Abstract
Background
FOLFOXIRI plus bevacizumab is considered a standard option in the upfront treatment of clinically selected patients with metastatic colorectal cancer irrespective of RAS and BRAF molecular status. The randomised MACBETH and VOLFI studies showed that a modified FOLFOXIRI regimen in combination with cetuximab or panitumumab, respectively, achieved high therapeutic activity in RAS and BRAF wild-type patients with an acceptable toxicity profile. Drawing from these considerations, we designed TRIPLETE study aiming at comparing two different chemotherapy backbones (mFOLFOXIRI or mFOLFOX6) in combination with panitumumab in the first-line treatment of patients with RAS and BRAF wild-type metastatic colorectal cancer.
Methods
This is a prospective, open-label, multicentre phase III trial in which initially unresectable and previously untreated RAS and BRAF wild-type metastatic colorectal cancer patients are randomised to receive a standard treatment with mFOLFOX6 plus panitumumab or an experimental regimen with modified FOLFOXIRI (irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, L-leucovorin 200 mg/m2, 5-fluoruracil 2400 mg/m2 48-hour continuous infusion) plus panitumumab up to 12 cycles, followed by panitumumab plus 5-fluorouracil and L-leucovorin until disease progression. The primary endpoint is overall response rate according to RECIST 1.1 criteria.
Discussion
The relative benefit of chemotherapy intensification when using an anti-EGFR-based regimen in molecularly selected patients is unknown; TRIPLETE study aims at filling this gap of knowledge. The study is sponsored by the Gruppo Oncologico Nord Ovest Cooperative Group and is currently ongoing at 42 Italian centres.
Clinical trial information
Keywords: folfoxiri, anti-egfr monoclonal antibody, panitumumab, metastatic colorectal cancer, triplet
Introduction
Selecting the most appropriate first-line treatment is a challenging issue in the management of metastatic colorectal cancer (mCRC). The paramount importance of this choice lies in the role of the upfront treatment in achieving disease control, thus obtaining symptoms’ relief, allowing further locoregional and systemic interventions and, even more relevantly, providing the unique opportunity to cure some metastatic patients.1
A growing amount of drugs is indicated for the first-line treatment of mCRC and, in the absence of contraindications, the association of a biological agent to a chemotherapy doublet is a standard upfront choice. Nevertheless, emerging evidence highlights the possibility to further personalise the treatment by modulating the intensity of the chemotherapy backbone from one- drug to three- drug regimens according to treatment’s objective, patient’s characteristics and tumour biology.2 3
To this regard, the phase III randomised TRIBE study evaluated the combination of bevacizumab with the three-drug regimen FOLFOXIRI (5-fluorouracil, oxaliplatin and irinotecan) or the doublet FOLFIRI (5-fluorouracil and irinotecan). The trial met its primary endpoint reporting significantly longer progression-free survival (PFS; 12.1 vs 9.7 months, HR: 0.75, p=0.003) in favour of the triplet. A significant advantage in terms of overall survival (OS; 29.8 vs 25.8 months, HR: 0.80, p=0.030) and objective response rate (ORR; 65% vs 53%, p=0.006) was also evident. The triplet was associated with increased grade 3 and 4 neutropaenia, diarrhoea and stomatitis but no higher incidences of febrile neutropaenia, serious adverse events or treatment-related deaths were reported.4 5 Other phase II randomised trials with the use of FOLFOXIRI plus bevacizumab were conducted, and consistent efficacy and safety results were achieved.6 Based on these results, the triplet FOLFOXIRI plus bevacizumab is now regarded by all major guidelines as a safe and efficacious first-line therapeutic option for selected patients with mCRC.2 3 7
Also the combination of FOLFOXIRI with an anti-EGFR monoclonal antibody has been investigated in some phase II trials, pointing out remarkable activity results, translating into high secondary resection rates (table 1),8–14 at the price of a substantial increase in chemotherapy-related toxicities, in particular grades 3 and 4 diarrhoea. A single-arm phase II study by Gruppo Oncologico Nord Ovest (GONO) assessed the activity and safety of the combination of panitumumab with a modified schedule of FOLFOXIRI (irinotecan was administered at 150 instead of 165 mg/m2) in a highly molecularly selected population of 37 patients with unresectable KRAS/NRAS/HRAS/BRAF wild-type (wt) disease. Based on the occurrence of severe diarrhoea and mucositis in two of the first three patients enrolled, the study was amended to reduce the dosage of the 48-hour continuous infusion of 5-fluorouracil (5-FU) from 3200 mg/m2 to 2400 mg/m2. After the amendment, the most common grades 3–4 adverse events were neutropaenia (48%), diarrhoea (35%), asthenia (27%), stomatitis (14%) and skin rash (14%).12 More recently, the phase II randomised MACBETH study tested the combination of cetuximab plus a modified FOLFOXIRI regimen (mFOLFOXIRI: irinotecan: 130 mg/m2 day 1, oxaliplatin: 85 mg/m2 day 1, L-Leucovorin (LV) 200 mg/m2 day 1, 5-FU 2400 mg/m2 48-hour continuous infusion) in 116 patients with RAS/BRAF-wt mCRC. The safety profile was acceptable (31% grades 3–4 neutropaenia, 18% grades 3–4 diarrhoea, 6% grades 3–4 stomatitis, 16% grades 3–4 skin rash and 3% febrile neutropaenia) and reassuring results about the feasibility of this combination were provided. Encouraging activity data emerged, with 72% and 91% ORR and disease control rate (DCR), respectively. In addition, 76% of patients achieved early tumour shrinkage (ETS), and the median depth of response (DoR) was 53%; R0 resection rate was 28% in the overall population and 50% in the liver-only subgroup. Four months of mFOLFOXIRI plus cetuximab followed by cetuximab maintenance achieved a median PFS of 10.1 months and an OS of 33.2 months.14 Therefore, data from MACBETH confirmed the feasibility of the modified triplet plus an anti-EGFR monoclonal antibody and remarked the notable activity of this treatment translating into a high secondary resection rate.
Table 1.
Trials with anti-EGFR plus triplet chemotherapy in patients with metastatic colorectal cancer
| Author | n | Molecular selection | Schedule | RR (%) | R0 resection rate (%) | Grades 3–4 diarrhoea (%) | mPFS | mOS |
| Garufi et al8 | 43 | No molecular selection | Chrono-IFLO+ cetuximab |
79 | 60 | 94–36 | 14 months | 37 months |
| Assenat et al9 | 42 | KRAS exon 2 wt | FOLFIRINOX+ cetuximab |
81 | Not reported | 52 | 9.5 months | 24.7 months |
| Folprecht et al10 | 20 | No molecular selection | mFOLFOXIRI+ cetuximab |
75 | Not reported | 25 | 16 months | 33 months |
| Saridaki et al11 | 30 | KRAS exon 2 wt | FOLFOXIRI+ cetuximab |
70 | 37 | 53 | 10.2 months | 30.3 months |
| Fornaro et al12 | 37 | KRAS, NRAS and BRAF wt | mFOLFOXIRI+ panitumumab |
89 | 35 | 33 | 11.3 months | Not reached |
| Geissler et al13 | 30 | RAS wt | mFOLFOXIRI+ panitumumab |
86 | 16 | Not reported | 10.8 months | Not reported |
| Cremolini et al14 | 143 | KRAS exon 2 then, RAS and BRAF wt | mFOLFOXIRI+ cetuximab |
72 | 28 | 18 | 10.1 months | 33.2 months |
mPFS, median progression free survival; mOS, median overall survival; RR, response rate.
Another recent phase II study, VOLFI, that randomised 96 patients with RAS wt mCRC to receive FOLFOXIRI±panitumumab, reported high ORR (86% vs 61% with FOLFOXIRI, p=0.0096) and DCR (97% vs 79%, p=0.0071) with the triplet plus the anti-EGFR. Secondary R0 resection rate was 16% in triplet plus anti-EGFR arm versus 9% in chemotherapy-only arm in the overall population and 50% vs 27% in the potentially resectable cohort.13
In spite of encouraging safety and activity results, it is not clear whether the intensification of the chemotherapy backbone in combination with the anti-EGFR may be beneficial and to what extent in properly selected patients. This gap of knowledge might be filled only by a phase III study. Drawing from this evidence, we designed the TRIPLETE study, a phase III randomised trial of first-line mFOLFOXIRI plus panitumumab versus mFOLFOX6 plus panitumumab in patients with RAS and BRAF wt unresectable mCRC.
Methods/design
Study design
The present study is a prospective, open-label, multicentre phase III randomised trial in which initially unresectable and previously untreated patients with RAS and BRAF wt mCRC are randomised in a 1:1 ratio to receive panitumumab plus mFOLFOX6 (arm A, standard treatment) or panitumumab plus mFOLFOXIRI (arm B, experimental arm) every 14 days up to 12 cycles, followed by panitumumab plus 5-FU/LV as maintenance in both arms until disease progression, unacceptable adverse events or consent withdrawal (figure 1).
Figure 1.
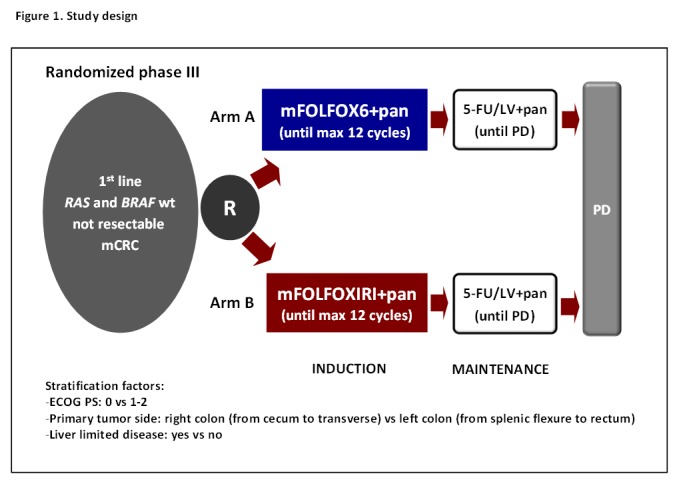
Study design. 5-FU, 5-fluorouracil; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LV, L-Leucovorin; PD, disease progression.
The feasibility of surgical radical resection of residual metastases in responsive patients is evaluated every 8 weeks. In the case of secondary resection of metastases, a postoperative therapy with the same chemotherapy regimen received before resection plus panitumumab is planned up to a total duration (preoperative plus postoperative treatment) of 12 cycles (figure 2). The postoperative treatment should start not earlier than 4 weeks after surgery.
Figure 2.
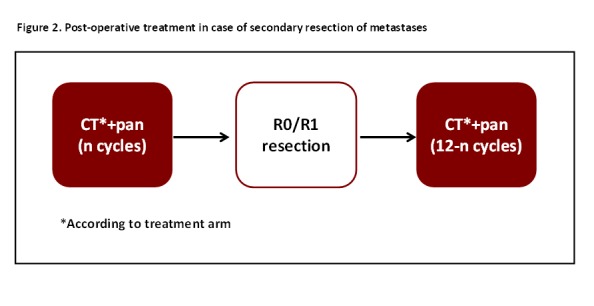
Postoperative treatment in case of secondary resection of metastases.
Study objectives and endpoints
The primary objective of this trial is to compare the activity of panitumumab in combination with mFOLFOX6 or with mFOLFOXIRI in patients with RAS and BRAF wt mCRC in terms of ORR according to RECIST 1.1 criteria.15 ORR is defined as the percentage of patients achieving a complete response (CR) or partial response (PR) during the study treatment period.
Secondary objectives of this study are to compare the two proposed treatments in terms of safety profile, PFS, OS, centrally assessed ORR, ETS, DoR and R0 resection rate.
PFS is defined as the time from randomisation to the first disease progression or death, whichever occurs first; OS is defined as the time from randomisation to the date of death due to any cause; centrally assessed ORR is defined as the percentage of patients relative to the total of enrolled subjects, achieving a CR or PR based on central re-evaluation of CT scan images; ETS is defined as the rate of patients, relative to the total of the enrolled subjects, achieving a ≥20% decrease in the sum of diameters of RECIST target lesions at week 8 compared with baseline; DoR is defined as the relative change in the sum of longest diameters of RECIST target lesions at the nadir, in the absence of new lesions or progression of non-target lesions, when compared with baseline; R0 resection rate is defined as the percentage of patients, relative to the total of enrolled subjects, undergoing secondary R0 resection of metastases.
Statistical design
The primary analysis of ORR will be performed in the intention-to-treat population. The proportion of patients with a best overall response of CR or PR with its 95% CI will be reported for each arm. The χ2 test for heterogeneity and the OR will be used for comparing the distributions of best overall response among the treatment groups. Patients are stratified according to the following factors: ECOG Performance Status (ECOG PS) (0 vs 1–2), primary tumour location (right [from cecum to transverse colon] vs left [from splenic flexure to rectum]) and spread of metastatic disease (liver limited vs no liver limited). The stratified analysis in the intention-to-treat population will be presented as primary analysis.
Under the assumption of a ORR in the control group equal to 60%, based on the results of the registrative trial of panitumumab in association with FOLFOX,16 a total sample size of 432 cases, randomised in a 1:1 ratio, provides approximately 90% power to a two-sided χ2 test for heterogeneity at the 0.05 significance level, in order to detect a ≥15% difference in ORR between the two treatment arm.
The Kaplan-Meier method will be used to performed survival analyses. Log-rank tests stratified by the same factors as used for randomisation will also be performed, as well as multivariable models including all the significant baseline variables. The median event times and corresponding two-sided 95% CIs for the median will be provided. A secondary analysis of all primary and secondary endpoints will be performed in the centrally assessed RAS/BRAF wt population.
Study population
The study has been approved by 58 ethics committees and is currently ongoing at 42 Italian oncology units. Consistent with previous FOLFOXIRI-based trials conducted by the GONO group, only patients aged <70 years with ECOG PS ≤2, or aged 71–75 years with ECOG PS 0, are eligible. Main inclusion criteria are: the availability of a tumour tissue sample (primary tumour and/or metastatic sites), RAS (codons 12, 13, 59, 61, 117 and 146 of KRAS and NRAS genes) and BRAF (V600E mutation) wt status of primary colorectal cancer or related metastasis, at least one measurable lesion according to RECIST 1.1, adequate liver, renal and bone marrow function. Previous oxaliplatin-based adjuvant chemotherapy is not permitted; adjuvant fluoropyrimidine monotherapy is allowed only if more than 6 months have elapsed between the end of adjuvant therapy and disease relapse. Other exclusion criteria are: previous treatment with anti-EGFR inhibitors, symptomatic peripheral neuropathy >1 according to NCI-CTCAE V.4.017 and contraindications to study drugs.
Study procedures
Patients randomised in arm A (standard arm) receive mFOLFOX6 plus panitumumab (panitumumab 6 mg/kg, oxaliplatin 85 mg/m2, LV200 mg/m2, 5-FU 400 mg/m2 intravenous bolus, 5-FU 2400 mg/m2 48-hour continuous infusion) every 2 weeks for a maximum of 12 cycles.
Patients randomised in arm B (experimental arm) receive mFOLFOXIRI plus panitumumab (panitumumab 6 mg/kg, irinotecan 150 mg/m2, oxaliplatin 85 mg/m2, LV 200 mg/m2, 5-FU 2400 mg/m2 48-hourh continuous infusion) every 2 weeks for a maximum of 12 cycles. If no progression occurs, maintenance with 5-FU/LV plus panitumumab is administered biweekly in both arms at the same dose used at the last cycle of the induction treatment until disease progression, unacceptable toxicity or patient’s refusal. The continuation of panitumumab until disease progression is recommended also if 5-FU is interrupted because of adverse events, patient’s refusal or at investigator’s choice.
The application of skin moisturisers, topical steroid (1% hydrocortison cream) and sunscreen protections is suggested in order to prevent skin acne-like rash. In addition, pre-emptive treatment with doxycycline 100 mg daily for 1 week starting from day 1 of cycle 1 and then in alternate cycles is recommended.
RAS and BRAF testing is locally or centrally performed at investigator’s choice. The independent central reassessment of RAS and BRAF mutational status by means of MALDI-TOF MassArray (Sequenom) is planned.18 19 Disease assessment is performed every 8 weeks by means of CT-scan.
Safety
All adverse events observed during the study treatment period are properly registered in the subjects’ medical records and in electronic case report forms. All serious adverse events (SAEs), that is, fatal or life-threatening adverse events or those requiring hospitalisation or resulting in persistent or significant disability/incapacity should be notified within 24 hours by the investigator to the sponsor according to local procedures, statutes and the European Clinical Trial Directive (when applicable). The sponsor will medically review all SAEs and is responsible for their notification to the appropriate ethics committees, competent authorities and participating Investigators.
Translational analyses
A wide programme of translational analyses is planned. The availability of tissue specimens (primary tumour or metastatic site) is mandatory for study entry. Tissue specimens are collected for the central assessment of RAS and BRAF status and for further molecular analyses. In the case of secondary resection of metastases, the collection of newly available specimens is required. Also blood and plasma samples are collected at baseline, at every CT scan performed during induction and at first evidence of disease progression. In the case of secondary resection of metastases, plasma samples will be collected also within 1 month before and after surgery, respectively.
Ethics and regulatory considerations
The procedures set out in the present study respect the Good Clinical Practice guidelines and the guiding principles detailed in the Declaration of Helsinki. The study is also carried out in keeping with applicable local law(s) and regulation(s).
It was registered in the EUDRACT database (EUDRACT NUMBER 2016-004394-40) in October 2016 and at clinicaltrials.gov (NCT03231722) in July 2017. Written informed consent to study procedures must be provided by all candidate patients before the enrolment.
Discussion
Doublets plus anti-EGFR are standard options for the upfront treatment of patients with RAS/BRAF wt mCRC. While phase II trials suggest interesting activity results with triple chemotherapy regimens plus an anti-EGFR, the added value of intensifying the chemotherapy backbone from doublets to the triplet, when using an anti-EGFR as upfront targeted agent, has never been estimated. TRIPLETE study aims at filling this current gap of knowledge.
Some key points of the trial design deserve consideration. First, with regard to patients’ inclusion criteria, primary tumour location was chosen as a stratification factor, based on the clear negative prognostic impact of right-sidedness20 21 but not as a selection factor. In fact patients with right-sided primary tumours were not excluded from this trial. Although recognising the lower sensitivity to anti-EGFRs of right-sided versus left-sided tumours when using a chemotherapy doublet,22 23 the lack of regulatory restrictions to the use of anti-EGFRs in right-sided tumours, as well as the lack of evidence about the usefulness of an intensified chemotherapy backbone in combination with the anti-EGFR in this poor-prognosis subgroup, drove this choice. In addition, though acknowledging the small sample sizes, in the VOLFI study, right-sided tumours achieved an ORR of 60% with FOLFOXIRI plus panitumumab as compared with 38% with triplet alone.13
Second, in the TRIPLETE study, ORR was chosen as primary endpoint. While the choice of OS as primary endpoint would have substantially hampered the feasibility of this academic study, the reliability of PFS as a surrogate endpoint of OS appears weakened when using upfront anti-EGFR-based treatments. However, a significant improvement in ORR translating into OS benefit was observed in recent phase III trials investigating anti-EGFR-containing regimens.16 24 An emblematic example of how activity parameters predict OS better than PFS in trials evaluating the efficacy of anti-EGFR agents is the FIRE-3 study. In this trial, a clear benefit was demonstrated in terms of OS in favour of FOLFIRI plus cetuximab with respect to FOLFIRI plus bevacizumab with no difference in terms of PFS. At the central independent radiological evaluation, significantly higher ORR, ETS and DoR were reported in RAS wt patients treated with cetuximab-based therapy. Also ETS and DoR correlated with survival.25 Moreover, as clearly stated by European Society for Medical Oncology guidelines, achieving cytoreduction is a relevant clinical objective in different scenarios of mCRC, including those cases in which the secondary resection of metastases is a pursuable objective and those in which the high tumour burden makes response needed in order to improve symptoms or to prevent their occurrence.2 To this purpose, the triplet plus an anti-EGFR might be a valuable option, as suggested by the choice of activity endpoints also in other currently ongoing trials evaluating similar regimens (table 2).
Table 2.
Ongoing trials with anti-EGFR plus triplet chemotherapy in patients with metastatic colorectal cancer
| Author | Phase | Estimated enrolment | Setting | Molecular selection | Experimental schedule | Endpoint |
| Deng et al33 | Phase II | 138 | Unresectable liver mestastases only | RAS wt | FOLFOXIRI± cetuximab |
Complete curative liver treatment (surgery and/or RFA) |
| Nakajima et al34 | Phase II | 360 | First line | RAS wt | FOLFOXIRI+ cetuximab or bevacizumab |
DoR |
| Folprecht et al35 | Phase II | 256 | Unresectable liver mestastases only | RAS/BRAF wt | FOLFOXIRI+ cetuximab |
ORR |
| Ychou et al36 | Phase II | 209 | First line | RAS/BRAF wt selected by cDNA analysis | Panitumumab+ mFOLFOX6 or FOLFIRINOX |
CRR |
cDNA, circulating DNA; CRR, complete response rate; DoR, best deepness of response; ORR, overall response rate; RFA, radiofrequency ablation.
Third, in the present study, a 6-month induction phase with chemotherapy plus panitumumab, followed by maintenance with 5-FU/LV plus panitumumab, is planned in both arms. Only a few data are currently available with regard to the role of maintenance after an anti-EGFR-based induction therapy and the ‘best’ maintenance to be administered. Our choice was driven by results of trials in the ‘chemotherapy-alone era’ underlining the possibility to de-potentiate the treatment without compromising patients’ prognosis26 27 and by results of two phase II trials, respectively showing the non-inferiority of maintenance with cetuximab alone versus the full treatment until progression28 29 and the feasibility of cetuximab maintenance versus a ‘stop&go’ strategy.29 This decision was also recently supported by the presentation of the Japanese phase II SAPPHIRE trial where patients not progressing after six cycles of FOLFOX plus panitumumab were randomised to receive 5-FU/LV and panitumumab as maintenance therapy or to continue induction treatment. Preliminary data showed similar 9-months PFS (primary endpoint of the study) in the two arms, thus supporting the use of anti-EGFR plus 5-FU/LV as maintenance in order to delay disease progression while preventing the occurrence of oxaliplatin-induced neuropathy.30
Finally, in order to improve the adherence to the therapy, the administration of doxycycline is recommended in both arms to prevent anti-EGFR-induced skin rash. Based on clinical experiences demonstrating a reduced incidence of acneiform rash in the case of pre-emptive rather than reactive administration of a tetracycline during anti-EGFR-based therapy,31 Multinational Association of Supportive Care in Cancer guidelines actually recommend the pre-emptive use of oral doxycycline or minocycline.32
Conclusions
In the era of personalised medicine, TRIPLETE study will throw light on the potential value of a modified schedule of FOLFOXIRI plus panitumumab as a valuable upfront option for some patients with mCRC, selected on the basis of a careful evaluation of patients’ characteristics, molecular features and treatment’s objective.
Acknowledgments
Thestudy is sponsored by GONO Cooperative Group
Footnotes
Contributors: RM, GM, ChC and AF contributed to the study design; all authors contributed to writing and revision of the paper.
Funding: The study is supported by GONO Cooperative Group and partially funded by AMGEN s.r.l.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: The study (protocol V. 1.2, 23 May 2017) was approved in June 2017 by the Ethics Committee of the Coordinating Center (Comitato Etico Area Vasta Nord Ovest) and then approved by the local ethics committees of participating centres.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Cremolini C, Schirripa M, Antoniotti C, et al. First-line chemotherapy for mCRC—a review and evidence-based algorithm. Nat Rev Clin Oncol 2015;12:607–19. 10.1038/nrclinonc.2015.129 [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 3. Network NCC. NCCN clinical practise guidelines in oncology, NCCN guidelines colon cancer (Version 2.2018), 2018. [Google Scholar]
- 4. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–18. 10.1056/NEJMoa1403108 [DOI] [PubMed] [Google Scholar]
- 5. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–15. 10.1016/S1470-2045(15)00122-9 [DOI] [PubMed] [Google Scholar]
- 6. Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol 2010;11:845–52. 10.1016/S1470-2045(10)70175-3 [DOI] [PubMed] [Google Scholar]
- 7. Salvatore L, Aprile G, Arnoldi E, et al. Management of metastatic colorectal cancer patients: guidelines of the Italian Medical Oncology Association (AIOM). ESMO Open 2017;2:e000147 10.1136/esmoopen-2016-000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer 2010;103:1542–7. 10.1038/sj.bjc.6605940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Assenat E, Desseigne F, Thezenas S, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist 2011;16:1557–64. 10.1634/theoncologist.2011-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folprecht G, Hamann S, Schütte K, et al. Dose escalating study of cetuximab and 5-FU/folinic acid (FA)/oxaliplatin/irinotecan (FOLFOXIRI) in first line therapy of patients with metastatic colorectal cancer. BMC Cancer 2014;14:521 10.1186/1471-2407-14-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saridaki Z, Androulakis N, Vardakis N, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer 2012;107:1932–7. 10.1038/bjc.2012.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fornaro L, Lonardi S, Masi G, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 2013;24:2062–7. 10.1093/annonc/mdt165 [DOI] [PubMed] [Google Scholar]
- 13. Geissler UMM M, Knorrenschield R. mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO-KRK0109). Ann Oncol 2017;28(Suppl 5):159. [Google Scholar]
- 14. Cremolini C, Antoniotti C, Lonardi S, et al. Activity and Safety of Cetuximab Plus Modified FOLFOXIRI Followed by Maintenance With Cetuximab or Bevacizumab for RAS and BRAF Wild-type Metastatic Colorectal Cancer: A Randomized Phase 2 Clinical Trial. JAMA Oncol 2018;4:529 10.1001/jamaoncol.2017.5314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 17. National Cancer Institute. Common terminology criteria for adverse events v4.0, 2009. [Google Scholar]
- 18. Arcila M, Lau C, Nafa K, et al. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn 2011;13:64–73. 10.1016/j.jmoldx.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fumagalli D, Gavin PG, Taniyama Y, et al. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer 2010;10:101 10.1186/1471-2407-10-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sunakawa Y, Ichikawa W, Tsuji A, et al. Prognostic Impact of Primary Tumor Location on Clinical Outcomes of Metastatic Colorectal Cancer Treated With Cetuximab Plus Oxaliplatin-Based Chemotherapy: A Subgroup Analysis of the JACCRO CC-05/06 Trials. Clin Colorectal Cancer 2017;16:e171–e180. 10.1016/j.clcc.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 22. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713–29. 10.1093/annonc/mdx175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 2017;70:87–98. 10.1016/j.ejca.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 24. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065–75. 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 25. Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426–34. 10.1016/S1470-2045(16)30269-8 [DOI] [PubMed] [Google Scholar]
- 26. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400. 10.1200/JCO.2005.03.0106 [DOI] [PubMed] [Google Scholar]
- 27. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33. 10.1200/JCO.2009.23.4344 [DOI] [PubMed] [Google Scholar]
- 28. García Alfonso P, Benavides M, Sánchez Ruiz A, et al. Phase II study of first-line mFOLFOX plus Cetuximab (C) for 8 cycles followed by mFOLFOX plus C or single agent (S/A) C as maintenance therapy in patients (P) metastatic colorectal cancer (mCRC): The MACRO-2 trial (Spanish Cooperative Group for Treatment of Digestive Tumours [TTD]). Ann Oncol 2014;25(Suppl 4):iv167–iv209. [Google Scholar]
- 29. Wasan H, Meade AM, Adams R, et al. Intermittent chemotherapy plus either intermittent or continuous cetuximab for first-line treatment of patients with KRAS wild-type advanced colorectal cancer (COIN-B): a randomised phase 2 trial. Lancet Oncol 2014;15:631–9. 10.1016/S1470-2045(14)70106-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura MMY, Takahashi M. SAPPHIRE: A randomized phase II study of mFOLFOX6 + panitumumab versus 5-FU/LV + panitumumab after 6 cycles of frontline mFOLFOX6 + panitumumab in patients with colorectal cancer. J Clin Oncol 2018;36:729. [Google Scholar]
- 31. Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:1351–7. 10.1200/JCO.2008.21.7828 [DOI] [PubMed] [Google Scholar]
- 32. Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer 2011;19:1079–95. 10.1007/s00520-011-1197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ClinicalTrials.gov. Medicine. UNLo. 2014. https://clinicaltrials.gov/ct2/show/NCT02063529
- 34. ClinicalTrials.gov. Medicine. UNLo. 2015. https://clinicaltrials.gov/ct2/show/NCT02515734
- 35. ClinicalTrials.gov. Medicine. UNLo. 2013. https://clinicaltrials.gov/ct2/show/NCT01802645
- 36. ClinicalTrials.gov. Medicine. UNLo. 2011. https://clinicaltrials.gov/ct2/show/NCT01328171


