Abstract
Background
Platelet activation reduces pulmonary microvascular blood flow and contributes to inflammation; these factors have been implicated in the pathogenesis of COPD and emphysema. We hypothesized that regular use of aspirin, a platelet inhibitor, would be associated with a slower progression of emphysema-like lung characteristics on CT imaging and a slower decline in lung function.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled participants 45 to 84 years of age without clinical cardiovascular disease from 2000 to 2002. The MESA Lung Study assessed the percentage of emphysema-like lung below –950 Hounsfield units (“percent emphysema”) on cardiac (2000-2007) and full-lung CT scans (2010-2012). Regular aspirin use was defined as 3 or more days per week. Mixed-effect models adjusted for demographics, anthropometric features, smoking, hypertension, angiotensin-converting enzyme inhibitor or angiotensin II-receptor blocker use, C-reactive protein levels, sphingomyelin levels, and scanner factors.
Results
At baseline, the 4,257 participants' mean (± SD) age was 61 ± 10 years, 54% were ever smokers, and 22% used aspirin regularly. On average, percent emphysema increased 0.60 percentage points over 10 years (95% CI, 0.35-0.94). Progression of percent emphysema was slower among regular aspirin users compared with patients who did not use aspirin (fully adjusted model: –0.34% /10 years, 95% CI, –0.60 to –0.08; P = .01). Results were similar in ever smokers and with doses of 81 and 300 to 325 mg and were of greater magnitude among those with airflow limitation. No association was found between aspirin use and change in lung function.
Conclusions
Regular aspirin use was associated with a more than 50% reduction in the rate of emphysema progression over 10 years. Further study of aspirin and platelets in emphysema may be warranted.
Key Words: COPD, CT, platelets
Abbreviations: COX, cyclooxygenase; MDCT, multidetector CT; MESA, Multi-Ethnic Study of Atherosclerosis; PD15, 15th percentile of lung density
FOR EDITORIAL COMMENT, SEE PAGE 3
COPD and emphysema are jointly the third leading cause of death in the United States and the world.1, 2 Emphysema is defined as destruction of alveolar walls distal to the terminal bronchioles on pathologic specimens3 and has also been measured on CT imaging as the percentage of emphysema-like lung (hereafter referred to as “percent emphysema”). Percent emphysema has been associated with lower left ventricular filling4 and reduced daily activity,5 as well as increased respiratory and all-cause mortality in COPD6 and in the general population without airflow obstruction.7, 8
The pathogenesis of COPD and emphysema is incompletely understood, but altered pulmonary blood flow and inflammation may be relevant factors.9, 10 Pulmonary capillaries are damaged in emphysematous lung in humans,11, 12 and factors that inhibit angiogenesis lead to emphysema in animals.13, 14 Platelet activation is increased in the setting of vessel injury and inflammation,15 and in animal models of acute lung injury, platelet activation reduces pulmonary microvascular blood flow and increases lung neutrophils,16, 17 findings that are improved with aspirin.17, 18 Additionally, platelet factor 4, which is released on platelet activation, increased the extent of neutrophil elastase-induced emphysema in an animal study.19 Finally, platelet activation was found to be increased in patients with COPD compared with control subjects20 and during exacerbation among those with COPD,21 and thrombocytosis was associated with increased mortality after hospitalization for COPD, a finding that was not present for those taking aspirin.22 These findings suggest a potential role of platelets in emphysema and COPD; however, it is unknown whether aspirin use alters the progression of emphysema or the decline in lung function.
We hypothesized that regular use of aspirin would be associated with slower progression of percent emphysema seen on CT over 10 years. We also examined the change in lung function over 5 years. We tested this hypothesis in a general population sample with mostly subclinical emphysema, as doing so may provide insights into strategies for treatment and prevention.
Methods
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study that recruited 6,814 participants in 2000 to 2002 from six US communities; they were aged 45-84 years and were free of clinical cardiovascular disease.23 The MESA Air Pollution Study recruited an additional 257 participants using the same criteria in 2004 to 2007.24 The MESA Lung Study enrolled 3,965 MESA participants in 2004 to 2006 (at the second and third visits).25 All MESA Air participants included were at one site. An additional 410 MESA participants were enrolled in 2010 to 2012 (e-Fig 1). The protocols of MESA and all studies described herein were approved by the institutional review boards of all collaborating institutions and the National Heart Lung and Blood Institute. All participants provided written informed consent.
Aspirin Use Assessment
Medication use was assessed at each visit by a medication inventory.26 Participants were instructed to bring all prescription and over-the-counter medications used in the preceding 2 weeks to the visit; staff recorded the name, strength, and frequency. Participants were separately asked whether they were taking aspirin, and if so how many days per week.
The primary exposure was regular use of aspirin at baseline, defined as use of any aspirin dose 3 or more days per week, as even 81 mg every 3 days inhibits platelet activation.27 Additional analyses evaluated any aspirin use at baseline, regular aspirin use at each visit, time-varying aspirin use, and doses of 81 and 300 to 325 mg.
Measurement of Percent Emphysema
All participants underwent cardiac CT scans at baseline following a standardized protocol at full inspiration on electron beam tomography and multidetector CT (MDCT) scanners.28 Participants were coached to total lung capacity, and two scans were obtained. Lung volumes on replicate cardiac CT scans were highly correlated (r = 0.95), and unless image quality differed, the scan with the greatest lung volume was selected for analysis. Follow-up cardiac CT scans used the same protocol. Forty-five off-protocol scans and 312 acquired on Aquilion scanners (Toshiba), which produce unreliable lung density measures, were excluded. Full-lung scans were performed on 3,204 MESA Lung participants at the 10-year follow-up examination at full inspiration on MDCT scanners following the Subpopulations and Intermediate Outcome Measures in COPD study (SPIROMICS) protocol.29
The percentage of emphysema-like lung (percent emphysema) was measured by trained readers at a reading center without knowledge of other participant information using modified Pulmonary Analysis Software Suite software for cardiac scans and Apollo 1.2 (an updated version of Pulmonary Analysis Software Suite; VIDA Diagnostics) for full-lung scans. Since cardiac CT images go from approximately the carina to the lung base, the upper third was excluded from full-lung scans to compare the same lung regions over time. Cardiac CT measures of percent emphysema are highly correlated with measures on full-lung scans from the same participants (r = 0.95 on MDCT scanners).30 Percent emphysema was defined as the percentage of lung voxels below –950 Hounsfield units, adjusted for the attenuation of air outside the chest to account for scanner variation. Sensitivity analyses use the lower 15th percentile of lung density (PD15) and a hidden Markov measure field model, which reduces variability in percent emphysema measures across different CT imaging protocols and levels of inspiration.31, 32 Monthly averages of outside air from all scans demonstrated little scanner drift over > 11 years, with one exception (e-Fig 2).
Spirometry
Spirometry was conducted between 2004 and 2007 and was repeated in 2010 to 2012 in accordance with American Thoracic Society/European Respiratory Society guidelines following the MESA Lung protocol; all examinations were reviewed by one investigator.33 At the time of the participants’ first spirometry measurement, airflow obstruction was defined as prebronchodilator FEV1/FVC < 0.70, and restrictive ventilatory defect was defined as FVC less than the lower limit of normal and FEV1/FVC ≥ 0.7.
Other Covariates
Age, sex, race/ethnicity, educational attainment, and smoking history were self-reported. Current smoking was defined as a self-report of smoking within 30 days or urinary cotinine levels > 100 ng/mL, measured at baseline and 10-year follow-up.25 Height, weight, BP, and C-reactive protein levels were measured using standard techniques.34 Plasma sphingomyelin levels were measured with a validated four-step enzymatic assay.35 Medication use was assessed by a medication inventory.26 Hypertension was defined as systolic or diastolic BP ≥ 140/90 mm Hg, or self-reported hypertension and antihypertensive medication use. Participants were asked about a physician diagnosis of asthma at each visit. The Framingham Risk Score was calculated36 and categorized as < 10% or ≥ 10%, as this was the indication for aspirin use in primary prevention of atherosclerotic events.37 The Agatston coronary artery calcium score was calculated on phantom-adjusted gated cardiac CT scans.28
Statistical Analysis
Participants were stratified by baseline regular aspirin use for descriptive purposes. Mixed linear regression growth curve models with random intercepts and slopes were used to assess the relationship between regular aspirin use and change in percent emphysema over time (ie, interaction term between aspirin use and time since baseline examination). The initial adjusted model included baseline age, sex, race/ethnicity, education, time-varying height, weight, scanner model, voxel size, milliamperes, and the interactions of sex and race/ethnicity with time. The subsequent model added baseline pack-years, time-varying cigarettes per day for current smokers, and the interaction of pack-years with time. The final model included baseline hypertension, C-reactive protein levels, sphingomyelin levels, angiotensin-converting enzyme (ACE)-inhibitor or angiotensin II-receptor blocker (ARB) use, and the interaction of ACE inhibitor or ARB use with time, which have been associated with the progression of percent emphysema.38, 39 Missing data were minimal except for pack-years (11% of ever smokers) and cigarettes per day (5% of current smokers) and were addressed by single imputation. Effect measure modification was assessed for sex, age, race/ethnicity, smoking status, airflow limitation, emphysema, and scanner manufacturer. Secondary analyses adjusted for other baseline medication use, including inhalers (inhaled steroids, beta-agonists, and anticholinergic agents alone or in combination), nonsteroidal antiinflammatory drugs, cyclooxygenase (COX)-2 inhibitors, adenosine diphosphate-receptor inhibitors, statin drugs, and diuretic agents), report of physician diagnosis of asthma, Framingham Risk Score > 10%, coronary artery calcium score, baseline emphysema, and the interaction of baseline emphysema and time. Sensitivity analyses were performed after excluding subjects with a change in smoking status and with propensity-score weighting for likelihood of aspirin use. Analyses for lung function used a similar statistical approach. Statistical significance was defined as a two-tailed P value < .05. Analyses were performed using SAS, version 9.3 (SAS Institute).
Results
Study Participants
Of 4,472 participants, 215 (5%) were excluded from the main analysis for self-reported irregular aspirin use. The 4,257 included participants were somewhat different from those not included (e-Table 1), but aspirin use was similar. Eighty-one percent had a follow-up cardiac CT scan and 3,041 (71% of total; 74% of those still living) underwent a full-lung CT scan at the 10-year follow-up. At baseline, participants were 61 ± 10 years of age, 49% men, 37% white, 27% black, 21% Hispanic, and 15% Chinese American. Forty-six percent were never smokers, 40% were former smokers, and 14% were current smokers.
At baseline, 22% reported taking aspirin regularly, with doses of 81 mg in 50%, 300 to 325 mg in 43%, alternative doses in 4%, and missing data in 3%. Compared with those not taking aspirin, participants taking aspirin regularly at baseline were more likely to be older white male former smokers with a higher education and greater hypertension, percent emphysema, and airflow limitation; follow-up time was similar in both groups (Table 1).
Table 1.
Selected Baseline Characteristics of Participants by Regular Aspirin Use
| Characteristic | Not Taking Aspirin at Baseline (n = 3,331) | Taking Aspirin Regularly at Baseline (n = 926) |
|---|---|---|
| Age, y | 60.2 ± 9.8 | 65.3 ± 8.9 |
| Male sex | 46.3 | 57.2 |
| Race | ||
| White, non-Hispanic | 32.2 | 53.7 |
| Black | 28.3 | 23.9 |
| Hispanic | 23.3 | 12.2 |
| Chinese American | 16.2 | 10.3 |
| Education | ||
| Did not complete high school | 16.9 | 12.1 |
| Completed high school | 18.6 | 16.7 |
| Some college | 28.5 | 26.2 |
| Completed college | 18.7 | 19.0 |
| Graduate degree | 17.2 | 25.8 |
| Height, cm | 166.0 ± 9.9 | 168.9 ± 10.0 |
| Weight, kg | 77.8 ± 17.2 | 80.8 ± 16.6 |
| BMI, kg/m2 | 28.1 ± 5.4 | 28.1 ± 4.7 |
| Smoking status | ||
| Never | 48.4 | 39.6 |
| Former | 37.2 | 48.5 |
| Current | 14.4 | 11.9 |
| Pack-yearsa | 24 ± 25 | 29 ± 28 |
| Cigarettes/db | 13 ± 21 | 14 ± 11 |
| Hypertension | 39.1 | 55.2 |
| Systolic BP, mm Hg | 124 ± 20 | 127 ± 20 |
| Total cholesterol, mg/dL | 195 ± 35 | 189 ± 34 |
| Diabetes | 9.9 | 15.7 |
| Medication use | ||
| ACE inhibitor or ARB | 10.5 | 17.6 |
| NSAID | 17.0 | 12.1 |
| COX-2 inhibitor | 5.9 | 8.5 |
| ADP-receptor inhibitor | 0.2 | 0.4 |
| Statin drug | 11.6 | 26.7 |
| Diuretic agent | 13.5 | 24.8 |
| Framingham Risk Score 10-y CHD risk ≥ 10% | 36.3 | 48.3 |
| FEV1, mLc | 2381 ± 732 | 2387 ± 712 |
| FEV1/FVC ratiod | 0.75 ± 0.08 | 0.74 ± 0.09 |
| Airflow limitation, %e | 23.1 | 30.0 |
| Asthma, self-report of physician diagnosis | 9.7 | 9.7 |
| Percent emphysema950, median (IQR) | 2.82 (1.17-5.55) | 3.47 (1.56-7.09) |
| Emphysema on CT, % | 7.3 | 10.7 |
| C-reactive protein, mg/dL | 3.6 ± 5.6 | 3.1 ± 4.4 |
| Sphingomyelin, mg/dL | 47.8 ± 15.7 | 47.9 ± 14.8 |
| Follow-up time, median (IQR), y | 9.3 (4.5-9.7) | 9.1 (4.6-9.6) |
Data are presented as mean ± SD or %, except where noted.
ACE = angiotensin converting enzyme; ARB = angiotensin II-receptor blocker, NSAID = nonsteroidal antiinflammatory drug (not including aspirin); ADP = adenosine diphosphate; CHD = coronary heart disease; IQR = interquartile range; percent emphysema950 = percent of emphysema-like lung below −950 Hounsfield units.
Among ever smokers reporting pack-years (1,511 not taking aspirin, 505 taking aspirin regularly).
Among current smokers reporting cigarettes per day (451 not taking aspirin, 105 taking aspirin regularly).
Among 3,759 subjects with FEV1 data (2,966 not taking aspirin, 793 taking aspirin regularly).
Among 3,742 subjects with FEV1/FVC data (2,951 not taking aspirin, 791 taking aspirin regularly).
Airflow limitation defined as prebronchodilator FEV1/FVC ratio < 0.7 among 3,431 subjects with FEV1/FVC data and no restrictive ventilatory defect (2,705 not taking aspirin, 726 taking aspirin regularly).
Regular Aspirin Use and Longitudinal Change in Percent Emphysema
The 4,257 participants underwent CT imaging a median of three times over a median of 9.3 years (interquartile range, 5.0-9.7), resulting in 11,465 measurements of percent emphysema. Median baseline percent emphysema was 2.97% (interquartile range, 1.23-5.83) (e-Table 1). The mean increase in percent emphysema was 0.60 percentage points over 10 years (95% CI, 0.30-0.90; P < .001).
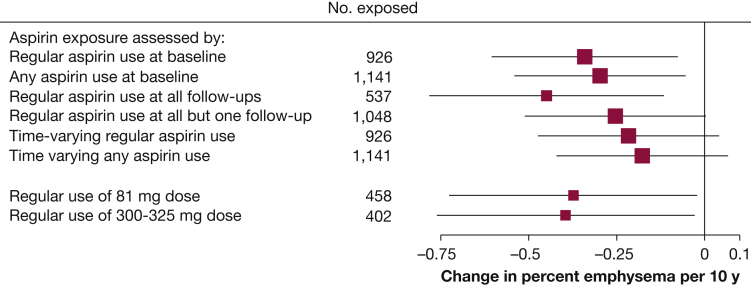
Regular aspirin use was associated with a significantly slower progression of percent emphysema in unadjusted and fully adjusted analyses (–0.34 percentage points over 10 years; 95% CI, –0.60 to –0.08; P = .01) (Table 2). Results were similar after propensity-score weighting (Table 2) and when the exposure was defined as any aspirin use at baseline (Fig 1). The association was of greater magnitude when considering regular aspirin use at all follow-up visits and attenuated for regular aspirin use at all but one follow-up visit and for time-varying aspirin use (Fig 1). The association for regular use of 81 mg aspirin was similar to that of a 300 to 325 mg dose (Fig 1). Results were similar using PD15 (e-Table 2) and attenuated with percent emphysema hidden Markov measure field (fully adjusted, –0.21 percentage points over 10 years; 95% CI, –0.51 to 0.08; P = .16).
Table 2.
Predicted Change in Percent Emphysema Over 10 Years for Participants Taking Aspirin Regularly Compared With Those Not Taking Aspirin, With and Without Propensity-Score Weighting
| Model | Change in Percent Emphysema Over 10 Years (95% CI) | P Value |
|---|---|---|
| Unweighted | ||
| Unadjusted | –0.42 (–0.70 to –0.14) | .003 |
| Model 1 | –0.35 (–0.62 to –0.09) | .009 |
| Model 2 | –0.36 (–0.63 to –0.10) | .007 |
| Model 3 | –0.34 (–0.60 to –0.08) | .011 |
| Weighted by propensity score | ||
| Unadjusted | –0.35 (–0.62 to –0.09) | .009 |
| Model 1 | –0.33 (–0.58 to –0.08) | .010 |
| Model 2 | –0.33 (–0.58 to –0.08) | .009 |
| Model 3 | –0.31 (–0.56 to –0.06) | .014 |
Model 1: Adjusted for baseline age, sex, race/ethnicity, sex × time and race/ethnicity × time interactions, education, time-varying height, weight, CT scanner model, milliamperes, and voxel size.
Model 2: Additionally adjusted for baseline pack-years, pack-years × time interaction, and time-varying cigarettes per day for current smokers.
Model 3: Additionally adjusted for baseline hypertension, C-reactive protein levels, sphingomyelin levels, ACE inhibitor or ARB use, and ACE-inhibitor or ARB use × time interaction.
See Table 1 legend for expansion of abbreviations.
Figure 1.
Association of different aspirin exposures with change in percent emphysema over 10 years. Analyses adjust for baseline age, sex, race/ethnicity, education, pack-years of smoking, hypertension, angiotensin-converting enzyme (ACE)-inhibitor or angiotensin II receptor-blocker (ARB) use, C-reactive protein levels, sphingomyelin levels, time-varying height, weight, cigarettes smoked per day, scanner model, milliamperes, voxel size, and interactions of sex, race/ethnicity, pack-years of smoking, and ACE-inhibitor or ARB use with time.
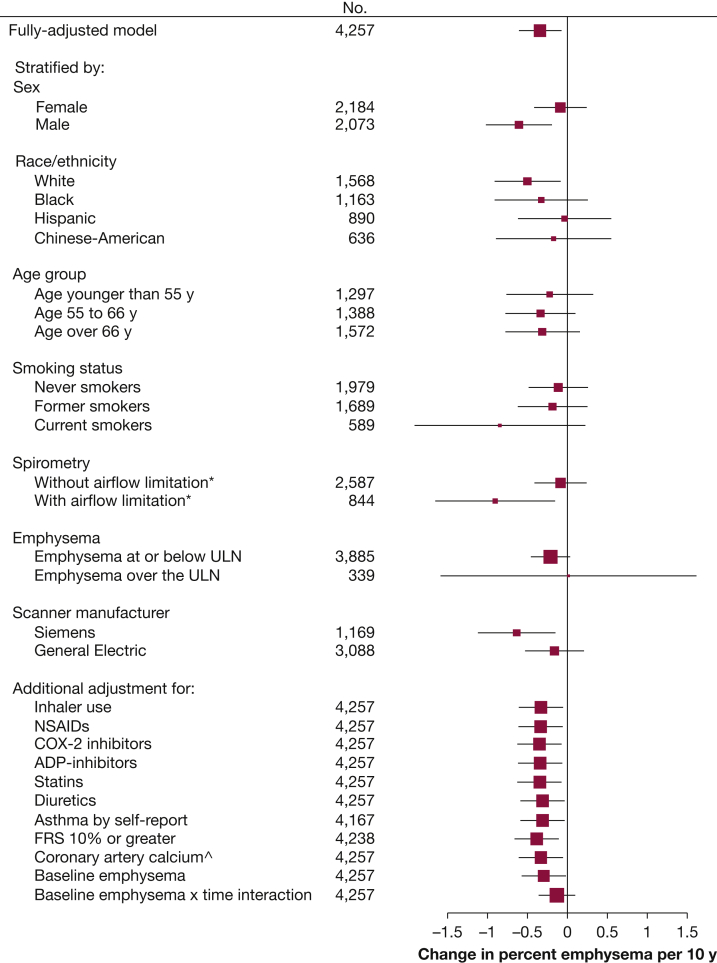
There was no evidence for effect modification of the association of regular aspirin use and percent emphysema by age, race/ethnicity, emphysema, or scanner manufacturer; however, the association was of greater magnitude among participants with airflow limitation (P interaction = .03), and there was a suggestion that results were stronger among men and current smokers (Fig 2).
Figure 2.
Sensitivity analysis showing the effect estimate of the change in percent emphysema over 10 years for those taking aspirin regularly compared with those not taking aspirin. Analyses adjust for baseline age, sex, race/ethnicity, education, pack-years of smoking, hypertension, angiotensin-converting enzyme-inhibitor or angiotensin II receptor-blocker use, C-reactive protein levels, sphingomyelin levels, and time-varying height, weight, cigarettes per day, scanner model, milliamperes, voxel size, and interactions of sex, race/ethnicity and pack-years of smoking with time. P value for interaction: sex, 0.09; race/ethnicity, 0.86; age, 0.84; smoking status, 0.09; airflow limitation, 0.03; emphysema, 0.40; and scanner manufacturer, 0.74. ∗Airflow limitation defined as FEV1/FVC < 0.7; ∧Coronary artery calcium measured as Agatston score on cardiac CTs. ADP = adenosine diphosphate; COX-2, cyclooxygenase-2; FRS = Framingham Risk Score; NSAIDs = nonsteroidal antiinflammatory drugs; ULN = upper limit of normal.
Results were similar after adjustment for inhaler use, nonsteroidal antiinflammatory drug use, COX-2-inhibitor use, adenosine diphosphate receptor-inhibitor use, statin drug and diuretic agent use, self-report of physician-diagnosed asthma, Framingham Risk Score > 10%, and coronary artery calcium and baseline emphysema, whereas the addition of baseline emphysema and time interaction weakened the results (Fig 2). Results were unchanged after excluding 340 participants (8%) who quit or resumed smoking (–0.34 percentage points over 10 years; 95% CI, –0.61 to –0.06; P = .017).
Regular Aspirin Use and Longitudinal Change in Lung Function
Valid spirometry measurements were obtained in 3,889 participants (98% of completed tests), and 68% had repeated valid tests a median of 4.8 years later. Thirty-four percent used aspirin regularly at the time of first spirometry measurements, and 3% were excluded due to self-reported irregular aspirin use. The mean decline in FEV1 was 28.9 mL/y (95% CI, –30.6 to –27.2; P < .001). There was no evidence for an association between regular aspirin use at time of first spirometry measurement and change in FEV1 or FEV1/FVC (e-Table 3).
Discussion
Regular aspirin use was associated with a > 50% slower progression of percent emphysema-like lung on CT imaging over 10 years in this general population sample. Results were similar across aspirin doses and many subgroups and were of greater magnitude among those with airflow obstruction. These findings, along with supportive results in animals, suggest that further study of aspirin and platelet activation in emphysema may be warranted.
This is the first study that we are aware of to show an association between aspirin use and longitudinal progression of percent emphysema. Prior studies have found platelet-related genes serotonin receptor 4 (HTR4), von Willebrand factor (VWF), and its platelet-receptor GP1BA to be associated with FEV1 and COPD.40, 41 Additionally, platelet factor 4 increased emphysema when added to a neutrophil elastase animal model of emphysema,19 and platelet activation was found to be greater in patients with COPD compared with control subjects and during exacerbations.20, 21
The consistency of the results for low-dose (81 mg) and full-strength (300 to 325 mg) aspirin suggests that inhibiting platelet activation is the most likely explanation for these findings, as 81 mg every 3 days impacts platelet activation.27 Platelet activation causes pulmonary microvascular constriction, which is reversed by aspirin.18 This may be relevant, as reduced pulmonary microvascular blood flow is seen in emphysema and even mild COPD,10 may contribute to disease pathogenesis, and early vascular changes in emphysema may be reversible.42 Additionally, platelet activation causes greater neutrophilic inflammation,17 which is also implicated in COPD.9
However, other mechanisms of aspirin such as the anti-inflammatory and potentially bronchodilatory effect of reducing COX-1-dependent prostaglandins cannot be ruled out.43 Although we did not perform expiratory CT scans to evaluate functional small airways disease, gas trapping is likely a minor contributor to the results for percent emphysema measured at full inspiration, and the normal results for lung function also suggest that this is unlikely.
The lack of an association between regular aspirin use and change in lung function may be due to the smaller sample size and shorter follow-up time in those with spirometry measurements. However, the result is consistent with normal results for aspirin and lung function in the National Health and Nutrition Examination Survey III.44 Importantly, progression of emphysema and airflow obstruction in COPD may represent distinct processes, as supported by findings from many biomarker and genetic studies.40, 41, 45, 46
Results were stronger in those with airflow limitation, and results were somewhat stronger in men and current smokers. These findings may be related, as people with airflow limitation are more likely to be male current and former smokers. However, smoking and sex may also contribute to differences in platelet activation and aspirin response.47, 48
Although the strengths of this study include repeated CT scans over 10 years, the large sample size, and multi-ethnic population-based sample, several limitations should be discussed. Confounding by indication is possible in this observational study. However, aspirin is not indicated for lung disease, and in this general population sample without clinical cardiovascular disease at baseline, the likely indication was primary prevention of atherosclerotic events. Adjustment for Framingham Risk Score > 10% and coronary artery calcium scores did not alter the results, and propensity score-adjusted results were similar. Other medication use was more common among those taking aspirin; however, the main analysis adjusted for ACE inhibitors and ARBs and further adjustment for other medication use did not impact the results. Nonetheless, residual confounding may exist given discrepancies between observational and interventional studies in respiratory disease.49, 50 Thus, small studies using an intermediate end point, such as pulmonary microvascular blood flow,10 may be a prudent next step.
Aspirin adherence is uncertain, as frequency was self-reported and aspirin was inevitably started and stopped over the nearly 10-year study. However, nonadherence would be expected to weaken the effect of aspirin, not strengthen it. The association for regular aspirin use at each follow-up was of greater magnitude and similar when considering any aspirin use at baseline, which was assessed by the more reliable medication inventory.26
The progression of percent emphysema was modest, likely due to the general population sample. Given the mostly subclinical emphysema, the presence of baseline emphysema was previously confirmed by visual assessment in subsets.46, 51 In this cohort, percent emphysema has also been associated with all-cause mortality,7 and a reduction of this magnitude may still be relevant in disease prevention. Importantly, aspirin use was associated with a > 50% reduction in the rate of overall progression.
At baseline, subjects taking aspirin regularly had greater percent emphysema compared with those not taking aspirin. Although the results were weakened with the interaction between baseline emphysema and time, there is disagreement about whether to adjust for baseline levels in longitudinal analyses,52 and there was not significant effect modification by baseline emphysema. Additionally, longitudinal associations are generally favored when results are discrepant.53
Percent emphysema was assessed in the lower two-thirds of the lungs, omitting the top portion of the upper lobes, a common location for centrilobular emphysema; however, measures of percent emphysema from this region of the lung are highly correlated with those from full-lung scans in MESA.30 Over the course of the study, advances in image acquisition and processing and changes in scanner models inevitably occurred, which may contribute to variation in quantitative emphysema. However, the attenuation of outside air was remarkably stable over more than a decade, and analyses of PD15 yielded similar results. Additionally, alterations in lung inflammation and perfusion, proposed mechanisms of aspirin, also alter CT lung attenuation. However, this effect would be small in a longitudinal analysis, particularly among those consistently taking or not taking aspirin.
Conclusions
Regular aspirin use was associated with a slower progression of percent emphysema-like lung on CT scans over 10 years in this general population sample. These findings suggest that further studies of platelet activation and aspirin in COPD and emphysema may be warranted.
Acknowledgments
Author contributions: C. P. A. drafted the manuscript, had full access to all the data, and takes responsibility for the integrity of the data and the accuracy of the data analysis. C. P. A., E. A. H., D. R. J., J. D. K., L. J. S., K. E. W., R. P. T., and R. G. B. contributed to study conception and design. C. P. A., J. E. S., E. A. H., E. A., J. H. M. A., M. C., D. R. J., J. D. K., A. L., L. J. S., J. Y., K. E. W., R. P. T., and R. G. B. contributed to data collection, critical revision, and final approval of the manuscript. C. P. A. and R. G. B. contributed to data analysis and interpretation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: C. P. A. reports research grants from the Alpha-1 Foundation and the Stony Wold-Herbert Fund. E. A. H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed in part at the University of Iowa. J. H. M. A. has received personal fees from PulmonX. R. G. B. reports research grants from the Alpha-1 and COPD Foundations and personal fees from UpToDate. None disclosed (J. E. S., E. A., M. C., D. R. J., J. D. K., A. L., L. J. S., J. Y., K. E. W., R. P. T.).
Other contributions: The authors thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Role of sponsors: The sponsor had no role in the collection, analysis or interpretation of the data, or the preparation of the manuscript. The views expressed in this document are solely those of the authors and not necessarily those of the NIH or EPA.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung, and Blood Institute [Grants HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, R01-HL077612, RC1-HL100543, R01-HL093081 and R01-HL098077]; by National Center for Advancing Translational Sciences [Grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420]; U.S. Environmental Protection Agency [Grant RD83169701]; and an Alpha-1 Foundation Research Grant.
Supplementary Data
References
- 1.Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1–96. [PubMed] [Google Scholar]
- 2.Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S., Committee G.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 4.Barr R.G., Bluemke D.A., Ahmed F.S. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo Cascio C.M., Quante M., Hoffman E.A. Percent emphysema and daily motor activity levels in the general population: Multi-Ethnic Study of Atherosclerosis. Chest. 2017;151(5):1039–1050. doi: 10.1016/j.chest.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannessen A., Skorge T.D., Bottai M. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187(6):602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 7.Oelsner E.C., Hoffman E.A., Folsom A.R. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161(12):863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oelsner E.C., Carr J.J., Enright P.L. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71(7):624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes P.J., Shapiro S.D., Pauwels R.A. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 10.Hueper K., Vogel-Claussen J., Parikh M.A. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD Study. Am J Respir Crit Care Med. 2015;192(5):570–580. doi: 10.1164/rccm.201411-2120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebow A.A. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80(1 Part 2):67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 12.Reid J.A., Heard B.E. The capillary network of normal and emphysematous human lung studied by injections of Indian ink. Thorax. 1963;18:201–212. doi: 10.1136/thx.18.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasahara Y., Tuder R.M., Taraseviciene-Stewart L. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106(11):1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrache I., Natarajan V., Zhen L. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11(5):491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichhorn M.E., Ney L., Massberg S., Goetz A.E. Platelet kinetics in the pulmonary microcirculation in vivo assessed by intravital microscopy. J Vasc Res. 2002;39(4):330–339. doi: 10.1159/000065545. [DOI] [PubMed] [Google Scholar]
- 17.Zarbock A., Singbartl K., Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song C., Suzuki S., Kubo H. Effects of antiplatelet agents on pulmonary haemodynamic response to fMLP in endotoxin primed rats. Thorax. 2004;59(1):39–44. doi: 10.1136/thx.2003.002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonky S.A., Wohl H. Stimulation of human leukocyte elastase by platelet factor 4. Physiologic, morphologic, and biochemical effects on hamster lungs in vitro. J Clin Invest. 1981;67(3):817–826. doi: 10.1172/JCI110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wedzicha J.A., Syndercombe-Court D., Tan K.C. Increased platelet aggregate formation in patients with chronic airflow obstruction and hypoxaemia. Thorax. 1991;46(7):504–507. doi: 10.1136/thx.46.7.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maclay J.D., McAllister D.A., Johnston S. Increased platelet activation in patients with stable and acute exacerbation of COPD. Thorax. 2011;66(9):769–774. doi: 10.1136/thx.2010.157529. [DOI] [PubMed] [Google Scholar]
- 22.Harrison M.T., Short P., Williamson P.A., Singanayagam A., Chalmers J.D., Schembri S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax. 2014;69(7):609–615. doi: 10.1136/thoraxjnl-2013-203996. [DOI] [PubMed] [Google Scholar]
- 23.Bild D.E., Bluemke D.A., Burke G.L. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J.D., Adar S.D., Allen R.W. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176(9):825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez J., Jiang R., Johnson W.C., MacKenzie B.A., Smith L.J., Barr R.G. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith N.L., Psaty B.M., Heckbert S.R., Tracy R.P., Cornell E.S. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52(2):143–146. doi: 10.1016/s0895-4356(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 27.Feldman M., Cryer B., Rushin K., Betancourt J. A comparison of every-third-day versus daily low-dose aspirin therapy on serum thromboxane concentrations in healthy men and women. Clin Appl Thromb Hemost. 2001;7(1):53–57. doi: 10.1177/107602960100700111. [DOI] [PubMed] [Google Scholar]
- 28.Carr J.J., Nelson J.C., Wong N.D. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 29.Sieren J.P., Newell J.D., Jr., Barr R.G. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman E.A., Jiang R., Baumhauer H. Reproducibility and validity of lung density measures from cardiac CT scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hame Y., Angelini E.D., Hoffman E.A., Barr R.G., Laine A.F. Adaptive quantification and longitudinal analysis of pulmonary emphysema with a hidden Markov measure field model. IEEE Trans Med Imaging. 2014;33(7):1527–1540. doi: 10.1109/TMI.2014.2317520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J.D., Angelini E.D., Balte P.P. Emphysema quantification on cardiac CT scans using hidden Markov measure field model: the MESA Lung Study. Med Image Comput Comput Assist Interv. 2016;9901:624–631. doi: 10.1007/978-3-319-46723-8_72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hankinson J.L., Kawut S.M., Shahar E., Smith L.J., Stukovsky K.H., Barr R.G. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MESA Manual of Operations. Field center and laboratory procedures. University of Washington; Seattle: 2008. http://www.mesa-nhlbi.org/publicDocs/MesaMop/MesaMop1-5-01.doc Accessed January 5, 2017. [Google Scholar]
- 35.Jiang X.C., Paultre F., Pearson T.A. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20(12):2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 36.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 37.Pearson T.A., Blair S.N., Daniels S.R. AHA Guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106(3):388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed F.S., Jiang X.C., Schwartz J.E. Plasma sphingomyelin and longitudinal change in percent emphysema on CT. The MESA Lung study. Biomarkers. 2014;19(3):207–213. doi: 10.3109/1354750X.2014.896414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh M.A., Aaron C.P., Hoffman E.A. Angiotensin-converting inhibitors and angiotensin II receptor blockers and longitudinal change in percent emphysema on computed tomography. The Multi-Ethnic Study of Atherosclerosis Lung Study. Ann Am Thorac Soc. 2017;14(5):649–658. doi: 10.1513/AnnalsATS.201604-317OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Repapi E., Sayers I., Wain L.V. Genome-wide association study identifies five loci associated with lung function. Nat Gen. 2010;42(1):36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu W., Baccarelli A., Carey V.J. Variable DNA methylation is associated with chronic obstructive pulmonary disease and lung function. Am J Respir Crit Care Med. 2012;185(4):373–381. doi: 10.1164/rccm.201108-1382OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer K.S., Newell J.D., Jr., Jin D. Quantitative Dual-energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med. 2016;193(6):652–661. doi: 10.1164/rccm.201506-1196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKeever T.M., Lewis S.A., Smit H.A., Burney P., Britton J.R., Cassano P.A. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med. 2005;171(9):966–971. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- 45.Coxson H.O., Dirksen A., Edwards L.D. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 46.Manichaikul A., Hoffman E.A., Smolonska J. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189(4):408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wennmalm A., Benthin G., Granstrom E.F., Persson L., Petersson A.S., Winell S. Relation between tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in young men. Circulation. 1991;83(5):1698–1704. doi: 10.1161/01.cir.83.5.1698. [DOI] [PubMed] [Google Scholar]
- 48.Becker D.M., Segal J., Vaidya D. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. 2006;295(12):1420–1427. doi: 10.1001/jama.295.12.1420. [DOI] [PubMed] [Google Scholar]
- 49.Sheehan W.J., Mauger D.T., Paul I.M. Acetaminophen versus ibuprofen in young children with mild persistent asthma. N Engl J Med. 2016;375(7):619–630. doi: 10.1056/NEJMoa1515990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Criner G.J., Connett J.E., Aaron S.D. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370(23):2201–2210. doi: 10.1056/NEJMoa1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donohue K.M., Hoffman E.A., Baumhauer H. Asthma and lung structure on computed tomography: the Multi-Ethnic Study of Atherosclerosis Lung Study. J Allergy Clin Immunol. 2013;131(2):361–368. doi: 10.1016/j.jaci.2012.11.036. e1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glymour M.M., Weuve J., Berkman L.F., Kawachi I., Robins J.M. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267–278. doi: 10.1093/aje/kwi187. [DOI] [PubMed] [Google Scholar]
- 53.Tager I.B. Outcomes in cohort studies. Epidemiol Rev. 1998;20(1):15–28. doi: 10.1093/oxfordjournals.epirev.a017969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.