Abstract
Surgical resection is the only option of cure for patients with metastatic colorectal cancer. Risk of recurrence after metastasectomy is around 75%. Use of adjuvant chemotherapy after metastasectomy is controversial.
Aim
To address whether adjuvant systemic therapy after colorectal cancer metastasectomy offers any survival benefit compared with surgery alone.
Methods
Systematic review of literature and meta-analysis of all available randomised evidence. Relative hazards (RHs) were summarised across trials and heterogeneity was assessed with the Q and I2 statistics.
Results
Five trials were eligible. Three trials, all using single-agent fluoropyrimidine chemotherapy, presented data valuable for analyses. 482 patients were included in the meta-analysis: 238 randomly assigned to receive postoperative chemotherapy and 244 to metastasectomy only. We found no overall survival (OS) benefit with the use of postoperative single-agent fluoropyrimidines compared with surgery alone, even if a trend for benefit was observed (relative hazard (RH)=0.781, 95% CI 0.593 to 1.030, p=0.080). Significant disease-free survival benefit with the use of postoperative chemotherapy was observed (RH=0.645, 95% CI 0.509 to 0.818, p=0.001). No quality of life (QL) data were available. All trials showed accrual delay, two stopped and one recruiting after 10 years. Long follow-up needs were evidenced since OS curves split only after 3.5 years.
Conclusions
No OS benefit was documented from the use of postoperative monochemotherapy. Metastasectomy alone continues to be the standard of care. Combination chemotherapy regimens should be evaluated along with QL assessment in future trials appropriately designed for long-term accrual and follow-up.
Keywords: postoperative chemotherapy, liver metastasectomy, colorectal cancer metastases, meta-analisys, fluoropyrimidines
Key questions.
What is already known about this subject?
Risk of recurrence after colorectal cancer liver metastasectomy for curative intent is around 75%.
The use of adjuvant chemotherapy after metastasectomy is controversial.
Only five small randomised trial from 1991 investigated the role of postoperative chemotherapy versus metastasectomy alone.
What does this study add?
Our systematic review of literature and meta-analysis of all available randomised trials evidenced no overall survival (OS) benefit with the use of postoperative single-agent fluoropyrimidines compared with surgery alone (RH=0.781, 95% CI 0.593 to 1.030, p=0.080).
Significant disease-free survival benefit with the use of postoperative chemotherapy was observed (RH=0.645, 95% CI 0.509 to 0.818, p=0.001), however no quality of life (QL) data were available to assess the quality of benefit.
In all the analysed trials, the OS curves were identical within the first 3.5 years after resection, and split later on with a trend for OS benefit.
All eligible randomised trials suffered from low accrual rates (two were stopped due to low accrual rate) and one is still struggling to recruit 300 patients 10 years after its initiation in 2007.
Key questions.
How might this impact on clinical practice?
Since no overall survival benefit was documented from the use of postoperative monochemotherapy, metastasectomy alone continues to be the standard of care.
Since significant disease-free survival benefit with the use of postoperative chemotherapy was observed, chemotherapy regimens in future trials should be evaluated along with quality of life assessment in order to quantify toxicity and the quality of survival benefit achieved.
Combination chemotherapy regimens in future trials should be appropriately designed considering the long-term requirements for accrual and follow-up.
Introduction
Colorectal cancer is the third most common cancer worldwide.1 2 Approximately 60% of patients will present with colorectal liver metastases during the course of disease.3 Surgical resection is the only option of cure for patients with metastatic colorectal cancer however the risk of recurrence after metastasectomy is around 75% with the liver being the most frequent site of relapse.4 5
Adjuvant chemotherapy improves disease-free survival (DFS), however evidence for overall survival (OS) benefit from clinical trials and meta-analyses studies are controversial,6–8 thus, its routine use is not universally recommended.
However, in previous contrasting analyses, both randomised data from preoperative, perioperative and postoperative trials as well as data from high-quality non-randomised trials were used.6–9 Moreover, the tegafur–uracil randomised trial was not included in any previous analyses since the follow-up data were not available yet.6
To address whether adjuvant systemic therapy after colorectal cancer metastasectomy offers any survival benefit compared with surgery alone, we performed a systematic review of literature and meta-analysis of all actually available cumulative randomised evidence from randomised trials. Only randomised evidence was included in the study.
Material and methods
Identification of randomised studies
We searched the Cochrane Central Trials Registry, Embase, Science Direct and PubMed databases without year and language restriction. The last search was updated in February 2018. We used the searching algorithm: (chemotherapy) AND (cancer OR carcinoma OR neopl*) AND (colon OR colorectal OR rectal) AND (metastasis OR metastases OR secondaris*) AND (metastasectomy OR resection OR surgical OR hepatectomy) AND (clinical trial OR randomized controlled trial OR double-blind OR single-blind OR single-blind OR random OR randomized OR placebo).
We also searched for the years 2012 through 2017 several oncology journals that publish randomised trials (Journal of Clinical Oncology, Annals of Oncology and Lancet Oncology) to ensure that electronic searches would not miss reports of eligible studies.10 The reference list of retrieved papers was further screened for additional publications and to minimise publication bias. Furthermore, as recent trials may still be unpublished, we also reviewed abstract books and presentations of major recent meetings in 2014–2017 of the American Society of Clinical Oncology, the European Society of Clinical Oncology, to identify any other trial that had presented final data and comparisons that would be eligible for consideration in the meta-analysis (American Society of Clinical Oncology ASCO, European Society of Medical Oncology ESMO, European Cancer Organisation ECCO annual meeting, ESMO World Congress on Gastrointestinal Cancer and ASCO Gastrointestinal Cancer Symposium). Earlier meeting abstracts were not included.
Eligibility criteria
We considered as eligible all randomised controlled trials that compared surgery alone versus adjuvant systemic therapy after colorectal cancer metastasectomy for curative intent in patients with advanced colorectal cancer. Trials were eligible regardless of the doses and schedules used for the regimens compared. We excluded earlier meeting abstracts because preliminary reports are opened to modification, as well as single-arm studies, dose-escalation studies, and non-randomised and pseudorandomised trials (eg, those with alternate allocation of subjects).
Trials that used other concomitant anticancer treatments (eg, radiofrequency ablation, radiotherapy or radioisotopic treatment) were eligible if these treatments did not differ systematically between the investigated arms. Trials in which the compared arms differed systematically in the use of these additional disease-related treatments were, however, excluded because the differences in survival could not necessarily be attributed to the comparison of adjuvant systemic chemotherapy with standard surgery alone. Whenever multiple reports pertained to overlapping groups of patients, we retained only the report with longest follow-up (largest number of events) for the meta-analysis calculations to avoid duplication of information. Data from interim analyses were eligible if no further final data were available.
Data extraction and outcomes
A literature search was performed by two independent reviewers: DM and GZr. independently searched medical libraries, LT and GZf. independently performed specific journals searches, PF and AP independently performed scrutinised 2014–2017 conferences. In case of disagreement between independent searches GP (gastrointestinal oncology expert) supervised the data.
From each eligible trial, we recorded the following items for both arms: authors' names; journal and year of publication; country of origin; years of patient enrolment; number of centres involved; number of patients randomly assigned and analysed per arm, age, site of metastases, the exact regimens used and their dose and schedule; the line of treatment; and any additional treatments given to both arms. We recorded study design items, including whether there was a description of the mode of randomisation, allocation concealment, the number of withdrawals per arm and blinding11 and whether any planned or unplanned interim analyses had been performed.12 We also recorded the median survival by arm and whether any statistically significant difference had been detected between the compared arms at a p value of 0.05.
Statistical analysis
For meta-analyses of the time-to-event outcomes (OS and DFS times), the most appropriate statistic is the HR. If provided in a trial report, the HR and associated variances were used directly in the meta-analysis. HRs and 95% CIs were pooled according to the inverse of variance method. An HR (relative hazard) <1 favoured use of postoperative chemotherapy with single-agent fluoropyrimidines.
The χ2 test of heterogeneity and the I2 statistic of inconsistency were used to assess heterogeneity among studies. Statistically significant heterogeneity was defined as a χ2 p value <0.1 or an I2 statistic >50%. In the absence of heterogeneity, pooled estimates of HRs with their 95% CIs were calculated using the Mantel-Haenszel method. In the presence of heterogeneity, the DerSimonian and Laird random effects method was used to pool primary study estimates. All statistical analyses were performed using STATA V.14.
Results
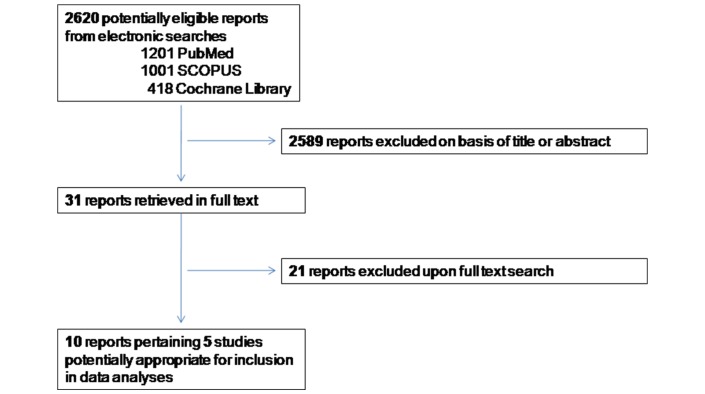
We identified 10 eligible reports pertaining 5 studies potentially appropriate for inclusion in data analyses (figure 1). The characteristic of the five trials that met our eligibility criteria are presented in table 1.13–21 Of these, two trials were excluded from analyses.13 14
Figure 1.
Flowchart diagram of study selection.
Table 1.
Randomised studies comparing adjuvant systemic chemotherapy versus surgery alone after curative resection of colorectal cancer liver metastases
| Author (study) |
Arm | Chemo regimen | TOT | N | Country (N centres) |
ADJ begin from surgery | Follow -up | Statistical aim (randomisation ratio) |
Primary outcome (secondary outcome) |
Enrolment (phase) |
| Saiura et al16 | Surgery+UFT/LV Surgery alone |
((Tegafur 300 mg/m2/day+leucovorin 75 mg/m2/day) three times per day, D1–28, q5 wks) ×5 | 180 | 90 90 |
Japan (11) |
Within 8 wks | 6 years | 15% increase in 3-year RFS (from 30% to 45%) two-sided 95% CI, power 75%, 180 patients needed (1:1) |
3-year RFS (OS, RFS liver, RFS other organs, toxicity) |
Completed 01.2004–12.2010 (phase III) |
| Langer et al19
(ENG) |
Surgery + 5-FU/FA Surgery alone |
(L-leucovorin 100/m2, 5-FU 370/m2 bolus D1–5/q28) ×6 | 129 | 62 67 |
Canada and Europe (67) |
Within 8 wks | NA | 15% increase in 5-year survival (from 30% to 45%) two-sided 95% CI power 90%, 418 patients needed (1:1) | OS (DFS) |
Stopped: slow accrual 2.1994–1.1998 (phase III) |
| Portier et al21
(FFCD) |
Surgery + 5-FU/FA Surgery alone |
(DL-leucovorin 200/m2, 5-FU 400/m2 bolus D1–5/q28)×6 | 173 | 86 87 |
France, Belgium and Switzerland (47) |
2–5 wks | 87 months | 20% increase in 2-year DFS (from 20% to 40%) two-sided, 95% CI power 90%, 200 patients and 134 events needed (1:1) |
DFS at 2 years (OS, toxicity) |
Stopped: slow accrual 12.1991–12.2001 (phase III) |
| Lopez-Ladron et al13 | Surgery+CT Surgery alone |
Not reported | 38 | 28 10 |
Spain (3) |
NA | 15 months | NA, study underpowered (3:1) |
OS | Completed (phase II) |
| Kanemitsu et al14
(JCOG0603) |
Surgery+mFOLFOX Surgery alone |
(Oxaliplatin 85 mg/m2, + L-leucovorin 200/m2,+5-FU 400/m2 bolus,+5-FU 2400 mg/m2 CI over 48 hours) q14×12 |
Still recruiting | Japan (38) |
6–10 wks | NA | 12% increase in 5-year DFS (from 25% to 37%), one-sided, 95% CI power 80%, 300 patients, 150 per arm, and 233 events needed (1:1) |
DFS (OS, toxicity) |
Still recruiting -analyses planned in 2024 (phases II and III) |
|
ADJ, adjuvant; DFS, disease-free survival; RFS, relapse-free survival, DL, dextro-levogyre; ENG, European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, Gruppo Italiano di Valutazione Interventi in Oncologia; FFCD, Federation Francophone de Cancerologie Digestive; 5-FU, fluorouracil; JCOG, Japan Clinical Oncology Group; LV, leucovorin; OS, overall survival; TOT, total; UFT, uracil–tegafur
The Lopez-Ladron et al’s study included a total of 38 patients, was reported only in abstract form at the ASCO meeting 2003, did not address adequate data for survival analysis (just months of median survival per arm), no adequate follow-up (just 15 months), randomisation was not clearly stated in the abstract (28 patients in arm A vs 10 patients arm B), and the chemotherapy regimen administered was not stated.13 The JCOG0603 (Japan Clinical Oncology Group) study started recruitment in March 2017 and is still ongoing and recruiting patients with last follow-up established in 2024. The JCOG0603 compares postoperative mFOLFOX vs liver metastasectomy alone, and it is the only study analysing a combination chemotherapy regimen versus surgery alone.14 15 Since its data are not mature, no data on combined chemotherapy regimen are actually available to be included in this meta-analysis.
Consequently, the meta-analysis included a total of three eligible trials and 482 patients16–21 of whom 238 had been randomly assigned to receive postoperative chemotherapy and 244 had been assigned to receive surgery only (resection of hepatic metastases). For the Japanese trial, 180 patients were recruited from 11 centres in Japan between February 2004 and December 2010.16–18 For the ENG trial, 129 patients were recruited from 69 centres in Canada and Europe between February 1994 and January 1998 (EORTC 20 patients, National Cancer Institute of Canada 54 patients, Gruppo Italiano di Valutazione Interventi in Oncologia 55 patients).19 20 For the FFCD trial (Federacion Francophone de Cancerologie Digestive), 173 patients were recruited from 47 centres in France, Belgium and Switzerland between December 1991 and December 2001.21
All three trials analysed in this meta-analysis used as postoperative chemotherapy single-agent fluoropyrimidines versus surgery alone. Thereafter this meta-analysis strictly pertains to the use single-agent fluoropyrimidine therapy and no implications may be extended to polychemotherapy regimens. In the Japanese trial, uracil–tegafur plus leucovorin was used,16 while in the FFCD and ENG trials, fluorouracil plus folinic acid was used.19 21
All trials analysed were multicentre and described in detail the mode of randomisation while withdrawals were described in detail in two trials.16 21 Table 2 presents patients’ demographics of the three studies included in the analyses. OS was primary outcome only in the Langer study,19 while in the Saiura and Portier trials it was a secondary outcome. Low accrual was documented in all trials and two studies were prematurely stopped for low accrual.19 21 In the ENG study, seven patients in the postoperative chemotherapy arm and six patients in the surgery alone arm received surgical treatment with metastasectomy for lung and not liver metastases, while in other four patients, the reason for metastasectomy was not reported (table 2).19 20
Table 2.
Characteristics of analysed studies
| Author Patients |
Saiura 180 |
ENG 129 |
FFCD 173 |
|||
| Arm | ADJ UFT/LV | Surgery alone | ADJ 5-FU/FA | Surgery alone | ADJ 5-FU/FA | Surgery alone |
| Randomised (analysed) |
90 (88) |
90 (89) |
62 (52) |
67 (55) |
87 (86) |
86 (85) |
| Male | 57 (64.8%) | 63 (70.8%) | 34 (65.4%) | 36 (65.4%) | 46 (53.5%) | 53 (62.4%) |
| Female | 31 (35.2%) | 26 (29.2%) | 28 (34.6%) | 31 (34.6%) | 40 (46.5%) | 32 (37.6%) |
| Median age | 62.3* (8.5 SD) |
64.4* (9.2 SD) |
63.5 (35-76) |
60 (20-82) |
63 (35-77) |
63 (36-76) |
| Primary tumour | ||||||
| Rectum | 36 (40.9%) | 31 (34.8%) | 14 (26.9%) | 17 (30.9%) | 35 (40.7%) | 34 (40.0%) |
| Colon | 52 (59.1%) | 58 (65.2%) | 32 (61.5%) | 35 (60.0%) | 50 (58.1%) | 51 (60.0%) |
| Unknown | – | – | 6 (11.5%) | 5 (9.1%) | 1 (1.2%) | – |
| Nodal status | ||||||
| Negative | 41 (48.2%) | 29 (33.0%) | 24 (46.1%) | 26 (47.3%) | 46 (53.5%) | 39 (45.8%) |
| Positive | 44 (51.8%) | 59 (67.0%) | 26 (50.0%) | 25 (45.4) | 39 (44.3%) | 43 (50.6%) |
| Unknown | – | – | 2 (3.9.%) | 4 (7.3%) | 1 (1.2%) | 3 (3.5%) |
| Disease-free interval, years† | ||||||
| <1 | NA | NA | 18 (34.6%) | 21 (38.2%) | 42 (48.8%) | 39 (45.9%) |
| >1 | NA | NA | 34 (65.3%) | 34 (61.8%) | 44 (51.2%) | 46 (54.1%) |
| Metastases | ||||||
| Single | 37 (42.0%) | 44 (49.4%) | 33 (63.5%) | 37 (67.3%) | 59 (68.6%) | 59 (69.4%) |
| Multiple | 51 (58.0%) | 45 (50.6%) | 19 (36.5%) | 18 (32.7%) | 27 (31.4%) | 26 (30.1%) |
| Synchronous | 39 (43.3%) | 40 (44.9%) | NA | NA | NA | NA |
| Metachronous | 49 (55.7%) | 49 (55.1%) | NA | NA | NA | NA |
| Maximum size, cm | ||||||
| >5 | 21 (23.3%) | 18 (20.0%) | NA | NA | 22 (25.6%) | 26 (30.6%) |
| <5 | 57 (76.7%) | 71 (80.0%) | NA | NA | 64 (74.4%) | 59 (69.4%) |
| Metastatic site | ||||||
| Liver | 88 (100%) | 89 (100%) | 44 (84.6%) | 46 (83.6%) | 86 (100%) | 85 (100%) |
| Lung | – | – | 7 (13.5%) | 6 (10.9%) | – | – |
| Unknown | – | – | 1 (1.9%) | 3 (5.4%) | – | – |
*mean, †DFS in ENG study was calculate as <vs > 6 months (and not 12 months as in FFCD study.
ADJ, adjuvant; DFS, disease-free survival; ENG, European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, Gruppo Italiano di Valutazione Interventi in Oncologia; FFCD, Federation Francophone de Cancerologie Digestive; 5-FU, fluorouracil; LV, leucovorin; UFT, uracil tegafur.
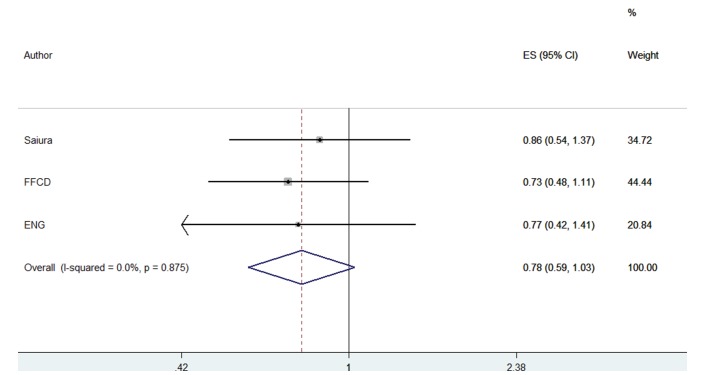
Overall survival
No OS benefit was documented with use of postoperative chemotherapy compared with surgery alone, although a trend for benefit was observed (fixed-effect model, RH=0.781, 95% CI 0.593 to 1.030, p=0.080) (figure 2). No between study heterogeneity was observed (heterogeneity Χ2=0.27 (d.f.=2) p=0.875, I2=0.0%). In all three trials, the OS curves were identical within the first 3.5 years after resection, and split later on.
Figure 2.
Forest plots of overall survival. ENG, European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, Gruppo Italiano di Valutazione Interventi in Oncologia; FFCD, Federation Francophone de Cancerologie Digestive.
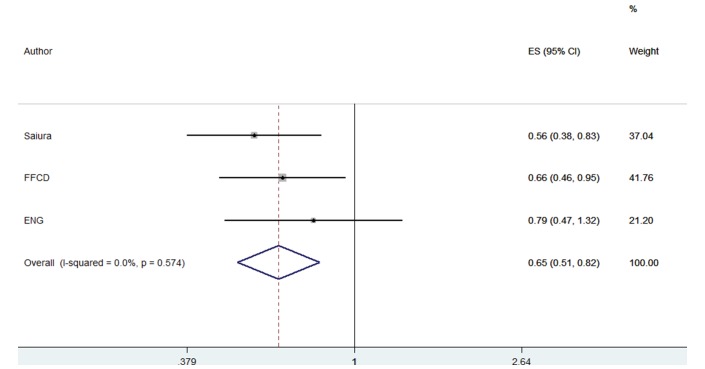
Disease-free survival
Significant DFS benefit with the use of postoperative single-agent fluoropyrimidines was documented compared with surgery alone (RH=0.645, 95% CI 0.509 to 0.818, p=0.001) (figure 3). No between study heterogeneity was observed (heterogeneity Χ2=1.11 (d.f.=2), p=0.574, I2=0.0%). No quality of life (QL) data were available from any trial to assess the quality of clinical benefit achieved with postoperative chemotherapy compared with metastasectomy alone.
Figure 3.
Forest plots of disease-free survival. ENG, European Organization for Research and Treatment of Cancer, National Cancer Institute of Canada, Gruppo Italiano di Valutazione Interventi in Oncologia; FFCD, Federation Francophone de Cancerologie Digestive.
Discussion
This meta-analysis showed that the actually available cumulative randomised evidence do not support the use of postoperative single-agent chemotherapy after liver metastasectomy. No OS benefit was documented with use of postoperative chemotherapy. However, since a trend for benefit was observed, a small benefit cannot be excluded. This study also strongly confirmed that use of adjuvant chemotherapy with single-agent fluoropyrimidines after resection of liver metastasis improves DFS compared with surgery alone. However, in the absence of OS differences, a DFS advantage cannot constitute by itself a reason to administer chemotherapy without having any information on the magnitude of QL gain. Thereafter, in light of the available randomised evidence surgery alone should continue to be the standard arm for studies evaluating the impact of chemo-regimens among patients with completely resected colorectal cancer liver metastases with curative intent.
We also found that the length of follow-up is an important issue to be taken in to account in future trials. In all trials analysed the OS curves were superimposable for at least the first 3.5 years of follow-up and split later on. The length of follow-up in the examined studies for OS analyses was 6 years (18) (table 1). Whether this phenomenon of late curve sharing is due to the long time needed for accrual, to early deaths among unfit treated patients in the chemo-arm or to other causes is unknown. A single- patient data meta-analysis might be probably able to focus on these variables.22 However, in light of the observed delay in curve sharing, shortening the accrual period should be imperative in future trials in this setting in order to have more timely results.
This meta-analysis included 482 randomised patients and summarises the actual available evidence for the use of postoperative chemotherapy after liver metastasectomy compared with liver metastasectomy alone. The number of patients analysed is of particular importance considering that since 2003 all randomised trials in this setting suffered from low accrual rates.14–21 Two of five studies were stopped due to low accrual rate,19–21 and one is still struggling to recruit 300 patients 10 years after its initiation in 2007.14 15 Thereafter the recruitment environment is particularly hostile.
For these reasons from 2002 to 2016, only two randomised trials, respectively published in 2002 and 2006,19 21 presented data for survival analysis. Data on 278 randomised patients from these trials were combined and analysed by Mitry et al.20 No randomised data were afterwards available for the use of chemotherapy in the posthepatic metastasectomy setting until 2016, when early survival data from the Saiura trial were released.17 Long-term survival data of the Saiura study were afterwards presented in 2017 by Hasegawa et al.18
Due to its sample size, the inclusion of the Saiura trial17 in the analyses for cumulative randomised evidence was of particular importance. Indeed, due to accrual problems in the Langer and Portier studies, only 278 patients were combined and analysed in the Mitry et al’s meta-analysis.20 The Saiura trial itself included ~66% of patients (new evidence) when compared with the Mitry et al’s study (180/278). This adds in study power in our meta-analysis and gives us the possibility to present more solid results and to reduce related CIs.
In the past, after 2008, many meta-analytic studies tried to answer the question for the use of postmetastasectomy chemotherapy among patients with resectable colorectal cancer liver metastases. Due to the paucity of randomised evidence, most of these studies generally powered their analyses with the inclusion of high-quality non-randomised trials6–9 and/or analysed the overall impact of systemic chemotherapy (preoperative, perioperative and postoperative). However, this led to controversial results and biases. Indeed, in these studies, the overall proportion of patients randomised for the use of postoperative chemotherapy versus non-use ranged from 12% to 45%, with the vast majority of patients being non-randomised 55%–88% (from cohort or retrospective matched controlled studies).6–9 The cumulative results were therefore largely driven by non-randomised trials and chemotherapy use in the perioperative setting. Conversely, summarising data from neoadjuvant, perioperative and postoperative chemotherapy for resectable liver metastases from colorectal cancer may lead to misleading conclusions since treatment in the preoperative setting may affect outcomes and cloud conclusions on the value of postoperative chemotherapy. Consequently, the cumulative evidence from these data cannot be generalised to the adjuvant setting and cannot provide answers on what physicians have to do in clinical practice for patients undergoing liver metastasectomy with curative intent.
Our study was strictly tailored to scrutinise whether postoperative systemic chemotherapy after colorectal cancer metastasectomy offers any survival benefit compared with metastasectomy alone. Since data from randomised trials are actually available in this setting, there is no place for the use of surrogates (data non-randomised or form ‘nearby-setting’) in the analysis. Randomised evidence is now firm itself with a nice sample size of ~500 randomized patients analysed in our study.
To the best of our knowledge, only one trial (on 300 randomised patients) is still ongoing in this setting (Kanemitsu JCOG0603), however it will be closed for analysis only in 2024.15 For this reason this meta-analysis presents the cumulative actually available evidence and will represent a landmark at least until 2024, or until new data will be published in this setting. Nonetheless considering the long time needed to accrual and follow-up for survival analysis, it is extremely improbable that future studies will give their results until 2024.
Since the role of postmetastasectomy chemotherapy among patients with colorectal cancer metastases is to eliminate micrometastases, it may be argued that the use of FOLFOX and CAPEOX regimens might be of greater benefit than the use of single-agent fluoropyrimidines. However, at the moment we have no data to support the use of combined chemotherapy. Indeed, we have to consider that many patients after liver metastasectomy are frail and not eligible for combination chemotherapy. The EORTC 40983 study on perioperative FOLFOX chemotherapy did not show an OS advantage,23 while the JCOG0603 study, evaluating the use of postoperative mFOLFOX versus metastasectomy alone, is planned to be closed for data analysis in 2024.
For these reasons, it will be better to enrol our patients in randomised trials in order to clarify which will be the best treatment option for these patients in the future.
Several limitations must be considered when interpreting these results. First, this meta-analysis is based on published data and a meta-analysis of individual level data might define more clearly treatment benefits.22 Second, many trials identified were reported in abstract form only, making complete data difficult to extract for analyses. Third, the results strictly pertain the use of single-agent fluoropyrimidines and may not be generalised to other combination chemotherapy regimens.
Allowing for these caveats, this meta-analysis showed that currently available randomised evidence does not support the hypothesis that adjuvant chemotherapy with single-agent fluoropyrimidines after resection of liver metastasis likely alters the natural course of the disease, because it does not affect OS. Until further evidence from new clinical trials becomes available, systemic adjuvant treatment after liver metastasectomy should not be routinely recommended. Modern combination chemotherapy regimens should be evaluated for efficacy in future trials appropriately designed for long follow-up with the QL as an endpoint.
Footnotes
Contributors: All authors participated in the design of the study. DM and GZar: literature search was performed by two independent reviewers. LT and GZaf: independently searched medical libraries. PF: independently performed specific journals searches. AP: independently performed scrutinized 2014-2017 conferences. GP: in case of disagreement between independent searches (gastrointestinal oncology expert) supervised the data. DM, PA and TL: performed data extraction. DM and GC: performed statistical analyses. DM, GZar, PA and GP: interpret results. DM and GP: wrote the manuscript. GZr, LT, GZf, PF, AP and GC: reviewed and commented on the final manuscript. All authors had full access to all data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. 10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 2. GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal (accessed Feb 2018).
- 3. Kemeny N. The management of resectable and unresectable liver metastases from colorectal cancer. Curr Opin Oncol 2010;22:364–73. 10.1097/CCO.0b013e32833a6c8a [DOI] [PubMed] [Google Scholar]
- 4. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 5. Penna C, Nordlinger B. Colorectal metastasis (liver and lung). Surg Clin North Am 2002;82:1075–90. 10.1016/S0039-6109(02)00051-8 [DOI] [PubMed] [Google Scholar]
- 6. Brandi G, De Lorenzo S, Nannini M, et al. Adjuvant chemotherapy for resected colorectal cancer metastases: Literature review and meta-analysis. World J Gastroenterol 2016;22:519–33. 10.3748/wjg.v22.i2.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang ZM, Chen YY, Chen FF, et al. Peri-operative chemotherapy for patients with resectable colorectal hepatic metastasis: A meta-analysis. Eur J Surg Oncol 2015;41:1197–203. 10.1016/j.ejso.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 8. Araujo RL, Gönen M, Herman P. Chemotherapy for patients with colorectal liver metastases who underwent curative resection improves long-term outcomes: systematic review and meta-analysis. Ann Surg Oncol 2015;22:3070–8. 10.1245/s10434-014-4354-6 [DOI] [PubMed] [Google Scholar]
- 9. Khoo E, O’Neill S, Brown E, et al. Systematic review of systemic adjuvant, neoadjuvant and perioperative chemotherapy for resectable colorectal-liver metastases. HPB 2016;18:485–93. 10.1016/j.hpb.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hopewell S, Clarke M, Lusher A, et al. A comparison of handsearching versus MEDLINE searching to identify reports of randomized controlled trials. Stat Med 2002;21:1625–34. 10.1002/sim.1191 [DOI] [PubMed] [Google Scholar]
- 11. The Cochrane Handbook. Systematic reviews of interventions. 2018. http://www.cochrane.org/resources/handbook/handbook.pdf
- 12. Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: a systematic review. JAMA 2005;294:2203–9. 10.1001/jama.294.17.2203 [DOI] [PubMed] [Google Scholar]
- 13. Lopez-Ladron A, Salvador J, Bernabe R, et al. Observation versus postoperative chemotherapy after resection of liver metastases in patients with advanced colorectal cancer. Proc Amer Soc Clin Oncol 2003;22:373. [Google Scholar]
- 14. Kanemitsu Y, Kato T, Shimizu Y, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol 2009;39:406–9. 10.1093/jjco/hyp035 [DOI] [PubMed] [Google Scholar]
- 15. JCOG0603, UMIN Clinical Trials Registry (UMIN-CTR). University hospital Medical Information Network (UMIN) Center. 2013. http://www.umin.ac.jp/ctr/index.htm (accessed Feb 2018).
- 16. Saiura A, Yamamoto J, Hasegawa K, et al. A combination of oral uracil-tegafur plus leucovorin (UFT + LV) is a safe regimen for adjuvant chemotherapy after hepatectomy in patients with colorectal cancer: safety report of the UFT/LV study. Drug Discov Ther 2014;8:48–56. 10.5582/ddt.8.48 [DOI] [PubMed] [Google Scholar]
- 17. Hasegawa K, Saiura A, Takayama T, et al. Adjuvant oral uracil-tegafur with leucovorin for colorectal cancer liver metastases: a randomized controlled trial. PLoS One 2016;11:e0162400 10.1371/journal.pone.0162400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasegawa K, Saiura A, Takayama T, et al. Oral adjuvant chemotherapy using uracil-tegafur (UFT) with leucovorin (LV) after resection of colorectal cancer liver metastases: long-term survival results of the phase III UFT/LV study. Journal of Clinical Oncology 2017;35:672 10.1200/JCO.2017.35.4_suppl.672 [DOI] [Google Scholar]
- 19. Langer B, Bleiberg H, Labianca R, et al. Fluorouracil (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): result of the ENG (EORTC/NCIC CTG/GIVIO). Proc. Am Soc Clin Oncol 2002;21:149a. [Google Scholar]
- 20. Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008;26:4906–11. 10.1200/JCO.2008.17.3781 [DOI] [PubMed] [Google Scholar]
- 21. Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006;24:4976–82. 10.1200/JCO.2006.06.8353 [DOI] [PubMed] [Google Scholar]
- 22. Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993;341:418–22. 10.1016/0140-6736(93)93004-K [DOI] [PubMed] [Google Scholar]
- 23. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–15. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]