Abstract
Background
Sleep apnea is an underdiagnosed condition in patients with heart failure. Efficient identification of sleep apnea is needed, as treatment may improve heart failure–related outcomes. Currently, use of portable sleep monitoring in hospitalized patients and those at risk for central sleep apnea is discouraged. This study examined whether portable sleep monitoring with respiratory polygraphy can accurately diagnose sleep apnea in patients hospitalized with decompensated heart failure.
Methods
Hospitalized patients with decompensated heart failure underwent concurrent respiratory polygraphy and polysomnography. Both recordings were scored for obstructive and central disordered breathing events in a blinded fashion, using standard criteria, and the apnea-hypopnea index (AHI) was determined. Pearson’s correlation coefficients and Bland-Altman plots were used to examine the concordance among the overall, obstructive, and central AHI values derived by respiratory polygraphy and polysomnography.
Results
The sample consisted of 53 patients (47% women) with a mean age of 59.0 years. The correlation coefficient for the overall AHI from the two diagnostic methods was 0.94 (95% CI, 0.89-0.96). The average difference in AHI between the two methods was 3.6 events/h. Analyses of the central and obstructive AHI values showed strong concordance between the two methods, with correlation coefficients of 0.98 (95% CI, 0.96-0.99) and 0.91 (95% CI, 0.84-0.95), respectively. Complete agreement in the classification of sleep apnea severity between the two methods was seen in 89% of the sample.
Conclusions
Portable sleep monitoring can accurately diagnose sleep apnea in hospitalized patients with heart failure and may promote early initiation of treatment.
Key Words: heart failure, inpatient sleep testing, polysomnography, sleep apnea
Abbreviations: AHI, apnea-hypopnea index
Sleep apnea is a highly prevalent condition that is associated with several clinical sequelae including hypertension,1, 2, 3 type 2 diabetes,4, 5 cardiovascular disease,6, 7 stroke,8, 9, 10 and heart failure.11, 12, 13 Diagnosis of sleep apnea can involve long wait times and significant resource utilization given that diagnostic testing traditionally has required an in-laboratory polysomnogram. Thus, it is not surprising that the vast majority of patients with sleep apnea do not receive a diagnosis.14, 15 The advent of portable sleep monitoring has led to a critical paradigm shift in the diagnostic strategy for sleep apnea. The use of relatively simple and portable devices for sleep testing has decreased the time, as well as the costs, associated with diagnosing sleep apnea.16, 17, 18 Despite the growing recognition of the associated clinical consequences, many patients with sleep apnea remain unaware or are dismissive of their symptoms and thus do not seek care.
Given its potential impact, the diagnosis of sleep apnea has clinical value, especially in high-risk patients. For example, patients with heart failure can have obstructive sleep apnea, central sleep apnea, or both. Epidemiological data show that sleep apnea can affect 70% to 80% of patients with heart failure and is also an independent risk factor for the development of heart failure.6 Moreover, untreated sleep apnea may be associated with an increased risk for death in patients with heart failure,19 and treatment can reduce the risk for death and hospitalization.20, 21 While the use of adaptive servo-ventilation therapy in patients with central sleep apnea and reduced ejection fraction heart failure is controversial and needs further assessment,22 studies using continuous positive airway pressure therapy for the treatment of sleep apnea in heart failure have mostly shown benefit, with improvement in left ventricular function,23 transplant-free survival,24 and even short-term readmission rates due to decompensated heart failure.21 Despite this, in a recent study of Medicare beneficiaries only 4% of patients with newly identified heart failure were suspected of having sleep apnea, and only 2% underwent testing for sleep apnea.25 Thus, efficient strategies are needed to diagnose sleep apnea in at-risk patients including those with heart failure. Hospitalization for decompensated heart failure may represent a unique opportunity for case identification of sleep apnea in the heart failure population, as patients are in a monitored setting where testing for sleep apnea can be conveniently deployed without additional patient burden and minimal staff effort. However, there is a paucity of evidence on diagnostic strategies for sleep apnea in the inpatient setting, and current recommendations discourage diagnosing sleep apnea in the hospitalized patient.26 Given the relevance of sleep apnea in heart failure, and the need for optimizing case identification and treatment approaches for sleep apnea, the overall aim of the current investigation was to determine whether portable respiratory polygraphy can be used to diagnose sleep apnea in hospitalized patients with decompensated heart failure.
Methods
Study Sample
A sample of adult patients ≥ 21 years of age admitted to the heart failure or general medical service at the Johns Hopkins Medical Institutions with a primary diagnosis of decompensated heart failure as determined by the inpatient team were considered for inclusion in the study. Inpatient provider teams identified patients who were medically stabilized, euvolemic, weaned off supplemental oxygen, and receiving a stable diuretic regimen on which they would be discharged. Exclusion criteria were as follows: current treatment for sleep apnea; oxygen use; right-sided heart failure without left-sided failure; systolic blood pressure less than 80 mm Hg; COPD exacerbation; status post–heart transplant or implantation of a left ventricular assist device; chronic hemodialysis; or uncorrected valvular heart disease. Patients could not be receiving standing sedative-hypnotic medication while in the hospital, and, specifically, patients could not take any sedative-hypnotic medication on the night of the sleep recordings. The aforementioned enrollment criteria were imposed to recruit a convenience sample of patients with heart failure at risk for sleep apnea while minimizing the confounding effects of other comorbid conditions. Typically, sleep studies were performed 24 h prior to discharge from the hospital. During the 12 months of recruitment, a total of 521 patients were admitted for decompensated heart failure from which a convenience sample of 57 patients was recruited for the current study. The protocol was approved by the Johns Hopkins Institutional Review Board (IRB00064193) and all patients provided informed consent.
Diagnosis of Sleep Apnea
Unattended polysomnography (ie, a type 2 sleep study) and respiratory polygraphy were used as the two independent approaches for diagnosing sleep apnea. Polysomnography was conducted with the Embletta MPR-PG system (Natus Medical Incorporated). The following signals were recorded: C3/A1 and C4/A2 electroencephalograms, bilateral electro-oculograms, a single bipolar electrocardiogram, a chin electromyogram, oxyhemoglobin saturation, chest and abdominal excursion by inductance plethysmography, and nasal airflow with a nasal pressure cannula. Concurrent with the unattended polysomnogram, respiratory polygraphy was conducted with a type 3 monitor (ApneaLink Plus; ResMed). Pulse oximetry was used to assess oxyhemoglobin saturation, and respiratory effort was measured with a pneumatic sensor attached to an effort belt. Nasal airflow was recorded with a nasal cannula connected to a pressure transducer. The nasal pressure transducers for polysomnography and respiratory polygraphy units were connected to one nasal cannula through a three-way valve for contemporaneous nasal airflow measurement. The two recording systems were synchronized such that both tests had equivalent total recording time. Both devices were applied to each patient by a certified polysomnographic technologist. The nursing staff were instructed not to disturb or awaken the patient between midnight and 6:00 a.m. However, nurses were allowed to remove the equipment in the morning after the patient awakened.
Scoring of the data acquired from polysomnography and respiratory polygraphy was conducted in a blinded fashion. All scoring was performed by trained technicians and reviewed by a board-certified sleep physician. The following criteria were used for scoring. For both polysomnography and respiratory polygraphy, apneas were identified if there was a 90% or greater reduction in airflow for at least 10 s. For both diagnostic approaches, hypopneas were identified if there was a ≥ 30% reduction in airflow for at least 10 s that was associated with an oxyhemoglobin desaturation of at least 3%. For polysomnography, hypopneas were also identified if there was an arousal associated with the ≥ 30% reduction in airflow. The apnea-hypopnea index (AHI), the disease-defining metric for sleep apnea, was the number of apneas and hypopneas per hour of total sleep time or total recording time for polysomnography and respiratory polygraphy, respectively. Hypopneas were scored as obstructive if snoring, and/or flow limitation was noted on the nasal pressure signal or if paradoxical movement was noted on respiratory inductance plethysmography during the event. In the absence of snoring, flow limitation, and paradoxical movement, the hypopnea was scored as a central event. An apnea was scored as obstructive if respiratory effort was present during the event or central in the absence of effort during the event.27 Indices corresponding to the severity of obstructive sleep apnea (obstructive AHI) and central sleep apnea (central AHI) were subsequently derived. Sleep apnea severity was assessed using the following commonly used cut-points: none (< 5 events/h), mild (5.0-14.9 events/h), moderate (15.0-29.9 events/h), and severe (≥ 30.0 events/h). The presence of Cheyne-Stokes respiration was determined if there were episodes of at least three consecutive central apneas and/or central hypopneas separated by a crescendo-decrescendo change in breathing amplitude.
Statistical Analyses
Agreement between results derived from respiratory polygraphy and full polysomnography was determined as follows. Bivariate scatter plots and Pearson’s product moment correlation coefficients were used for the overall, obstructive, and central AHI to quantify the level of agreement between the two diagnostic methods. Because all patients were concurrently assessed by respiratory polygraphy and polysomnography and those two assessments are the only assessments, the Pearson’s product moment correlation coefficients are mathematically equivalent to the intraclass correlation coefficients that would be determined by the two-way linear mixed-effects model to assess agreement as proposed by Shrout and Fleiss.28 Differences between the two methods were also examined by computing the average AHI difference between respiratory polygraphy and polysomnography, and using the method proposed by Bland and Altman.29 Misclassification of sleep apnea severity (none, mild, moderate, and severe) was examined using contingency tables and percent agreement. All analyses were performed with the SAS 9.4 software system (SAS Institute).
Results
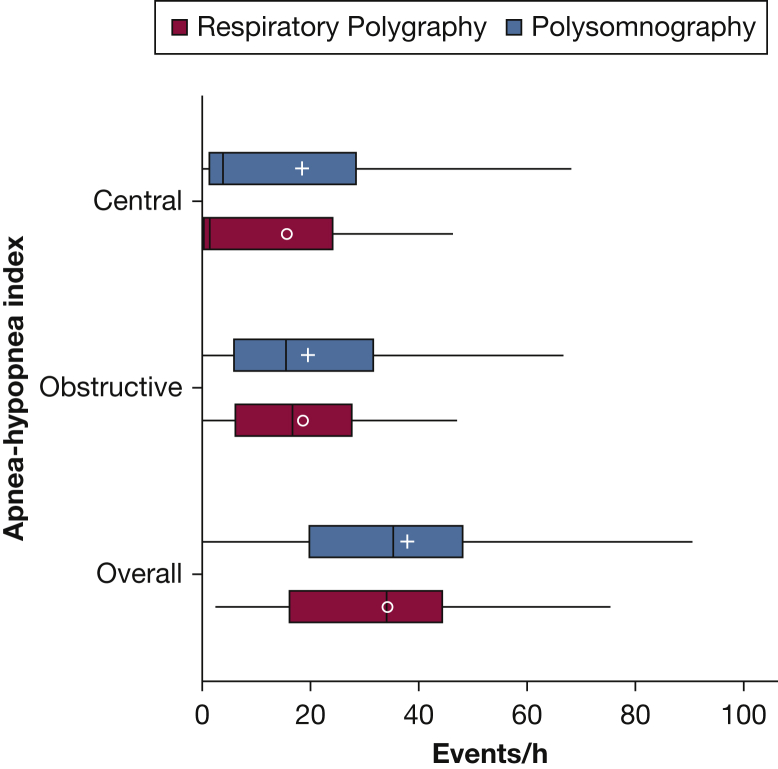
The study sample was composed of 57 patients hospitalized with decompensated heart failure. Two patients were considered to have suboptimal polysomnography, and two additional patients were noted to have respiratory polygraphy that was not amenable to scoring (ie, 3.5% failure for each type of recording). Thus, the sample size consisted of 53 patients with concurrent recordings of respiratory polygraphy and polysomnography. The demographic and anthropometric characteristics of the sample are shown in Table 1. The mean age of the sample was 59.0 years (SD, 12.9). Fifty-three percent of the patients were male and the mean ejection fraction was 35.7%. The majority of the patient sample (60.7%) had a reduced ejection fraction (≤ 40%) whereas the remaining 39.3% had a preserved ejection fraction. The average total sleep time from polysomnography was 5.4 h (SD, 2.0) and the average recording time was 7.5 h (SD, 2.4). Because respiratory polygraphy and polysomnography were synchronized, the average total recording time of 7.5 h was similar for both approaches. Figure 1 displays the distributions of the overall, obstructive, and central AHI values derived from respiratory polygraphy and polysomnography. No statistically significant differences were noted in the distributions of the overall AHI, obstructive AHI, or central AHI values when comparing the two diagnostic methods (all P values > .05). In addition, the proportion of patients noted to have Cheyne-Stokes respiration was exactly similar (37.4% with both methods).
Table 1.
Characteristics of the Study Sample
| Characteristic | Mean (SD) or Percentage |
|---|---|
| Age, y | 59.0 (12.9) |
| BMI, kg/m2 | 37.1 (18.8) |
| Male sex | 52.8 |
| Race | |
| White | 49.1 |
| Black | 39.6 |
| Other | 11.3 |
| Hypertension | 81.1 |
| Type 2 diabetes mellitus | 50.9 |
| Atrial fibrillation | 52.8 |
| CABG | 22.6 |
| LVEF | 35.7 (10.0) |
| HFrEF | 60.7 |
| HFpEF | 39.3 |
| Heart failure etiology | |
| Ischemic | 26.4 |
| Nonischemic | 52.8 |
| Other | 20.8 |
| AHI ≥ 5 events/h | 90.6 |
| Obstructive AHI ≥ 5 events/h | 75.5 |
| Central AHI ≥ 5 events/h | 58.5 |
AHI = apnea-hypopnea index; CABG = coronary artery bypass graft; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricle ejection fraction.
Figure 1.
Boxplots for central, obstructive, and overall apnea-hypopnea index (AHI) derived from respiratory polygraphy and polysomnography Hypopneas were scored using the 3% desaturation or arousal criteria for polysomnography and 3% desaturation only for respiratory polygraphy. (+, mean AHI respiratory polygraphy; ○, mean AHI polysomnography).
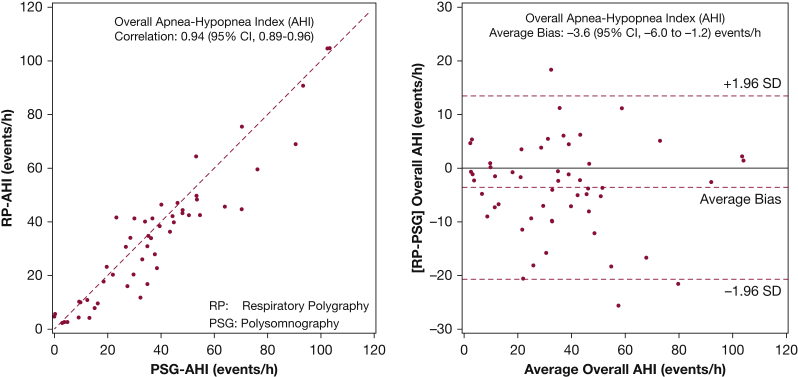
Figure 2 shows the bivariate and associated Bland-Altman plots for the overall AHI, comparing respiratory polygraphy with polysomnography, using a hypopnea definition of 3% desaturation or arousal for polysomnography and 3% desaturation for respiratory polygraphy. The correlation coefficient for the overall AHI across the two diagnostic methods was 0.94 (95% CI, 0.89-0.96). Despite the high degree of correlation, respiratory polygraphy underestimated the overall AHI by 3.6 events/h (95% CI, −6.0 to −1.2) when compared with polysomnography. Sensitivity analyses were also performed using a hypopnea definition of 4% desaturation or arousal for polysomnography and 4% desaturation for respiratory polygraphy. A high degree of agreement between the two methods was again noted. Specifically, the correlation coefficient for the AHI between polysomnography and respiratory polygraphy was 0.96 and the average bias was −4.2 events/h, with respiratory polygraphy underestimating the AHI compared with polysomnography. In the subset of nine patients where respiratory polygraphy underestimated the overall AHI by at least 10 events/h or more when compared with polysomnography, the overall AHI was recomputed for respiratory polygraphy using the total sleep time from the polysomnogram instead of the total recording time. Not surprisingly, in these nine patients the average difference in the overall AHI improved from −17.8 events/h (SD, 4.8) to 2.6 events/h (SD, 6.2), indicating that a significant portion of the underestimation in the assessment of the AHI by respiratory polygraphy was due to the use of total recording time instead of total sleep time.
Figure 2.
Scatter plot (left) with line of identity (---) and Bland-Altman plot (right) for overall apnea-hypopnea index with mean bias (---) along with the ±1.96 SD limits of bias (---). Hypopneas were scored using the 3% desaturation or arousal criteria for polysomnography and 3% desaturation only for respiratory polygraphy.
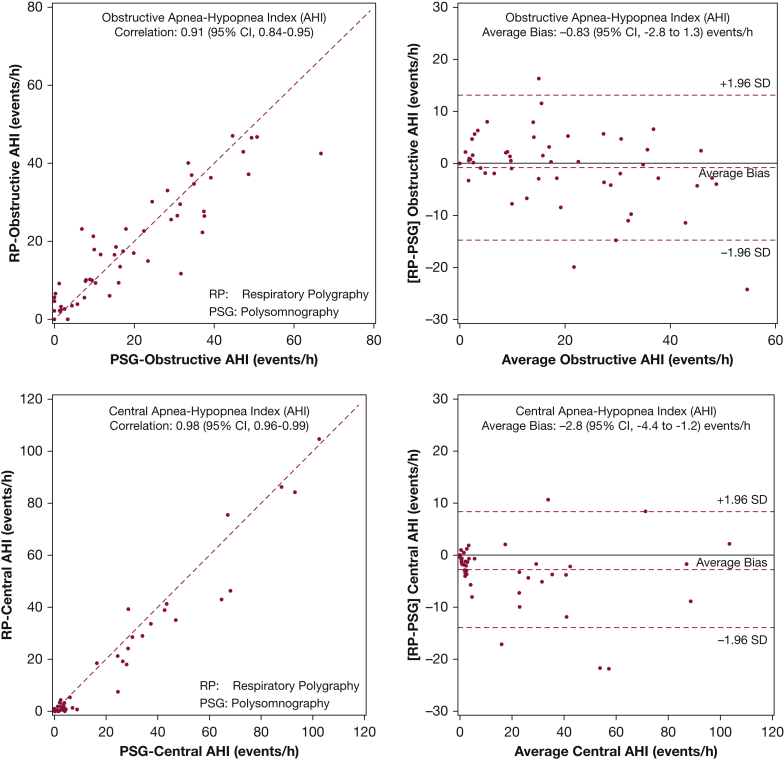
Analyses examining the differences between respiratory polygraphy and polysomnography were also conducted uing the obstructive and central AHI values. Figure 3 displays the bivariate and associated Bland-Altman plots for the obstructive AHI (top panels) and central AHI (bottom panels). The correlation coefficients for the obstructive and central AHI were 0.91 (95% CI, 0.84-0.95) and 0.98 (95% CI, 0.96-0.99), respectively. The average differences between respiratory polygraphy and polysomnography for obstructive and central AHI were −0.83 events/h (95% CI, −2.8 to 1.3) and −2.8 events/h (95% CI, −4.4 to −1.2), respectively. As with the overall AHI, calculating the obstructive AHI and central AHI values using total sleep time from polysomgraphy instead of total recording explicated all of the major differences between values derived from respiratory polygraphy and polysomnography.
Figure 3.
Scatter plot (left) and Bland-Altman plot (right) for obstructive (top) and central (bottom) apnea-hypopnea index with mean bias (---) along with the ±1.96 SD limits of bias (---). Hypopneas were scored using the 3% desaturation or arousal criteria for polysomnography and 3% desaturation only for respiratory polygraphy.
Agreement in the classification of sleep apnea severity as none, mild, moderate, or severe disease, using the overall AHI between the two diagnostic approaches, was 88.7%, indicating that respiratory polygraphy was able to accurately classify disease severity in most of the patients when compared with polysomnography. Robust agreement was also observed in the classification of disease severity for obstructive (77.4%) as well as central (94.3%) sleep apnea. The diagnostic accuracy of portable monitoring for the overall AHI, using a cut-point of 5 events/h, was strong, with a sensitivity and specificity of 95.8% and 80.0%, respectively. The positive and negative predictive values were 97.9% and 66.7%, respectively. Sensitivity and specificity measures were even more robust when only the central AHI (cut-point of ≥ 5 events/h) was considered, with values of 90.9% and 100%, respectively. Positive and negative predictive values for central AHI were 100% and 93.9%, respectively. Finally, the sensitivity, specificity, and positive and negative predictive values for an obstructive AHI of ≥ 5 events/h were as follows: 97.5%, 76.9%, 92.9%, and 90.9%, respectively.
Discussion
The current investigation demonstrates that sleep apnea can be accurately and efficiently identified in the inpatient setting by respiratory polygraphy. In fact, respiratory polygraphy was performed successfully in patients hospitalized for decompensated heart failure and demonstrated robust agreement with the concurrently performed full polysomnography. When examined separately, a high degree of agreement was also noted for metrics of both obstructive and central sleep apnea, and no material differences were observed in the level of agreement between the two diagnostic methods irrespective of the criteria used for scoring hypopneas.
The available literature on inpatient sleep testing is limited but demonstrates consistency in results, with a high level of concordance seen between portable sleep testing used in the inpatient setting and subsequent outpatient polysomnography for the diagnosis of obstructive sleep apnea.30, 31, 32, 33 Previous studies have shown that inpatient sleep monitoring, using either high-resolution pulse oximetry33, 34 or portable sleep monitors, is technically feasible21, 30, 35, 36 with failure rates ranging between 2% and 19%. Findings from the current study are congruent with previous reports, demonstrating a technical failure rate of 3.5% for respiratory polygraphy. More importantly, the present study extends the evidence on inpatient sleep testing by demonstrating a high level of agreement between respiratory polygraphy and full polysomnography performed concurrently in the inpatient environment despite the differences in hypopnea criteria, with arousals being an additional criterion on polysomnography as EEG data were available. Executing simultaneous recordings of both types of studies is of considerable value, as it has been previously observed that a significant number of central respiratory events initially noted on inpatient portable monitoring subsequently abated on outpatient polysomnography performed at a later date.31 Indeed, the uncertainty regarding the reliability of portable sleep monitoring in identifying central sleep apnea has been a limitation in its use. Previous studies have primarily reported agreement on obstructive sleep apnea as the samples had predominantly obstructive disordered breathing events, and agreement for central vs obstructive events was not reported.30, 33 Thus, there is a glaring dearth of information on the concurrence between portable sleep monitoring and full polysomnography for detecting and classifying central respiratory events. The only study reporting on concordance for central respiratory events between portable sleep monitoring and full polysomnography in an in-laboratory setting demonstrated robust agreement between the two modalities, with an accuracy of 91.4% for the portable device.37 The study sample for the present investigation, which included patients with heart failure with reduced ejection fraction, was chosen to increase the likelihood of detecting central events and assessing agreement between the two diagnostic modalities for both obstructive and central sleep apnea in a complex environment—an inpatient setting at a tertiary care medical center. Thus, the current investigation challenges existing recommendations dissuading providers from the use of portable sleep monitoring for central sleep apnea or in hospitalized patients.26, 38 The results of this study also suggest that for most patients admitted with heart failure, portable sleep monitoring provides information for clinical decision making, whether a patient has obstructive, central, or both types of respiratory events. The ability to accurately and efficiently diagnose central and obstructive sleep apnea in patients with heart failure is of substantial value given that both central and obstructive sleep apnea have been found to independently predict heart failure–related readmissions39 and postdischarge mortality even at 36 months out.35 While there may be a propensity for longitudinal change in the severity and type of sleep apnea after convalescence, the low burden associated with respiratory polygraphy and the finding that it is reasonably comparable to the full polysomnography conducted in an inpatient setting support its use. Given that therapy with positive airway pressure seems to mitigate the risk of readmission21 and possibly mortality,35 associated with sleep apnea, early identification is of value.
Several strengths and weakness in the current study merit discussion. Strengths include the inclusion of both sexes, racial/ethnic minorities, patients with both reduced and preserved ejection fraction, and patients with both ischemic and nonischemic heart failure, all of which make the results more broadly applicable. Unique to the present study is the simultaneous collection of data from a portable monitor and full polysomnography in an inpatient setting. Finally, assessing agreement for central and obstructive sleep apnea addresses a major concern in the use of portable sleep monitoring—the reliable diagnosis of central sleep apnea by portable sleep monitoring. Limitations of the current study include the modest sample size, lack of event-by-event agreement, and stringent inclusion and exclusion criteria that limited generalizability of the findings. In addition, results from a specific monitoring device cannot be extrapolated to other portable monitoring devices, especially type 4 devices, as these latter devices typically do not include data on respiratory effort that are needed to differentiate obstructive and central respiratory events. Furthermore, there may be differences in the number and type of sensors between different devices. For example, some portable monitoring devices may utilize a pneumatic band while other manufacturers provide impedance plethysmography. However, the potential of limited generalizability does not hinder the internal validity of our results given that respiratory polygraphy was compared with the reference standard of polysomnography in a time-synchronized manner. Moreover, the current study helps fill existing knowledge gaps, addresses major concerns, and potentially expands the utility of portable sleep monitoring. More specifically, this study provides empirical evidence that a simple approach (ie, respiratory polygraphy) has value for diagnosing sleep apnea in heart failure by providing sufficient information which approximates the information that is derived from a more complex and expensive test (ie, a polysomnogram). Determination of specific therapy once a diagnosis is made should be based on the totality of clinical data and may require an in-laboratory assessment to determine the most optimal means for treatment. Finally, the results presented in this study could improve the designs of health care paradigms and treatment plans for sleep apnea in heart failure.
Acknowledgments
Author contributions: N. M. P. is the guarantor of the manuscript and has had full access to all of the data reported from the study and takes responsibility for the integrity and the accuracy of the data analysis. R. N. A. contributed to data collection, statistical analysis and interpretation, initial drafting of the manuscript, critical review for important intellectual content, and final approval of the manuscript. S. P. P. contributed to data collection, critical review for important intellectual content, and final approval of the manuscript. N. M. P. contributed to study conception and design, statistical analysis and interpretation, initial drafting of the manuscript, critical review for important intellectual content, and final approval of the manuscript.
Financial/nonfinancial disclosure: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Adarsh Menon, Alex Stryzak, and Greg Dennis for the acquisition and scoring of sleep recordings.
Footnotes
FUNDING/SUPPORT: Supported by the National Institutes of Health [Grant R01-HL075078, R01-HL117167, and K23-HL118414] and the American Sleep Medicine Foundation [113-SR-15].
References
- 1.Nieto F.J., Young T.B., Lind B.K. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor G.T., Caffo B., Newman A.B. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. doi: 10.1164/rccm.200712-1809OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard P.E., Young T., Palta M. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Nagayoshi M., Punjabi N.M., Selvin E. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med. 2016;25:156–161. doi: 10.1016/j.sleep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw J.E., Punjabi N.M., Wilding J.P. Sleep-disordered breathing and type 2 diabetes: a report from the International Diabetes Federation Taskforce on Epidemiology and Prevention. Diabetes Res Clin Pract. 2008;81(1):2–12. doi: 10.1016/j.diabres.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb D.J., Yenokyan G., Newman A.B. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somers V.K., White D.P., Amin R. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Arzt M., Young T., Finn L. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redline S., Yenokyan G., Gottlieb D.J. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaggi H.K., Concato J., Kernan W.N. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 11.Bitter T., Faber L., Hering D. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 12.Herrscher T.E., Akre H., Overland B. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Oldenburg O., Lamp B., Faber L. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9(3):251–257. doi: 10.1016/j.ejheart.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Kapur V., Strohl K.P., Redline S. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 15.Young T., Evans L., Finn L. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 16.Safadi A., Etzioni T., Fliss D. The Effect of the transition to home monitoring for the diagnosis of OSAS on test availability, waiting time, patients’ satisfaction, and outcome in a large health provider system. Sleep Disord. 2014;2014:418246. doi: 10.1155/2014/418246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiao W., Durr M.L. Trends in sleep studies performed for Medicare beneficiaries. Laryngoscope. 2017;127(12):2891–2896. doi: 10.1002/lary.26736. [DOI] [PubMed] [Google Scholar]
- 18.Kim R.D., Kapur V.K., Redline-Bruch J. An economic evaluation of home versus laboratory-based diagnosis of obstructive sleep apnea. Sleep. 2015;38(7):1027–1037. doi: 10.5665/sleep.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Parker J.D., Newton G.E. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625–1631. doi: 10.1016/j.jacc.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Kasai T., Narui K., Dohi T. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. 2008;133(3):690–696. doi: 10.1378/chest.07-1901. [DOI] [PubMed] [Google Scholar]
- 21.Kauta S.R., Keenan B.T., Goldberg L. Diagnosis and treatment of sleep disordered breathing in hospitalized cardiac patients: a reduction in 30-day hospital readmission rates. J Clin Sleep Med. 2014;10(10):1051–1059. doi: 10.5664/jcsm.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowie M.R., Woehrle H., Wegscheider K. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aurora R.N., Chowdhuri S., Ramar K. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arzt M., Floras J.S., Logan A.G. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP) Circulation. 2007;115(25):3173–3180. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 25.Javaheri S., Caref E.B., Chen E. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 26.Collop N.A., Anderson W.M., Boehlecke B., Portable Monitoring Task Force of the American Academy of Sleep Medicine Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 27.Berry R.B., Budhiraja R., Gottlieb D.J. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 29.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 30.Khayat R.N., Jarjoura D., Patt B. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagubadi S., Mehta R., Abdoh M. The accuracy of portable monitoring in diagnosing significant sleep disordered breathing in hospitalized patients. PLoS One. 2016;11(12):e0168073. doi: 10.1371/journal.pone.0168073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma S., Mather P., Efird J.T. Photoplethysmographic signal to screen sleep-disordered breathing in hospitalized heart failure patients: feasibility of a prospective clinical pathway. JACC Heart Fail. 2015;3(9):725–731. doi: 10.1016/j.jchf.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S., Mukhtar U., Kelly C. Recognition and treatment of sleep disordered breathing in obese hospitalized patients may improve survival: the HoSMed database. Am J Med. 2017;130(10):1184–1191. doi: 10.1016/j.amjmed.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 34.Sharma S., Mather P.J., Chowdhury A. Sleep Overnight Monitoring for Apnea in Patients Hospitalized with Heart Failure (SOMA-HF Study) J Clin Sleep Med. 2017;13(10):1185–1190. doi: 10.5664/jcsm.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khayat R., Jarjoura D., Porter K. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur Heart J. 2015;36(23):1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Povitz M., Kimoff R.J. Use of a level 3 portable monitor for the diagnosis and management of sleep disordered breathing in an inpatient tertiary care setting. Can Respir J. 2014;21(2):96–100. doi: 10.1155/2014/214943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinreich G., Armitstead J., Topfer V. Validation of ApneaLink as screening device for Cheyne-Stokes respiration. Sleep. 2009;32(4):553–557. doi: 10.1093/sleep/32.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur V.K., Auckley D.H., Chowdhuri S. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khayat R., Abraham W., Patt B. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail. 2012;18(7):534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]