Footnotes
Presented at: Abstract of GOAL study results was published at the 77th American Diabetes Association Scientific session in 9–13 June 2017 (No. 2359-PUB).
Contributors: All authors were involved in the analysis and interpretation of data, contributed to the discussion and writing of the manuscript, and approved the final version of the article.
Funding: The study and data analysis was sponsored by Sanofi, Paris, France.
Competing interests: KD is employee at Sanofi. MF, YO, AAIM, AH and DD were national investigators for the study in their respective countries. AAIM, YO, NI, MF, AH, MM, TC received support from Sanofi to discuss the design, analysis, and interpretation of the study.
Patient consent: Not required.
Ethics approval: Ethics approval was obtained from institutional boards of each country.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data statement: No additional data available.
Author note: GOAL (non-interventional on the therapeutic strategy). There was no EUDRACT or NCT number as for clinical trials. Protocol was not disclosed to CT.gov. Study identifications numbers were: ClubNet number (LANTUR06314) and the OPTIMEnumber (OBS13527).
References
- 1. International Diabetes Federation. IDF Diabetes Atlas – Seventh Edition [online]. 2015. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html (accessed 19 Dec 2017).
- 2. World Health Organization (WHO). Global Report on Diabetes [online]. World HealthOrganization, Geneva (Switzerland). 2016. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (accessed 19 Dec 2017).
- 3. van Dieren S, Beulens JW, van der Schouw YT, et al. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 2010;17(Suppl 1):S3–8. 10.1097/01.hjr.0000368191.86614.5a [DOI] [PubMed] [Google Scholar]
- 4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203. 10.2337/dc08-9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 8. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–12. 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerstein HC, Pogue J, Mann JF, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 2005;48:1749–55. 10.1007/s00125-005-1858-4 [DOI] [PubMed] [Google Scholar]
- 10. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–9. 10.2337/dc14-2441 [DOI] [PubMed] [Google Scholar]
- 11. Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–7. 10.2337/dc13-0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009;32:227–33. 10.2337/dc08-0435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bannister CA, Poole CD, Jenkins-Jones S, et al. External validation of the UKPDS risk engine in incident type 2 diabetes: a need for new type 2 diabetes-specific risk equations. Diabetes Care 2014;37:537–45. 10.2337/dc13-1159 [DOI] [PubMed] [Google Scholar]
- 14. Sandbaek A, Griffin SJ, Rutten G, et al. Stepwise screening for diabetes identifies people with high but modifiable coronary heart disease risk. The ADDITION study. Diabetologia 2008;51:1127–34. 10.1007/s00125-008-1013-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skriver MV, Sandbæk A, Kristensen JK, et al. Relationship of HbA1c variability, absolute changes in HbA1c, and all-cause mortality in type 2 diabetes: a Danish population-based prospective observational study. BMJ Open Diabetes Res Care 2015;3:e000060 10.1136/bmjdrc-2014-000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alba LH, Bastidas C, Vivas JM, et al. [Prevalence of glycemic control and associated factors in type 2 diabetes mellitus patients at the Hospital Universitario de San Ignacio, Bogotá-Colombia]. Gac Med Mex 2009;145:469–74. [PubMed] [Google Scholar]
- 17. Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–25. 10.7326/M13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarez Guisasola F, Mavros P, Nocea G, et al. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab 2008;10(Suppl. 1):8–15. 10.1111/j.1463-1326.2008.00881.x [DOI] [PubMed] [Google Scholar]
- 19. Noureddine H, Nakhoul N, Galal A, et al. Level of A1C control and its predictors among Lebanese type 2 diabetic patients. Ther Adv Endocrinol Metab 2014;5:43–52. 10.1177/2042018814544890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Al-Rasheedi AA. Glycemic Control among Patients with Type 2 Diabetes Mellitus in Countries of Arabic Gulf. Int J Health Sci 2015;9:339–44. 10.12816/0024701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khunti S, Davies MJ, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes 2015;15:65–9. 10.15277/bjdvd.2015.019 [DOI] [Google Scholar]
- 22. Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab 2016;18:401–9. 10.1111/dom.12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 2002;26(Suppl. 3):S18–24. 10.1038/sj.ijo.0802173 [DOI] [PubMed] [Google Scholar]
- 24. Polonsky WH, Fisher L, Guzman S, et al. Psychological insulin resistance in patients with type 2 diabetes: the scope of the problem. Diabetes Care 2005;28:2543–5. 10.2337/diacare.28.10.2543 [DOI] [PubMed] [Google Scholar]
- 25. Nam S, Chesla C, Stotts NA, et al. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care 2010;33:1747–9. 10.2337/dc10-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yousefzadeh G, Shokoohi M, Najafipour H. Inadequate control of diabetes and metabolic indices among diabetic patients: A population based study from the Kerman Coronary Artery Disease Risk Study (KERCADRS). Int J Health Policy Manag 2014;4:271–7. 10.15171/ijhpm.2015.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarkadi A, Rosenqvist U. Field test of a group education program for type 2 diabetes: measures and predictors of success on individual and group levels. Patient Educ Couns 2001;44:129–39. 10.1016/S0738-3991(00)00170-1 [DOI] [PubMed] [Google Scholar]
- 28. Kadowaki T, Ohtani T, Odawara M. Baseline predictive factors for glycemic control in Japanese type 2 diabetes patients treated with insulin glargine plus oral antidiabetic drugs: ALOHA study subanalysis. Diabetol Int 2013;4:16–22. 10.1007/s13340-012-0087-6 [DOI] [Google Scholar]
- 29. Healy SJ, Black D, Harris C, et al. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care 2013;36:2960–7. 10.2337/dc13-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajeshwar Y, Eticha T, Mulu A, et al. Factors Associated with Poor Glycemic Control in Type 2 Diabetic Patients Investigated at Ayder Referral Hospital Mekelle, Ethiopia. Ijppr Human 2016;6:160–71. [Google Scholar]
- 31. Crowley MJ, Holleman R, Klamerus ML, et al. Factors associated with persistent poorly controlled diabetes mellitus: clues to improving management in patients with resistant poor control. Chronic Illn 2014;10:291–302. 10.1177/1742395314523653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kendall DM, Bergenstal RM. Comprehensive management of patients with type 2 diabetes: establishing priorities of care. Am J Manag Care 2001;7(10 Suppl):S327–43. quiz S344-8. [PubMed] [Google Scholar]
- 33. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–72. 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 35. Cefalu WT. Glycemic targets and cardiovascular disease. N Engl J Med 2008;358:2633–5. 10.1056/NEJMe0803831 [DOI] [PubMed] [Google Scholar]
- 36. UK Prospective Diabetes Study Group (UKPDS). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- 37. Fonseca V, Gill J, Zhou R, et al. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab 2011;13:814–22. 10.1111/j.1463-1326.2011.01412.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul SK, Klein K, Thorsted BL, et al. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:100 10.1186/s12933-015-0260-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ali A, Cheng AY, Yu CH. Breaking down barriers to initiating insulin: Insulin prescription pad. Can Fam Physician 2015;61:445–7. [PMC free article] [PubMed] [Google Scholar]
- 40. Food and Drug Administration. 2016. Collection of Race and Ethnicity Data in Clinical Trials. Guidance for Industry and Food and Drug Administration Staff https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-gen/documents/document/ucm126396.pdf (accessed 03 Apr 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000519supp001.docx (29.1KB, docx)


