Abstract
Typhoid fever is a life-threatening disease, but little is known about the molecular bases for its unique clinical presentation. Typhoid toxin, a unique virulence factor of Salmonella Typhi (the cause of typhoid fever), recapitulates in an animal model many symptoms of typhoid fever. Typhoid toxin binding to its glycan receptor Neu5Ac is central, but, due to the ubiquity of Neu5Ac, how typhoid toxin causes specific symptoms remains elusive. Here we show that typhoid toxin displays in vivo tropism to cells expressing multiantennal glycoprotein receptors, particularly on endothelial cells of arterioles in the brain and immune cells, which is in line with typhoid symptoms. Neu5Ac displayed by multiantennal N-glycans, rather than a single Neu5Ac, appears to serve as the high-affinity receptor, as typhoid toxin possesses five identical binding pockets per toxin. Human counterparts also express the multiantennal Neu5Ac receptor. Here we also show that mice immunized with inactive typhoid toxins and challenged with wild-type typhoid toxin presented neither the characteristic in vivo tropism nor symptoms. These mice were protected against a lethal-dose toxin challenge, but Ty21a-vaccinated mice were not. Cumulatively, these results reveal remarkable features describing how a bacterial exotoxin induces virulence exclusively in specific cells at the organismal level.
Salmonella enterica serovar Typhi (S. Typhi), the cause of typhoid fever, remains a major global health concern, resulting in more than 200,000 deaths annually1–3. Little is known about the molecular bases for its unique clinical presentation4–10. Currently available vaccines against S. Typhi offer incomplete protection11. Typhoid toxin is abundantly produced and secreted during S. Typhi infection of humans12–14. This toxin recapitulates many typhoid fever symptoms in mice, including leukopenia and signs of lethargy, malaise and stupor9. Both S. Typhi and typhoid toxin are adapted to humans, as reflected in the current lack of an optimal animal model to study typhoid toxin in the context of S. Typhi infection. However, C57BL/6 mice naturally express Neu5Ac, thus allowing for the recreation of key features of the typhoid fever disease. We administered highly purified typhoid toxin intravenously into mice to mimic the toxin in circulation9,15–18 (Fig. 1a). Typhoid toxin has an unusual A2B5 architecture consisting of two enzymatically active A subunits, CdtB (DNase) and PltA (mono ADP-ribosyl transferase), which are linked to a homopentamer of its receptor-binding B subunit, PltB (Fig. 1a)9. Each PltB possesses one binding pocket that recognizes Neu5Ac terminally located on glycans such as in the trisaccharide consensus (Fig. 2a), totalling five identical receptor-binding sites per toxin9 and potentially implying multivalent binding with multiantennal N-glycans. Unlike other bacterial AB toxins, which are constitutively expressed without infecting host cells, typhoid toxin is exclusively produced by intracellular S. Typhi within the infected cell19,20, implying that attenuated S. Typhi vaccine strains (for example, Ty21a) might not be effective in protecting against typhoid toxin. After being produced by intracellular S. Typhi, instead of directly entering into the cytoplasm of the infected cell and harming that cell, typhoid toxin traffics out from the Salmonella-containing vacuole (SCV) within the infected cell to the extracellular milieu21. Following export, the toxin in circulation can reach target cells by interacting with the Neu5Ac-terminated glycan receptor on the cell surface membrane9,15. However, the in vivo trafficking mechanisms of typhoid toxin to its target cells remain elusive. In light of the fact that the CdtB subunit of the typhoid toxin is almost identical to that of other cytolethal distending toxins (CDTs)9, it is crucial to understand the mechanism underlying the in vivo tropism of typhoid toxin to comprehend the unique pathogenesis of typhoid fever.
Fig. 1|. Typhoid toxin causes neurological complications associated with motor function deficits where both PltB and CdtB subunits play critical roles.
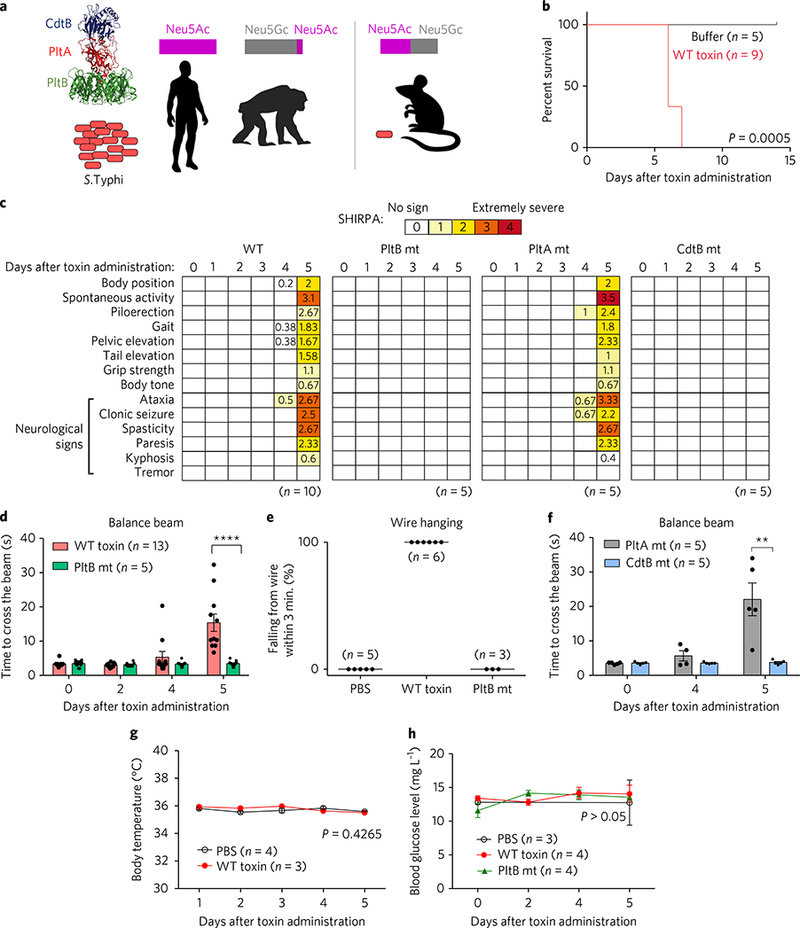
a, A schematic depicting the structure and organization of typhoid toxin, as well as the typhoid toxin receptor expression and S. Typhi permissiveness of humans and animal models. Humans and chimpanzees are permissive to S. Typhi but mice do not support S. Typhi infection. Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid. Magenta and grey bars represent approximate ratios of sialic acid expression in different hosts. b, Survival graph of mice administered typhoid toxin. Comparison of survival curves was performed with a log-rank test. P = 0.0005. c-f, A group of C57BL/6 mice were administered with indicated toxin preparations and analysed by comprehensive SHIRPA (c), balance beam (d,f) and wire hanging (e) tests. Impairments of motor functions began to be exhibited from day 4 after toxin administration (c,d,f). Average pathology scores are shown in the colour blocks. SHIRPA tests were performed on a daily basis. White rectangles represent a score of ‘0’ (no symptoms). Note severe neurological signs in SHIRPA by day 5 (c). Similarly, note the significantly delayed beam walking time (d,f) and lack of wire hanging capability (e) in the intoxicated mice with WT toxin or a PltAE133A catalytic mutant (mt) toxin. In contrast, neurological complications were not observed in mice administered either a PltBS35A-binding-defective mutant toxin or a CdtBH160Q catalytic mutant toxin. We also tried a fivefold higher dose of PltBS35A and CdtBH160Q mutants but this resulted in no neurological signs. Data were analysed by unpaired Student’s t-test. ****P< 0.0001, **P< 0.01. g,h, To examine whether there were fever and systemic metabolic changes in typhoid-toxin-administered mice, we determined body temperature (g) and blood glucose levels (h). We found no significant changes among mice administered the indicated toxin preparations. Bars represent means±.e.m. (d,f-h). At least three independent experiments were performed for b-g. For h, two independent experiments were performed.
Fig. 2|. Typhoid toxin has in vivo tropism to endothelial cells of arterioles in the brain and to immune cells.
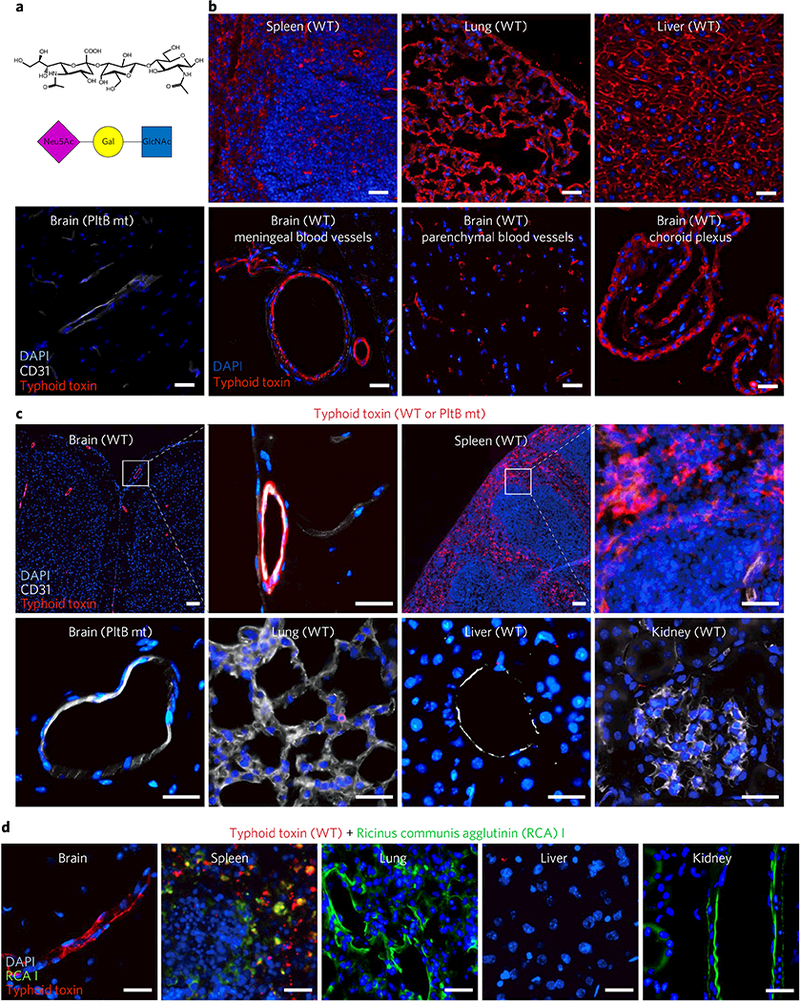
a, A schematic showing the trisaccharide consensus recognized by typhoid toxin PltB9–15. Neu5Ac, N-acetylneuraminic acid; Gal, galactose; GlcNAc, N-acetylglucosamine. b, Visualization of the expression of the typhoid toxin glycan receptor (red). To investigate receptor expression, the indicated mouse tissue sections of untreated C57BL/6 mice were probed for Alexa555-conjugated typhoid toxin (n = 3) or Alexa555-conjugated PltB-binding-defective mutant (n = 3). Scale bar, 100 μm. These results indicate that the glycan receptor for typhoid toxin is ubiquitously expressed in the tissue sections tested. Images shown are representative of 30 images in at least three independent experiments. c, Alexa555-conjugated typhoid toxin (red), either WT toxin (n = 5) or a PltB binding defective mutant toxin (n = 3), was systemically administered into live C57BL/6 mice and toxin localization in various tissues was investigated 2 h after administration. Typhoid toxin localized in the brain and the spleen but did not localize in lung, liver and kidney. CD31 is a specific cell surface marker for endothelial cells, while DAPI stains DNA. A PltB mutant toxin conjugated to Alexa555 was defective for the binding to the brain endothelial cells, indicating toxin signal is specific. Scale bar, 100 μm. d, Ricinus communis agglutinin I (RCAI) conjugated to FITC (green) that has binding specificity to terminal galactose was co-administered along with typhoid toxin-Alexa555 into live C57BL/6 mice (n = 2). RCAI localized in spleen, lung and kidney but not in brain and liver, supporting the specific in vivo trafficking of typhoid toxin. Images shown are representative of 30 images in at least two independent experiments. Scale bars, 100 μm.
To better understand the in vivo function of typhoid toxin, we performed the clinical scoring SHIRPA test22,23, following toxin administration into mice. Consistent with our previous observation, administration of the toxin did not lead to the development of fever, but caused weight loss, a decrease in the number of circulating white blood cells, and a near-complete depletion of neutrophils9. Intoxicated mice also showed signs of lethargy, malaise and stupor—symptoms associated with the acute phase of typhoid fever—and the mice ultimately died by day 6 after administration (Fig. 1b)9. By performing SHIRPA tests, we were able to objectively evaluate typhoid symptoms previously overlooked, such as serious impairment of motor functions by days 4.5–5 after administration of wild-type (WT) toxin, which further developed to clonic seizure by days 5–6 (Fig. 1c and Supplementary Videos 1 and 2). Mice dosed with a PltA catalytic mutant showed results comparable to those of WT toxin. However, the motor function deficits were not observed in mice administered with a PltB binding-defective mutant or a CdtB catalytic mutant toxin, indicating that both the host receptor-binding capability (PltB) and the nuclease activity (CdtB) of typhoid toxin are required to cause these neurological complications (Fig. 1c), agreeing with observations in our previous report concerning different symptoms9. Balance beam and wire-hanging tests further demonstrated that PltB and CdtB are essential to cause motor function deficits (Fig. 1d-f and Supplementary Videos 3–8). Toxin administration into TLR4 null mice resulted in a comparable susceptibility to typhoid toxin, confirming that the purified typhoid toxin is free of lipopolysaccharide (LPS) contamination, and that our results are bona fide toxin effects (Supplementary Fig. 1). We also noted a lack of fever and systemic metabolic changes, as measured by the body temperature and blood glucose levels of the mice (Fig. 1g,h). Moreover, to investigate the specificity of these neurological signs, we infected C57BL/6 mice orally with a lethal dose of S. Typhimurium, which does not produce typhoid toxin as a control. The infected mice showed severe clinical signs, including hunched posture, lethargy and neutrophilia, and died by 6 days after infection, but did not show any neurological signs (measured by SHIRPA and balance beam tests) or neutropenia signs (Supplementary Fig. 2 and Supplementary Video 9).
Based on these and previous findings9, we hypothesized that direct trafficking of typhoid toxin to the brain and to immune cells may be responsible for clinical signs related to the central nervous system and the immune system. To investigate this hypothesis, we prepared typhoid toxin conjugated via amine coupling to Alexa555, dosed mice with the fluorescent-labelled toxin, analysed its localization, and found toxin on the endothelial cells of arterioles in the brain and immune cells (Fig. 2c and Supplementary Fig. 3a-g). To rule out the possibility that this in vivo tropism is simply due to the difference in Neu5Ac/Neu5Gc presentation in different cells and tissues of mice, we performed the same experiment using CMAH null mice and found comparable in vivo tropism of typhoid toxin (Supplementary Fig. 4). Cytidine monophosphate-N-acetylneur-aminic acid hydroxylase (CMAH) converts typhoid toxin receptor Neu5Ac to non-toxin-binding Neu5Gc in other mammals such as chimpanzees; thus, CMAH null mice exclusively express Neu5Ac, as in humans (CMAH is a pseudogene in humans)15·16. Moreover, a PltB-binding defective mutant toxin administered into mice was not seen in any tissues examined, indicating that the toxin signal is specific (Fig. 2b,c). Strikingly, in contrast to the endothelial cells of arterioles in the brain, typhoid toxin administered into live mice did not localize in the endothelial cells of blood vessels of other organs, such as lung, liver and kidney, in live mice (Fig. 2c). However, in agreement with the ubiquity of Neu5Ac, endothelial cells of those tissues highly express Neu5Ac, as demonstrated by strong typhoid toxin binding to the tissue sections of lung and liver from untreated mice (Fig. 2b). In support of this distinct in vivo tropism of typhoid toxin, directed by PltB binding to the glycan receptor terminated with Neu5Ac, we did not observe ricinus communis agglutinin I (RCA I, which has binding specificity to terminal galactose24) in the brain. To observe this we co-administered RCA I-FITC and typhoid toxin-Alexa555 simultaneously into live mice (Fig. 2d). Despite the abundant expression of glycan terminated with galactose in the mouse brain (Supplementary Fig. 5), RCA I did not interact with the brain in this co-administration model. This indicates that typhoid toxin’s localization in the brain is not due to non-specific damage/leakage of the brain endothelium, because this leakage would enable RCA I-FITC to reach into the brain (Fig. 2d). Furthermore, the localization of RCA I-FITC in the spleen, lung and kidney, but excluding the brain, supports the conclusion that different toxins have their unique B-subunit-mediated in vivo tropisms. Cumulatively, these findings demonstrate that typhoid toxin has an in vivo tropism to the endothelial cells of arterioles in the brain and to immune cells, which is in good agreement with the typhoid toxin-mediated symptoms. However, as Neu5Ac is ubiquitously expressed across many tissues and cell types, the underlying molecular mechanism behind this tropism remains a conundrum.
Based on these findings and our previous glycan microarray screening studies9, we hypothesized that endothelial cells of arterioles in the brain and immune cells display Neu5Ac primarily in the context of multiantennal N-glycans, and thus probably serve as a higher-affinity multivalent binder, as opposed to the lower-affinity interaction with glycans displaying a single Neu5Ac (Fig. 3a). To investigate this hypothesis, we explored several glycan-binding proteins known as lectins, whose preferred glycan-binding specificity has been characterized. For instance, Maackia amurensis lectin I (MAL-I) binds preferentially to the trisaccharide consisting of Neu5Ac-α2–3-galactose-ß1–4-N-acetylglucosamine, whereas MAL-II prefers receptors displaying Neu5Ac-α2–3-galactose-ß 3-N-acetylgalactosamine, and Sambucus nigra bark lectin (SNA) exhibits a preferred recognition for receptors that are Neu5Ac α 2–6-linked to galactose (Fig. 3a and Supplementary Fig. 6)25. In contrast, Phaseolus vulgaris (PHA)-E and -L preferentially bind multiantennal N-glycans—the internal part of multiantennal N-glycans—instead of recognizing terminal Neu5Ac (Fig. 3a). Thus, unlike typhoid toxin, PHA lectins can bind both sialylated multiantennal N-glycans and their asialylated counterparts (although less commonly in this multiantennal form), implying that PHA lectins are one of the best tools available to test our hypothesis, albeit with the expectation of some variation. We found that PHA lectins largely phenocopied typhoid toxin, while other lectins did not (Fig. 3a and Supplementary Fig. 6). PHA lectins also showed weak binding to the endothelia of lung and kidney (Supplementary Fig. 6), which presumably represents the expression of asialylated multiantennal N-glycans on those cells. These results indicate that Neu5Ac on the endothelial cells of arterioles in the brain and immune cells is displayed largely in the context of the multiantennal N-glycans and primarily with an α2–3 configuration linked to the underlying glycans.
Fig. 3|. Typhoid toxin binds preferentially to Neu5Ac displayed in the context of multiantennal N-glycans over linear N-glycans.
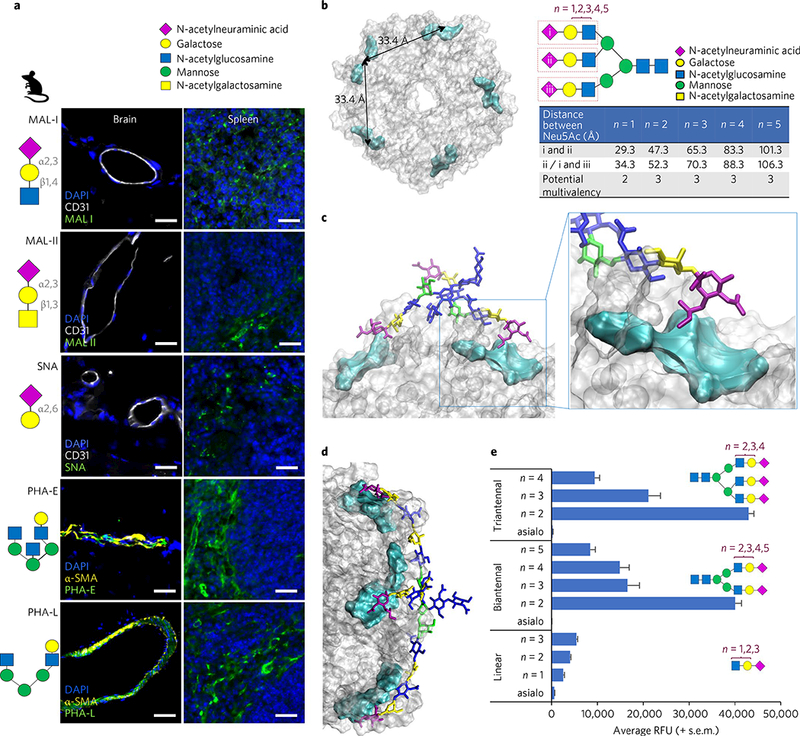
Glycan symbol and colour coding recommended by the Consortium for Functional Glycomics are used. a, Glycan binding preferences of five plant lectins, indicated schematically. Unlike the others, PHA-E and -L lectins phenocopied the in vivo tropism of typhoid toxin, indicating that endothelial cells of arterioles in the brain and immune cells express multiantennal N-glycans. Shown are representative images of at least two independent experiments. Mouse numbers used are: Mal-I (n = 2), Mal-II (n = 2), SNA (n = 2), PHA-E (n = 4) and PHA-L (n = 4). Scale bars, 100 μm. b, Top view of the PltB homopentamer. Cyan: location of the receptor binding pocket in the PltB subunits. The two adjacent binding pockets are separated by 33.4 Å. The distance is measured between Neu5Acs of the triantennal glycan with LacNAc sialosides (n = 1,2,3,4,5). In the schematic depicted in b, triantennal N-glycan contains the trisaccharide consensus recognized by typhoid toxin (red rectangles)9. n = 1,2,3,4,5, elongated forms of N-acetyllactosamine (LacNAc; galactose β1–4 linked to N-acetylglucosamine). c,d, Depiction of the mono-LacNAc sialoside interacting with two receptor binding sites within a single homopentamer (c), while the bi-LacNAc sialoside interacts with three receptor binding sites (d). e, Glycan microarray indicating that triantennal, bi-LacNAc sialoside (no. 61, the 61st glucan in the microarray that contains 130 glycans) shows the strongest binding to typhoid toxin (see Supplementary Fig. 7 for the results for all 130 glycans). These results also imply that sialosides with the unnecessarily long extension of LacNAc are unlikely to be favourable for typhoid toxin binding. Glycans shown are linear glycans (asialo (glycan with no terminal Neu5Ac) (no. 1), n = 1 (no. 17), n = 2 (no. 18), n = 3 (no. 19)), biantennal glycans (asialo (no. 7), n = 2 (no. 55), n = 3 (no. 56), n = 4 (no. 57), n = 5 (no. 58)) and triantennal glycans (asialo (no. 9), n = 2 (no. 61), n = 3 (no. 62), n = 4 (no. 63)). Data presented as mean±s.e.m.
To further support our hypothesis we conducted computational simulations, and found that two branches of N-glycans can bridge the binding sites of two protomers on the same PltB pentamer (Fig. 3c), which are separated by 33.4 À (Fig. 3b). Elongated forms of multiantennal N-glycans are also found in human cells, where N-acetyllactosamine (LacNAc; galactose β1–4 linked to N-acetylglcosamine, Fig. 3a) can be repeated multiple times26. Next, to investigate whether three PltB subunits could simultaneously bind three Neu5Acs in the triantennal glycan, we calculated and summarized the distance between Neu5Acs, which indicated that three PltB subunits of a single pentamer could recognize all three Neu5Acs at once when the repeat of LacNAc is more than two (Fig. 3b). Both the simulation and experimental glycan microarray results support the prediction (Fig. 3d,e). In this customized glycan microarray, typhoid toxin showed the highest affinity to triantennal extended (n = 2) N-glycans, with Neu5Ac α2–3-linked to its underlying glycans, among 130 glycans tested (Fig. 3e, Supplementary Figs. 7 and 8 and Supplementary Table 5). Next, to investigate whether human brain endothelial cells of arterioles and immune cells also express the multiantennal glycan receptor of typhoid toxin, we carried out a human tissue microarray and found that human counterparts also express multiantennal N-glycans (Supplementary Fig. 9). We quantified the human brain tissue microarray results, which indeed demonstrated that 100% of the α-smooth muscle actin (αSMA)+ vessels (arterioles) were stained with both PHA lectins and typhoid toxin (table in Supplementary Fig. 9). Cumulatively, these results indicate that typhoid toxin has an in vivo tropism to cells expressing multiantennal N-glycan receptors terminated with Neu5Ac with an α2–3 configuration linked to its underlying glycans, and this tropism probably also occurs in humans.
Finally, we also investigated whether the S. Typhi Ty21a vaccine strain11,27 can protect against typhoid toxin-associated in vivo tropism and clinical signs. S. Typhi Ty21a is an auxotrophic mutant of S. Typhi Ty227, which is weakly invasive but unable to survive within host cells. We thus hypothesized that S. Typhi Ty21a poorly expresses antigens that are exclusively produced by the S. Typhi-infected cell, such as typhoid toxin. In agreement with this hypothesis, we found that S. Typhi Ty21a has poor ability to both infect and survive within the cells (Fig. 4a,b). Consistently, infection of cells with S. Typhi Ty21a did not induce G2 cell cycle arrest, a readout of typhoid toxin intoxication in host cells (Fig. 4c). Due to the attenuation that S. Typhi Ty21a presents, we used 20-fold higher multiplicity of infection (MOI) for Ty21a than for WT S. Typhi (Fig. 4a-c). Next, to investigate the vaccine potential of genetically engineered inactive typhoid toxin (toxoid) and to further validate the causality between typhoid toxin and its in vivo tropism as well as related clinical signs, we immunized mice with typhoid toxoid via a standard prime-boost regimen. Prominent antibody titres were detected after immunization (Fig. 4d). Next, to investigate whether the antibodies can neutralize the toxicity of typhoid toxin, we performed cell cycle analysis of human epithelial cells that were treated with purified WT toxin or infected with S. Typhi that secretes typhoid toxin during infection, and we found that addition of the immunized mouse sera resulted in the absence of G2/M cell cycle arrest (Fig. 4e,f). In in vivo protection assays, where we challenged those immunized mice with a lethal dose of typhoid toxin, we found that the immunized mice were completely protected from typhoid toxin-mediated effects (for example, lethality, weight loss, neurological symptoms and immune cell depletion; Fig. 4g-j and Supplementary Video 10). To develop a more simple vaccine platform, we investigated whether the PltB homopentamer was equally sufficient for protection and found it was (Fig. 4d,g-j). Consistent with the absence of neurological signs in vaccinated and toxin-challenged mice (Fig. 4i), we found that typhoid toxin failed to bind to the endothelial cells of arterioles in those mouse brains (Fig. 4k and Supplementary Fig. 11). In contrast, mice immunized with S. Typhi Ty21a showed no or little humoral immune responses to typhoid toxin (Supplementary Fig. 10) and subsequently exhibited typhoid toxin-mediated in vivo tropism and clinical signs (Fig. 4g-k), leading to eventual failure to protect mice against a lethal dose of typhoid toxin challenge. Cumulatively, these results indicate that S. Typhi strain Ty21a induces efficacious protective immunity against the bacterium, but not against typhoid toxin, and support the vaccine potential of typhoid toxoid to prevent typhoid toxin-mediated in vivo tropism and clinical signs. Of note, WT S. Typhi infection in human volunteers showed marginal protection against re-infection10, which seemingly contradicts the abundant antibody titres against typhoid toxin in the sera of convalescent patients who have recovered from S. Typhi infection in typhoid-endemic regions12–14. Human volunteer studies, which comply with stringent regulations, are often interrupted early before the full development of infection and disease. In contrast, typhoid convalescent patients in typhoid-endemic regions probably experienced the disease without interruption and were exposed to S. Typhi multiple times, which is in agreement with our findings regarding the need for boosting to observe increased antibody titres. Moreover, unlike inactive toxoids, active typhoid toxin produced by WT S. Typhi during infection in human volunteers probably altered the immune cell functions required for significant antibody production.
Fig. 4|. Vaccination of naive mice with genetically engineered inactive typhoid toxin or PltB subunit alone completely protects the mice against a lethal-dose toxin challenge.
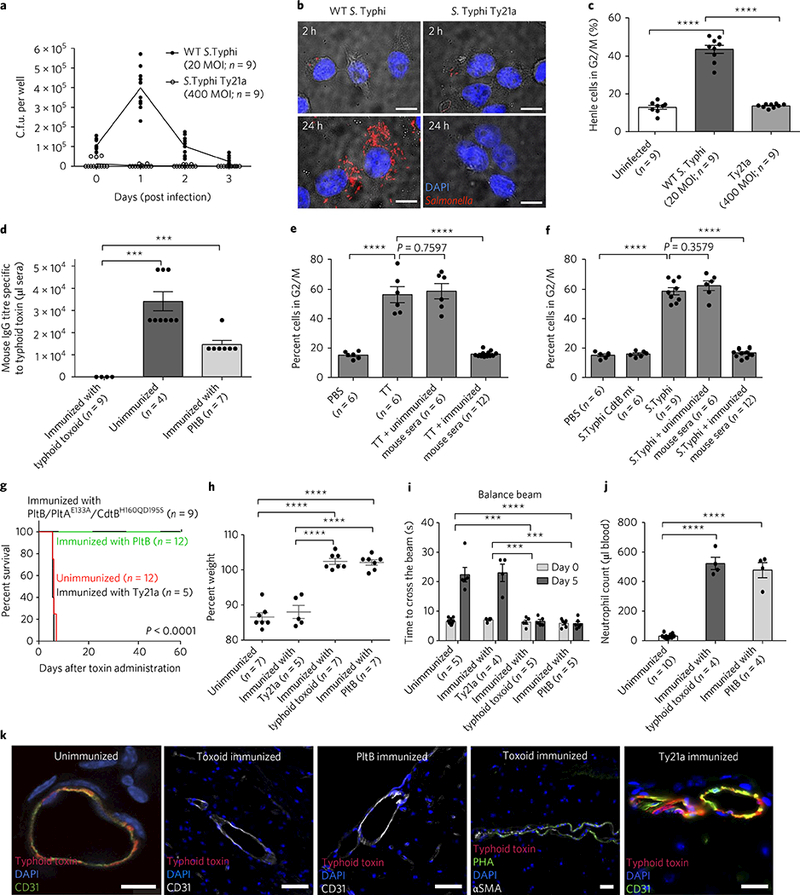
a-c, Determination of intracellular S. Typhi c.f.u. at indicated time points by plating (a) or fluorescent microscopy (b), and evaluation of typhoid-toxin-mediated toxicity of infected cells by flow cytometric cell cycle analysis 3 days after infection (c). The results indicate the limitations of S. Typhi Ty21a in producing typhoid toxin. Mice were immunized with typhoid toxoid, PltB pentamer or S. Typhi Ty21a, and PBS was used for the unimmunized control group. d, Relative serum antibody levels were determined by standard endpoint ELISA titration. e,f, Evaluation of typhoid-toxin-mediated toxicity (G2/M cell cycle arrest) in immunized sera-treated human cells. TT, WT typhoid toxin. In f, S. Typhi CdtB catalytic mutant (producing CdtB mutant typhoid toxin during infection, an experimental control) or WT S. Typhi (producing WT typhoid toxin) was used as a source of typhoid toxin. g-j, Both immunization strategies— with either typhoid toxoid (all three subunits) or PltB alone—efficiently protected mice against a lethal-dose typhoid toxin challenge, as shown by survival (g), no weight loss (h), no neurological complications (i) and no immune-cell-associated symptoms (j). In contrast, Ty21a did not protect mice against a typhoid toxin challenge. Antibody titres of Ty21a-immunized mouse sera are shown in Supplementary Fig. 10. k, Brain images of vaccinated and toxin-challenged mice. Typhoid toxin’s in vivo tropism to the brain was observed in both Ty21a-vaccinated mice (n = 5) and PBS-treated mice (n = 3), but not typhoid toxoid- or PltB subunit-vaccinated mice (n = 3 each) (see Supplementary Fig. 11 for a quantitative analysis of images). Note that immunized mice expressed multiantennal N-glycans (PHA+): CD31 for endothelial cells (green or white); PHA lectins for multiantennal N-glycans; αSMA for smooth muscle cells; DAPI for DNA. Images shown in b and k are representative of the finding. Scale bars, 10μm (b) and 100μm (k). Unpaired Student’s f-test was used for c-f and h-j (***P< 0.001, ****p< 0.0001) and a low-rank test for g.
Overall, our results present a remarkable feature of an exotoxin where virulence is induced exclusively in selected cells at the organ-ismal level. Our results provide insights and may help the development of potential prophylactics and therapeutics to prevent and treat typhoid fever.
Methods
Bacterial strains, plasmids, mammalian cells and other materials.
WT Salmonella enterica serovar Typhi strain ISP2825 has been described previously28. S. Typhi Ty21a vaccine strain was purchased from ATCC. All the plasmids used in this study are listed in Supplementary Table 1. For infection experiments, S. Typhi strains were grown at 37 °C in 2 ml LB broth containing 0.3 M NaCl to an optical density at 600 nm (OD600) of ~0.9 after inoculation from overnight cultures at a dilution of 1:50. Human intestinal epithelial Henle-407 cells were cultured at 37 °C under an atmosphere of 5% CO2, using DMEM (Invitrogen) supplemented with 10% FBS (Atlanta Biologicals). Plant lectins were purchased from Vector Laboratories (Supplementary Table 3). Human tissue microarray slides were purchased from US Biomax.
Authentication of key biological resources.
Newly prepared typhoid toxin was validated via multiple criteria including size-exclusion chromatography, SDS-PAGE and western blot. In addition to these methods, the subunits of the toxin preparation were validated by mass spectrometry. All the required subunit mutants were built on the same backbone, for which protein products were previously validated via mass spectrometry. We used a very sensitive Limulus amebocyte lysate assay to validate that the toxin preparation was free from LPS endotoxin contamination. Salmonella strains were genotyped via a PCR-based assay before and after the completion of experiments. We used primer sets to amplify typhoid toxin gene plt A for S. Typhi strains ISP2825, Ty2 and Ty21a and Type 3 secretion system genes (avrA, spvB and sspH1) for S. Typhimurium SL1344. The PCR products were further sequence-confirmed. A mycoplasma test was carried out regularly to confirm that all of the cells were free of mycoplasma contamination. To validate Henle-407 cells, we used the cell surface markers Podxl-1 and/or intestinal alkaline phosphatase (IAP) for Henle-407 cells. Breeding pairs of mice were originally purchased from the Jackson Laboratory, and newborn knockout mice were genotyped, with primer sets posted on the Jackson Laboratory website, before and after the completion of experiments.
Typhoid toxin expression and purification.
Expression and purification of His6-epitope-tagged typhoid toxin were carried out as previously described9. Briefly, Escherichia coli strains carrying the different plasmids were grown to an OD600 of 0.6–0.7 at 37 °C; expression of typhoid toxin was subsequently induced by the addition of 0.5 mM IPTG and induced cultures were incubated overnight at 29 °C. Bacterial cells were pelleted by centrifugation and bacterial cells were resuspended in a buffer containing 15 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mg ml−1 lysozyme, 10 μg ml−1 DNase and 1× protease inhibitor cocktail (Roche), lysed via sonication, pelleted and affinity-purified using a Nickel-resin (Qiagen) according to the vendor’s recommendation. The eluates were diluted in 20 mM MES, pH 6.0, buffer and loaded onto a Hi-trap SP ion-exchange column. Fractions from the ion-exchange chromatography were monitored by SDS-PAGE, concentrated, and further purified using a Superdex 200 column. Final fractions were examined for purity on 15% SDS-PAGE. In the case of PltB pentamer purification, the eluates were diluted in 15 mM Tris-HCl, pH 8.0 buffer and loaded onto a Hi-trap Q column ion-exchange column, followed by size-exclusion chromatography using a Superdex 200 column. Purified toxin preparations were further subjected to Pierce high-capacity endotoxin removal resin (Thermo Scientific). LPS levels of all of the toxin preparations were determined using a ToxinSensor chromogenic LAL endotoxin assay kit (GenScipt; <0.2 EU ml−1).
Alexa Fluor-555 typhoid toxin labelling.
Indicated typhoid toxin preparations were fluorescently labelled with Alexa Fluor-555 dye (Thermo Fisher) according to the vendor’s recommendation. Alexa Fluor-555 dye has a succinimidyl ester moiety that reacts with primary amines of proteins to form stable dye-protein conjugates. Purified toxin preparations (0.5 mg ml−1) were incubated with reactive dye for 1 h at room temperature and applied to a size-exclusion chromatography column provided by the vendor to separate the dye-protein conjugates from free dye. The degree of labelling was determined by measuring the absorbance of the conjugate solution at 280 nm and 555 nm, which yielded the toxin labelling degree with 4–6 dye/holotoxin ratios. The typhoid holotoxin’s predicted extinction coefficient is 191,400 M−1 cm−1.
Mouse experiments.
All animal experiments were conducted according to protocols approved by Cornell University’s Institutional Animal Care and Use Committee. Age- and sex-matched 5- to 8-week-old WT C57BL/6, Cmah−/− or Tlr4−/− mice were randomly allocated to each group, and numbers of animals per group were estimated to obtain statistically significant data after a pilot experiment to obtain a sense of the potential variability. Groups of mice were injected with 100 μl solutions containing PBS buffer alone, or 2 μg of the indicated purified toxin preparations (endotoxin-free) via retro-orbital injections. Changes in the behaviour, weight and survival of the toxin-injected mice were closely monitored. The mice used in this study were originally purchased from the Jackson laboratory and bred in a vivarium in the animal facility at Cornell University. All the knockout mice used were genotyped before and after the experiments. When indicated, C57BL/6 mice were orally injected with 100 μl of bicarbonate buffer to neutralize stomach acid and then infected with 107 S. Typhimurium using a gavage needle. For immunization studies, C57BL/6 mice were immunized by subcutaneous injections of 2 μg genetically engineered inactive typhoid toxin (typhoid toxoid) or PltB pentamer, followed by a boost injection after two weeks. When indicated, the immunized mice were challenged with WT toxin two weeks after the boost. To investigate whether typhoid toxin localization in the brain is associated with astrocytes, we used S100beta-EGFP transgenic mice (Jackson Laboratory). All mice experiments were conducted in a double-blind manner.
Motor function tests.
Modified SHIRPA test.
The mice were subjected to a series of behavioural evaluations based on a modified SHIRPA protocol (European Mouse Phenotyping Resource of Standardized Screens) as described previously22,23. To minimize bias, double-blind end-point assessment was applied to examine the behaviour of the animals after toxin administration. Each of the tests generated an observational rating score to record the neurological and physiological deficits. Briefly, body position was assessed qualitatively by monitoring the mice in a viewing jar, and was recorded as completely flat (4), lying on side (3), lying prone (2), sitting or standing (1) and rearing on hind legs (0). An assessment of gait was scored on a scale from incapacity (4), to limited movement only (2.7), fluid but abnormal (1.3) and normal (0). Pelvic elevation was used as the measure of hind limb integrity and was rated on a scale from markedly flattened (4), to barely touched (2) and normal as ~3 mm elevation (0). Other observable neuromuscular signs include ataxia (unsteady walk or a tendency to stumble while walking), clonic seizure (limbic or facial muscle twitching), spasticity (involuntary tail or back muscle spasms with sustained muscle contraction toward unusual orientation), paresis (lying or crawling in the cage) and thoracic kyphosis (abnormally excessive curvature of the spine). Each score was converted to a colour block corresponding to the severity of the symptoms. Average scores from three independent determinations were written in the colour block.
Balance beam test.
Before the tests, mice were trained to walk along a ruler (80 cm long, 3 cm wide), elevated 30 cm above the bench, to a goal box. Multiple sponges were placed below the beam to cushion any falls. After the mice had been trained to walk on a ruler, they were encouraged to walk on the balance beam. At least three trials were performed on each mouse until they learned to cross rapidly from the beginning point to the finish point without any additional encouragement. After this behaviour was stable, we then started the test and data collection. During the test, the mice were placed on the beam at one end and allowed to walk to the goal box. The time to cross the beam and video was recorded for each mouse. Each mouse participated in three trials, with 1 min recovery period between trials. If the mice were unable to cross, or fell from the beam, a score of 60 s was given and no further test was performed.
Wire hanging test.
The mice were held by their tails and allowed to grip the middle part of the wire with their fore limbs. The tail was then released while the mouse was still grasping the wire with its front paws. The timer was started upon release. The latency to fall was recorded. The maximum time per trial was set to 180 s.
Blood neutrophil counts and blood glucose level measurements.
Blood samples were collected by submandibular bleeding in Microtainer tubes coated with EDTA as an anticoagulant (BD), kept at room temperature, and analysed within 2 h after blood collection using a Hemavet 950FS haematology analyser (Drew Scientific) or a haematology analyser (ADVIA 2120) in the Animal Health Diagnostic Center at Cornell University. Blood glucose levels were measured using a Bayer Contour Next EZ glucose meter on a daily basis (Bayer).
Frozen tissue preparation.
The mice were anaesthetized with isoflurane, followed by sacrifice-perfusion, which was conducted by sequentially administering 10% sucrose and 4% paraformaldehyde using a pressure-controlled Perfusion One system (Leica Biosystems) at indicated time points. After perfusion, the mice brain, lung, spleen, liver and kidney tissues were extracted and fixed in 4% paraformaldehyde for 24 h at 4 °C. Tissues were washed with PBS and immersed in 30% sucrose solution overnight for cryoprotection. The brain tissue was trimmed into coronal sections and placed in cassettes for Tissue-Tek OCT (optimum cutting temperature) embedding. The embedded tissues were flash-frozen in isopentane cooled to −80 °C. Cryosections of frozen tissue samples were cut to be 8 μm thick and stored in −80 °C until staining.
Immunofluorescent staining.
The frozen tissue sections were fixed with 4% paraformaldehyde for 10 min, washed with PBS and blocked in 3% BSA/PBS for 30 min. The slides were incubated with primary antibodies to detect CD31 (BD bioscience, clone MEC 13.3), α-SMA (Abcam), CD45 (eBioscience, clone 30-F11), Iba-1(Abcam) or PDGF receptor beta (R&D Systems) for 1 h at room temperature or overnight at 4 °C. Then sections were washed with PBS and incubated with fluorochrome-conjugated secondary antibodies (Molecular Probes) for 1 h at room temperature in the dark. The nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) and the slides were mounted in ProLong antifade mounting solution (Molecular Probes). Digital photomicrographs were taken using a Leica DMI6000B/DFC340 FX fluorescence microscope system, or a Leica TCS SP5 confocal laser scanning microscope.
Mammalian cell intoxication assay.
Cell-cycle arrest after typhoid toxin intoxication was examined by flow cytometry as previously described9,15. Briefly, after treatment with His6-tagged typhoid toxin or bacterial infection for different time points (as indicated), cells were trypsinized, collected, washed, and fixed overnight at −20 °C in ~70% ethanol/PBS. Fixed cells were washed with PBS and resuspended in 500 μl PBS containing 50 μgml−1 propidium iodide, 0.1 mg ml−1 RNase A and 0.05% Triton X-100. After incubation for 40 min at 37 °C, cells were washed with PBS, resuspended in 500 μl PBS, filtered, and analysed by flow cytometry. DNA contents of cells were determined using FlowJo software (Treestar).
Bacterial infections and colony-forming unit assay.
Human intestinal epithelial Henle-407 cells were infected with WT S. Typhi (MOI of 20), or S. Typhi Ty21a (MOI of 400) for 1 h, treated with 100 μg ml−1 gentamicin (to remove all extracellular bacteria) for 45 min, washed with PBS. Infected cells were then incubated in complete cell culture medium containing 10 μgml−1 gentamicin for indicated durations, and infected cells were lysed by incubating the cells in 1 ml of sterile PBS containing 0.1% sodium deoxycholate for 15 min at room temperature. Bacterial numbers were determined by plating tenfold serial dilutions of homogenates on LB agar plates and incubating them overnight at 37 °C. Colonies were counted and the number of total colony-forming units (c.f.u.) recovered was calculated. The colonies were validated by conducting a PCR-based genotyping assay.
Measurement of antibody responses specific to Salmonella or typhoid toxin.
Antibody levels in the sera of immunized mice were examined using a direct enzyme-linked immunosorbent assay (ELISA). ELISA plates were coated with a Salmonella total cell lysate prepared as follows. S. Typhi Ty21a was cultured, heat-killed at 70 °C for 20 min, centrifuged, and resuspended in 50 mM Tris-HCl buffer, pH 8.0 containing 1% Triton X-100 and a protease inhibitor cocktail (Roche). The bacterial cells were then sonicated, and unbroken cells were removed by centrifugation; 96-well plates (Maxisorp, Nunc) were coated with 1 μg bacterial cell lysates or 50 ng purified typhoid toxoid in 100 μl plate-coating buffer (50 mM carbonate-bicarbonate buffer, pH 9.6), and incubated overnight at 4 °C. Wells were washed with PBS containing 0.05% Tween 20 and blocked with PBS containing 1% BSA for 1 h at 37 °C. A twofold serial dilution of the sera contained in 50 μl PBS/0.05% Tween 20/0.5% BSA was added to each well and incubated for 2 h at 37 °C. After washing, bound antibodies were detected with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin IgG (Southern Biotech) at a 1:10,000 dilution in PBS/0.05% Tween/0.5% BSA. Wells were then incubated with an HRP substrate, tetramethylbenzidine (Sigma), for 10–30 min, and the reaction was stopped by addition of 100 μl of 1 M H3PO4. Relative serum antibody levels were determined by standard endpoint ELISA titration.
Image acquisition, equipment and settings.
Immunofluorescent images were acquired with a Leica DMI6000B/DFC340 FX fluorescence microscope system. For imaging of typhoid toxin and other lectin distributions in the tissue, 1,600×1,200-pixel full-frame pictures of various channels were recorded as 16-bit TIFF files with ×20 (numerical aperture (NA) 0.5) or ×40 (NA 0.75) objectives. The filter wavelengths were as follows: Alexa Fluor-488 (plant lectins, CD31): excitation filter 470/40 nm, emission filter 525/50 nm; Alexa Fluor-555 (typhoid toxin): excitation filter 545/30 nm, emission filter 610/75 nm; DAPI: excitation filter 340–380 nm, emission filter 425 nm. Recorded images were merged using the Image J (NIH) merge channels function. Confocal images were acquired with a Leica TCS SP5 confocal laser-scanning microscope. For imaging of typhoid toxin localization in the cells of the blood-brain barrier, z-stacks of 1,024-pixel-dimension pictures of the typhoid toxin, CD31, α-SMA and DAPI channels were recorded as RGB TIFF files using 12 slices of ~1 μm thickness with a ×63 (NA 1.4) objective. The fluorochrome settings were as follows: Alexa Fluor-488 (CD31): excited by 488 nm argon laser, emission wavelength 499 nm; Alexa Fluor-555 (typhoid toxin): excited by 561 nm mercury-arc lamp, emission wavelength 568 nm; Alexa fluor-633 (α-SMA): excited by 633 He-Ne laser; emission wavelength 648 nm; DAPI: excited by 345 UV laser, emission wavelength 455 nm. Recorded images were processed further with Adobe Photoshop to adjust the brightness and contrast equally for all recordings. Image stacks were merged to create a four-channel RGB stack. Stacks were subjected to orthogonal view using the Image J stack function.
Simulation.
All simulations were performed in the laboratory of R. Nussinov using the high-performance computational facilities of the Biowulf PC/Linux cluster at the National Institutes of Health, Bethesda, MD.
System preparation.
Initial conformations for the triantennal N-glycan depicted in Fig. 3d were built and energy-minimized using GLYCAM-Web (www.glycam.org). The initial conformation for Neu5Ac, the PltB pentamer and the Neu5Ac-PltB pentamer complex were obtained from the crystal structure of 4RHR15. The missing residues according to the pdb file were rebuilt and modelled using the Swiss model server29. To build the triantennal N-glycan-PltB complex, we first built a complex between the PltB pentamer and two Neu5Acs, in which the two neighbouring toxin protomers were occupied. We chose two structures with lowest minimized energy for complex preparation. As the tip glycan of the triantennal N-glycan is the same as the tip of Neu5Ac, the two tips of the triantennal N-glycan were aligned to the tips of the two Neu5Acs by using the GS-align software package (www.glycanstructure.org/gsalign). The coordinates of the tips of the triantennal N-glycan were constrained during the following energy minimization. Determination and assignment of the correct glycan type based on its geometry and stereochemistry and generation of CHARMM inputs were performed using Glycan Reader30.
Molecular dynamics.
The N and C termini of the PltB homopentamer were charged as NH3+ and COO− groups, respectively. The conserved intradomain disulfide bonds were constructed. The systems were then solvated by TIP3P water molecules, and sodium and chlorides were added to neutralize the system and to achieve a total concentration of ~150 mM. The resulting solvated systems were energy-minimized for 5,000 conjugate gradient steps, where the protein and glycans were fixed and water molecules and counterions were allowed to move, followed by an additional 5,000 conjugate gradient steps, where all atoms were allowed to move. In the equilibration stage, each system was gradually relaxed by performing a series of dynamic cycles, in which the harmonic restraints on proteins and glycans were gradually removed to optimize the protein-water and protein-glycans interactions. In the production stage, all simulations were performed using the NPT ensemble at 310 K. All MD simulations were performed using NAMD software with the CHARMM36 force field. MD trajectories were saved every 2 ps for analysis. The C4 atoms of each glycan were used to measure the distance between glycans. To estimate the distance between the tips of those elongated glycans, we evaluated the length of all possible LacNAcs (Gal-GlcNAc) in the triantennal N-glycan and found that the length of LacNAc averaged ~9.1 Â. We then added an extra 9.1 Â to the distances depending on the number of LacNAc added (n = 1, 2, 3, 4, 5). Moreover, as the i and ii terminal Neu5Acs are equivalent (Fig. 3c; to demonstrate the distance between the terminal Neu5Acs, we labelled each as i, ii and iii), we measured the distance between i and ii, and between i/ii and iii (listed in the Fig. 3c table). A summary of all simulation systems is provided in Supplementary Table 4.
Glycan microarray and glycan ELISA.
A glycan microarray and glycan ELISA were performed in the laboratory of J.C. Paulson.
Glycan array.
Alexa Fluor-555-labelled typhoid toxin stock was diluted in 1 × PBS-T (50 μgml−1 final) and incubated on the array surface for 1 h at room temperature. Following sequential washes in PBS-T, PBS and sterile dH2O, bound toxin was detected using an Innoscan1100AL (Innopsys) fluorescent microarray scanner. Fluorescent signal intensity was measured using Mapix (Innopsys), and the mean intensity minus mean background of four replicate spots was calculated. A complete list of the glycans on the array is presented in Supplementary Table 5 and Supplementary Fig. 7.
Glycan ELISA.
Purified typhoid toxin was pre-complexed with anti-HIS mouse IgG (Invitrogen) and HRP-conjugated goat anti-mouse IgG (Pierce), then diluted in series, in PBS containing 1% BSA (wt/vol), to the required assay concentrations (40–0.05 μgml−1 final). Preparation of streptavidin-coated plates with biotinylated glycans, incubation, and washing of pre-complexed typhoid toxin dilutions was exactly as described previously26.
Statistical analysis.
P values were calculated using a two-tailed, unpaired Student’s t-test for two-group comparisons in GraphPad Prism (GraphPad software) unless otherwise specified. P values <0.05 were considered significant.
Supplementary Material
Acknowledgements
The authors thank J. E. Galan for his suggestion on the typhoid toxoid vaccination study, which was initiated while J.S. was at Yale, N. Nishimura for her comments on brain images, and X. Gao for plasmid pET21a-pltB-His6. This work was supported by a Cornell University Startup, PCCW Affinito-Stewart Award and the USDA/NIFA Hatch project 1010701 (to J.S.), the NIH, NCI, under contract no. HHSN261200800001E (to R.N.), the NIH, NIDCD intramural research programme (to J.Z.), and NIH R01 AI114730 to J.C.P. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
Y.-A.Y. conducted the experiments shown in Figs. 1, 2, 3a and 4d,g-k, Supplementary Figs. 1–6 and 9–11 and Supplementary Videos 1–10. S.L. conducted the experiments in Figs. 4a-c,e,f. J.Z. conducted the analyses and simulations in Figs. 3b-e. R.N. supervised the computational project. L.D. contributed to Fig. 3. A.J.T. conducted the experiments in Supplementary Fig. 8. R.M. conducted experiments in Fig. 3f and Supplementary Fig. 7. B.T. contributed to Supplementary Figs. 7 and 8. J.C.P. supervised the glycan microarray project. J.S. was involved in the design, interpretation and supervision of this study. Y.-A.Y. and J.S. wrote the paper and all the authors commented on the manuscript.
Competing interests
The authors declare no competing financial interests.
Life Sciences Reporting Summary. Further information on experimental design is available in the Life Sciences Reporting Summary.
Data availability. The data that support the findings of this study are available from the corresponding author upon request.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41564-017-0076-4.
Reprints and permissions information is available at www.nature.com/reprints.
Correspondence and requests for materials should be addressed to J.S.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dougan G & Baker S Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol 68, 317–336 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH & Ochiai RL Typhoid fever. Lancet 385, 1136–1145 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Crump JA & Mintz ED Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis 50, 241–246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump JA, Sjolund-Karlsson M, Gordon MA & Parry CM Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin. Microbiol. Rev 28, 901–937 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali G, Rashid S, Kamli MA, Shah PA & Allaqaband GQ Spectrum of neuropsychiatric complications in 791 cases of typhoid fever. Trop. Med.Int. Health 2, 314–318 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Lutterloh E et al. Multidrug-resistant typhoid fever with neurologic findings on the Malawi-Mozambique border. Clin. Infect. Dis 54, 1100–1106 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Sejvar J et al. Neurologic manifestations associated with an outbreak of typhoid fever, Malawi-Mozambique, 2009: an epidemiologic investigation. PLoS ONE 7, e46099 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo I et al. Neurologic complications and sequellae of infectious diseases in Uganda and Kenya: analysis of 288 cases from two rural hospitals. Neuro. Endocrinol. Lett 34, 28–31 (2013). [PubMed] [Google Scholar]
- 9.Song J, Gao X & Galan JE Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 499, 350–354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornick RB et al. Typhoid fever: pathogenesis and immunologic control. New Engl. J. Med 283, 686–691 (1970). [DOI] [PubMed] [Google Scholar]
- 11.Fraser A, Paul M, Goldberg E, Acosta CJ & Leibovici L Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine 25, 7848–7857 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Liang L et al. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci. Rep 3, 1043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles RC et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin. Vaccine Immunol. 17, 1188–1195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran Vu Thieu N et al. An evaluation of purified Salmonella Typhi protein antigens for the serological diagnosis of acute typhoid fever. J. Infect 75, 104–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng L et al. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell 159, 1290–1299 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou HH et al. Inactivation of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc. Natl Acad. Sci. USA 99, 11736–11741 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J et al. A mouse model for the human pathogen Salmonella Typhi. Cell Host Microbe 8, 369–376 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong A, Lee S, Yang YA & Song J The role of typhoid toxin in Salmonella Typhi virulence. Yale J. Biol. Med 90, 283–290 (2017). [PMC free article] [PubMed] [Google Scholar]
- 19.Haghjoo E & Galan JE Salmonella Typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl Acad. Sci. USA 101, 4614–4619 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spano S, Ugalde JE & Galan JE Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 3, 30–38 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Chang SJ, Song J & Galan JE Receptor-mediated sorting of typhoid toxin during its export from Salmonella Typhi-infected cells. Cell Host Microbe 20, 682–689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatcher JP et al. Development of SHIRPA to characterise the phenotype of gene-targeted mice. Behav. Brain Res 125, 43–47 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Rogers DC et al. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci. Lett 306, 89–92 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Rutenber E, Ready M & Robertus JD Structure and evolution of ricin B chain. Nature 326, 624–626 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Geisler C & Jarvis DL Effective glycoanalysis with Maackia amurensis lectins requires a clear understanding of their binding specificities. Glycobiology 21, 988–993 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng W et al. Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity. Cell Host Microbe 21, 23–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cryz SJ Jr et al. Construction and characterization of a Vi-positive variant of the Salmonella Typhi live oral vaccine strain Ty21a. Infect. Immun 57, 3863–3868 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galan JE & Curtiss R III Distribution of the invA, -B, -C, and -D genes of Salmonella typhimurium among other Salmonella serovars: invA mutants of Salmonella Typhi are deficient for entry into mammalian cells. Infect. Immun 59, 2901–2908 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biasini M et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo S, Song KC, Desaire H, MacKerell AD Jr, Glycan Im W. Reader: automated sugar identification and simulation preparation for carbohydrates and glycoproteins. J. Comput. Chem 32, 3135–3141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


