Abstract
Background and Objective
Breast cancer is the commonest cancer in women worldwide. Many patients are frequently admitted to the operating theaters for mastectomies. Thoracic paravertebral block (PVB) is increasingly used as an effective means for post-operative pain relief. The present study aimed at evaluating the effectiveness and safety of dexmedetomidine and nalbuphine as an adjuvant to bupivacaine local anesthetic in thoracic paravertebral block in breast cancer surgeries.
Methods
A total of 60 female patients aged 18 to 78 were included in the study, and ASA I, II, III were scheduled for mastectomy. These patients were unsystematically assigned into three 20-member groups: group PB received bupivacaine (0.3 mL/ kg) + 1 mL (0.9% sodium chloride) normal saline; group PBD received bupivacaine (0.3 mL/kg) + dexmedetomidine 1 μg/kg; and Group PBN received bupivacaine (0.3 mL/kg) and 10 mg (1 mL) nalbuphine. Demographic data, intraoperative SPO2, ETCO2, HR, SBP and DBP, pain scores (at rest and movement), and sedation scores were recorded every 30 minutes during the initial 2 hours and 4, 8, 24, and 48 hours from T0. Also, postoperative tramadol consumption, the time to the first analgesic request, and any complications were also recorded.
Results
There were no statistically significant differences among the three groups regarding demographic data, SPO2, ETCO2, HR, SBP and DBP intraoperatively. Moreover, no significant difference was found in HR, SBP and DBP postoperatively. Postoperative pain scores were significantly higher in group BP, whether at rest or movement. The sedation was significantly higher in PBD group in the first 12 hours postoperatively. There was a significantly lower postoperative tramadol consumption in PBN group and a significantly longer time to the first analgesic request than other groups. No complications were reported in any group.
Conclusions
Addition of nalbuphine 10 mg as an adjuvant to bupivacaine local anesthetic in PVB improved the quality of the block and decreased postoperative analgesic requirements than the bupivacaine only group and dexmedetomidine and bupivacaine group. However, adding dexmedetomidine to bupivacaine increased the time to the first analgesic request and more sedation than bupivacaine and bupivacaine and nalbuphine.
Keywords: Dexmedetomidine, Nalbuphine, Paravertebral Block, Postoperative Pain
1. Background
Breast cancer is the commonest cancer in women that frequently requires surgical intervention and they suffer from pain postoperatively (1, 2). Most of the responses of the human body to postsurgical pain have been proven to be detrimental to the patient's homeostasis and recovery (3). Hence, a number of therapeutic maneuvers had been accepted as a part of the “multimodal approach” to control postoperative pain (4). Thoracic paravertebral block (PVB) is used to relieve pain after thoracotomy and mastectomy. There are few systematic researches on the effectiveness and safety of adding adjunctive analgesic agents in paravertebral blockades. If superiority and time length of painlessness due to PVB could be value-added by adjuncts to local anesthetic (LA), its good outcomes can be substantially magnified (5).
Dexmedetomidine is found to be a vastly discriminating α-2 adrenoreceptor agonist that is confirmed to possess both analgesic and sedative properties. While has solely been accepted for intravenous sedation (IV), it has also been broadly utilized for various other purposes (including neuraxial and peripheral nerve blocks) with respectable outcomes. A noteworthy prolongation of time length of analgesia had been stated when dexmedetomidine was supplemented in epidural blockades LA (6), caudal blocks (7), subarachnoid blocks (8, 9), PVB (10), brachial plexus blocks (11), ulnar nerve blocks (12), and greater palatine nerve blocks (13).
Nalbuphine, derived from 14-hydroxymorphine, is considered a potent analgesic possessing a mixture of k agonist and μ competitor profiles. The pain-relieving potency considering nalbuphine had been reported to be identical to morphine but dissimilar from it in exhibiting a top limit effect on respiratory depression. Nalbuphine possesses the effect of maintaining or even augmenting the opioid μ receptor centered analgesia and at the same time mitigating the μ-opioid side effects. Also, it had been used successfully and safely in epidural and intrathecal blocks (14).
The present study aimed at evaluating the efficacy and safety of dexmedetomidine and nalbuphine as local anesthetic adjuvants in thoracic paravertebral block (TPVB).
2. Methods
This study was performed on 66 adult patients who were subjected to modified radical mastectomy (MRM) or breast conservative surgery with axillary lymph node clearance. The ethical and scientific committee of El Fayoum University hospitals approved this study, and written informed consent was obtained from the patients. Moreover, this study was registered in the Pan African clinical trial registry (www.pactr.org) database (identification number: PACTR201710002161189). Females of American society of anesthesiologists (ASA) physical status I, II and III, aged 18 to 78 years, who were scheduled for MRM or breast conservative surgery with dissection of axillary lymph nodes were included in this study. However, those subjected to surgery on both sides or reconstruction of the breast, patients with any contra-indications regarding PVB, eg, patients’ refusal, infection at the site ofinjection, anticoagulant use, coagulopathy, or hypersensitivity to LA, those on chronic antiemetics or chronic pain drugs, pregnant women, those with central neuropathy, or those with renal or hepatic diseases, alcohol or drug addicts, and individuals with psychiatric disorders,overweight patients with a body mass index (BMI) > 30 kg/m2, and those with hypotension were excluded from the study. The participants were randomly assigned into three 20-member groups using a computer-generated random number table, moreover, closed cover method was used for distribution camouflage.
2.1. Anesthetic Technique
Preoperatively, patients were shown how to assess their own pain intensity by means of the numerical rated scale (NRS), ranging from 0 to 10, where 0 = no pain and 10 = the poorest pain possible.The patients in PB, PBD, and PBN groups were injected in the sitting position, with a single level PVB injection, at the fourth thoracic vertebral level. A staff anesthesiologist not involved in the management of the patient or the study prepared the injectate according to randomization.
2.2. Block Technique
Group PB patients were given PVB consisting ofbupivacaine 0.5%0.3 mL/kg plus normal saline 1 mL; patients in PBD group were given PVB consisting of bupivacaine 0.5% 0.3 mL/kg, and dexmedetomidine 1 μ g/kg, which is 1 mL volume; and those in PVN group were given PVB with bupivacaine 0.5% 0.3 mL/kg and 10 mg nalbuphine in 1 mL before going under GA. Blocks were done behindcurtains, and the patients, surgeons, and anesthesiologists who gave GA were blinded to the division of the groups. The patients were placed in the sitting position, and sedation in the form of 2 mg IV midazolam and 50 μ g iv fentanyl were given when needed using in-plane single injection technique. General anesthesia was induced with a dose of propofol 1 - 2 mg/kg and 1 μ g/kg fentanyl in all groups. Intubation of the trachea was done by 0.5 mg/kg atracurium. General anesthesia was maintened by isoflurane 1% to 2% in 100% oxygen, or as required. Mechanical ventilation was adjusted to maintain ETCO2 between 33 and 36 mmHg. No doses of fentanyl were required intraoperatively in any patient. Bradycardia, defined as HR less than 50 beats/min, was corrected by 0.6 mg of atropine and hypotension, defined as SBP less than 90 mmHg, managed by fluids and 3 - 9 mg ephedrine shots, or by adjusting the depth of isoflurane, if required. Neuromuscular blockade was neutralized with neostigmine 0.04 mg/kg and extubation was achieved after regaining full neuromuscular function.
2.3. Measured Parameters
Intraoperative, heart rate, ETCO2, systolic and diastolic blood pressures, and SPO2 were recorded preoperatively and after 30, 60, and 120 minutes. Postoperatively, SpO2, HR, BP sedation scores, and pain scores were documented every 30 minutes for the first 2 hours, and at 4, 8, 24 and 48 hours from T0. Documentation of pain scores at relaxation and on ipsilateral arm movement was done by NRS, and Richmond agitation sedation score (RASS) sedation was used to evaluate sedation from +4 combative to -5 unarousable sedation. Pain on movement was evaluated by arm abduction at a 90 angle. Time to the first analgesic request, which was defined as time from completion of the paravertebral block injection till the time to the first analgesic demand, was documented, and analgesia was delivered at whatever time NRS was greater than 3 or when the patients asked for a painkiller. Then, PCA was started with on demand tramadol doses of 50 mg and increased according to patient’s requirements. Incidents of PONV were documented and dealt with; then, metoclopramide was given intravenously, and if necessary, dexamethasone. Primary outcome measure was to aggregate IV tramadol consumption over 48 hours. Secondary outcome measures involved pain scores at rest and active movement of ipsilateral arm, intraoperative fentanyl requirements, time to first analgesic request, bradycardia, hypotension, sedation scores, nausea/vomiting, pruritus, and patient satisfaction.
2.4. Statistical Analysis
Sample size was estimated to be at least 15 patients in each group assuming = 0.05, power = 80%, and effect size d = 0.7. Enrollment of 20 patients in each group was done to adjust for possible dropouts. Sample size was calculated using G*Power 1.3.7. Software. Microsoft Access and SPSS software version 18 in windows 7 was used to perform data analysis. For quantitative parametric data, one-way ANOVA test was used to compare more than two independent groups of quantitative data. Benferroni post-hoc was used to test the significance between the two groups. For quantitative non- parametric data, Kruskal Wallis test was used to compare more than two independent groups. For qualitative data, chi square test was used to compare two of more than two qualitative groups. P value equal to or less than 0.05 was considered to be significant.
3. Results
A total of 70 patients were admitted for this study; of them, 4 refused this regional technique and were excluded from the study. The final cohort included 66 patients who were randomly assigned into three groups: Group PB: 2 patients were further excluded because of failure of insertion. The remaining 20 patients were included in the final analysis. Group PBD: 2 patients were further excluded, one due to failure of insertion and one was lost to follow- up. Group PBN: 2 Patients were further excluded due to lost follow-up. There was no statistically significant differences among the study groups regarding demographic data (Table 1), SpO2 level before and during the operation, and ETCO2 level before and during the operation.
Table 1. Demographic Data of Different Study Groupsa.
| Variables | Study Groups | P Value | Sig. | ||
|---|---|---|---|---|---|
| PB | PBN | PBD | |||
| Age, y | 55.8 ± 5.8 | 55.2 ± 5.7 | 55.9 ± 6 | 0.91 | NS |
| Anthropometric measures | |||||
| Height, cm | 169.9 ±3.5 | 169.5 ± 3.1 | 169.5 ± 3.1 | 0.88 | NS |
| Weight, kg | 80.3 ± 8.9 | 80.6 ± 9.6 | 79.7 ± 9.4 | 0.95 | NS |
| BMI, kg/m2 | 27.8 ± 3.2 | 28.1 ± 3.6 | 27.7 ± 3.5 | 0.95 | NS |
aValues are expressed as mean ± SD.
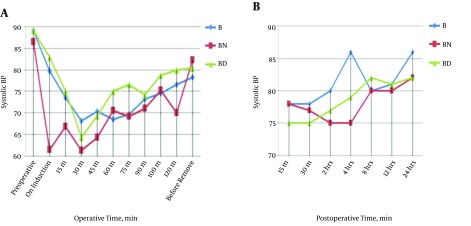
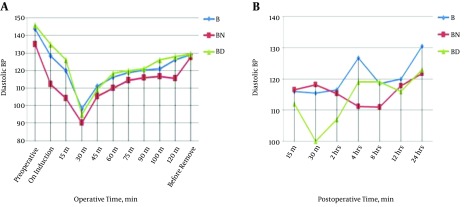
Changes in intraoperative heart rate are demonstrated in Table 2. Changes in systolic blood pressure pre, intra, and postoperative values are demonstrated in Figure 1. Also, changes in diastolic blood pressure pre, intra, and postoperatively are illustrated in Figure 2.
Table 2. Comparisons of Heart Rate Follow Up Before and During Operation in Different Study Groups.
| HR, beats /min | Study Groups | P Value | Sig. | |||||
|---|---|---|---|---|---|---|---|---|
| PB | PBN | PBD | ||||||
| Median | IQR | Median | IQR | Median | IQR | |||
| Preoperative | 90 | 8 | 96 | 10 | 85 | 9 | 0.36 | NS |
| Post induction | 81 | 10 | 80 | 20 | 79 | 9 | 0.14 | NS |
| After 15 min | 78 | 8 | 74 | 17 | 76 | 8 | 0.003a | HS |
| 0.9b | NS | |||||||
| 0.01c | S | |||||||
| After 30 min | 74 | 3 | 67 | 14 | 66 | 7 | < 0.001a,b | HS |
| 0.9c | NS | |||||||
| After 45 min | 75 | 3 | 77 | 5 | 72 | 11 | 0.03a | S |
| 0.01b | S | |||||||
| 0.9c | NS | |||||||
| After 60 min | 75 | 4 | 76 | 5 | 74 | 9 | 0.01a | S |
| 0.002b | HS | |||||||
| 0.9c | NS | |||||||
| After 75 min | 78 | 5 | 79 | 11 | 75 | 7 | 0.9a | NS |
| 0.02b | S | |||||||
| 0.1c | NS | |||||||
| After 90 min | 77 | 5 | 81 | 18 | 75 | 6 | 0.01a | HS |
| 0.07b | ||||||||
| 0.5c | ||||||||
| After 105 min | 81 | 13 | 77 | 9 | 76 | 7 | 0.001a | HS |
| 0.4b | NS | |||||||
| 0.4c | NS | |||||||
| After 120 min | 83 | 17 | 81 | 20 | 77 | 5 | 0.09 | NS |
| Before removal | 82 | 8 | 78 | 15 | 77 | 5 | < 0.001a,b | HS |
| 0.9c | NS | |||||||
Abbreviations: HS, highly significant difference; IQR, interquartile range; NS, no statistically significant difference; PB, Bupivacaine; PBD, Bupivacaine and Dexmedetomedine; PBN, Bupivacaine and Nalbuphine; S, statistically significant difference.
aSignificance between PB, and PBN.
bSignificance between PB, and PBD.
cSignificance between BD, and PBN.
Figure 1. Comparisons of systolic blood pressure follow up before, A, during and B, postoperative among different study groups.
Figure 2. Comparisons of diastolic blood pressure follow up before, A, during and B, postoperative among different study groups.
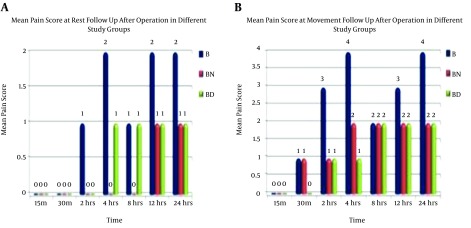
Pain scores at rest showed improvement of pain scores ain PBN and PBD groups compared to group PB. It was noticed that group PB started with higher values which decreased after four hours and then increased again. On the other hand, PBN and PBD groups showed more stable pain scores with no obvious difference between PBN and PBD groups (Figure 3A).
Figure 3. Comparisons of pain scores at A, rest and B, movement follow up after operation in different study groups.
With respect to pain scores at movement follow- up after operation in different study groups, the results showed improvement of pain scores in PBN and PBD groups compared to PB group. It was also found that group PB started with higher values then decreased after four hours then raised again. On the other hand, PBN and PBD groups showed more stable pain scores with no obvious difference between PBN and PBD groups (Figure 3B).
There was a statistically significant difference (P value > 0.05) between study groups with respect to RASS follow- up after 15 minutes, 30 minutes, and 2, 4, 8, and 24 hours after operation. In the first four hours after the operation, patients of group PB were more agitated than those in PBD and PBN groups (P value < 0.001). However, there was no statistically significant difference (P value > 0.05) in R.A.S.S 12 hours after the operation. There was also a statistically significant difference (P value < 0.05) among the study groups in tramadol consumption, with the highest mean in PB group (75 ± 9.2 mg), the lowest mean in PBN group (22.5 ± 7.7 mg), and (35 ± 5.3 mg) in PBD. There was a statistically significant difference (P value < 0.05) between study groups in time to the first analgesic request, with the highest mean in the PBD group (7.8 ± 0.68 hours), the lowest mean in the PB group (3.25 ± 0.56 hours), and 6.8 ± 0.91 hours in PBN group.No complications occurred in different study groups except in one case of a pleural puncture in each group.
4. Discussion
The first paravertebral block was implemented in 1905 for obstetric surgeries as an alternative to neuraxial block (15, 16).
Dexmedetomidine has peripheral in hands with central actions. In the spinal cord, α2-C and α2-ARs are situated in the neurons of superficial dorsal horn, especially lamina II (17-19), and their stimulation directly diminishes pain conduction via decreasing the release of pronociceptive transmitter, substance P, and glutamate from primary afferent terminals and by hyperpolarizing spinal interneurons via G-protein-mediated activation of potassium channels (18). The peripheral analgesic action of α-2 agonist is enabled by decreasing the release of norepinephrine and by α-2 receptor independent inhibition of nerve fiber action potentials.
Nalbuphine, a 14-hydroxymorphine derivative, is a sturdy analgesic with combined opioid receptor k agonist and opioid receptor μ antagonist properties. Nalbuphine possesses the prospective to maintain or even augment μ- receptor opioid-based analgesia while concurrently modifying the μ-receptor opioid side effects (14). Nalbuphine has the onset of action of two and three minutes, duration of action of 3. 6 hours with cardiovascular stability, and minimal side effects in the dose of 0.2. - 0.4 mg/kg (20, 21). Nalbuphine has a reasonable analgesic outcome when paralleled to morphine. Apart from μ opioids based spinal and supraspinal analgesia, suppression of serotonin uptake in the neurons leads to amplification of the inhibitory pathways in the spinal cord for pain (22). Opiate receptors stimulation on the central nervous system neurons leads to suppression of intracellular adenylyl cyclase, an opening of potassium channels, and closing of the calcium channels. This results in hyperpolarization of the cell membrane potential and suppression of action potential spread of ascending pain pathways (23). No statistically significant differences were found among the three groups relating to demographic data and operative features. There was a significant decline in pulse rate at 30 minutes in the three groups, but it was more evident in PBD group and least evident in PVB group; then, it started to rise nearly to preoperative values, and this might have beendue to unilateral sympathetic block and bradycardic and the hypotensive effect of dexmedetomidine. However, it was managed effectively with boluses of ephedrine, atropine, and changing the depth of anesthesia without any morbidity. Our study was also consistent with the study of Hazem et al. (23) in hemodynamics. No intraoperative fentanyl required in the three study groups, indicating the adequacy of analgesia produced by TPVB, which was consistent with Moller et al. (24) and Mohta et al. (25).
The time to the first analgesic request was only after 3.25 ± 65 hours in PVB, it was 7.8 ± 0.68 hours in PVD, and 6.8 ± 0.91 hours in PVN, which was significantly longer in PVN and PVD compared to PVB, but it was not statistically significant between PVN and PVD. Our result was consistent with that of Mohamed et al. (5), Mohta et al. (25), and Shaikh et al. (26), who studied the addition of dexmedetomidine and clonidine to bupivacaine in epidural anesthesia in patients who underwent orthopedic lower limb surgery, and they showed the time to the first analgesic request was prolonged to 340 minutes.
Our study showed that addition of nalbuphine 10 mg in a volume of 1 mL to 15 mL 0.5% bupivacaine in PBN group improved the quality of the block in the form of improved pain scores and prolonged the time to the first analgesic request to 6.8 hours, with a high statistical significance (P = 0.005) compared to group PVB, which was 3.2 hours. This was consistent with Gupta et al. (27), who studied the effect of adding nalbuphine 10 mg to 20 mL 0.5% bupivacaine with the quality of supraclavicular nerve block at upper arm surgery. Their results showed enhanced sensory and motor block time length. The postoperative analgesia time length was 481.53 ± 42.45 minutes in nalbuphine group and 341.31 ± 21.42 minutes in bupivacaine only group, with a high statistically significant difference (P < 0.001). Also, our result was consistent with that of Abdelhaq et al. (28) who used higher dose of nalbuphine in supraclavicular block, which led to better results than previous studies, indicating that higher doses lead to better quality of the block and showing a significant increase in the length of analgesic effect in nalbuphine group (835.18 ± 42.45 minutes) compared to the control group (708.14 ± 54.57 minutes) (P value less than 0.001). Similar results were also reported by Chatrath et al. (14). On the other hand, postoperative tramadol consumptions were significantly lowered in PVD (35 ± 5.3 mg) and PVN (22.5 ± 7.7 mg) than PVB (75 ± 7.2 mg) in the first 24 hours, which was in agreement with Mohamed et al. (5), Mohta et al. (25) and Das et al. (29) findings. Our study revealed that total tramadol consumption was lower in PBN group than in PBD group with no statistical significance, which may be due to the analgesic effect of nalbuphine. Also, the low dose of nalbuphine (10 mg) explains the lesser time to the first analgesic request in PBN group postoperatively; thus, the time to the first analgesic request may change in PBN group if the dose is increased to 20 mg.
In the first four hours after operation, patients of the PB group were more agitated than those in PBD group and PBN group (P value < 0.001), measured by RASS. Later on, there was no significant alteration in the sedation score among the three groups. This may be due to administration of tramadol as an analgesic and, on the other hand, the sedating effect of dexmedetomidine. These results were also consistent with those of Almarakbi et al. (30). Also, Abdallah and Brull (31) found similar results.
In our study, there was no evidence of intraoperative or postoperative complications except for three cases of pleural puncture without occurrence of pneumothorax, especially with the direct visualization of the site of injection and real time injection of the local anesthetic under ultrasound guidance.
4.1. Conclusions
We conclude that addition of dexmedetomidine and nalbuphine to bupivacaine at thoracic PVB in breast surgeries provide intense sensory blockade and opioid sparing effect without serious side effects. Time to first analgesic request was longer in dexmedetomidine group, but tramadol consumption was lower in nalbuphine group, but both were statistically insignificant.
4.2. Limitation and Recommendation
More studies with larger sample sizes will be needed to confirm our results. We recommend using higher doses of nalbuphine (20 mg) to provide more intense block and longer period of postoperative analgesia. Also, we recommend further studies on combination of dexmedetomidine and nalbuphine in the same group to gain benefits of both drugs.
References
- 1.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59(1):27–41. doi: 10.3322/caac.20008. [DOI] [PubMed] [Google Scholar]
- 2.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7(9):626–34. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imani F. Postoperative pain management. Anesth Pain Med. 2011;1(1):6–7. doi: 10.5812/kowsar.22287523.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemati K, Zaman B, Hassani V, Imani F, Dariaie P. Efficacy of fentanyl transdermal patch in the treatment of chronic soft tissue cancer pain. Anesth Pain Med. 2015;5(1):e22900. doi: 10.5812/aapm.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed SA, Fares KM, Mohamed AA, Alieldin NH. Dexmedetomidine as an adjunctive analgesic with bupivacaine in paravertebral analgesia for breast cancer surgery. Pain Physician. 2014;17(5):E589–98. [PubMed] [Google Scholar]
- 6.Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth. 2011;5(4):365–70. doi: 10.4103/1658-354X.87264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103(2):268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 8.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55(4):347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to Bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27(3):339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha S, Mukherjee M, Chatterjee S, Vijay MK, Hazra A, Ray M. Comparative study of analgesic efficacy of ropivacaine with ropivacaine plus dexmedetomidine for paravertebral block in unilateral renal surgery. Anaesth Pain Intens Care. 2012;16:38–42. [Google Scholar]
- 11.Fritsch G, Danninger T, Allerberger K, Tsodikov A, Felder TK, Kapeller M, et al. Dexmedetomidine added to ropivacaine extends the duration of interscalene brachial plexus blocks for elective shoulder surgery when compared with ropivacaine alone: a single-center, prospective, triple-blind, randomized controlled trial. Reg Anesth Pain Med. 2014;39(1):37–47. doi: 10.1097/AAP.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 12.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013;110(3):438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 13.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27(3):280–4. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 14.Chatrath V, Attri JP, Bala A, Khetarpal R, Ahuja D, Kaur S. Epidural nalbuphine for postoperative analgesia in orthopedic surgery. Anesth Essays Res. 2015;9(3):326–30. doi: 10.4103/0259-1162.158004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eid HEA. Paravertebral block: An overview. Curr Anaesth Crit Care. 2009;20(2):65–70. [Google Scholar]
- 16.Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia. 1979;34(7):638–42. doi: 10.1111/j.1365-2044.1979.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 17.Hui Yun S, Suk Choi Y. The Effects of Dexmedetomidine Administration on Postoperative Blood Glucose Levels in Diabetes Mellitus Patients Undergoing Spinal Anesthesia: A Pilot Study. Anesth Pain Med. 2016;6(6):e40483. doi: 10.5812/aapm.40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84(4):873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Nelson LE, You T, Maze M, Franks NP. Evidence that the mechanism of hypnotic action in dexmedetomidine and muscimol-induced anesthesia converges on the endogenous sleep pathway. Anesthesiology. 2001;95:A1368 [Google Scholar]
- 20.Amin SM, Amr YM, Fathy SM, Alzeftawy AE. Maternal and neonatal effects of nalbuphine given immediately before induction of general anesthesia for elective cesarean section. Saudi J Anaesth. 2011;5(4):371–5. doi: 10.4103/1658-354X.87265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen RI, Edwards WT, Kezer EA, Ferrari DA, Liland AE, Smith ER. Serial intravenous doses of dezocine, morphine, and nalbuphine in the management of postoperative pain for outpatients. Anesth Analg. 1993;77(3):533–9. doi: 10.1213/00000539-199309000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Acharya R, Jena M, Mishra S, Rath SK. Effect of butorphanol versus placebo as adjuvant to bupivacaine for supraclavicular brachial plexus blockade. Int J Appl Pharm. 2014;6(1):8–10. [Google Scholar]
- 23.Moawad HE, Mousa SA, El-Hefnawy AS. Single-dose paravertebral blockade versus epidural blockade for pain relief after open renal surgery: A prospective randomized study. Saudi J Anaesth. 2013;7(1):61–7. doi: 10.4103/1658-354X.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: a randomized double-blind study. Anesth Analg. 2007;105(6):1848–51. doi: 10.1213/01.ane.0000286135.21333.fd. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30(2):252–60. doi: 10.1007/s00540-015-2123-8. [DOI] [PubMed] [Google Scholar]
- 26.Shaikh SI, Mahesh SB. The efficacy and safety of epidural dexmedetomidine and clonidine with bupivacaine in patients undergoing lower limb orthopedic surgeries. J Anaesthesiol Clin Pharmacol. 2016;32(2):203–9. doi: 10.4103/0970-9185.182104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta K, Jain M, Gupta PK, Rastogi B, Zuberi A, Pandey MN. Nalbuphine as an adjuvant to 0.5% bupivacaine for ultrasound-guided supraclavicular brachial plexus blockade. Indian J Pain. 2016;30(3):176. doi: 10.4103/0970-5333.198024. [DOI] [Google Scholar]
- 28.Abdelhaq MM, Adly Elramely M. Effect of Nalbuphine as Adjuvant to Bupivacaine for Ultrasound-Guided Supraclavicular Brachial Plexus Block. Open J Anesthesiol. 2016;06(03):20–6. doi: 10.4236/ojanes.2016.63004. [DOI] [Google Scholar]
- 29.Das A, RoyBasunia S, Mukherjee A, Biswas H, Biswas R, Mitra T, et al. Perineural Nalbuphine in Ambulatory Upper Limb Surgery: A Comparison of Effects of Levobupivacaine with and without Nalbuphine as Adjuvant in Supraclavicular Brachial Plexus Block - A Prospective, Double-blinded, Randomized Controlled Study. Anesth Essays Res. 2017;11(1):40–6. doi: 10.4103/0259-1162.200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammar AS, Mahmoud KM. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6(3):229–33. doi: 10.4103/1658-354X.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110(6):915–25. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]